Abstract
Correlations between brain or brain component size and behavioral measures are frequently studied by comparing different animal species, which sometimes introduces variables that complicate interpretation in terms of brain function. Here, we have analyzed the brain composition of honey bees (Apis mellifera) that have been individually tested in an olfactory learning paradigm. We found that the total brain size correlated with the bees’ learning performance. Among different brain components, only the mushroom body, a structure known to be involved in learning and memory, showed a positive correlation with learning performance. In contrast, visual neuropils were relatively smaller in bees that performed better in the olfactory learning task, suggesting modality-specific behavioral specialization of individual bees. This idea is also supported by inter-individual differences in brain composition. Some slight yet statistically significant differences in the brain composition of European and Africanized honey bees are reported. Larger bees had larger brains, and by comparing brains of different sizes, we report isometric correlations for all brain components except for a small structure, the central body.
Keywords: Brain size, Mushroom body, Africanized bees
1. Introduction
Nervous systems generate and control behavior. More elaborate behaviors should therefore require more complex and/or larger brains or brain components. The best evidence supporting this idea comes from animals that show specialized and highly advanced sensory or motor skills supported by elaborate and enlarged brain components, of which there are many examples (Aboitiz, 1996), such as: echolocating bats feature an enlarged auditory cortex (Suga & Jen, 1976), weakly electric fish have giant cerebelli that process electro-sensory information (Nieuwenhuys & Nicholson, 1969), the vibrissae of rodents (Woosley & Van der Loos, 1970) or the nose appendages of star-nosed moles have enlarged representation in somatosensory cortex (Catania & Kaas, 1995), and songbirds have specialized forebrain areas analogous to primary auditory cortex of mammals that serve the production and learning of complex songs (reviewed by Brainard & Doupe, 2002).
Within most species, however, differences in brain composition and behavior are less conspicuous, and it is much harder to correlate particular brain components with an animal’s behavioral performance. While the size of a brain structure alone reveals little about its function, it can still be informative to compare total brain volumes (Deaner, Isler, Burkat, & Schaik, 2006; Jerison, 1973; Rensch, 1956) or the relative size of particular brain components with certain behaviors or behavioral repertoires across related species (in paper wasps: Molina, Harris, & O’Donnell, 2009). This approach has been used extensively across many vertebrate taxa, most notably to determine the contribution of brain or brain component size to the evolution of social behavior in primates (Dunbar, 2003, 2009; reviewed by Roth & Dicke, 2005) or corvid birds (reviewed by Emery & Clayton, 2004). Such comparative studies are at the core of the difficult question regarding the association between brain and intelligence. This is not only a controversial topic, but it is also fraught with the difficulty of measuring and ranking intelligence, or, more generally, behavioral complexity across different species. Does a given species solve a particular task better than another species because it is cognitively more advanced or ‘smarter’, or because the task is more appropriate for that species, given its ecological background?
This problem does not apply to the comparison of individuals of a single species. Behavioral performance across individuals of the same species raised in the same environment can be easily compared and represents natural variation within a population rather than ecological constraints affecting different species differently. Under these conditions, differences in behavioral performance can be correlated with differences in brain composition and may help in understanding the significance of particular brain components for certain behaviors. Honey bees seem particularly suited for this approach: within a colony, bee workers are highly related (they are all sisters from the same mother, although they may have different fathers). They are also reared in the same nest, thus having almost identical experiences until they leave the nest and start foraging.
Here, we focus on a particular learning behavior, olfactory proboscis extension conditioning, as a behavioral measure to compare individual bees. We ask the question: does performance in a simple associative learning paradigm correlate with some aspects of brain composition? Is the size of the antennal lobes (primary olfactory centers) or mushroom bodies (central brain structures involved in learning and memory; Erber, Masuhr, & Menzel, 1980; reviewed by Fahrbach, 2006; Strausfeld, Hansen, Li, Gomez, & Ito, 1998) associated with a bee’s odor learning performance? We analyze the brains of honey bees that have been the subject of a recent learning study (Couvillon, DeGrandi-Hoffman, & Gronenberg, 2010) and we describe differences in brain composition that correlate with the bees’ performance in the olfactory learning task. Olfactory proboscis extension conditioning is a standard paradigm (Bitterman, Menzel, Fietz, & Schäfer, 1983) that can be easily quantified. Bees learn to associate an odor stimulus with a sugar reward; they perform well in this paradigm, requiring only 1–5 learning trials on average (depending on odor and reward concentration; Getz & Smith, 1991; reviewed by Hammer & Menzel, 1995). This learning paradigm closely mimics the experience that bees have when landing on a flower: they perceive the flower’s odor as they start drinking the nectar, and they learn this association, presumably as it helps them to find more similarly rewarding flowers.
We also consider the effect of body size, a topic usually ignored in the majority of studies dealing with learning and memory in honey bees. While differences in body size are more pronounced in some other social bees [e.g. stingless bees Ramalho, Imperatriz-Fonseca, and Giannini (1998) or bumblebees Heinrich (1979)], honey bees do vary in body size, especially between subspecies, and this variation may contribute to task specialization (Riveros & Gronenberg, submitted for publication; Waddington, 1989) and is also the basis for discriminating European honey bees (Apis mellifera) from Africanized honey bees (A. mellifera scutellata hybrid; Sylvester & Rinderer, 1987). Does brain size correlate with body size in honey bees? And if so, do bees with larger brains perform better in learning tasks? We here report a correlation between brain and body size and, importantly, between brain size and learning performance. We also describe general correlations among the size of different brain components in the context of brain size, and differences in brain composition between European and Africanized honey bees.
2. Materials and methods
The current study focuses on brain morphometry of bees that have previously undergone behavioral experiments. Here, we just recapitulate the origin and handling of the bees as well as the behavioral procedures that have been described in more detail in the previous study (Couvillon et al., 2010).
2.1. Bees
Honey bees (A. mellifera) returning from foraging trips and not carrying pollen loads (presumed to be nectar foragers) were caught in front of their hives. European (EHB) and Africanized (AHB) bee colonies were raised and managed at the United States Department of Agriculture Carl Hayden Bee Laboratory in Tucson, Arizona. AHB colonies were established from local swarms, as all feral bees are Africanized in Southern Arizona (Rabe, Rosenstock, & Nielsen, 2005). EHB colonies were raised from queens imported from Hawaii where no Africanized bees exist. Bees were collected and tested on six different days between December and January from one EHB and one AHB colony per day (altogether six colonies each) to avoid colony specific effects or weather/time-related biases.
2.2. Behavior
Bees were briefly cooled on ice and harnessed in tubes with their proboscis (tongue) free to move. After recovery, bees were allowed to feed on 50% (w/w) sucrose solution to satiation and then to rest over night.
Bees were conditioned the following day. Olfactory conditioning followed established procedures (Bitterman et al., 1983; Giurfa, 2007): an air current carrying vapors of jasmine essential oil was presented for 7 s and the antennae were touched with sucrose solution 3 s after the onset of the still ongoing odor stimulus. Bees that spontaneously responded to the first odor presentation or that did not respond to the subsequent sucrose stimulus with a proboscis extension were discarded. The odor/sucrose conditioning sequence (training trial) was then repeated every 30 min for seven test trials. Bees that showed a proboscis extension during the first 3 s of a given odor presentation (before the sucrose stimulation) were identified as having learned the association in the previous conditioning trial. Memory was tested 24 h later by presenting just the odor stimulus and recording which bees showed the conditioned proboscis extension.
In the analysis and figures, learning performance corresponds to the number of positive responses (conditioned proboscis extensions) out of the seven test trials. Thus, a bee responding to every odor presentation had a score of 100%. Another measure used was the ‘speed’ of learning, which refers to the number of training trials required for a bee to show the first conditioned response to an odor stimulus.
2.3. Histology
After the behavioral experiments, bees were cooled to immobility and weighed on a balance (Scientech SA 80) to the nearest 0.1 mg to determine the total fresh weight and the fresh weight of the combined head and thorax, a particularly well suited measure to discriminate European from Africanized honey bee colonies according to the “FABIS” test (“Fast Africanized Bee Identification System”; Sylvester & Rinderer, 1987). The head width (maximum distance between the outer edges of the left and right compound eye) was measured using digital calipers under a microscope. The bee heads were fixed in alcoholic Bouin’s fixative overnight and then rinsed and stored in 70% ethanol until further processing.
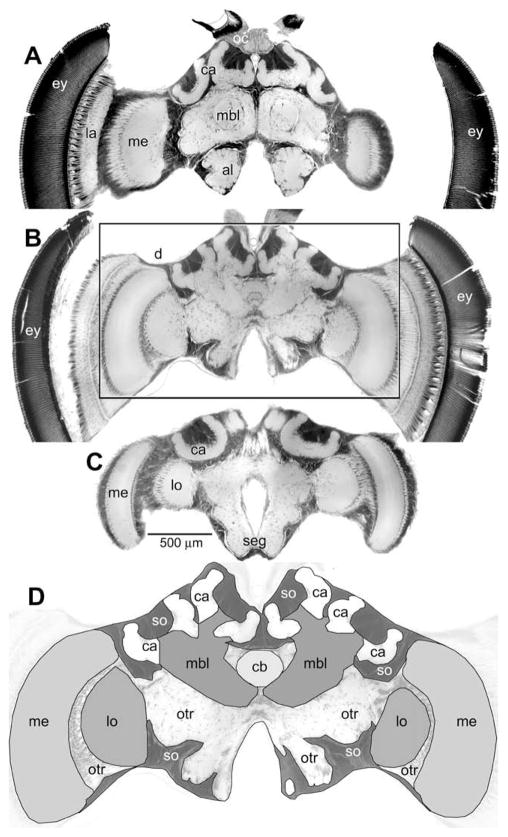
Heads were embedded in wax, opened frontally and the brains dissected under 50% ethanol, then transferred to water and block-stained in toluidine blue (1% toluidine blue in 1% aqueous borax solution) overnight at room temperature on a rotator. The staining was then differentiated in water for 3–6 h, transferred to 50% ethanol and then dehydrated in acidified 2,2-dimethoxypropane (Thorpe & Harvey, 1979), embedded in Spurr’s low viscosity embedding medium (Electron Microscopy Science; Hatfield, PA) and polymerized at 70 °C. Brains were sectioned on a sliding microtome at 20 μm thickness, mounted and cover-slipped. Three such sections representing different antero-posterior depths are shown in Fig. 1.
Fig. 1.
Photomicrographs of a bee brain. Three sections taken at different depth from the frontal brain surface: 100 μm (A), 300 μm (B) and 480 μm (C) showing the different brain components examined. The area boxed in (B) is enlarged in (D) outlining the different brain structures and highlighting the regions measured in the study. al, antennal lobe; ca, mushroom body calyx; cb, central body; lo, lobula; me, medulla; mbl, mushroom body lobe (includes vertical lobe, medial lobe and peduncle); otr, other brain neuropils (including the subesophageal ganglion (seg)); so, somata regions; the compound eye (ey), the ocelli and ocellar neuropil (oc) and the lamina (la) were not included in the analysis; scale bar applies to (A–C).
2.4. Morphometry
Outlines of the brains and brain components were traced on paper from the sections using a projection microscope (Ken-A-Vision, Kansas City, MO) at 100× overall magnification. Using a flatbed scanner, drawings were then scanned to a computer and respective areas of the digitized images were measured using the Photoshop (Adobe) pixel counting routine. Brain volumes were calculated from the area measurements multiplied by the section thickness. Every second section was thus measured, probing the brains at 40 μm intervals. Controls (Mares, Ash, & Gronenberg, 2005) have shown this method to be well below the error mark of 5%, deemed acceptable in other studies on honey bee brains (Fahrbach, Giray, & Robinson, 1995; Withers, Fahrbach, & Robinson, 1995).
The volumes of each brain (including the suboesophageal ganglion, but excluding the retina and lamina; Fig. 1D) and its components (antennal lobes, medulla, lobula, mushroom body, central body; see Fig. 1) were thus measured. For some calculations, we combined the volume of the medulla and lobula and referred to is as the ‘optic lobes’, even though this measure does not comprise the lamina. The mushroom body is composed of a medial and a lateral calyx, which were measured separately, and a peduncle and lobes, which were not discriminated and are here jointly referred to as the mushroom body lobe (Fig. 1A and D). The brain also comprises additional central neuropil that is not as conspicuously compartmentalized as the other brain neuropils mentioned above. It is here referred to as ‘other’ neuropil and also includes the subesophageal ganglion and fiber tracts (Fig. 1C and D). In addition, the volume occupied by cell bodies (somata in Fig. 1D), which are rendered dark in Fig. 1, was measured. Relative volumes of brain components were calculated dividing the respective volume (e.g., the medulla volume) by the overall brain volume. The learning performance as well as the brain composition was measured in a total of 67 EHB and 54 AHB.
2.5. Data analysis
European and Africanized bees were compared using Student’s t-test. Multivariate outliers were determined using Jackknife distances based on the different brain components. This procedure led to normal distributions of all variables (based on Shapiro–Wilk Test). Univariate comparisons were then conducted using two-tailed Student’s t-tests. All statistical analyses were performed using JMP v 7.0 (SAS Institute, Inc., Cary, NC). Type one errors (false positives) associated with multiple comparisons were corrected for using the false discovery rate control procedure (Benjamini & Hochberg, 1995). This procedure reduces false negatives, a common problem with procedures such as Bonferroni correction (Verhoeven, Simonsen, & McIntyre, 2005). The respective values are here referred to as “FDR α” (false discovery rate).
3. Results
3.1. Correlation with body size
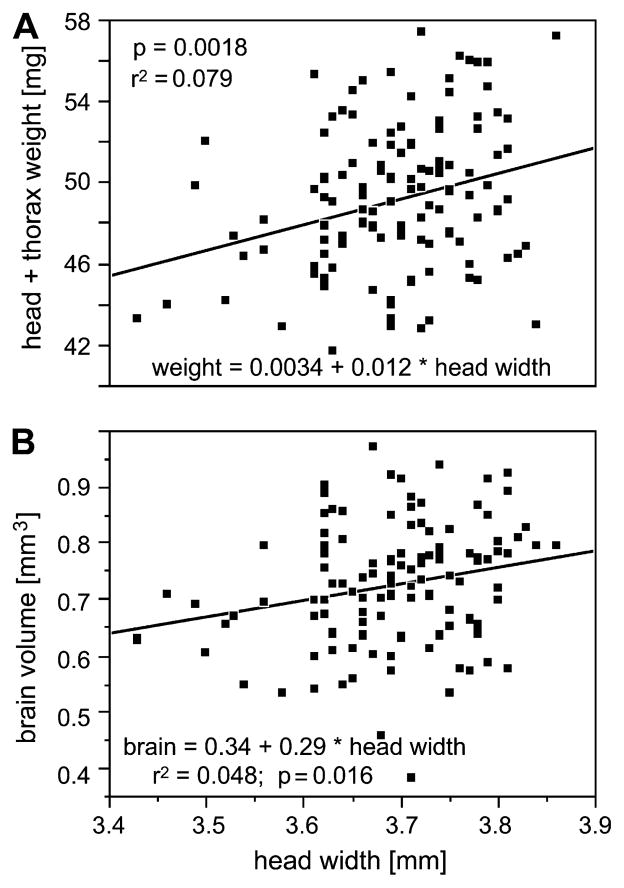
Compared to other social bees such as bumblebees, honey bees do not differ much in body size. However, some size variation does exist in honey bees (Waddington, 1989). In our sample (N = 121 bees; combining EHB and AHB), we found the weight (head plus thorax) to vary by about 26% (42–57 mg) and head width by about 12% (3.42–3.87 mm). The correlation between head width and head plus thorax weight was highly significant (p = 0.002; FDR α = 0.004), although confounded by considerable variance (r2 = 0.08; Fig. 2A). The correlation between total body weight and head width was not significant as the amount of nectar the bees carried in their crop, hence the weight of the abdomen, varied considerably (overall body weight 79–175 mg).
Fig. 2.
Correlations between the bees’ head widths and their body weights (head and thorax weight) (A) and their total brain volumes, respectively (B); N = 121.
We found a significant correlation between head width and brain volume (p = 0.016; FDR α = 0.016; r2 = 0.05; Fig. 2B), but not between brain volume and other measures of size (body weight or head plus thorax weight). The finding that brain size does not vary randomly but correlates with head width may seem trivial as the brain fills the entire width of the head. However, this correlation of brain and head size has not been previously described, and the brain size variation is the basis for the subsequent sections.
3.2. Correlations among brain components
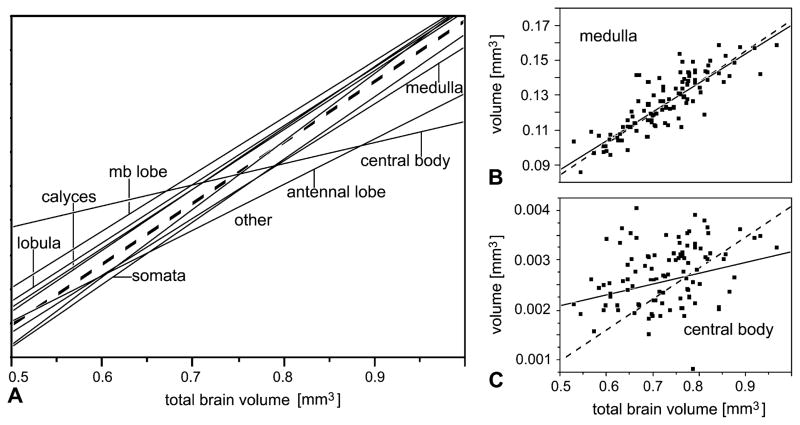
As expected, the absolute volumes of all examined brain components were highly correlated: the larger a given brain, the larger were its components. Most of the brain components (medulla, lobula, antennal lobe, mushroom body components, the cell body layers around the brain neuropil and the less compartmentalized ‘other’ brain neuropil) scaled isometrically with the entire brain volume. This is indicated by the slopes of regression lines in Fig. 3A and B, which are close to 1 (broken lines) and by the actual slope values and the confidence intervals in Table 1. Only the central body (Fig. 3C) had a lower slope (0.56) with a confidence interval not approaching 1.0 (Table 1), suggesting that bees with larger brains do not require a corresponding increase in central body size.
Fig. 3.
Correlations between brain components (absolute volumes). (A) shows the slopes of the linear correlations for the different brain components (y-axis not given; it differs for each component). Note almost isometrical increase of most brain components with total brain size, except for the central body. Detailed graphs including data points are given for the medulla (B) and central body (C) (note almost 50-fold difference in y-axis). Data describing these correlations are given in Table 1. Broken lines indicate true isometric correlation (slope = 1). mb, mushroom body; somata, the volume occupied by cell bodies; other, neuropil other than optic and antennal lobes, mushroom and central body. N = 101.
Table 1.
Correlations of individual brain components with total brain volume; slope of linear fits (log–log transformed) in bold type; N = 59 EHB; 42 AHB; after correction for false discovery rate, all α values are below 0.001 (not shown).
| Brain component | Bivariate fit | 95% Confidence | r2 | p |
|---|---|---|---|---|
| Cell body regions | log(cell body) = −1.17 + 1.10 × log(brain) | 0.98–1.22 | 0.77 | <0.0001 |
| Other neuropil | log(other) = −1.33 + 0.99 × log(brain) | 0.89–1.09 | 0.79 | <0.0001 |
| Medulla | log(medulla) = −1.77 + 0.96 × log(brain) | 0.85–1.08 | 0.73 | <0.0001 |
| Lobula | log(lobula) = −2.89 + 0.99 × log(brain) | 0.83–1.15 | 0.62 | <0.0001 |
| MB total | log(MB) = −1.87 + 0.92 × log(brain) | 0.83–1.02 | 0.79 | <0.0001 |
| Medial calyx | log(med cal) = −3.14 + 0.91 × log(brain) | 0.79–1.04 | 0.68 | <0.0001 |
| Lateral calyx | log(lat cal) = −3.09 + 0.95 × log(brain) | 0.8–1.09 | 0.63 | <0.0001 |
| MB lobes | log(MB lobe) = −2.71 + 0.92 × log(brain) | 0.74–1.11 | 0.50 | <0.0001 |
| Antennal lobe | log(ant lobe) = −3.25 + 0.96 × log(brain) | 0.76–1.15 | 0.48 | <0.0001 |
| Central body | log(central body) = −5.76 + 0.56 × log(brain) | 0.29–0.82 | 0.13 | <0.0001 |
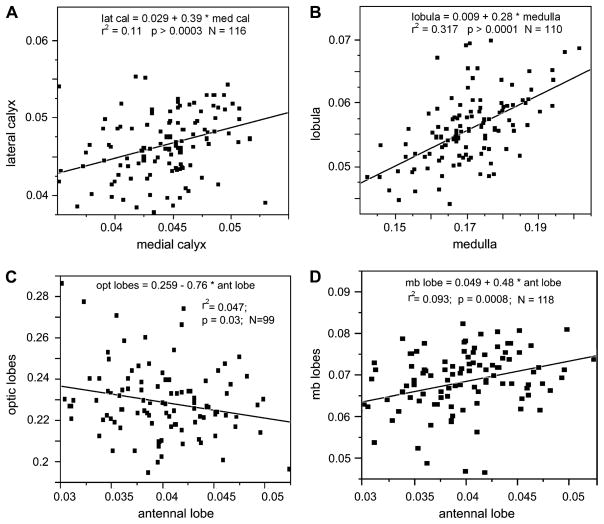
More interesting from a functional point of view are correlations among the relative volumes of individual brain components. For instance, one might expect functionally closely related brain components to correlate in their relative size. However, we found statistically significant correlations only between four pairs of structures (Fig. 4):
Fig. 4.
Correlations between pairs of brain components. The two mushroom body calyces (A), the two optic lobes (B) and the combination of mushroom body lobes and antennal lobes (D) show significant correlations when comparing their relative size. The combined volume of medulla and lobula (‘optic lobes’) correlates negatively with the relative volume of the antennal lobes (C).
The median and lateral mushroom body calyces, which are morphologically very similar and generally considered identical in function, strongly correlated with each other in relative size (p = 0.0003; FDR α = 0.0006; r2 = 0.11; N = 116; Fig. 4A).
Likewise, the medulla and lobula strongly correlated in relative size (p < 0.0001; FDR α = 0.0001; r2 = 0.32; N = 110; Fig. 4B). This was to be expected as they share several classes of neurons and most of the information processed by the lobula is derived from the medulla.
A third significant correlation was less expected: the mushroom body lobes (but not the calyces) significantly correlated with the relative size of the antennal lobes (p = 0.0008; FDR α = 0.0024; r2 = 0.09; N = 118; Fig. 4D) even though the former do not receive direct input from the antennal lobes.
Trends were found for the relative lobula (p = 0.1) and medulla (p = 0.08) to negatively correlate with the relative antennal lobe volume. When combining the volumes of lobula and medulla (here referred to as the “optic lobes”), a significant negative correlation was found between the relative optic lobe and antennal lobe volume (p = 0.03; r2 = 0.05; N = 99; Fig. 4C). This suggests a certain trade-off between visual and olfactory processing: bees with larger optic lobes appear to have relatively smaller antennal lobes and vice versa. However, when correcting for multiple comparisons, this trend was not significant (FDR α = 0.12).
We did not find significant correlations between the relative sizes of any of the other brain components, considering all possible permutations. This is interesting, as one might have expected correlations between the mushroom body calyces and their sensory input regions (optic lobes and antennal lobes) or between the mushroom body calyces and mushroom body lobes, as the same neurons (Kenyon cells) give rise to both structures (the calyces comprising the Kenyon cells’ dendrites and the lobes comprising their axons).
3.3. European vs. Africanized bees
We found European honey bees to be significantly heavier when comparing the combined head and thorax weight (Fig. 5A). When considering the entire body weight, there was a trend (p = 0.08) for EHB to be heavier than AHB, but this was not statistically significant, probably because of the large variation in their crop filling status (see above). EHB also had larger heads compared to AHB (Fig. 5B). As EHB and AHB are discriminated by body size – related morphological measures (Sylvester & Rinderer, 1987), these data confirm that our samples are representative of EHB and AHB in general. We did not find any significant differences regarding the brain volumes of EHB and AHB (Fig. 5D). Interestingly, we found differences in the learning performance of EHB and AHB that were more pronounced than the morphological differences (compare p values in Fig. 5C with those of Fig. 5A and B). These differences have been reported and discussed in more detail in the previous study (Couvillon et al., 2010).
Fig. 5.
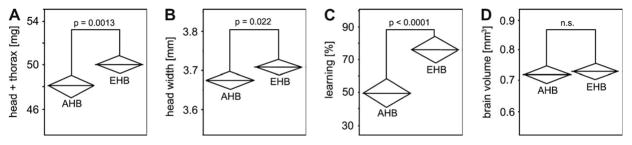
Comparison of head plus thorax weight (A), head width (B), learning performance (C) and total brain volume (D) of European bees (EHB; N = 64) and Africanized bees (AHB; N = 57). Diamonds indicate means and 95% confidence intervals; statistically significant differences (t-test) are indicated.
Some statistically significant differences were also found when comparing the brain composition of EHB and AHB (Table 2): cell bodies occupied more volume in EHB than in AHB in relative terms (t = 3.02; p = 0.003; FDR α = 0.003), and EHB had relatively less volume occupied by the lobula (t = 2.63; p = 0.01; FDR α = 0.02) and by the less compartmentalized ‘other’ neuropil (t = 2.45; p = 0.016; FDR α = 0.05).
Table 2.
Differences between the fastest learners (requiring only one training trial) and slower learners (requiring 2–7 trials) regarding different body and brain components. Significant differences after correction for false discovery rate (t-test) in boldface; N = 94.
| Body weight | Head + thor. weight | Cell body (rel.) | Medulla (rel.) | Lobula (abs.) | Lobula (rel.) | Antennal lobe (rel.) | |
|---|---|---|---|---|---|---|---|
| t | 2.45 | 1.95 | 3.24 | 2.18 | 2.68 | 4.45 | 1.96 |
| p | 0.016 | 0.054 | 0.002 | 0.032 | 0.0087 | 0.0001 | 0.053 |
| FDR, α | 0.016 | 0.006 | 0.02 | 0.02 |
3.4. Correlations between learning performance and brain composition
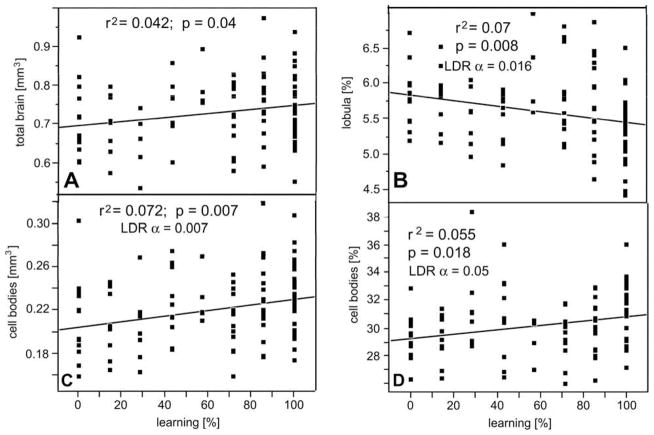
Having described size- and race-related sources of variation in brain composition, we can now address the potential effect of brain composition on learning performance. Brain and brain component volumes were compared with the respective bees’ learning scores (Fig. 6) as published in the previous study (Couvillon et al., 2010). We found a significant correlation between the overall brain size and the number of correct (learned) proboscis extension responses (Fig. 6A). This correlation is not a consequence of the fact that EHB, which were larger and had larger brains, were better learners overall as we also found a significant correlation between brain size and learning when comparing AHB alone (% learning = −56.8 + 144.3 × total brain; r2 = 0.11; p = 0.03; N = 41). Likewise, the absolute and relative portion of the brain volume occupied by cell bodies correlated with learning performance (Fig. 6C and D). In contrast, negative correlations with learning performance were found for the relative volume of the lobula (Fig. 6B). Note that these statistical relationships are weak, even if highly significant. Correlations of learning performance with other brain components were not significant after correction for false discovery rate.
Fig. 6.
Correlation between brain (A) or brain component size (B–D) and the learning performance of all bees excluding outliers (N = 101). Volumes are absolute (A and C) or relative to total brain volume (B and D). Linear fits (lines), r2, p and α values (corrected for multiple comparisons) indicated for each brain component. Abscissa represents the percentage of correct (learned) proboscis extensions in response to odor presentations (0%, no learning; 100%, response to all odor presentations).
We also compared the brain composition with learning performance separately for EHB and AHB. However, in our entire sample we only had two EHBs that did not learn the odor association during the seven training cycles, hence learning performance was skewed for EHB. Perhaps for that reason we did not find any significant correlation between brain composition and learning in EHB. We only found a trend for the relative lobula volume of EHB to be smaller in better learners (% learned responses = 150.9–1382 × relative lobula volume; r2 = 0.054; p = 0.076; N = 59). This same correlation was significant when not excluding the outliers or when comparing all bees (EHB and AHB combined; see above), but a similar trend was not apparent in AHB.
In contrast to EHB, we found correlations between olfactory learning performance and the size of brain components in AHB, where our sample included more non-learners and was thus less skewed towards learners. Significant correlations existed for the total brain volume (r2 = 0.11; F = 5.1; p = 0.03) and for the absolute volume of the mushroom bodies (r2 = 0.19; F = 9.21; p = 0.004; FDR α = 0.008) and the mushroom body calyces in particular (r2 = 0.21; F = 10.9; p = 0.002; FDR α = 0.002). More importantly, in EHB, the mushroom body calyces were the only brain components whose relative volume showed a significant correlation with olfactory learning after controlling for multiple comparisons (r2 = 0.14; F = 6.34; p = 0.016; FDR α = 0.05). Hence the mushroom body calyces seem to correlate with learning performance beyond the general tendency of the learning performance to increase with brain size (Fig. 6A).
In addition to using the cumulative number of correct (learned) odor responses, we also compared the brain component volumes with the number of trials it took a bee to associate odor and sucrose reward (learning speed). Using this measure and combining EHB and AHB, we found significant correlations between learning speed and relative cell body volume (number of trials required for learning = 7.73–18.4 × relative cell body volume; r2 = 0.069; p = 0.01; FDR α = 0.02; N = 94) and between learning speed and the relative lobula volume (number of trials required for learning = −3.14 + 95.3 × relative lobula volume; r2 = 0.107; p = 0.001; FDR α = 0.001; N = 94). Other correlations were not significant.
We also compared the brains of the fastest learners (which showed a conditioned response after a single pairing of odor and sucrose reward) with those of all other learners (bees that required 2–7 sucrose/odor pairings to associate the stimuli) and found significant differences (t-test; Table 2): the fastest learners were heavier, had larger relative cell body volume and smaller absolute and relative lobula volumes than slower learners.
In addition, we compared the brains in the context of memory (N = 94). Among the bees that had learned, those that remembered the odor/sucrose association after 24 h differed in two respects from those bees that did not: they had larger cell body volumes in absolute terms (t = 2.74; p = 0.007; FDR α = 0.007) and relative terms (t = 2.78; p = 0.007; FDR α = 0.004) and their lobula were relatively smaller compared to the bees that did not show memory after 24 h (t = 2.99; p = 0.004; FDR α = 0.012).
4. Discussion
Here we will focus on those findings that we consider most important and interesting in terms of their functional implications: relationships between body and brain size and interrelations between brain components and, most importantly, associations between brain or brain component size and learning performance. We will not try to make functional sense of every correlation that we found. Too little is known regarding physiological and other differences between EHB and AHB to interpret the enlargement or reduction of a particular brain component in one race or the other. Moreover, the fact that our EHB sample only contained two non-learners makes it difficult to compare the two races, even though some of the differences in brain composition are statistically significant.
4.1. Body and brain size
We found considerable differences in body size among the bees of our sample (Fig. 2A), as one would expect for almost any animal species. AHBs were significantly smaller than EHBs in our sample, which was previously reported for much larger samples (Sylvester & Rinderer, 1987). In behavioral as well as in neurobiological studies on honey bees, individual size differences are generally neglected. An exception is the work by Waddington (1989), who found that honey bee workers tend to recruit other workers of the same body size, and a recent study by Riveros and Gronenberg (submitted for publication) who tried to establish a connection between body size-related traits and foraging preferences. The dependence of task allocation on body size (Kapustjanskij, Streinzer, Paulus, & Spaethe, 2007), and of learning behavior in particular (Worden, Skemp, & Papaj, 2005), has been best studied in bumblebees, where body size variation within a colony is much more pronounced (Heinrich, 1979). Our findings show that body size should be taken into account by future learning studies not only in bumblebees, but in honey bees, too, as it might explain some of the inter-individual variation in learning performance.
Importantly, we found substantial variation in brain size, which was not random but correlated with body size (in particular, head width; Fig. 2B). This may seem obvious, but such a correlation has not been previously established (an earlier study by Mares et al., 2005, did not find a significant correlation, probably because of a much smaller sample size). This correlation between body and brain size may be a major reason for the behavioral differences associated with body size of bumblebees mentioned above (Worden et al., 2005; Kapustjanksij et al., 2007). Larger bees have, on average, larger brains, which may allow them to surpass their smaller nestmates in certain tasks presumably requiring more complex neural processing, such as orientation or learning and memory.
A case in point is our finding that bees with larger brains performed better in the olfactory learning paradigm (Fig. 6A). This supports the general idea that larger brains facilitate more complex behavior (Deaner et al., 2006; Jerison, 1973; Rensch, 1956) and may thus seem trivial. However, the idea that larger brains enable more complex behavior is derived from studies comparing different species rather than individuals of the same species. In this respect, our result seems very interesting indeed, as it allows linking a single, well defined behavioral capacity (learning an olfactory association) with overall brain size. As mentioned in the Introduction, many cases are known where a particular behavioral capacity (e.g. the ability of bats to use ultrasound for echolocation; reviewed by Pollak & Casseday, 1989) manifests itself in the enlargement of the particular brain region controlling this behavior.
However, the current findings show that beyond these explicit relationships, the overall size of a brain can have very specific effects on the performance of individual behaviors. We would expect to find similar correlations between brain size and behavioral performance when testing other paradigms, such as visual learning or orientation in honey bees (reviewed by Menzel & Giurfa, 2006). While we tested only a very specific aspect of bee behavior, we assume that having a larger brain would profit many advanced behaviors in the same way that it seems to support olfactory learning, thus yielding a high payoff for the increased metabolic costs that a larger brain incurs (which may be substantial; Laughlin, 2001). The analogous question whether individual brain size correlates with intelligence in humans has been the topic of scientific debate for more than 150 years, and recent meta-analyses of large data samples suggest that the correlation is indeed significant (McDaniel, 2005). We have nothing to contribute to this controversial topic, but at least in insects, individual brain size seems to have implications for behavioral performance.
4.2. Brain component size
Most brain components seem to increase in size isometrically (proportionally) with overall brain volume (Fig. 3), suggesting that larger brains have basically the same composition than smaller ones. There is one exception to this trend: the central body shows allometric growth; it increases in size more slowly than the overall brain volume (Fig. 3 and Table 1). The same has been found to be the case in bumblebees, where the range of brain sizes is much larger than in honey bees (Mares et al., 2005). The central body is supposed to be involved in the control of walking (Strauss, 2002) and more complex motor control in general (Strausfeld, 1999). However, other studies suggest an involvement of the central body in certain aspects of visual processing (Heinze, Gotthardt, & Homberg, 2009; Homberg, 2008), or formation of memory for visual patterns (Liu et al., 2006). The assumed function of the central body as a motor control center might explain why in larger bees the central body is relatively smaller than in small ones: motor functions are probably less affected by body size. Large and small bees have the same number of muscles and motor neurons. Hence, the control of movements should not require substantially more neurons in a larger bee compared to a small one. In contrast, it does require integration of more sensory input originating from more photoreceptors in the larger eyes (Spaethe & Chittka, 2003) or more mechanoreceptors from the larger body surface and more chemo-receptors from the larger antennae (Spaethe, Brockmann, Halbig, & Tautz, 2007). This increased amount of sensory information is one of the reasons why the total brain volume should increase in larger bees.
4.3. Correlations among brain components
The absolute sizes of all brain components correlate with each other, which is trivial: the bigger a brain is, the larger are all of its components. More interesting are correlations of the brain components’ relative sizes. As previously mentioned, positive correlations between the two calyces (Fig. 4A) or between the optic lobes medulla and lobula (Fig. 4B) are to be expected. One might also have expected correlations between the relative size of the calyces and the optic lobes or the antennal lobes, as the calyces receive massive input from both sensory structures, but our data set may not have been large enough to reveal such connections. Instead, we found a significant correlation between the volume of the antennal lobe and the mushroom body lobes, which was unexpected as no direct connections exist between these structures. We have no explanation for this finding, other than that in general the mushroom bodies are heavily involved in olfactory information processing in most insects and for relating contextual and multi-modal information to odor signals (reviewed by Strausfeld et al., 1998).
Of particular interest is the negative correlation (highly significant, although not very strong) that we found between the relative volume of the optic and antennal lobes (Fig. 4C). Irrespective of brain or body size, bees that have larger visual centers seem to have relatively smaller olfactory centers, and vice versa. This suggests that a certain degree of sensory specialization might exist among individual honey bees. Some bees might have advanced visual capabilities (such as temporal or spatial resolution, color discrimination, etc.), while others may more invest in odor sensitivity or discrimination. This is interesting in the context of division of labor in social insects. The response threshold hypothesis (Pankiw & Page, 2000; reviewed by Fewell & Beshers, 2001) suggests that bees perform particular tasks according to certain thresholds. Our findings would support this hypothesis well: depending on their respective sensory ‘preference’, individual bees would have different thresholds for certain tasks, which would help to establish division of labor within a colony. These same sensory ‘preferences’ might also affect learning and memory and one might expect that some bees perform better in visual learning paradigms while others might score higher in olfactory tests. However, behavioral evidence from honey bees also indicates that the sensitivities for different sensory modalities (sucrose, odor, light and touch) are correlated (Erber, Hoormann, & Scheiner, 2006; Scheiner, Page, & Erber, 2001), suggesting that bees particularly sensitive to sucrose are also more light and odor sensitive. Our idea that individual bees may have sensory preferences is only based on the morphometric data presented here, but it might encourage further behavioral studies testing individual bees with different sensory modalities.
4.4. Correlations with learning
As discussed above, the most striking finding is probably that learning performance is reflected by overall brain size. But there is also an interesting correlation between learning performance and the size of the mushroom body, and the calyces in particular. These were only found in AHB, probably because we had almost no ‘non-learners’ among the EHB. The fact that the mushroom body and its calyces showed a strong correlation with learning performance is reassuring, as it is the brain structure that has been shown to be involved in learning and memory in many other studies (in honey bees using lesion and developmental ablation as well as electrophysiological techniques and neurochemical treatments; e.g. Erber et al., 1980; Hammer & Menzel, 1998; Komischke, Sandoz, Malun, & Giurfa, 2005; Mauelshagen, 1993; in Drosophila using many different genetic and molecular strategies; reviewed by Davis, 2005; Heisenberg, 2003).
Mushroom body calyces have been shown to increase in size in the context of foraging: a certain increase seems to be a prerequisite for bees to be able to forage (Fahrbach, Moore, Capaldi, Farris, & Robinson, 1998), and an additional increase in calyx size is a consequence of foraging experience (Durst, Eichmüller, & Menzel, 1994; Farris, Robinson, & Fahrbach, 2001; Withers, Fahrbach, & Robinson, 1993). However, to our knowledge, this is the first time that mushroom body size has been linked to a quantifiable measure of learning in ‘normal’ insects (as opposed to genetically modified or otherwise ‘handicapped’ insects). While mere volumetric findings do not contribute much to our understanding of the neuronal processing in the mushroom body, this is another strong indication for its involvement in associative learning.
Interestingly, we found a negative relationship between olfactory learning performance and the size of the optic lobes (Fig. 6B and Table 2): better learners have smaller optic lobes. We think that this correlation represents the finding that we have discussed above: that antennal lobes and optic lobes have an inverse relationship (Fig. 4C). Bees are not better learners because they have smaller optic lobes; rather, we think that those bees that performed best in our olfactory learning task were bees that had invested more in olfactory processing (developmentally and metabolically speaking). Because of their relatively reduced visual processing capacities we would expect these bees to perform worse in visual learning tasks. By the same token, we would expect to find a positive correlation between optic lobe size and learning performance (and a negative correlation with antennal lobe size) in a visual learning paradigm.
In summary, our findings confirm the idea that absolute brain size contributes strongly to differences in individual cognitive abilities; that the mushroom bodies are important brain centers for learning in insects; and that advanced social insects such as honey bees might show individual sensory biases based on their brain composition.
Acknowledgments
We thank Gloria DeGrandi-Hoffman for her advice regarding Africanized honey bees and for providing the bees, Andre Riveros for advice and help with the conditioning experiments and statistical analysis, him and two anonymous reviewers for helpful suggestions on the manuscript, and Elizabeth Collier, Jennifer Heller, Allie Moriarty and Alexandra Stepczynski for help with sectioning and tracing the brains. This work was supported by grants from NSF (IOB 0519483 to W.G.) and from NIH [Postdoctoral Excellence in Research and Teaching (PERT) fellowship] to M.J.C.
References
- Aboitiz F. Does bigger mean better? Evolutionary determinants of brain size and structure. Brain Behavior and Evolution. 1996;47:225–245. doi: 10.1159/000113243. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;57:289–300. [Google Scholar]
- Bitterman M, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) Journal of Comparative Psychology. 1983;97:107–119. [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Catania KC, Kaas JH. Organization of the somatosensory cortex of the star-nosed mole. Journal of Comparative Neurology. 1995;351:549–567. doi: 10.1002/cne.903510406. [DOI] [PubMed] [Google Scholar]
- Couvillon MJ, DeGrandi-Hoffman G, Gronenberg W. Africanized honey bees are slower learners than their European counterparts. Naturwissenschaften. 2010 doi: 10.1007/s00114-009-0621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annual Reviews of Neuroscience. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Isler K, Burkat J, Schaik CV. Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain, Behavior and Evolution. 2006;70:115–124. doi: 10.1159/000102973. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain: Mind, language, and society in evolutionary perspective. Annual Reviews of Anthropoogy. 2003;32:163–181. [Google Scholar]
- Dunbar RIM. Darwin and the ghost of Phineas Gage: Neuro-evolution and the social brain. Cortex. 2009;45:1119–1125. doi: 10.1016/j.cortex.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Durst C, Eichmüller S, Menzel R. Development and experience lead to increased volume of subcompartments of the honey bee mushroom body. Behavioral and Neural Biology. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Clayton NS. The mentality of crows: Convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–1907. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- Erber J, Hoormann J, Scheiner R. Phototactic behaviour correlates with gustatory responsiveness in honey bees (Apis mellifera L.) Behavioral Brain Research. 2006;174:174–180. doi: 10.1016/j.bbr.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Erber J, Masuhr T, Menzel R. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiological Entomology. 1980;5:343–358. [Google Scholar]
- Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annual Reviews of Entomology. 2006;51:209–232. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Giray T, Robinson GE. Volume changes in the mushroom bodies of adult honeybee queens. Neurobiology of Learning and Memory. 1995;63:181–191. doi: 10.1006/nlme.1995.1019. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Moore D, Capaldi EA, Farris SM, Robinson GE. Experience-expectant plasticity in the mushroom bodies of the honeybee. Learning and Memory. 1998;5:115–123. [PMC free article] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Fahrbach SE. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honey bee. Journal of Neuroscience. 2001;21:6395–6404. doi: 10.1523/JNEUROSCI.21-16-06395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JH, Beshers SN. Models of division of labor in social insects. Annual Reviews of Entomology. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Getz WM, Smith KB. Olfactory perception in honeybees: Concatenated and mixed odorant stimuli, concentration, and exposure effects. Journal of Comparative Physiology A. 1991;169:215–230. [Google Scholar]
- Giurfa M. Behavioral and neural analysis of associative learning in the honeybee: A taste from the magic well. Journal Comparative Physiology A. 2007;193:801–824. doi: 10.1007/s00359-007-0235-9. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Learning and memory in the Honeybee. Journal of Neuroscience. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local microinjections of octopamine in honeybees. Learning and Memory. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- Heinrich B. Bumblebee economics. Cambridge, MA: Harvard University Press; 1979. p. 245. [Google Scholar]
- Heinze S, Gotthardt S, Homberg U. Transformation of polarized light information in the central complex of the locust. Journal of Neuroscience. 2009;29:11783–11793. doi: 10.1523/JNEUROSCI.1870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: From maps to models. Nature Review Neuroscience. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Homberg U. Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Structure and Development. 2008;37:347–362. doi: 10.1016/j.asd.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of the brain and intelligence. New York: Academic Press; 1973. [Google Scholar]
- Kapustjanskij A, Streinzer M, Paulus HF, Spaethe J. Bigger is better: Implications of body size for flight ability under different light conditions and the evolution of alloethism in bumblebees. Functional Ecology. 2007;21:1130–1136. [Google Scholar]
- Komischke B, Sandoz JC, Malun D, Giurfa M. Partial unilateral lesions of the mushroom bodies affect olfactory learning in honeybees Apis mellifera L. European Journal of Neuroscience. 2005;21:477–485. doi: 10.1111/j.1460-9568.2005.03879.x. [DOI] [PubMed] [Google Scholar]
- Laughlin SB. Energy as a constraint on the coding and processing of sensory information. Current Opinion in Neurobiology. 2001;11:475–480. doi: 10.1016/s0959-4388(00)00237-3. [DOI] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- Mares S, Ash L, Gronenberg W. Brain allometry in bumblebee and honeybee workers. Brain, Behavior, Evolution. 2005;66:50–61. doi: 10.1159/000085047. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J. Neural correlates of olfactory learning in an identified neuron in the honey bee brain. Journal of Neurophysiology. 1993;69:609–625. doi: 10.1152/jn.1993.69.2.609. [DOI] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Menzel R, Giurfa M. Dimensions of cognition in an insect, the honeybee. Behavioral and Cognitive Neuroscience Reviews. 2006;5:24–40. doi: 10.1177/1534582306289522. [DOI] [PubMed] [Google Scholar]
- Molina Y, Harris RM, O’Donnell S. Brain organization mirrors caste differences, colony founding and nest architecture in paper wasps (Hymenoptera: Vespidae) Proceedings of the Royal Society B. 2009;276:3345–3351. doi: 10.1098/rspb.2009.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Nicholson C. A survey of the general morphology, the fiber connections and the possible functional significance of the gigantocerebellum of mormyrid fishes. In: Llinás R, editor. Neurobiology of cerebellar evolution and development. American Medical Association; 1969. pp. 107–134. [Google Scholar]
- Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honeybees. Behavioral Ecology and Sociobiology. 2000;47:265–267. [Google Scholar]
- Pollak GD, Casseday JH. Zoophysiology. Vol. 25. Berlin: Springer; 1989. The neural basis of echolocation in bats. [Google Scholar]
- Rabe MJ, Rosenstock SS, Nielsen DI. Feral Africanized honey bees (Apis mellifera) in Sonoran Desert habitats of southwestern Arizona. Southwestern Naturalist. 2005;50:307–311. [Google Scholar]
- Ramalho M, Imperatriz-Fonseca VL, Giannini TC. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera) Apidologie. 1998;29:221–228. [Google Scholar]
- Rensch B. Increase of learning capability with increase of brain-size. American Naturalist. 1956;90:81–94. [Google Scholar]
- Riveros AJ, Gronenberg W. Body size, foraging task specialization and resource exploitation in honeybees. Behavioral Ecology and Sociobiology submitted for publication. [Google Scholar]
- Roth G, Dicke U. Evolution of the brain and intelligence. Trends in Cognitive Sciences. 2005;9:250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behavioral Brain Research. 2001;120:67–73. doi: 10.1016/s0166-4328(00)00359-4. [DOI] [PubMed] [Google Scholar]
- Spaethe J, Brockmann A, Halbig C, Tautz J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften. 2007;94:733–739. doi: 10.1007/s00114-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Spaethe J, Chittka L. Interindividual variation of eye optics and single object resolution in bumblebees. Journal of Experimental Biology. 2003;206:3447–3453. doi: 10.1242/jeb.00570. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. A brain region in insects that supervises walking. Progress in Brain Research. 1999;123:273–284. doi: 10.1016/s0079-6123(08)62863-0. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery, and interpretation of arthropod mushroom bodies. Learning and Memory. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Current Opinion in Neurobiology. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Suga N, Jen PHS. Disproportionate tonotopic representation for processing CF-FM sonar signals in the mustached bat auditory cortex. Science. 1976;194:542–544. doi: 10.1126/science.973140. [DOI] [PubMed] [Google Scholar]
- Sylvester HA, Rinderer TE. Fast Africanized bee identification system. (FABIS) manual. American Bee Journal. 1987;127:511–516. [Google Scholar]
- Thorpe JR, Harvey DMR. Optimization and investigation of the use of 2,2-dimethoxypropane as a dehydration agent for plant tissue in TEM. Journal of Ultrastructural Research. 1979;68:186–194. doi: 10.1016/s0022-5320(79)90153-9. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre M. Implementing false discovery rate control: Increasing your power. Oikos. 2005;108:643–647. [Google Scholar]
- Waddington KD. Implications of variation in worker body size for the honey bee recruitment system. Journal of Insect Behavior. 1989;2:91–103. [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomial plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Effects of experience and juvenile hormone on the organization of the mushroom bodies of honeybees. Journal of Neurobiology. 1995;26:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]
- Woosley TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Research. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Worden BD, Skemp AK, Papaj DR. Learning in two contexts: The effects of interference and body size in bumblebees. Journal of Experimental Biology. 2005;208:2045–2053. doi: 10.1242/jeb.01582. [DOI] [PubMed] [Google Scholar]