Abstract
Background and Objectives:
We sought to develop a simulation model that accurately replicates the challenges of the thoracoscopic intrathoracic anastomosis. This model is intended to serve as a teaching tool during the introduction to, and development of, the skills required to perform a thoracoscopic intrathoracic anastomosis during an Ivor Lewis minimally invasive esophagectomy.
Methods:
The simulation model uses porcine tissue placed within an artificial hemithorax and covered with a synthetic skin. The model is draped to simulate a realistic operative setting, and ports are placed in standard surgical fashion. Dissection of the esophagus from the mediastinum is then performed, followed by the creation of an esophagogastric anastomosis. The effectiveness of the training model was evaluated using volunteer general and thoracic surgery residents at varying stages of surgical training. The quality of the anastomoses created were evaluated using both objective and subjective criteria, and successful anastomoses were tested for leaks using hydrostatic pressure.
Results:
Objective evaluation showed that successful completion of the anastomosis task increased with the number of attempts, with 100% of participants successfully completing an anastomosis by the final attempt. The time to completion of a successful anastomosis also improved across successive attempts. Moreover, objective measures also showed improvement over time based on the graded quality of the completed anastomosis.
Conclusion:
As surgical techniques continue to evolve, so must the means by which they are taught. This simulation model shows effectiveness in the training of general and thoracic surgery residents performing thoracoscopic intrathoracic anastomosis during the Ivor Lewis minimally invasive esophagectomy.
Keywords: Esophageal, Esophageal cancer, Minimally Invasive Esophagectomy (MIE), Anastomoses, Simulation
INTRODUCTION
Surgical approaches for esophageal resection have evolved to include various open and minimally invasive esophagectomy (MIE) procedures. One such evolution has been the development of the Ivor Lewis MIE. The anastomosis is often considered the most critical portion of this technically challenging procedure because of the morbidity and mortality associated with leaks and strictures.1–3 Currently, there are a paucity of training models for esophageal surgical procedures in the adult patient.
We sought to develop a surgical simulation model that allows participants to practice the steps required to successfully complete a thoracoscopic intrathoracic anastomosis (TIA) and also allow for evaluation of the participants' performance.
MATERIALS AND METHODS
The simulation model consists of a bovine tissue block composed of an aorta, heart, lungs, trachea, esophagus, and stomach from Animal Lab (Tyler, TX). In preparation of the simulation model, a neo-esophagus is created from the stomach using GIA staplers (Covidien, New Haven, CT) along the lesser curve. Once the neo-esophagus is complete, the tissue block is placed within a synthetic hemithorax (VATS Trainers, Okemos, MI), which includes an artificial skin covering (Figure 1). Surgical ports are placed in fairly standard positions (Figure 2).4 The entire model is then draped in standard surgical fashion to simulate the operative setting as closely as possible. The tissue sample costs between $80 and $100 depending on its size. The staples used in initial trials were expired, and all surgical instruments and materials were donated by the host institution for the purpose of this project. The hemithorax and synthetic skin covering are not yet available for commercial purchase, and therefore no price can be quoted. All trials were performed on a single specimen and model, and all trials took place on the same day.
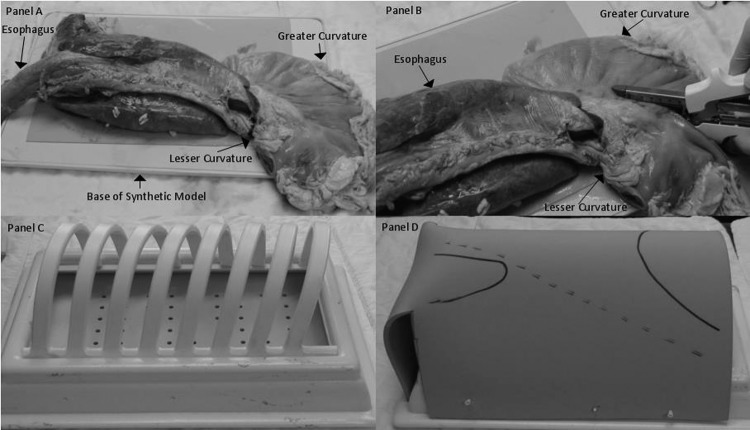
Figure 1.
Assembly of thoracoscopic intrathoracic anastomosis model. A, Bovine aorta, heart, lungs, trachea, esophagus, and stomach. B, Creation of neo-esophagus. C, Synthetic hemithorax. D, Hemithorax with artificial skin covering.
Figure 2.
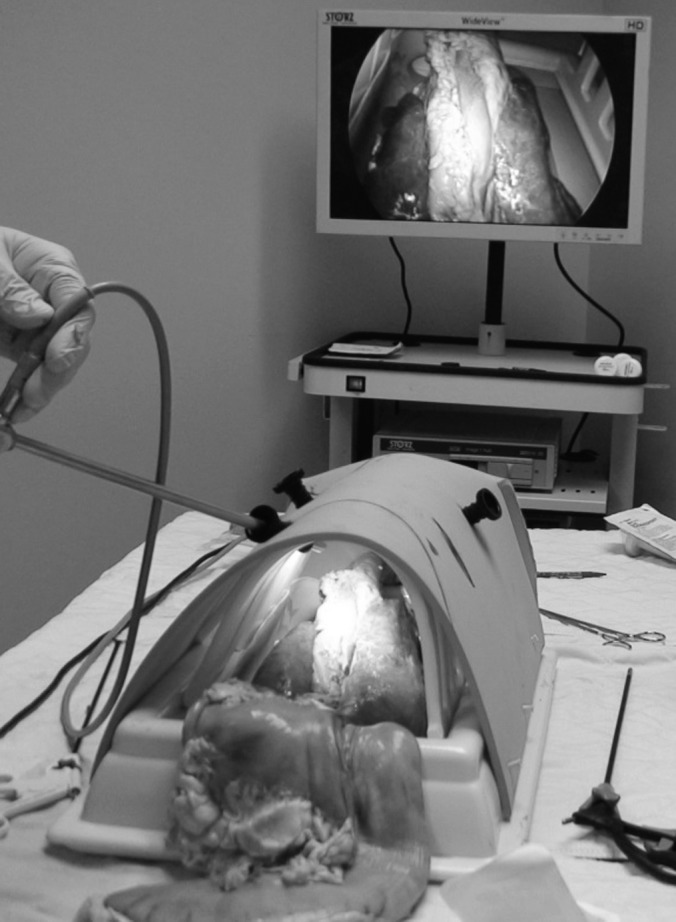
Fully assembled thoracoscopic intrathoracic anastomosis model with surgical ports and laparoscopic instruments.
Evaluation of this anastomosis model was performed using volunteer surgical residents in various stages of their training. Five participants between postgraduate year (PGY)–5 and PGY-7 volunteered to participate. No attending physicians were included as subjects. The PGY status of the participants was used as a means of quantifying surgical experience.
Participants were shown an instructional video on the Ivor Lewis MIE technique for creating an esophageal TIA. After the video, they were encouraged to ask technical questions regarding the technique. No instructions were given after the completion of questions, and the subjects received no assistance during the actual testing procedure.
Participants were tasked with placing a purse-string suture (Figures 3A and 3B) to secure a 25-mm EEA stapler (Covidien, New Haven, CT) and create an anastomosis (Figures 3C and 3D). Each of the 5 subjects performed 5 attempts. Each attempt was evaluated with objective and subjective criteria. The objective criteria included (1) ability to complete the task and (2) time to successful completion. The subjective criteria included evaluation of the quality of the purse-string suture as failure, poor, good, or very good by a nonblinded thoracic surgeon instructor. The criteria for each grade were as follows: failure, inability to create a functional anastomosis; poor, suboptimal anastomosis with technical deficits that would preclude the anastomosis from healing adequately; good, well-created anastomosis with minor technical irregularities that would most likely result in successful healing; and very good, anastomosis created without deficits.
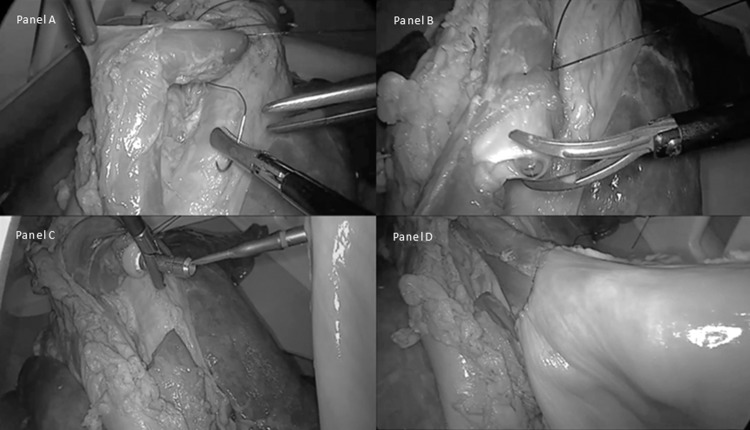
Figure 3.
A, Placement of purse-string suture on esophagus. B, Transection of esophagus after placement of purse-string suture. C, Alignment of EEA stapler and anvil. D, Successful anastomosis and creation of neo-esophagus.
In cases in which the participants completed the task with a very good evaluation, they were then asked to complete the anastomosis and fire the EEA stapler. After firing the EEA stapler, the anastomoses were evaluated for leaks using hydrostatic pressure of 60 mm Hg.
RESULTS
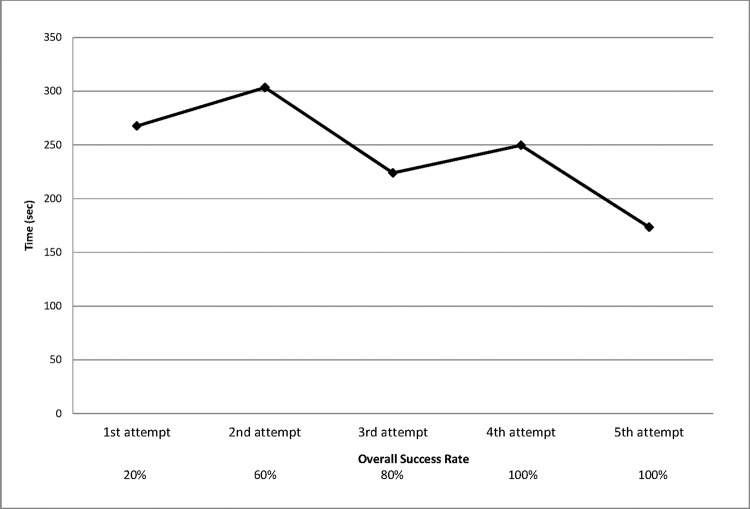
Successful completion of the purse-string task increased with the number of attempts. During the first attempt to perform the purse-string task, 1 of 5 participants (20%) was able to complete the task. On the second attempt, 3 participants (60%) successfully completed the task; on the third attempt, 4 participants (80%); and on the fourth and fifth attempts, 5 of 5 participants (100%). The overall time to complete the task improved with successive attempts (Figure 4). For the PGY-7 participant, with the largest number of successful trials, the time to the first successful attempt was 4 minutes 46 seconds and the time to the last successful attempt was 2 minutes 46 seconds. This trend of improvement was consistent across all participants.
Figure 4.
Time to completion of placement of anvil and purse string.
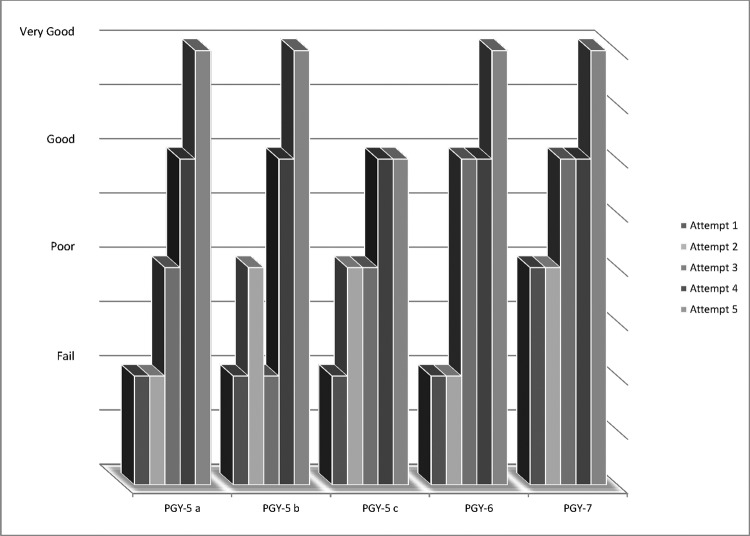
Each participant showed improvement in the subjective measurement of the completed task with repetition. All 5 participants were given a subjective grade of failure or poor on each of their first 2 attempts at the task, whereas 5 of 5 participants (100%) received a grade of good or very good on each of their last 2 attempts (Figure 5). On successful completion of anastomosis, the leak rate was 0% when measured with hydrostatic pressure of 60 mm Hg.
Figure 5.
Subjective grading of completed task based on postgraduate year.
DISCUSSION
Surgical simulation has increasingly been shown to be an important part of surgical education.5,6 As the technical complexity of surgical procedures increases, simulation will become more important for trainees. In the case of MIE, patients' outcomes are directly related to leak rates.7,8 As such, simulation may play an even more important role in teaching the techniques required to successfully perform a TIA.
This model provides a realistic reproduction of the unique challenges of performing a TIA. In an attempt to establish construct validation of the model, an experimental group composed of 3 PGY-5 general surgery residents and 2 thoracic surgery residents was asked to perform a critical portion of the TIA—specifically, placing a retrograde 25-mm EEA stapler and, in turn, securing the anastomosis with a purse-string suture as described by Bizekis et al.4 The level of training of each subject became apparent because the PGY-7 participant showed success earlier than his less experienced counterparts. Not surprisingly, the PGY-7 cardiothoracic surgery resident with the most experience was able to complete the task on the first attempt whereas none of the PGY-5 residents were able to do so.
The results indicate that use of the model and repetition of the exercise lead to (1) adoption of the skills necessary to complete the task, (2) improved speed to task completion, and (3) improved quality of the task performed. These points are demonstrated by our finding that 4 of 5 participants (80%) failed to complete the task on their first attempt whereas all participants were able to successfully complete the task by their third attempt. The trend seen in time improvement (Figure 4) and the subjective grade received on the task (Figure 5) are both indicative of improvement in the previously mentioned skill measures.
One concern is whether the task studied, placement of a retrograde anvil and purse-string suture, is of value. It is our opinion that in real-life surgery, although there are many technically challenging portions of Ivor Lewis MIE, the creation of the TIA is consistently challenging. This is true not only for intraoperative performance but also for its teaching. It is important to note that mobilization and transection of the esophagus, as well as correct positioning of the esophagus and stomach, are challenging maneuvers in the thoracoscopic setting. However, these steps were not individually identified as targets for evaluation in the initial testing of the model.
Use of this model allows for the education and development of the skills required to complete the described task in the operating theater. With repetition this should translate into confidence and precision in the actual operative setting. In future trials a larger participant group composed of individuals of comparable experience should be tested, starting at the beginning of training and continually as they gain both operative experience and further practice with the model. These longitudinal data would ideally be compared with data from trainees who did not use the model regularly during training. Subjective grading of intraoperative performance by an attending physician could also be used to evaluate the effectiveness of the training regimen. Another concern is whether time or subjective grading is an adequate measurement of improvement. Time itself is not a direct measurement of skill, but in this study it was used as an objective measure of skill development. Subjective grading may be construed as a weakness in the study design; however, in preparation for this study, we found that all completed anastomoses performed after purse-string failure or poor-quality purse-string suture placement resulted in leaks on hydrostatic testing. In addition, leaks were uncommon after good purse-string suture placement, and they were nonexistent after very good purse-string placement. Finally, the small size of this study limits the utility of analysis for statistical significance. These trials are meant to test the applicability of the simulation model for performing the task intended. Further testing is required to establish a sizable and reliable dataset and assess statistical significance.
CONCLUSION
As new surgical techniques evolve, acquisition of the new skills required to perform them is paramount. We believe that this novel surgical simulation model can contribute to the training of surgical residents and attending surgeons alike as they learn, practice, and perform the TIA technique.
Contributor Information
Thomas Fabian, Albany Medical College, and Section of Thoracic Surgery, Albany Medical Center, Albany, NY, USA..
Owen S. Glotzer, Albany Medical College, and Section of Thoracic Surgery, Albany Medical Center, Albany, NY, USA..
Charles T. Bakhos, Albany Medical College, and Section of Thoracic Surgery, Albany Medical Center, Albany, NY, USA..
References:
- 1. Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc. 2012;26(7):1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 3. Bakhos CT, Fabian T, Oyasiji TO, et al. Impact of the surgical technique on pulmonary morbidity after esophagectomy. Ann Thorac Surg. 2012;93(1):221–226; discussion 226–227. [DOI] [PubMed] [Google Scholar]
- 4. Bizekis C, Kent MS, Luketich JD, et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2006;82(2):402–406; discussion 406–407. [DOI] [PubMed] [Google Scholar]
- 5. Dawe SR, Pena GN, Windsor JA, et al. Systematic review of skills transfer after surgical simulation-based training. Br J Surg. 2014;101(9):1063–1076. [DOI] [PubMed] [Google Scholar]
- 6. Dawe SR, Windsor JA, Broeders JA, Cregan PC, Hewett PJ, Maddern GJ. A systematic review of surgical skills transfer after simulation-based training: laparoscopic cholecystectomy and endoscopy. Ann Surg. 2014;259(2):236–248. [DOI] [PubMed] [Google Scholar]
- 7. Yamagata Y, Kawashima Y, Yatsuoka T, et al. Surgical approach to cervical esophagogastric anastomoses for post-esophagectomy complications. J Gastrointest Surg. 2013;17(8):1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters RT, Goh YL, Veitch JM, Khalil BA, Morabito A. Morbidity and mortality in total esophagogastric dissociation: a systematic review. J Pediatr Surg. 2013;48(4):707–712. [DOI] [PubMed] [Google Scholar]