Abstract
The ability of the liver to regenerate and adjust its size after two/third partial hepatectomy (PH) is impaired in old rodents and humans. Here, we investigated by microarray analysis the expression pattern of hepatic genes in young and old untreated mice and the differences in gene expression profile following PH. Of the 10,237 messenger RNAs that had detectable expression, only 108 displayed a greater than 2-fold modification in gene expression levels between the two groups. These genes were involved in inflammatory and immune response, xenobiotics, and lipid and glucose metabolism. To identify the genes responsible for the different regenerative response, 10-week and 18-month-old mice subjected to PH were sacrificed at different time intervals after surgery. The results showed that 2463 transcripts had significantly different expression post PH between the two groups. However, in spite of impaired liver regeneration in old mice, cell cycle genes were similarly modified in both groups, the only exception being cyclin D1 gene which was up-regulated soon after PH in young mice, but mostly down-regulated in aged animals. Surprisingly, while in young hepatectomized mice, Yap messenger RNA (mRNA) expression was not significantly enhanced and protein expression essentially reflected the progression into cell cycle, its mRNA and protein levels were robustly increased in the liver of aged animals. Furthermore, a significant change of the age-related expression of the size regulator Yes-associated protein (YAP) was observed. Unexpectedly, while in young hepatectomized mice, Yap mRNA expression was not significantly enhanced and protein expression essentially reflected the progression into cell cycle, its mRNA and protein levels were robustly increased in the liver of aged animals. Moreover, when PH was performed on mitogen-induced enlarged livers, the earlier restoration of the original liver mass compared to animals subjected to PH only led to YAP down-regulation concomitantly with cyclin D1 up-regulation. Our data suggest that YAP activation is a size-dependent homeostatic mechanism that does not necessarily reflect cell cycle progression.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-015-9796-7) contains supplementary material, which is available to authorized users.
Keywords: YAP, Cyclin D1, Microarray, Liver
Introduction
Aging is a genetically programmed process characterized by a progressive reduction in the efficacy of a biological function in most organ systems. Among the different hypotheses raised to justify the biological alterations associated with aging, two, in particular, seem to provide the best explanation for aging process: the gene’s silencing theory (Burzynski 2003, 2005) and the free radical theory (Praticò 2002); the gene’s silencing hypothesis is based on the finding that 90 % of human genes are silenced in adult life through deacetylation of the histones, methylation of genes promoter sequences, and the chromatin remodeling. According to the free radical hypothesis, senescence is the consequence of alteration of hepatic oxidation and reduction of detoxifying enzymes that lead to accumulation of reactive oxygen species due to free radical incomplete destruction by the appropriate endogenous defense systems.
The progressive reduction in biological functions associated with aging includes immunoresponsiveness, tissue growth, cell proliferation, and organ capacity to respond to intrinsic and extrinsic stimuli. Several age-related changes have been well documented, including the increased development of many pathologies of the liver. The most documented age-related changes in the liver consist in a decline in organ volume (in men liver, weight is reduced by about 6.5 % and in women 14.3 %), decrease in hepatic blood flow, increased dense bodies volume, reduced concentration of smooth-surfaced endoplasmic reticulum, and of hepatocyte number (Schmucker 2005; Sheedfar et al. 2013). The most important functional effect of all these aging-related changes is a decrease in the regenerative capacity of the hepatocytes in rodents and humans (Bucher et al. 1964; Fry et al. 1984; Zhu et al. 2014). Adult liver is a quiescent organ with no basal hepatocyte proliferation; however, in response to injury, hepatocytes can be recruited into an active cell cycle (liver regeneration). The best experimental model for the study of liver regeneration is the 2/3 partial hepatectomy (PH) in rodents in which two thirds of the liver is removed leading to entry into S phase of 99 % of hepatocytes which, in 5–7 days, reconstitute the basal liver mass (Higgins and Anderson 1931). Several studies have characterized the mechanisms associated to liver regeneration (Fausto et al. 1995; Taub 1996; Michalopoulos and DeFrances 1997). On a molecular level, the entry of hepatocyte into cell cycle is associated with a cascade activation of cytokines and immediate early genes, including tumor necrosis factor alpha (Tnfa), interleukin-6 (Il-6), and Jun, leading to transcription factors, signal transducer, and CCAAT/enhancer-binding protein-β (C/EBPβ) activation which are responsible for delayed early gene induction that leads to synthesis of cell cycle regulatory proteins (cyclins and cyclin-dependent kinase). Different cyclins exhibit distinct expression and degradation patterns which contribute to the temporal coordination of each mitotic event (Grana and Reddy 1995). Several studies have demonstrated that in old rodents, the liver’s ability to regenerate and to adjust its size after PH is reduced and delayed (Bucher et al. 1964; Fry et al. 1984). Indeed, while in young rats, DNA synthesis peaks at 24 h after PH, in old animals, the S phase peak is reached at 36 h. Different explanation has been proposed to justify this proliferation defect observed in aged rodents. Among these, Wang et al. (2001) showed that aging process is associated with reduced expression of the cell cycle gene Forkhead Box M1 (Foxm1). Foxm1 has been suggested to be essential in regulating expression of genes involved in cell proliferation since increased levels of this transcription factor stimulate expression of cyclin D1, A2, B1, and B2 and are sufficient to restore cell cycle by promoting gene expression and to potentiate hepatocyte proliferation in aged mice. Furthermore, Iakova et al. (2003) demonstrated that C/EBPα, a transcription factor highly expressed in rodent liver and a strong inhibitor of cell proliferation through inhibition of cyclin-dependent kinases, forms a complex with Rb-E2F4 which binds to E2F, repressing its gene expression and leading to a loss in the proliferative response to PH in old mice.
More recently, the Hippo signaling pathway has been identified to play a key role in restricting organ size by controlling both cell proliferation and apoptosis (Dong et al. 2007; Camargo et al. 2007; Pan 2010). In mammals, the Hippo pathway is represented by a kinase cascade leading to regulation of the transcriptional co-activator Yes-associated protein (YAP) and transcriptional co-activator (TAZ) with PDZ-binding motif. In rodent liver, YAP overexpression leads to a dramatic increase in size (Dong et al. 2007; Camargo et al. 2007). Moreover, the finding that YAP activation occurs during liver regeneration after PH and terminates when the liver to body weight ratio reaches homeostatic levels further supports its crucial role in this process. The present study was designed to investigate, by microarray technology, possible differences in gene expression between young and old livers during compensatory regeneration after 2/3 PH. The old livers were characterized by an impaired hepatic regenerative response after surgery but showed nevertheless increased expression of the cell cycle regulator cyclin D1 and YAP. This discrepancy prompted us to investigate on YAP regulation in an opposite condition, namely liver regeneration in mitogen pretreated enlarged livers, whereby the restoration of original liver mass is achieved earlier than in the livers subjected to PH only.
Materials and methods
Animals and treatment
Female CD-1 mice (10 weeks and 18 months old), purchased from Charles River (Milano, Italy), were used. Procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the animal ethics committee of this university. Hepatocyte proliferation was induced by 2/3 partial hepatectomy (PH), according to Higgins and Anderson (1931). Three to six mice per group were used in animal experiments. Mice were killed 3, 6, 12, 24, and 36 h after PH.
To analyze the regenerative response of the liver after 2/3 PH, two additional groups of mice (10 weeks and 18 months old) were given bromodeoxyuridine (BrdU, Sigma Chem. Co., Milan, Italy) continuously in drinking water (1 mg/ml) and were sacrificed 48 h thereafter.
To analyze YAP expression after PH in hyperplastic livers, 10-week-old CD1-mice were administered a single dose of the hepatomitogen 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (3 mg/kg body weight, Sigma Chem. Co.) 72 h prior to 2/3 PH. All animals received BrdU and were sacrificed 72 h after surgery. Controls were either untreated or subjected to surgery in the absence of mitogen pretreatment.
Histology and BrdU labeling
Immediately after killing of the mice, liver sections were fixed in 10 % buffered formalin and processed for staining with hematoxylin-eosin or immunohistochemistry. The remaining liver was snap-frozen in liquid nitrogen and kept at −80 °C until use. For determination of hepatocyte proliferation, mouse monoclonal anti-BrdU antibody was obtained from Becton Dickinson (Becton Dickinson, San Jose, CA), and the peroxidase method was used to stain BrdU-positive hepatocytes. Peroxidase-conjugated goat anti-mouse immunoglobulin was obtained from Dako (Dako EnVision Peroxidase Mouse; Dako Corporation, Carpinteria, CA). Four-micron-thick sections were deparaffinized, treated with 2 N HCl for 1 h, then with 0.1 % trypsin type II (crude from porcine pancreas; Sigma Chem. Co.) for 20 min, and treated sequentially with normal goat serum 1:10 (Dako Corporation), mouse anti-BrdU 1:100, and Dako EnVision Peroxidase Mouse ready-to-use. The sites of peroxidase binding were detected by 3,3′-diaminobenzidine. The labeling index (LI) was expressed as number of BrdU-positive nuclei/100 nuclei. Results are expressed as means ± SE of four to five mice per group. At least 2000 hepatocyte nuclei per liver were scored.
Microarray analysis
Total RNA was isolated from livers using the guanidium isothiocyanate method (TRIzol; Invitrogen, San Giuliano Milanese, Italy) and purified with RNeasy clean up kit (Qiagen, Hilden, Germany). The young (10 weeks old) and old (18 months old) mice were sacrificed before (0 h) and 3, 6, 12, 24, and 36 h after PH. For each time point, three to six mice were used. RNA concentration was determined with a NanoDrop (NanoDrop, Wilmington, Delaware, USA) spectrophotometer, and its quality was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Milano, Italy). For each time point, 5 μg purified RNA/animal was pooled together to minimize sample-to-sample variations and analyzed together. For each pools, 500 ng of total RNA was synthesized to biotinylated complementary RNA (cRNA) using the Illumina RNA Amplification Kit (Ambion, Inc., Austin, TX). Synthesis was carried out according to the manufacturers’ instructions. cRNA concentration and the quality were assessed out as described above.
From each pool, technical replicates were produced and 750 ng cRNA was hybridized for 18 h to MouseRef-8 Expression BeadChips (Illumina Inc., San Diego, CA, USA) according to the protocol provided by the manufacturer. Hybridized chips were washed and stained with streptavidin-conjugated Cy3 (GE Healthcare Milano, Italy). BeadChips were dried and scanned with an Illumina BeadArray Reader (Illumina Inc.).
For data analysis, the intensity files were loaded first into the GenomeStudio Software (Illumina Inc.) to extract the fluorescence values (AVG_Signal), quality control, and gene expression analysis. First, the quantile normalization algorithm was applied on the dataset to correct systematic errors. Using a normalization based upon quantiles, this method normalizes a matrix of probe-level intensities.
For each sample, three technical replicates grouped together and probes with a detection p value below 0.05, corresponding to a false-positive rate of 5 %, were considered as detected. Then, after multiple testing corrections using Benjamini and Hochberg false discovery rate, differential expression analysis was performed and probes with DiffScore ≥20 or ≤−20 (corresponding to a q value of 0.01) and fold differences (FD) ≥2 or ≤ −2 were considered as differentially expressed.
In particular, to examine the effect of aging on hepatic gene expression, RNA extracted before 2/3 PH from the liver of young (10 weeks control) and old (18 months control) mice were compared. While, to evaluate the effect of liver regeneration 3, 6, 12, 24, and 36 h after 2/3 PH, samples were compared with their age-matched controls.
Raw microarray data have been deposited in the EBI ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) with accession number E-MTAB-3374.
Functional annotation analysis
Transcripts were classified according to their role in biological process, cellular components, and molecular function using Database for Annotation, Visualization, and Discovery (DAVID), Mouse Genome Informatics (The Jackson Laboratory), and GeneCards (Weizmann Institute of Science). For those differentially modulated by aging (0 h old vs 0 h young), Molecular Function and InterPro functional characterization were performed using FIDEA, a server for the functional interpretation of differential expression analysis. Canonical pathways were also examined utilizing PathwayStudio 5.0 (Ariadne Genomics, USA).
Real-time PCR
RNA was retro-transcribed using the High Capacity Kit (Life Technology, Monza, Italy). Analysis of Ccnd1, Cdk4, Jun, Fos, Cdkn1a, and Cebpa was performed using specific TaqMan probes: Mm00432359_m1, Mm00726334_s1, Mm 0495062_s1, Mm00487425_m1, Mm00432448_m1, and Mm00514283_s1 (Life Technologies). Gapdh was used as endogenous control. The experiments were carried out in triplicate on a pool of livers per group/time. Relative differences in gene expression were evaluated according to the comparative ∆∆Ct method using untreated old and young mice as calibrators to which all hepatectomized old and young mice, respectively, were compared.
Western blot analysis
Total cell extracts were prepared from frozen livers as described previously (Ledda-Columbano et al. 2004). For cyclin D1, D2, D3, and YAP, 100 mg of total extracts was prepared. The following antibodies were used: mouse monoclonal antibodies against cyclin D1 (72–13), cyclin D2, cyclin D3, and proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology, CA); actin (clone AC-40) (Sigma Chem. Co) and albumin (Bethyl Laboratories, Montgomery, TX, USA); and rabbit polyclonal antibodies directed against Yap and Phospho-Yap (Cell Signaling Technology, Danvers, MA, USA). Anti-mouse and anti-rabbit horseradish peroxidase-conjugated IgGs were from Santa Cruz Biotechnology.
Results
Age-related gene expression profile in young and aged untreated mouse liver
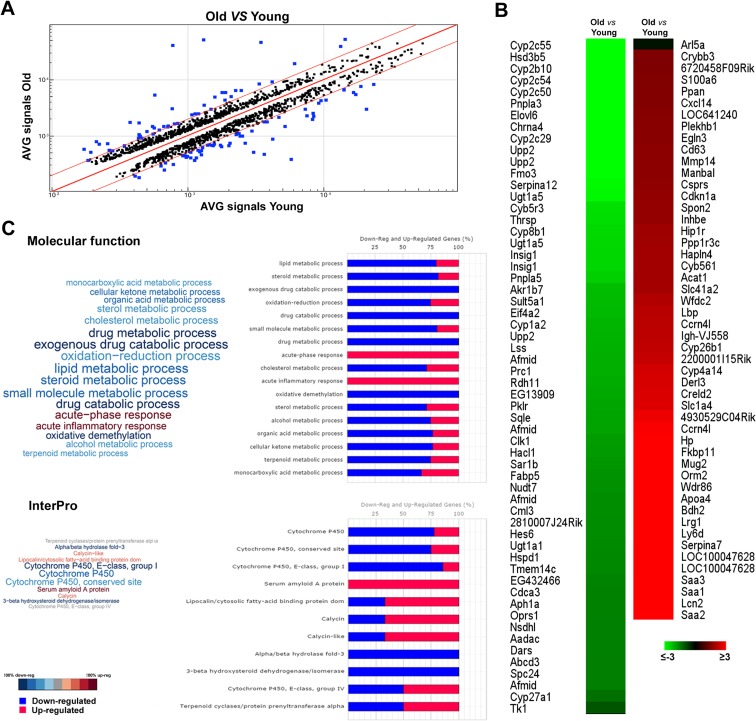
To examine the effect of aging on hepatic gene expression, RNA was extracted from the liver of young (10 weeks) and old (18 months) mice and the analysis of the gene expression profile was performed by RNA microarrays. A comparison of livers from young and old mice revealed that aging is associated with minor modification at the RNA level (Fig. 1a); indeed, of 10,237 transcripts, only 108 (1.05 %) displayed a greater than 2-fold (FDR adjusted P value of ≤0.05) modification in gene expression levels compared to young mice. Among these genes, 49 were up-regulated with age and 59 were down-regulated (Fig. 1b and Supplementary Table 1). Functional annotation analysis of deregulated transcripts obtained comparing young and old mice livers results in enriched molecular function ontology categories (Fig. 1c, upper panel and Supplementary Table 2); the pathways most significantly modified by aging were those related to inflammation and immune response (up-regulated), with xenobiotic and several other metabolic pathways, such as those involved in lipid and glucose metabolism being down-regulated. In particular, six genes associated with inflammation showed an age-associated increase, namely serum amyloid A1-, 2-, and 3- (Saa1, Saa2, Saa3); orosomucoid 2-; haptoglobin-; and lipopolysaccharide-binding protein. Serum Amyloid A (Saa) is a component of a group of major acute phase proteins, and its expression increases in response to infection or systemic inflammation. In agreement with previous works (Vranckx et al. 1995; Ballou et al. 1996), the present study found that the expression levels of this gene family (consisting, in mouse, of three active genes, Saa1, Saa2, Saa3) were 9- to 55-fold higher in old mice compared to young animals. These results suggest that inflammation is a component of aging process in the liver, according to the concept of the existence in aged animals of a state of chronic inflammation in the absence of “stressors.” Genes involved in immune response were also up-regulated in aged liver; particularly striking was the 40-fold increase in the expression of lipocalin 2, a novel autocrine and paracrine adipokine, which acts as an antagonist of inflammatory molecules. Our data also showed that aging is associated with changes in the expression of several genes related to cholesterol metabolism; indeed, we observed down-regulation of insulin-induced gene 1 (Insig1), squalene epoxidase (Sqle), and lanosterol synthase (Lss). Insig1 plays an important inhibitory role in the SREBP-mediated regulation of cholesterol biosynthesis, associated with a decreased expression of hydroxymethylglutaryl-CoA reductase (Hmgcr), the rate controlling enzyme of the mevalonate pathway that produces cholesterol. Finally, gene expression analysis also showed a strong down-regulation of biosynthetic enzymes (Fig. 1c lower panel), such as cytochrome P450-IIC55, P450-IIC54 (steroid metabolism), patatin-like phospholipase domain containing lipid metabolism, fatty acid-binding protein 5 (glucose metabolism), and uridine phosphorylase 2 (nucleoside metabolism). Down-regulation of these genes indicates a reduced capacity of the liver to maintain biosynthetic homeostasis, in the elderly.
Fig. 1.
Gene expression profile in old and young mice. a Scatter plot showing comparison between all expressed transcripts in old vs young mice livers. Blue dots represent transcripts differentially expressed between the two groups above the fold difference cutoff 2 represented by red lines. b Heat map showing differentially expressed transcripts between old vs young mice livers. c Functional annotation analysis of deregulated transcripts obtained comparing young and old mice livers. Transcripts were classified according to biological process (upper panel) and InterPro (lower panel) ontology categories using FIDEA bioinformatics tool
Liver regeneration after PH
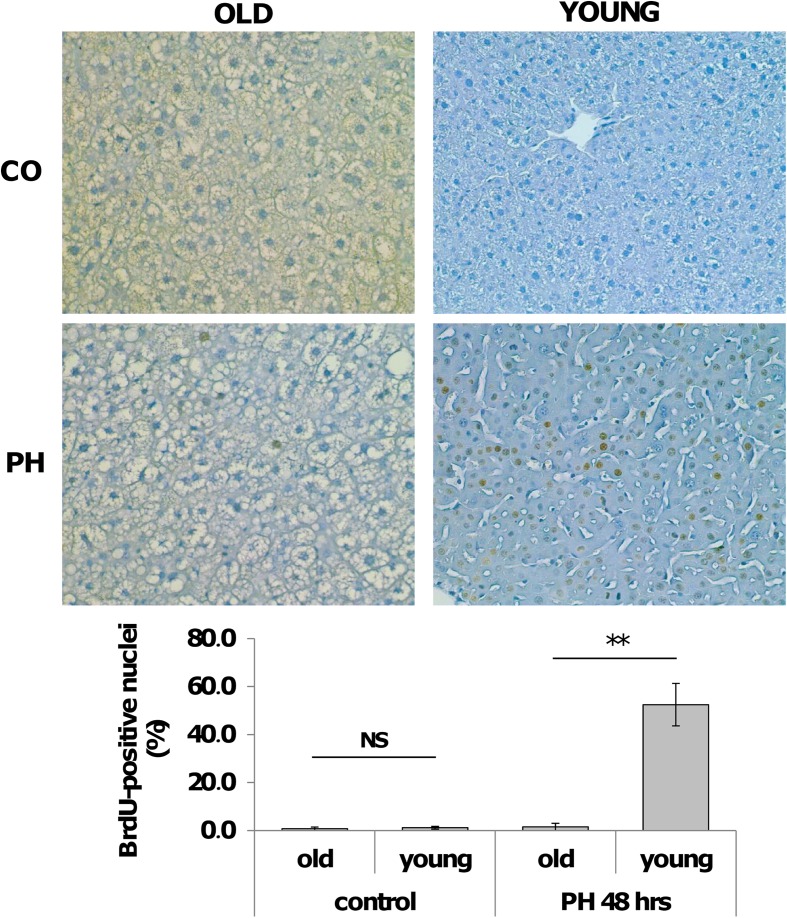
To investigate how aging could influence the expression of cell-cycle-associated genes following a proliferative stimulus, experiments were performed in young and old mice sacrificed 48 h after PH, a time point when most hepatocytes have entered into S phase. According to previous results (Bucher et al. 1964; Fry et al. 1984; Wang et al. 2001), while a strong proliferative response, as detected by BrdU incorporation, was observed in young mice (Fig. 2a, b), (LI of 52.5 % ± 8.8), liver regeneration was severely impaired in old mice; indeed, virtually, no hepatocyte nuclei were BrdU-positive at the same time point (LI of 1.5 % ± 0.5). No major differences in the labeling index were found in untreated young and old mice (0.56 and 0.71 %, respectively).
Fig. 2.
Hepatocyte proliferation after PH in old and young livers. a Representative photomicrographs showing BrdU incorporation of hepatocytes in old and young mice after 2/3 PH (sections counterstained with hematoxylin, X200). Mice were subjected to PH and sacrificed 48 h later. All mice were given BrdU (1 mg/ml) in drinking water until the time of sacrifice. CO controls. b Labeling index of hepatocytes from old and young mice subjected to 2/3 PH. At least 2000 hepatocyte nuclei per liver were scored. LI was expressed as number of BrdU-positive hepatocyte nuclei/100 nuclei. Results are expressed as means ± SE of four to five mice per group **Significantly different for p < 0.001; NS not significant
Aging-related gene expression profile in mouse liver after PH
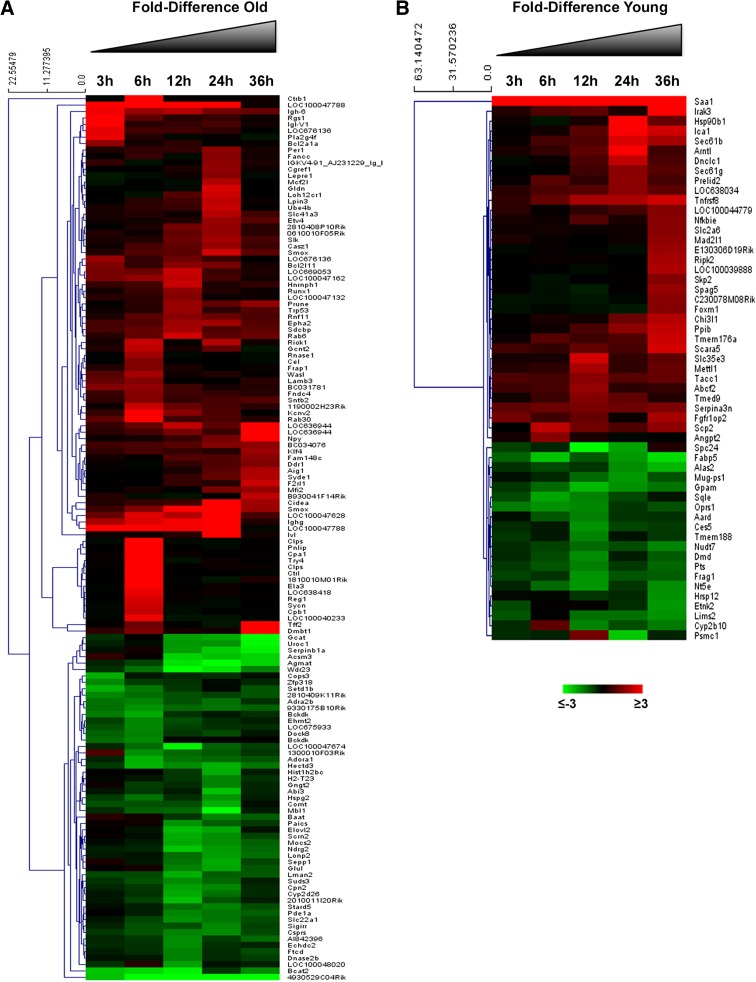
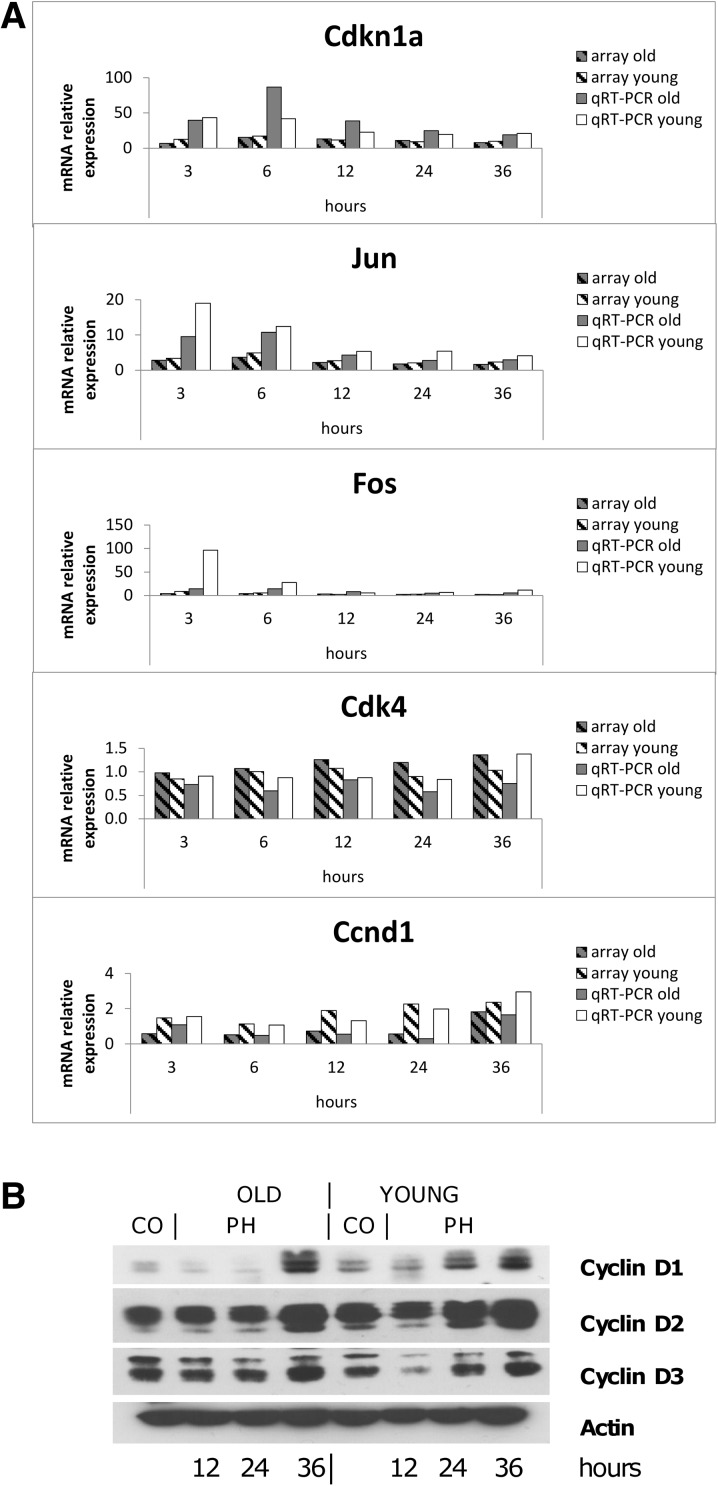
RNAs from liver of young and old mice extracted 3, 6, 12, 24, and 36 h after PH were hybridized to arrays. The results showed that out of 12,751 detected transcripts, 2463 were significantly dysregulated in young and aged animals vs. controls, for at least one of the time points (FD cutoff 2.0 and FDR-adjusted P value of ≤0.05); in particular, 2270 transcripts (Supplementary Table 3) were differently expressed in both old and young animals; 138 were differently expressed only in old mice (Fig. 3a and Supplementary Table 4) and 55 genes only in young mice (Fig. 3b and Supplementary Table 5). These genes are involved in different biological processes, including cell cycle, cell biosynthesis, protein metabolism, and others. Due to our interest in identifying critical genes responsible for the impaired regenerative response of the liver in old animals, we focused our attention on cell cycle genes. Table 1 shows the expression of representative genes involved in the priming phase, immediate early genes, and genes involved in the G1 to S phase transition of hepatocytes. The results indicate that the expression of genes coding for cytokines involved in liver regeneration (Tnfa, Il-6), or for growth factors, such as hepatocyte growth factor (Hgf) and Transforming growth factor alpha (Tgfa), was not modified during the first 36 h after PH; as to transcription factors, while no change in the expression of nuclear factor-κB (NF-κB) was observed, signal transducer and activator of transcription factor 3 (Stat3) did show some increase compared to resting liver, but no age-related difference after PH. Moreover, no differences between young and old mouse livers were observed in the expression of Cebpa transcription factor which plays an inhibitory role in liver regeneration. A decreased expression of the cell cycle regulator Foxm1 has been suggested to be responsible for the impaired regenerative response of aged livers (Wang et al. 2001). In agreement with these findings, our results showed that Foxm1 messenger RNA (mRNA) levels were increased only in young livers at 36 h after PH while no significant differences in gene expression were observed between hepatectomized and untreated old mice. Immediate early genes, such as early growth response-1 (Egr-1), Fos and Jun showed a similar expression profile in aged and young mice, although the levels of mRNA were significantly higher in the liver of young mice. Cdk4 and Cdk6 expression was unchanged compared to controls, while Cdk2 was upregulated at 36 h after surgery in both old and mice animals. Interestingly, while cyclin E was up-regulated in both groups, the regulation of cyclin D1 after PH was completely different from all other genes examined. Indeed, while in young mice, cyclin D1 was up-regulated 12 h after PH, and its mRNA levels were found significantly elevated at all time points; thereafter, down-regulation of this gene was observed in the liver of old mice as early as 6 h after surgery and persisted for the first 24 h. qRT-PCR validation studies performed on cell cycle genes confirmed the results of the microarray analysis (Fig. 4a), including cyclin D1 gene expression that was increased after PH in the liver of young mice but was down-regulated in old animals during the first 24 h after surgery. Accordingly, as shown in Fig. 4b, increased protein content of cyclin D1 was observed starting from 24 h after PH in young mice while, in old mice, it exhibited a decrease compared to control values for the first 24 h, its levels being elevated only at 36 h after PH. Notably, increased protein levels of cyclin D2 and D3 did not show major differences between old and young mice, their increase being evident only 36 h after PH in both groups.
Fig. 3.
Gene expression profiling in old and young mice after PH. Heat map showing specific transcripts regulated in at least one time point, 3, 6, 12, 24, and 36 h after PH in old (a) or young mice (b). Transcripts that showed an increase >2 times (red) or reduction >2 times (green) compared to the 0 time point are displayed
Table 1.
Expression of cell cycle genes in old vs young mice after PH. Livers of hepatectomized young or old mice were analyzed at indicated time points
| Accession number | Gene symbol | Description | Old | Young | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 12 h | 24 h | 36 h | 3 h | 6 h | 12 h | 24 h | 36 h | |||
| NM_008689.1 | NF-κB | Nuclear factor of kappa light polypeptide gene enhancer in B cells | Unchanged | |||||||||
| NM_013693 | TNF-α | Tumor necrosis factor alpha | Unchanged | |||||||||
| NM_011609.2 | TNFRSF1a | Tumor necrosis factor receptor superfamily, member 1a (Tnfrsf1a) | NS | 2.1 | 1.7 | 1.6 | 1.4 | NS | 1.7 | 1.3 | NS | 1.3 |
| NM_013749.1 | TNFRSF12a | Tumor necrosis factor receptor superfamily, member 12a (Tnfrsf12a) | 5.34 | 10.7 | 23.0 | 20.4 | 13.8 | 6.7 | 17.4 | 19.8 | 15.2 | 13.9 |
| NM_031168.1 | IL-6 | Interleukin-6 | Unchanged | |||||||||
| NM_010427.2 | HGF | Hepatocyte growth factor | Unchanged | |||||||||
| NM_007678.1 | C/EBPα | CCAAT/enhancer-binding protein alpha | 0.7 | 0.6 | 0.5 | 0.5 | 0.4 | 0.5 | 0.5 | 0.4 | 0.5 | 0.4 |
| NM_031199.1 | TGF-α | Transforming growth factor alpha | Unchanged | |||||||||
| NM_007913.2 | Egr-1 | Early growth response 1 | 2.98 | 4.3 | 4.1 | 5.7 | 4.6 | 5.6 | 6.0 | 3.3 | 2.7 | 5.1 |
| NM_011486.2 | Stat3 | Signal transducer and activator of transcription 3 | NS | 2.0 | 1.7 | 2.2 | 1.7 | 1.6 | 2.6 | 2.1 | 1.3 | 1.9 |
| NM_010234.2 | Fos | FBJ osteosarcoma oncogene | 3.93 | NS | 3.1 | 2.1 | 2.5 | 9.0 | 5.4 | 2.4 | 2.7 | 1.9 |
| NM_010591.1 | Jun | Jun oncogene | 2.82 | 3.7 | 2.2 | 1.8 | 1.6 | 3.4 | 4.9 | 2.7 | 2.1 | 2.3 |
| NM_010849.2 | Myc | Myelocytomatosis oncogene | Unchanged | |||||||||
| NM_007631.1 | Ccnd1 | Cyclin D1 | NS | 0.5 | 0.7 | 0.6 | 1.8 | 1.5 | NS | 1.9 | 2.3 | 2.4 |
| NM_009870.2 | Cdk4 | Cyclin-dependent kinase 4 | Unchanged | |||||||||
| NM_009873.1 | Cdk6 | Cyclin-dependent kinase 6 | Unchanged | |||||||||
| NM_007891.1 | E2f1 | E2F transcription factor 1 | NS | 0.4 | 0.4 | 0.5 | 3.2 | NS | 0.5 | 0.5 | NS | 3.9 |
| NM_007633.1 | Ccne1 | Cyclin E1 | NS | NS | NS | 2.6 | 2.8 | NS | NS | 1.6 | 1.7 | 4.5 |
| NM_011640.1 | Trp53 (P53) | Transformation related protein 53 | NS | NS | 2.2 | 1.4 | 1.7 | NS | NS | NS | NS | NS |
| NM_007669.2 | Cdkn1a(p21) | Cyclin-dependent kinase inhibitor 1A (P21) | 6.67 | 15.3 | 13.1 | 11.2 | 7.9 | 12.5 | 17.1 | 11.7 | 9.1 | 9.9 |
| NM_009877.1 | Cdkn2a(p16) | Cyclin-dependent kinase inhibitor 2A (Cdkn2a) | Unchanged | |||||||||
| NM_016679.2 | Keap1 | Mus musculus kelch-like ECH-associated protein 1 (Keap1) | Unchanged | |||||||||
| NM_010902.2 | NRF2 | Mus musculus nuclear factor, erythroid derived 2, like 2 (Nfe2l2) | unchanged | |||||||||
Results are expressed as fold difference (FD) in respect to untreated young or old (control) livers, respectively. Differentially expressed genes were selected with DiffScore cutoff ≥20 or ≤−20, corresponding to a q value of 0.01 and fold difference cutoff ±2. Unchanged: gene expression with DiffScore cutoff >20 but not significantly modifies in respect to control (0.5 < FD > 2). NS: gene expression with DiffScore cutoff >−20 or <20
Fig. 4.
Cell cycle-related gene modification after PH in old and young livers. a mRNA expression was determined in old and young mice after PH by qRT-PCR and gene expression microarray. Gene expression is reported as relative quantification (RQ) or fold difference (FD) relative to age-matched controls. b Western blot analysis of cell-cycle-associated proteins. Old and young mice were sacrificed 12, 24, and 36 h after 2/3 PH. Total extracts (100 to 150 mg/lane) were prepared, and Western analysis was performed as described in “Materials and methods” section. Appropriate loading was confirmed by staining the gel with Coomassie Blue and using anti-actin antibody as loading control. Each lane represents a pool of three livers. CO controls
YAP expression in liver regeneration of young and old mice
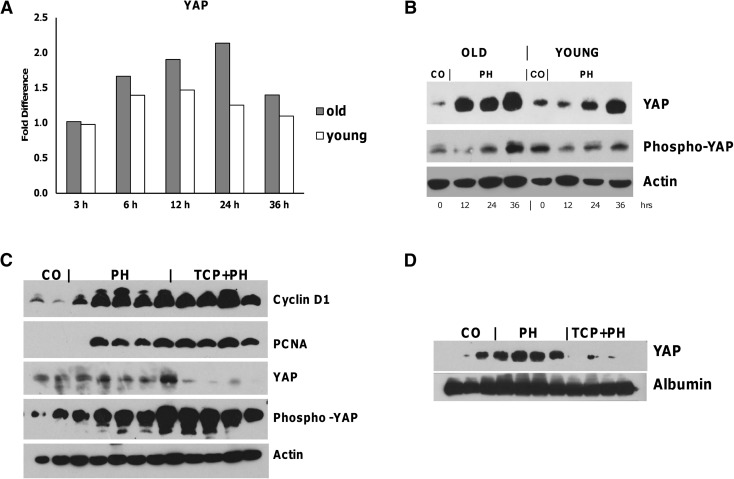
Previously, it has been shown that YAP overexpression leads to a dramatic increase in liver size (Camargo et al. 2007). Moreover, an increase of YAP protein has been shown during liver regeneration post-PH (Grijalva et al. 2014). These findings together with YAP-induced hepatomegaly in rodent liver (Dong et al. 2007; Camargo et al. 2007; Pan 2010) strongly suggest that YAP overexpression is associated with the re-entry of hepatocytes into the cell cycle. Microarray analysis performed in the present study showed that Yap mRNA levels did not change during the active liver regeneration occurring in young mice; however, they were increased from 6 to 24 h after PH, with a return to normal values at 36 h in old animals, despite hepatocyte proliferation was almost completely blunted (Figs. 5a and 2b). In agreement with mRNA levels, YAP protein content was strongly enhanced in the liver of aged animals as soon as 12 h after surgery, while a weaker and delayed increase was observed in young mice. Less dramatic changes in the two groups were observed for phospho-YAP protein content (Fig. 5b).
Fig. 5.
YAP expression in old and young livers after PH. a Microarray analysis of Yap mRNA. mRNA expression was determined in old and young mice after PH. Gene expression is reported as fold difference relative to age-matched controls. b Western blot analysis of YAP and phospho-YAP proteins. Old and young mice were sacrificed 12, 24, and 36 h after 2/3 PH. Total extracts (100 to 150 mg/lane) were prepared as described in “Materials and methods” section. Appropriate loading was confirmed by staining the gel with Coomassie Blue and using anti-actin antibody as loading control. Each lane represents a pool of three livers. CO controls. c Western blot analysis of cyclin D1, PCNA, YAP, and phospho-YAP in livers from mice subjected to PH with or without TCPBOP pretreatment (TCP + PH and PH, respectively). Young mice were subjected to PH 3 days after treatment with the mitogen TCP, as described in “Materials and methods” section. Animals were sacrificed 72 h after PH. Total extracts (100 to 150 mg/lane) were prepared from the livers, and Western analysis was performed as described in “Materials and methods” section. Appropriate loading was confirmed by staining the gel with Coomassie Blue and using anti-actin antibody as loading control. Each lane represents a pool of three livers. CO controls. d Western blot analysis of nuclear YAP in TCP + PH or PH livers. Nuclear extracts for YAP (100 to 150 mg/lane) were prepared, and Western analysis was performed as described in “Materials and methods” section. Appropriate loading was confirmed by staining the gel with Coomassie Blue and using anti-albumin antibody as loading control. Each lane represents a pool of three livers. CO controls
YAP expression is inhibited when surgery is performed in enlarged livers
The finding of a sustained YAP overexpression in aged livers characterized by impaired regenerative capacity is indicative of a dissociation between cell cycle genes and YAP. Therefore, we investigated whether the enhanced YAP expression observed in the liver of old mice is the result of mechanisms that “sense” the decreased liver mass and signal a proliferative response in an attempt to re-establish the original organ size. To better understand whether liver size dictates YAP expression, we evaluated its protein content in young mice subjected to surgery after induction of hepatomegaly by treatment with the hepatomitogen TCPOBOP (Columbano et al. 2008). Animals were sacrificed 72 h after PH, since under this condition, hepatic mass reaches the values of the liver of intact animals within 3 days (Columbano et al. 2008).
As shown in Fig. 5c, while cell cycle proteins, such as cyclin D1 and PCNA, showed a similar expression pattern in PH and PH + TCPOBOP animals, YAP protein levels were drastically decreased in PH-enlarged livers; notably, YAP inhibition was associated with a strong increase of phospho-YAP protein levels in the same group (Fig. 5c). Western blot analysis performed on nuclear extracts confirmed the decrease of the active form of YAP in TCPOBOP-induced enlarged livers subjected to PH (Fig. 5d), but not in PH alone.
Discussion
Several studies have shown that the liver, an organ with a wide range of functions, undergoes specific age-related changes, including increased hepatocyte size, number of binucleated cells, and reduction in mitochondrial number (Premoli et al. 2009; Gan et al. 2011). These changes may significantly affect liver morphology, physiology, and oxidative capacity. To further probe into the molecular changes occurring in the elderly, in the present study, we have analyzed the gene expression profile in the liver of young and aged mice. The results showed that only minor modifications take place at a transcriptional level during aging (108 genes/12,751). Most of these genes were associated with pathways, such as those related to inflammation and immune response (up-regulated) and xenobiotic and lipid and glucose metabolism (down-regulated), supporting previous data that suggest that an unbalanced stimulation/response of the immune system, characterized by increased levels of pro-inflammatory molecules, may act as a driver of the aging process (Franceschi et al. 2000). Our present data showing that aged liver is characterized by a striking increase in several genes associated with response to infection or systemic inflammation thus fit with the hypothesis that pathways involved in inflammation/immune response play a relevant role in hepatic cell aging.
The loss of regenerative capacity is the most dramatic age-related alteration (Timchenko 2009). Indeed, several studies on the regenerative response of the liver that follows 2/3 PH have shown a striking difference in both the magnitude of the peak response in DNA synthesis and the time at which the maximal DNA synthesis occurs, between young (4–8 weeks) and old (12–15 months) rats and mice (Bucher et al. 1964; Fry et al. 1984; Wang et al. 2001). Among the possible causes, an age-related switch from cyclin-dependent kinase inhibition to repression of E2F transcription (Iakova et al. 2003) and/or a decrease in expression of a gene coding for a critical Forkhead Box has been correlated with reduced proliferation of regenerating hepatocytes from old mice (Wang et al. 2001). However, to date, the effect of aging on liver regeneration has not been fully elucidated and the understanding of the molecular early changes occurring during liver regeneration in young vs. old mice remains elusive.
In particular, the finding that aged hepatocytes thoroughly maintain their replicative capacity following mitogenic stimuli (Ledda-Columbano et al. 2004) suggests that the depressed replicative response observed in aged rodent liver after PH results from lowered levels of extra-cellular factors, such as growth factors and/or hormones, rather than from intrinsic changes within the cell. In agreement with this, our present work found that the expression of several early changes, considered to be essential for liver regeneration (Yamada et al. 1997; Cressman et al. 1996; Michalopoulos and DeFrances 1997), is essentially similar in regenerating (young mouse) and non-regenerating (old mouse) liver. Indeed, the expression of immediate early genes, such as Jun, Fos, and Egr-1, was not significantly different in aged vs. young animals when compared to age-matched controls. Moreover, no significant differences were observed in the expression of cytokine genes such as Tnfa and Il-6 or growth factors such as Hgf or Tgfa. PathwayStudio analysis also showed no difference in transcription factor-dependent pathway, such as those classically involved in liver regeneration (NF-κB, Stat3, AP-1). In this context, the NRF2-Keap1 pathway recently involved in liver regeneration after PH (Beyer et al. 2008; Köhler et al. 2014) was also not differently affected in aged vs. young livers, as indicated by a similar expression of target genes such as thioredoxin, heme oxygenase-1, and Nqo2.
It was recently reported that elevated interferon gamma signaling contributes to impaired regeneration in the aged liver (Singh et al. 2011). Our present study was unable to confirm this finding; it is likely that the different results may depend on the different strain of mice (CD-1 vs. CB6F1) or to the different gender used. On the other hand, we found that while the hepatic expression of cyclin D1, determined either as mRNA or protein, was up-regulated 24 h after PH in young mice, it was profoundly down-regulated in aged livers at the same time point. Interestingly, no such difference was observed for cyclin D2 and cyclin D3, Cdk4 and Cdk6, and for the cell cycle inhibitors p21 and p16 mRNA levels. These results suggest that down-regulation of cyclin D1 may play a critical role in the impaired regenerative response in elderly.
A novel finding of the present study was the increased expression of YAP in the livers of old mice after surgery, in spite of the impaired regeneration observed in the elderly. The co-transcriptional activator YAP has been shown to be involved in proliferative signals, and its increase leads to hepatomegaly and, when sustained, to HCC development. However, the role of YAP in liver regeneration after 2/3 PH remains elusive. Indeed, only a few studies have analyzed YAP expression during liver regeneration in not genetically modified animals (Grijalva et al. 2014; Wang et al. 2012). Thus, while YAP activation is thought to simply reflect the cell cycle progression, a strict correlation between YAP expression and liver size in non-transgenic mice has not been clearly established. In our view, the finding that YAP expression is increased in non-regenerating aged livers and is inhibited when PH is performed on enlarged livers suggests that YAP expression does not necessarily mirror cell cycle gene expression but is more likely organ size-dependent. The finding that a robust and persistent YAP expression is found in the liver of aged animals, in spite of impaired hepatocyte regeneration, is intriguing; although no clear explanation at present is available, one may speculate that the impairment of liver regeneration during aging could lead to a sustained recruitment of proteins, such as components of the Hippo pathway that sense the decreased liver mass and signal a proliferative response in an attempt to re-establish the original liver size. In support to the concept of a role of YAP as size and not cell cycle regulator, when we analyzed YAP expression after PH performed on enlarged livers, at a time at which liver size is already restored, we found that YAP was inhibited, despite a massive proliferative response, concomitantly with a strong increase of the phospho-YAP protein.
In conclusion, the present study showed that (i) only a relatively small number of genes are transcriptionally modified during the aging process in mouse liver, (ii) modification of the expression of genes classically associated with the entry into the cell cycle with the exception of cyclin D1 is unlikely responsible for the impaired liver regeneration observed in the elderly, and (iii) sustained YAP activation occurring during liver regeneration in aged mice is likely a homeostatic mechanism triggered to compensate for the liver inability to rapidly recover its original mass.
Electronic supplementary material
(XLS 37 kb)
(XLS 123 kb)
(XLS 646 kb)
(XLS 58 kb)
(XLS 43 kb)
(DOC 27 kb)
Acknowledgments
Work supported by Italian Ministry of Health (Grant Young Researcher GR-2011-02350476 to M.R. and GR-2011-02347781to G.N.), Italian Ministry for Education, University and Research (Grants PRIN 2010LC747T), National Research Council Flagship Project Interomics, University of Salerno (FARB 2014), and Fondazione Banco di Sardegna to A.P.; G.N. is supported by a ‘Mario e Valeria Rindi’ fellowship of the Italian Foundation for Cancer Research. M.A.K. is a fellow of the Associazione Italiana Ricerca sul Cancro.
Abbreviations
- AP-1
Activating protein-1
- BrdU
Bromodeoxyuridine
- Cebp
CCAAT/enhancer-binding protein (C/EBP)
- egr 1
Early growth response-1
- Foxm1
Forkhead box M1
- HGF
Hepatocyte growth factor
- Keap1
Kelch-like ECH-associated protein 1
- IL-6
Interleukin-6
- IHC
Immunohistochemistry
- NF-κB
Nuclear factor-κB
- Nqo2
NAD(P)H dehydrogenase quinone 2
- NRF2
Nuclear factor (erythroid-derived 2)-like 2
- PCNA
Proliferating cell nuclear antigen
- PH
Partial hepatectomy
- qRT-PCR
Quantitative reverse transcriptase polymerase chain reaction
- STAT3
Signal transducer and activator of transcription factor 3
- TAZ
Transcriptional co-activator
- TCPOBOP
1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene
- TGF-β
Transforming growth factor-β
- TNF-α
Tumor necrosis factor-α
- YAP
Yes-associated protein
Footnotes
Monica Pibiri and Pia Sulas contributed equally to this work.
Contributor Information
Monica Pibiri, Phone: +39-070-6758346, Email: mpibiri@hotmail.com.
Maria Ravo, Phone: +39-089-9698286, Email: mravo@unisa.it.
References
- Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25:224–230. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher NLR, Swaffield MN, Di Troia JF. The influence of age upon the incorporation of thymidine-2-C14 into the DNA of regenerating rat liver. Cancer Res. 1964;24:509–512. [PubMed] [Google Scholar]
- Burzynski SR. Gene silencing a new theory of aging. Med Hypotheses. 2003;60:578–583. doi: 10.1016/S0306-9877(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Burzynski SR. Aging: gene silencing or gene activation? Med Hypotheses. 2005;64:201–208. doi: 10.1016/j.mehy.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Columbano A, Simbula M, Pibiri M, Perra A, Pisanu A, Uccheddu A, Ledda-Columbano GM. Potential utility of xenobiotic mitogens in the context of liver regeneration in the elderly and living-related transplantation. Lab Investig. 2008;88:408–415. doi: 10.1038/labinvest.2008.3. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Laird AD, Webber EM. Role of growth factors and cytokines in hepatic regeneration. Faseb J. 1995;9:1527–1536. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fry MF, Silber J, Loeb LA, et al. Delayed and reduced cell replication and diminishing levels of DNA polymerases-α in regenerating liver of aging mice. J Cell Physiol. 1984;118:225–232. doi: 10.1002/jcp.1041180302. [DOI] [PubMed] [Google Scholar]
- Gan L, Chitturi S, Farrell GC (2011) Mechanisms and implications of age-related changes in the liver: nonalcoholic fatty liver disease in the elderly. Curr Gerontol Geriatr Res 831536 [DOI] [PMC free article] [PubMed]
- Grana X, Reddy PE (1995) Cell cycle control in mammalian cells, role of cyclins, cyclin-dependent kinases (CDKs), growth suppressor genes and cyclin dependent kinase inhibitors (CKIs) 11:211–219 [PubMed]
- Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, Sadri-Vakili G, Vakili K. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–G204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- Higgins GM, Anderson RM. Experimental pathology of the liver. Restoration of the liver of the white rat following partial removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- Iakova P, Awad SS, Timchenko N. Aging reduced proliferative capacities of liver by switching pathway of C/EBPα growth arrest. Cell. 2003;113:495–506. doi: 10.1016/S0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- Köhler UA, Kurinna S, Schwitter D, Marti A, Schäfer M, Hellerbrand C, Speicher T, Werner S. Activated Nrf2 impairs liver regeneration in mice by activation of genes involved in cell-cycle control and apoptosis. Hepatology. 2014;60:670–678. doi: 10.1002/hep.26964. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Pibiri M, Cossu C, Molotzu F, Locker J, Columbano A. Aging does not reduce the hepatocyte proliferative response of mice to the primary mitogen TCPOBOP. Hepatology. 2004;40:981–988. doi: 10.1002/hep.1840400429. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D (2002) Lipid peroxidation and the aging process. Sci Aging Knowl Environ (50)re5 [DOI] [PubMed]
- Premoli A, Paschetta E, Hvalryg M, Spandre M, Bo S, Durazzo M. Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol. 2009;55:71–78. [PubMed] [Google Scholar]
- Schmucker DL. Age-related changes in liver structure and function: implications for disease? Exp Gerontol. 2005;40:650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: friends or foes? Aging Cell. 2013;12:950–954. doi: 10.1111/acel.12128. [DOI] [PubMed] [Google Scholar]
- Singh P, Goode T, Dean A, Awad SS, Darlington GJ. Elevated interferon gamma signaling contributes to impaired regeneration in the aged liver. J Gerontol A Biol Sci Med Sci. 2011;66:944–956. doi: 10.1093/gerona/glr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. Transcriptional control of liver regeneration. Faseb J. 1996;10:413–427. [PubMed] [Google Scholar]
- Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20(4):171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Vranckx R, Savu L, Lambert N, de Conchard GV, Grosse R, Mourey MS, Corman B. Plasma proteins as biomarkers of the aging process. Am J Physiol. 1995;268:R536–R548. doi: 10.1152/ajpregu.1995.268.2.R536. [DOI] [PubMed] [Google Scholar]
- Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age related proliferation defects in regenerating liver. PNAS. 2001;20:11468–11473. doi: 10.1073/pnas.201360898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang L, He Q, Feng X, Zhu J, Xu Z, Wang X, Chen F, Li X, Dong J. Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol Med Rep. 2012;5:410–414. doi: 10.3892/mmr.2011.640. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N (1997) Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 94(4):1441–1446 [DOI] [PMC free article] [PubMed]
- Zhu C, Ikemoto T, Utsunomiya T, Yamada S, Morine Y, Imura S, Arakawa Y, Takasu C, Ishikawa D, Shimada M. Senescence-related genes possibly responsible for poor liver regeneration after hepatectomy in elderly patients. J Gastroenterol Hepatol. 2014;29:1102–1108. doi: 10.1111/jgh.12468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS 37 kb)
(XLS 123 kb)
(XLS 646 kb)
(XLS 58 kb)
(XLS 43 kb)
(DOC 27 kb)