Abstract
Purpose:
To evaluate two drug delivery systems, a nonbiodegradable poly(2-hydroxyethyl methacrylate) (P[HEMA]) system with mitomycin C (MMC) and a biodegradable poly(lactic-co-glycolic acid) (PLGA) system with 5-fluorouracil (5-FU) with and without MMC for their ability to reduce fibrosis when attached to an Ahmed glaucoma valve (AGV) and implanted in a rabbit model.
Methods:
New Zealand albino rabbits (48) were divided into six equal groups, and AGVs, modified as described below, were implanted in the right eye of each rabbit. The groups included (1) PLGA alone; (2) P(HEMA) plus MMC (6.5 μg); (3) PLGA plus 5-FU (0.45 mg); (4) PLGA plus 5-FU (1.35 mg); (5) PLGA plus 5-FU and MMC (0.45 mg and 0.65 μg, respectively); (6) PLGA plus 5-FU and MMC (1.35 mg and 0.65 μg, respectively). The rabbits were followed for 3 months prior to euthanasia.
Results:
The bleb wall thickness was significantly less in groups 2, 5, and 6 compared to the rest. At 3 months, the PLGA polymer had completely disappeared, while the P(HEMA) polymer remained intact. There were no statistical differences in the degree of clinically graded conjunctival injection, histologic inflammation, or histologic fibrosis among the six groups.
Conclusions:
We successfully created a sustained-release drug delivery system that decreased the postoperative fibrosis using both a nonbiodegradable P(HEMA) polymer and a biodegradable (PLGA) polymer. Both systems appear to work equally well with no side effects.
Translational Relevance:
These results are supportive of the antifibrotic effect of the slow-release drug delivery system following glaucoma drainage device implantation, thus paving the way for human pilot studies.
Keywords: glaucoma slow-release drug delivery system, glaucoma drainage device, bleb fibrosis
Introduction
Management of intraocular pressure (IOP) is the mainstay of glaucoma treatment. Surgical treatment options include trabeculectomy and glaucoma drainage devices (GDDs). The Tube Versus Trabeculectomy Study recently demonstrated both short- and long-term advantages of GDDs over trabeculectomy,1,2 but success remains limited to approximately 70% at 1 year and 40% to 50% at 5 years, primarily because of the development of fibrosis, which may limit sufficient egress of shunted intraocular fluid. Strategies to attenuate fibrosis after trabeculectomy include 5-fluorouracil (5-FU) and mitomycin C (MMC) from Streptomyces caespitosus, and these antifibrotics have been shown to improve surgical outcomes of that procedure.3 However, use of antifibrotics solely at the time of GDD surgery has not been demonstrated to improve outcomes.4 The ideal tube implant would be immediately effective, show consistent behavior, and have long-term efficacy. More recent reports have demonstrated that adjunctive use of the antifibrotics MMC and 5-FU, both intraoperatively and postoperatively, with weekly injections for 5 weeks, resulted in a higher long-term success rate, lower rate of the hypertensive phase in the 3-week to 6-month period, and fewer complications.5 However, intraoperative application may be imprecise, and repeated postoperative interventions are both uncomfortable and risky for the patient, with the possibility of infection and postneedling bleb leaks. There is a clear need for a drug delivery system that can be installed intraoperatively to deliver antifibrotic drugs during the period of wound healing after GDD implantation.
Our laboratories have previously reported a process for loading drugs into poly(2-hydroxyethyl methacrylate) (P[HEMA]) hydrogels with demonstration of controlled, sustained release and inhibition of human fibroblast proliferation.6 We subsequently reported in vitro and in vivo experiments pairing P(HEMA) hydrogels loaded with various concentrations of MMC (0–0.80 mg/g of P[HEMA]) with Ahmed glaucoma valves (AGVs), a type of GDD, in a rabbit model. A favorable MMC release profile was found, and a statistically significant decrease in the thickness of the bleb fibrous capsule roof was demonstrated at all concentrations of MMC tested (total n = 22; P ≤ 0.05).7
The P(HEMA) hydrogel is a nonbiodegradable polymer, and the effects of this polymer on the bleb tissue architecture in the long term (beyond 3 months) remain unknown. We have therefore developed a biodegradable drug delivery system suitable for implantation in association with GDDs. Our laboratories have also recently reported the synthesis of a porous, poly(lactic-co-glycolic acid) (PLGA) film, summarized briefly in the Materials and Methods section below. This biodegradable film also had favorable in vitro release characteristics, and the released drug retained its ability to inhibit fibroblast proliferation.8 The present study continues this research with an in vivo analysis of the effect of this PLGA-based drug delivery system on bleb fibrosis in a rabbit model.
Materials and Methods
Drug Dosages
The P(HEMA) implants were infused with MMC at a concentration previously shown to reduce fibrosis in this rabbit model (0.17 mg of MMC per gram of dry gel, equivalent to 6.5 μg MMC in the polymer piece attached to the Ahmed valve).7
Early experiments designed to incorporate MMC into the PLGA matrix were unsuccessful because of the lability of this drug; 5-FU, in contrast, was stable when suspended as particles in PLGA.8 The doses of 5-FU used in this study were based upon the relative toxicity of MMC and 5-FU to fibroblasts in a tissue culture system8 and on our previous experiments showing that 6.5 μg of MMC infused into the P(HEMA) drug delivery system was sufficient to inhibit bleb fibrosis in the rabbit model.7 Based on these relative toxicity data, the 5-FU concentrations added to the PLGA films (0. 45 and 1.35 mg/polymer piece) were calculated to be equivalent to a dosage of 6.5 and 19.5 μg of MMC, respectively.
The amount of MMC loaded on the surface of the PLGA film was reduced tenfold as compared to the P(HEMA) system because of the differences in its release rate in the two drug delivery systems. Our previous experiments showed that MMC was released over an approximately 10-day period in the P(HEMA) system,7 while the surface-loaded MMC in the PLGA system was released in approximately 1 to 2 days.8
Implant Preparation
Polymers were prepared as previously described.6–8 The P(HEMA) polymer was prepared by redox polymerization of 2-hydroxyethyl methacrylate (130 Da) with 1% N,N′-methylene-bisacrylamide as cross-linker. The polymer was cast as a sheet between sealed glass plates. Impurities were removed and the P(HEMA) sheet (∼2 mm thick) was cut into semicircular discs measuring 5 × 6 mm to fit the lower half of the Ahmed valve plate (model FP-7). A 1-mm trephine was used to punch a hole in the middle of each semicircle so that the polymer could be attached to the valve with a silicon rivet.
The PLGA films were spin cast onto 8-mm silicon discs as described previously.8 The bottom layer was cast using 12.5% Resomer 506 (96 kDa; Boehringer Ingelheim Chemicals, Inc., Petersburg, VA). An appropriate weight of finely ground 5-FU (12 mg/mL to provide 0.9 mg per disc) was added and dispersed by bath sonication. These dispersions were subsequently spun onto 8-mm silicone discs (75 μL dispersion/disc; 200 rpm; 6 minutes) under humid conditions. As the solvent in the polymer solution evaporated, the surface cooling induced by the heat of vaporization led to the condensation of water droplets on the surface of the thickening solution. These droplets sank into the polymer film and created a porous surface with release characteristics different from a conventionally cast PLGA film (see Fig. 1C).9 A second, sealing layer was then coated by applying 50 μL of 15% Resomer RG 504 (56 kDa; Boehringer Engelheim Chemicals, Inc.) to the disc and spin-coating at 1000 rpm for 25 seconds under humid conditions. The thickness of the final double-layered PLGA film was 157 + 21 μm (n = 5). The films were removed from the 8-mm silicon disk, and MMC (1.3 μg in the total disc, 0.65 μg in the half disc) was subsequently loaded onto pores of the sealing layer in a subset of pieces. The MMC loading was achieved by dissolving the appropriate amount of MMC in CH2Cl2/tetrahydrofuran (5:1) and applying it to the top of the film. The circular films were cut in half, and a 1-mm hole was then made in the center of each semicircle using a trephine to permit attachment to the Ahmed valve. The films were sterilized by UV irradiation and then attached to the Ahmed valve using a medical grade silicone rivet.
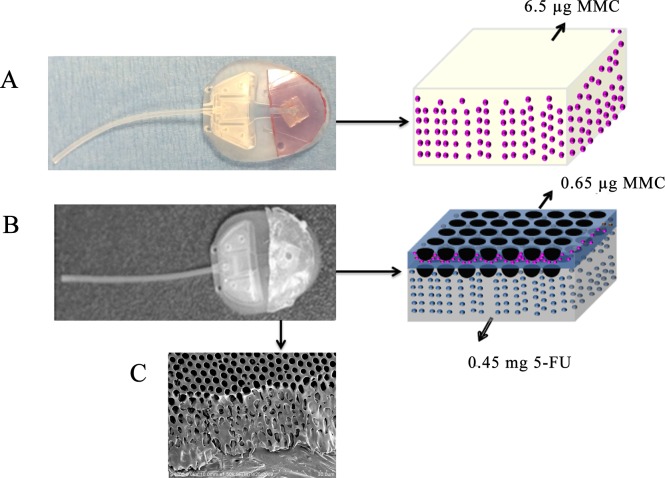
Figure 1.
Ahmed glaucoma valves modified with drug delivery devices. (A) Photo of MMC-loaded P(HEMA) attached to a model FP-7 AGV and schematic of MMC loading (pink dots) into the P(HEMA). (B) Photo of PLGA film loaded with MMC and 5-FU and attached to a model FP-7 AGV and schematic of drug loading (MMC, pink dots; 5-FU, blue dots). (C) Scanning electron micrograph of PLGA film cast using the breath figure method, demonstrating the porosity of the layers.
In Vivo Experiment
Previous in vitro experiments with the double-layered PLGA films have demonstrated that the surface-loaded MMC is released during the first 1 to 2 days after the film is hydrated; the 5-FU is subsequently released in a continuous fashion during the next 4 to 21 days.8 The cytotoxic effects of the released drug were also confirmed in this study using cultured fibroblasts, and the 5-FU plus MMC formulations showed more consistent effects, compared to PLGA films that contained only 5-FU, especially in the first 8 days of drug release.8
In vivo experiments were therefore performed to test the PLGA formulations for their effects on bleb fibrosis in order to verify their safety in an animal model and to compare the activity of the PLGA devices to the previously reported P(HEMA) device that contained only MMC.7 Our previous experiments have shown that the P(HEMA) polymer with no loaded drug had no effect on conjunctival fibroblast proliferation and that the P(HEMA) polymer, when attached to model FP-7 Ahmed valve, had no deleterious effects on the bleb or the rabbit.6,7 Because of these published results, a control group of animals implanted with an Ahmed valve modified with P(HEMA) alone was omitted from the current study. All animal experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, to regulations of the National Institutes of Health, and to the guidelines set forth by the Tulane University Institutional Animal Care and Use Committee.
Forty-eight male albino New Zealand rabbits (Covance, Denver, PA) were used in these experiments. The right eye of each rabbit was used for implantation of the AGV, and its IOP was measured prior to implantation using the Tonopen XL pneumatonometer (Reichert Ophthalmic Instruments, Buffalo, NY). The rabbits were divided into six groups. Group 1 (n = 8) received AGVs with a PLGA-coated semicircle without added drugs (control). Group 2 (n = 8) received AGVs with P(HEMA) polymers infused with 6.5 μg MMC. Group 3 (n = 8) received AGVs with PLGA containing 0.45 mg of 5-FU. Group 4 (n = 8) received AGVs with PLGA containing 1.35 mg 5-FU. Group 5 (n = 8) received AGVs with PLGA containing 0.45 mg of 5-FU and 0.65 μg of surface-loaded MMC. Group 6 (n = 8) received AGVs with PLGA containing 1.35 mg of 5-FU and 0.65 μg of surface-loaded MMC. Figure 1 shows photographs of the polymer-modified valves and a schematic of how the drugs were loaded into the polymers.
Surgical Procedure
The surgery was performed as previously described.10,11 After administering adequate anesthesia using ketamine hydrochloride, the right eye of the rabbit was prepared with povidone-iodine (Betadine; Purdue Pharma LP, Stamford, CN). A 7-0 polyglactin 910 (Vicryl; Ethnicon, Somerville, NJ) suture was passed through the supratemporal limbus to rotate the eye downward. Conjunctival peritomy was performed at the limbus in the supratemporal quadrant, followed by posterior dissection in the same plane. The dissection was carried further between the superior and lateral rectus muscles posteriorly. The AGV was brought to the operative site and primed with balanced salt solution. The end plate was tucked into the supratemporal quadrant and secured to the underlying episclera with two interrupted 10-0 nylon sutures approximately 7 mm from the limbus, as shown in Figure 2. The silicone tube was cut 0.5 mm anterior to the limbus using Westcott scissors. A 23-gauge butterfly needle was used to enter the anterior chamber 0.25 mm posterior to the limbus, and 0.1 mL of viscoelastic (Healon; Advanced Medical Optics, Inc., Santa Ana, CA) was injected. The silicone tube was inserted into the anterior chamber through the needle tract and secured to the surrounding sclera with a 10-0 nylon suture. The conjunctiva was secured to the limbus with an interrupted 10-0 polyglactin 910 suture. The same surgeon (RSA) performed all the surgeries in this study.
Figure 2.
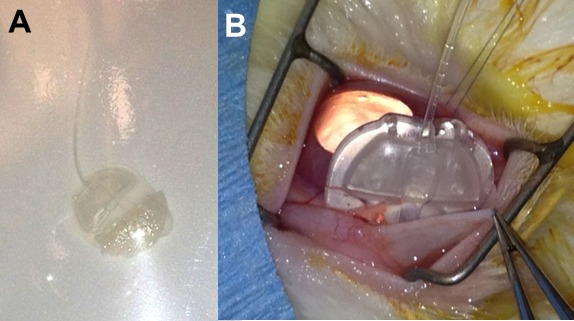
Surgical implantation of PLGA-modified Ahmed valve. (A) Ahmed valve ready for insertion. (B) Implantation of valve in subconjunctival space.
Postoperative Care
The eye was treated with ciprofloxacin drops, tobramycin-dexamethasone ointment (Tobradex; Alcon Laboratories, Inc., Fort Worth, TX) and cyclopentolate hydrochloride, 1% (Cyclogel; Falcon Pharmaceuticals, Ltd., Fort Worth, TX) four times a day for 1 week. All animal eyes were examined for signs of infection and tube erosion on days 1, 3, 7, and 14 and monthly thereafter. At 3 months after implantation, a blinded observer otherwise external to the study graded each surgical eye for conjunctival vascular hyperemia (conjunctival injection) as follows: grade 0, no hyperemia; grade 1, mild hyperemia; grade 2, moderate hyperemia; grade 3, severe hyperemia. Intraocular pressures were measured by Tonopen XL pneumatonometer (Reichert Ophthalmic Instruments) at 1, 2, and 3 months.
Enucleation and Examination
The animals were euthanized 3 months after implantation using U.S. Food and Drug Administration (FDA)– and Institutional Animal Care and Use Committee–approved procedures. The eyes were then enucleated, and care was taken not to disturb the bleb and the implant. The bleb was then sectioned through the middle of its roof and the AGV removed. The thickness of the bleb roof in each section was measured with digital calipers (resolution 10 μm, accuracy ±30 μm; VWR International, Radnor, PA) as shown in Figure 3. The AGV was then visually examined for the presence of attached polymer. Ten percent formalin was injected into the vitreous cavity, and the enucleated eye was stored overnight in 10% formalin. Histologic sections of the bleb and surrounding tissue were prepared and examined using light microscopy by a pathologist (CEM) who was blinded to the different groups.
Figure 3.
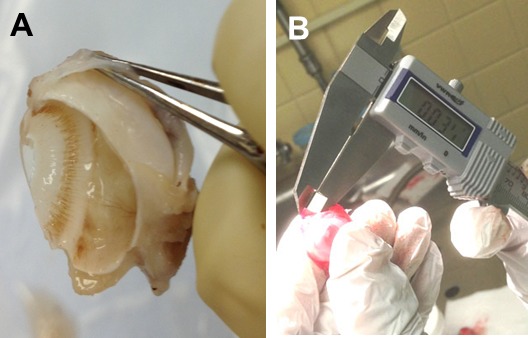
(A) Enucleation of eye and bleb after 3 months and (B) measurement of bleb roof with calipers.
Statistical Analysis
Statistical analysis was performed by a statistician (DZ) who was blinded to the different groups. One-way analysis of variance (ANOVA) followed by a posttest based on Student-Newman-Keuls analysis was used to compare the experimental groups to the group with a GDD modified with PLGA alone.
Results
There were no instances of infections, unexpected animal deaths, or other complications. Of the 48 eyes, 47 operations resulted in the formation of an elevated, vascular bleb; one operation, in the PLGA 1.35-mg 5-FU group, did not, due to improper placement of the AGV tube at the time of surgery. This eye, which failed due to surgical error unrelated to the experimental model, was excluded from this published analysis; however, including it in an auxiliary analysis did not change any measures of statistical significance.
Intraocular pressure showed significant group differences (F = 2.98, P = 0.02) preoperatively in control versus P(HEMA) (mean difference −4.5 mm Hg; P = 0.013) and in control versus PLGA 0.45-mg 5-FU plus 0.65-μg MMC group (mean difference −5.75 mm Hg; P = 0.002). At 1 month, significant group differences (F = 5.01, P < 0.001) were seen in control versus PLGA 1.35-mg 5-FU (mean difference +4.86 mm Hg; P = 0.004) and in control versus PLGA 1.35-mg 5-FU plus 0.65-μg MMC group (mean difference +6.70 mm Hg; P < 0.001). No group differences were seen at 2 months (F = 0.160, P = 0.18) and 3 months (F = 1.45, P = 0.23). As discussed below, the IOP group differences found, while statistically significant in some comparisons, do have mean differences near the range of error for Tonopen XL in albino New Zealand rabbits and therefore may not represent true variation.12
Conjunctival injection, graded by the blinded observer prior to anesthesia and enucleation, did not differ significantly between groups (ANOVA, F = 1.22, P = 0.32; see Fig. 4A). Inflammation, graded on a scale from 0 to 5 by light microscopy, was highly variable within each group, and there were no significant differences among the groups (ANOVA, F = 0.83, P = 0.53; Fig. 4B). Similarly, fibrosis, graded on a scale from 0 to 5 by light microscopy, was variable within groups and did not differ significantly (ANOVA, F = 1.79, P = 0.14; Fig. 4C). Due to shrinkage of the bleb capsule during the fixation process after sectioning the bleb roof for immediate measurement of fresh tissue, reliable measurements of the fibrous capsule lining the bleb, via light microscopy, was not possible.
Figure 4.
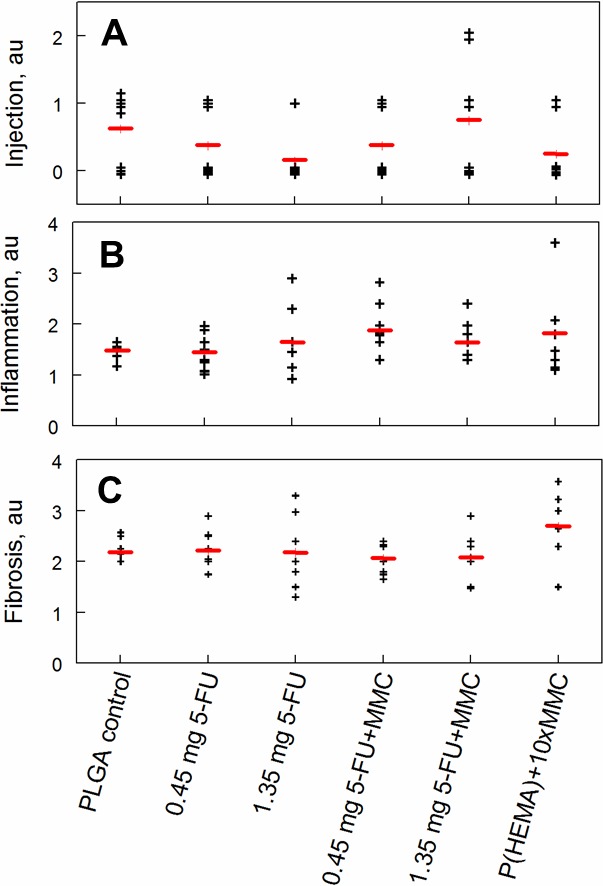
Conjunctival injection, bleb inflammation, and bleb fibrosis 3 months after GDD implantation in control and experimental groups. The black crosses represent scores for individual animals, and the red lines show the mean for each group. (A) Conjunctival vascular hyperemia (injection) was graded as follows: grade 0, no hyperemia; grade 1, mild hyperemia; grade 2, moderate hyperemia; grade 3, severe hyperemia. No significant differences were noted (ANOVA, F = 1.22, P = 0.317). (B), Inflammation was graded from 1 to 5 by a pathologist who was blinded to the groups. No significant differences were observed among the groups (ANOVA, F = 0.83, P = 0.53). (C) Fibrosis in the wall of the blebs was graded from 1 to 5 by a pathologist who was blinded to the groups. No significant differences were observed (ANOVA, F = 1.79, P = 0.14). au, arbitrary units.
Thickness of the bleb roof as measured by digital calipers showed significant group differences (Fig. 5). Bleb roof thickness of the control PLGA group was 544 μm (±170 μm). The P(HEMA) 65-μg MMC group bleb roof thickness was significantly thinner than control at 373 μm (±143 μm; P = 0.006). Both combination 5-FU plus MMC PLGA groups were significantly thinner than control. PLGA 0.45-mg 5-FU plus 0.65-μg MMC was 316 μg (±55 μm; P < 0.001). PLGA 1.35-mg 5-FU plus 0.65-μg MMC was 320 μm (±44 μm; P < 0.001).
Figure 5.
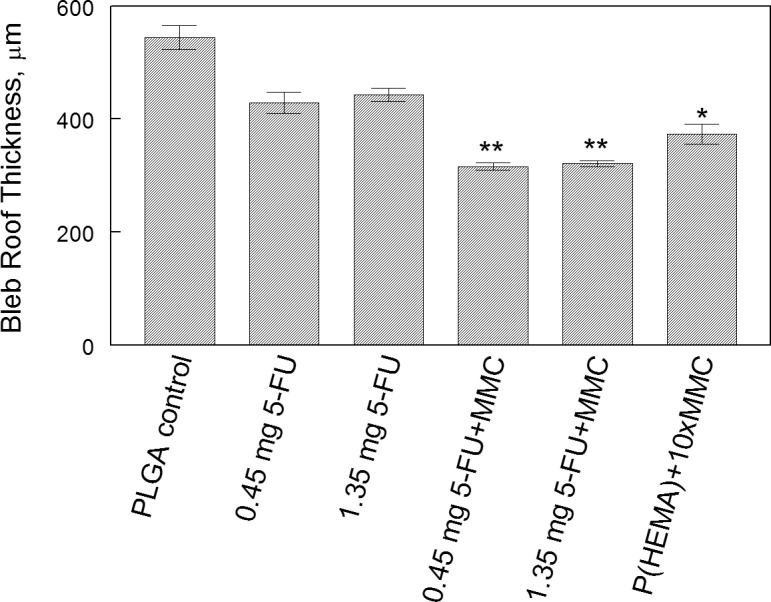
Bleb roof thickness by group. Data are expressed as mean ± SEM. Pairwise comparisons were performed, and groups that were significantly different from the PLGA control are indicated by asterisks. *P = 0.006; **P < 0.001.
At 3 months, the PLGA films had completely dissolved in all samples while the P(HEMA) samples remained intact. All P(HEMA) implants had lost their purple color and were colorless, indicating the release of MMC.7
Discussion
Postoperative fibrosis is the main cause of glaucoma surgery failure.13 The risk of glaucoma drainage device failure due to fibrosis is 10% per year. Excessive collagen around the end plate of the GDD leads to encapsulation that prevents aqueous humor from accessing the conjunctival blood vessels. Decreasing postoperative fibrosis has the potential not only to increase the success rate of the surgery, but also to potentially achieve lower IOP. Various techniques have been attempted to achieve this goal, including one-time intraoperative application of MMC at the time of surgery and sub-Tenon's injection of Kenalog in the surgical site, but neither was successful.4,14 Serial injections of initial MMC followed by 5-FU has been shown to be of some efficacy by Alvarado and coworkers.5 However, serial injections over several months are not an optimal solution for this problem given the expense, increased chair time, patient discomfort, and risks inherent in repeated needle injection. A slow-release drug delivery system that can release low but sustained concentrations of antifibrotic agents such as MMC and 5 FU over an extended period of time may attenuate the postoperative fibrosis, leading to better surgical results.
Hydrogels, hydrophilic polymer networks that are cross-linked into a three-dimensional biomaterial, have the ability to absorb thousands of times their dry weight in water, allowing them to mimic soft tissues.8 These types of polymers can be manipulated to encapsulate drugs and enhance biocompatibility. These polymers, by their nature, cannot be used as end plates, but can be used to coat existing end plates and deliver drugs that can decrease the postoperative fibrosis. The hydrogel P(HEMA) is commonly used in tissue engineering, contact lenses, and even drug delivery systems.9,3 This hydrogel contains water-filled pores that facilitate the passage of solutes. Additionally, P(HEMA) has a hydrophilic surface that minimizes interaction with body fluids and adhesion of proteins and cells. These qualities have made P(HEMA) a popular choice in the development of polymers with a range of biological applications, including adsorption of heparin, scaffolding of bone tissue, and selective albumin adsorption.10
Poly(lactic-co-glycolic acid) has become a popular polymer used in sustained drug delivery applications because it is biodegradable, biocompatible, and FDA approved. Degradation of PLGA is triggered under physiological conditions, resulting in breakdown products of lactic and glycolic acid, which can be eliminated via the tricarboxylic acid metabolic pathways.11 The recent innovation of “breath figures” has made it possible to create thin films of PLGA with extremely porous structures.12 These breath figure polymers are prepared by casting the polymer film under humid conditions. As the solvent in the PLGA solution evaporates, the resultant cooling leads to the condensation of water droplets onto the surface of the thickening solution. The greater density of the water droplets causes them to sink, generating nascent pores in the gel-like system, which continues to evolve to the solid polymer state with solvent evaporation. Eventually the solvent and the water evaporate, leaving pores in the polymer matrix. The number of pores, the size of the pores, and organization of the pores can be modified to modulate the rate of drug delivery.12 Poly(lactic-co-glycolic acid) breath figures have been successfully produced and have the potential to be a biocompatible slow-release drug delivery system that can be easily modulated.12 Both polymer systems can be used to promote the release of antifibrinogenic drugs in response to biological triggers, thus minimizing scar tissue formation and lengthening the lifetime of GDDs.
Our previous work focused on the development of a slow-release drug delivery device using P(HEMA) as the vehicle. This device worked well in decreasing the postoperative fibrosis in the animal model.7 However, P(HEMA) is a nonbiodegradable polymer and, as such, will remain attached to the end plate through the life of the GDD. Since P(HEMA) is an inert and biocompatible polymer, it worked well with the GDD model of plate and tube. However, to reduce the long-term hardware burden of the implant and to develop a system that might also be compatible with the newer plateless devices that drain the aqueous humor into the subconjunctival space (e.g., Ex-Press shunt) and/or suprachoroidal space (e.g., Gold shunt),15 we turned our attention to biodegradable polymers. The FDA has previously approved PLGA as a biocompatible, biodegradable polymer. The ideal drug delivery system would deliver the drug over approximately 1 month (the critical period of maximum postoperative fibrosis) and biodegrade. In addition, we wanted to limit the negative potential side effects of high concentrations of MMC, particularly the development of cystic avascular blebs, which predispose to bleb leaks and infection.16–18
The current rabbit study was designed to compare the efficacy of our P(HEMA)-MMC system to a new biodegradable PLGA-based drug delivery system that contained a tenfold lower dose of MMC in combination with 5-FU. Two other objectives of the present study were to study the potential toxic effects of PLGA as it degraded within the forming bleb and to confirm that the polymer actually degraded and fully disappeared after release of its embedded antifibrotics. The results presented here suggest that PLGA was not toxic to the bleb and that it did completely degrade and disappear by 3 months. This result is consistent with that reported by Peng et al.,19 who also reported the absence of toxicity/inflammation 6 months after subconjunctival implantation of PLGA films in rabbits. Incorporation of 5-FU alone in the PLGA polymer was not effective in reducing postoperative fibrosis in the bleb. This could have been due either to its lower potency or to the fact that its release was delayed until 4 to 6 days after hydration.8 However, when augmented with MMC, which was surface loaded and released during the first 1 to 2 days of hydration, the double-loaded films led to bleb walls that were significantly thinner than that of the control, as shown in Figure 3. Thus the PLGA delivery system was as effective as the P(HEMA)-MMC, even though it contained fivefold less MMC.
As more than 90% of the postsurgical inflammatory reaction is over by 3 months, it is not surprising to see that the inflammatory reaction was not different among the different groups. Conjunctival injection is a marker of vascular toxicity, and the lack of intergroup differences suggests that neither the concentration of the drugs nor the degradation of the PLGA polymer caused significant toxicity. This is supported as well by the lack of adverse events in this study, including scleral melt, bleb melt, bleb leak, or endophthalmitis. No rabbit developed an avascular bleb during the study. Thus, it appears that we have achieved the ideal state of reduced bleb wall thickness, without compromising the surface vasculature, using either the P(HEMA)-MMC system or the PLGA-MMC plus 5-FU system.
In the present study, we measured the thickness of the entire bleb roof immediately after tissue harvest rather than just the fibrous layer after fixation, sectioning and staining as was done in the previously described P(HEMA) rabbit study.7 We were still able to discern differences between the control valves and those modified with our drug delivery devices. The technique that we used to measure the roof thickness using a digital caliper was similar to the technique described previously by Ayyala et al.20 There are advantages to this technique because the tissue was unaltered by fixation and sectioning and was a true reflection of bleb fibrosis. This difference in analytical method also explains the differences in the bleb roof thickness between previous experiments and those reported herein. For example, the thickness of the fibrous capsule at the roof of the bleb in previously described P(HEMA) experiments was 20.8 μm in the control group versus 6.6 μm in the modified group.7 In the current experiments, the bleb wall (including the conjunctiva, tenons, and the fibrous capsule) was measured after sacrifice and before fixation. The thickness of the intact bleb wall was 544 μm (±170 μm) in the control group and 373 μm (±143 μm; P = 0.006) in the MMC-modified P(HEMA) group.
The limitation of this proof-of-concept study was the lack of long-term follow-up, since the rabbits were sacrificed at 3 months. In conclusion, we successfully created a sustained-release drug delivery system that decreased the postoperative fibrosis using both a nonbiodegradable P(HEMA) polymer and a biodegradable (PLGA) polymer. The systems appear to work equally well, with no side effects. Clinical trials will be necessary to demonstrate and validate the efficacy of these modified GDDs in humans.
Decisions have not yet been made regarding which system will ultimately be selected for clinical trials because so many other factors are involved in that process (ease of GMP manufacturing procedures, sterilization protocols, stability of product, etc.). The fact that both systems appear viable at this stage of development enhances the chances that some form of drug-modified GDD will ultimately reach the clinics.
Acknowledgments
Supported by a grant from the Louisiana Board of Regents (LEQSF(2010-2012)-RD-B-05) to DAB and by the Tulane Glaucoma Research Fund.
Disclosure: E.D. Schoenberg, None; F.B. Swann, None; A.W. Parlin, None; D. Zurakowski, None; C.E. Margo, None; D.A. Blake, (P); V.T. John, (P); T. Ponnusamy, (P); R.S. Ayyala, (P, R, I)
References
- 1. Gedde SJ,, Singh K, Schiffman JC,, Feuer WJ. Group TVTS. The Tube Versus Trabeculectomy Study: interpretation of results and application to clinical practice. Curr Opin Ophthalmol. 2012; 23: 118–126. [DOI] [PubMed] [Google Scholar]
- 2. Gedde SJ, Schiffman JC,, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012; 153: 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spaeth G,, Mutlukan E. The use of antimetabolites with trabeculectomy: a critical appraisal. J Glaucoma. 2001; 10: 145–151. [DOI] [PubMed] [Google Scholar]
- 4. Costa V, Azuara-Blanco A,, Netland P, Lesk M,, Arcieri E. Efficacy and safety of adjunctive mitomycin C during Ahmed Glaucoma Valve implantation: a prospective randomized clinical trial. Ophthalmology. 2004; 111: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 5. Alvarado J, Hollander D,, Juster R, Lee L. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: long-term outcomes. Am J Ophthalmol. 2008; 146: 276–284. [DOI] [PubMed] [Google Scholar]
- 6. Blake DA, Sahiner N,, John VT, et al. Inhibition of cell proliferation by mitomycin C incorporated into P(HEMA) hydrogels. J Glaucoma. 2006; 15: 291–298. [DOI] [PubMed] [Google Scholar]
- 7. Sahiner N,, Kravitz D, Qadir R,, et al. Creation of a drug-coated glaucoma drainage device using polymer technology: in vitro and in vivo studies. Arch Ophthalmol. 2009; 127: 448–453. [DOI] [PubMed] [Google Scholar]
- 8. Ponnusamy T, Yu H,, John VT, Ayyala RS,, Blake DA. A novel anti-proliferative drug coating for glaucoma drainages devices. J Glaucoma. 2014; 23: 526–534. [DOI] [PubMed] [Google Scholar]
- 9. Ponnusamy T, Lawson LB,, Freytag LC, Blake DA,, Ayyala RS, John VT. In vitro degradation and release characteristics of spin coated thin films of PLGA with a “breath figure” morphology. Biomatter. 2012; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ayyala R, Harman L,, Michelini-Norris B, et al. Comparison of different biomaterials for glaucoma drainage devices. Arch Ophthalmol. 1999; 117: 233–236. [DOI] [PubMed] [Google Scholar]
- 11. Ayyala R,, Michelini-Norris B, Flores A,, Haller E, Margo C. Comparison of different biomaterials for glaucoma drainage devices: part 2. Arch Ophthalmol. 2000; 118: 1081–1084. [DOI] [PubMed] [Google Scholar]
- 12. Lim K, Wickremasinghe S,, Cordeiro M, Bunce C,, Khaw P. Accuracy of intraocular pressure measurements in New Zealand white rabbits. Invest Ophthalmol Vis Sci. 2005; 46: 2419–2423. [DOI] [PubMed] [Google Scholar]
- 13. Rao K, Ahmed I,, Blake DA,, Ayyala RS. New devices in glaucoma surgery. Expert Rev Ophthalmol. 2009; 4: 491–504. [Google Scholar]
- 14. Hong C, Arosemena A,, Zurakowski D, Ayyala R. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005; 50: 48–60. [DOI] [PubMed] [Google Scholar]
- 15. Ayyala R, Duarte J,, Sahiner N. Glaucoma drainage devices: state of the art. Expert Rev Med Devices. 2006; 3: 509–521. [DOI] [PubMed] [Google Scholar]
- 16. Kessing SV, Flesner P,, Jensen PK. Determinants of bleb morphology in minimally invasive clear-cornea micropenetrating glaucoma surgery with mitomycin C. J Glaucoma. 2006; 15: 84–90. [DOI] [PubMed] [Google Scholar]
- 17. Mochizuki K,, Jikihara S, Ando Y,, Hori N, Yamamoto T,, Kitazawa Y. Incidence of delayed onset infection after trabeculectomy with adjunctive mitomycin C or 5-fluorouracil treatment. Br J Ophthalmol. 1997; 81: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand N, Arora S,, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity transconjunctival oozing, and leaks. Br J Ophthalmol. 2006; 90: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng Y,, Ang M, Foo S,, et al. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS One. 2011; 6: e22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ayyala R, O'Brien C,, Grierson I, Maltby P. Transscleral flow of aqueous humor: an in vitro experiment. Ophthalmic Surg Lasers. 1996; 27: 66–69. [PubMed] [Google Scholar]