Abstract
Purpose:
To examine the relationship between Motor Vehicle Collisions (MVCs) in drivers with glaucoma and standard automated perimetry (SAP), Useful Field of View (UFOV), and driving simulator assessment of divided attention.
Methods:
A cross-sectional study of 153 drivers from the Diagnostic Innovations in Glaucoma Study. All subjects had SAP and divided attention was assessed using UFOV and driving simulation using low-, medium-, and high-contrast peripheral stimuli presented during curve negotiation and car following tasks. Self-reported history of MVCs and average mileage driven were recorded.
Results:
Eighteen of 153 subjects (11.8%) reported a MVC. There was no difference in visual acuity but the MVC group was older, drove fewer miles, and had worse binocular SAP sensitivity, contrast sensitivity, and ability to divide attention (UFOV and driving simulation). Low contrast driving simulator tasks were the best discriminators of MVC (AUC 0.80 for curve negotiation versus 0.69 for binocular SAP and 0.59 for UFOV). Adjusting for confounding factors, longer reaction times to driving simulator divided attention tasks provided additional value compared with SAP and UFOV, with a 1 standard deviation (SD) increase in reaction time (approximately 0.75 s) associated with almost two-fold increased odds of MVC.
Conclusions:
Reaction times to low contrast divided attention tasks during driving simulation were significantly associated with history of MVC, performing better than conventional perimetric tests and UFOV.
Translational Relevance:
The association between conventional tests of visual function and MVCs in drivers with glaucoma is weak, however, tests of divided attention, particularly using driving simulation, may improve risk assessment.
Keywords: glaucoma, driving, visual function, visual field, perimetry
Introduction
Inability to drive is a major concern for patients with glaucoma, especially as in many regions driving is important for maintaining independent living and quality of life.1 In fact driving cessation is associated with higher risk of depressive symptoms,2 social isolation, and entry into long-term care,3 and patients with glaucoma are more likely to limit or cease driving compared with healthy individuals.1,4,5 On the other hand, continued driving in the presence of impaired vision increases the risk of involvement in a motor vehicle collision (MVC).6,7 A previous study reported drivers with glaucoma to have a three-fold increase in odds of accident compared with controls.8 It is therefore important to correctly identify drivers with glaucoma at high risk of MVC, while avoiding penalization of low risk drivers.
The routine evaluation of visual function in glaucoma is based on visual field testing using standard automated perimetry (SAP). Although driving is a highly visual task, studies have shown only weak correlation between MVCs and conventional tests of visual function such as SAP.5,9,10 This is likely because conventional visual sensory tests are performed under artificial conditions with minimal visual distractions, whereas the ability to deal with visual distractions, to “divide attention,” or “multi-task,” is essential for most daily activities, including complex cognitively demanding activities such as driving.5,7
Divided attention specifically requires processing and/or responding to information from one task while simultaneously conducting another, which in the case of driving involves continuously monitoring information from the roadway to control the vehicle, while simultaneously maintaining awareness of potential hazards surrounding the vehicle.11 This requires attention to be distributed across the driving scene. As the cognitive system has a limited amount of attentional resources, the quality and efficiency of a particular task may be compromised if performed under a divided attention situation. Indeed, failures to divide attention have been identified as a leading cause of MVCs, accounting for up to 50% of incidents,7,12,13 and also are a powerful predictor of impaired ability to perform other daily activities such as walking.5,7,11,14
Difficulties with divided attention tasks seem to be related to slowing of the visual processing speed,9 which can be defined as the amount of time needed to make a correct judgment about a visual stimulus.15,16 The Useful Field of View (UFOV) Test (Visual Awareness, Inc., Chicago, IL) is a computerized test developed by Ball et al.9 to evaluate processing speed with and without conditions of divided attention.11,17 The UFOV test is based on the findings from behavioral studies that suggested that older adults struggle with visual search due to a reduction in the size of the perceptual window. This results in subjects needing to take smaller samples of a visual scene and scan each sample more slowly, effectively reducing their field of useful view. Initial studies suggested that slower divided attention processing speed on the UFOV test could be predictive of increased risk of MVC,9,11,13,17 however, recently there has been evidence contrary to this.16,18,19 Driving simulation is an alternative method for evaluation of ability to divide attention as related to driving that may have potential advantages over UFOV, especially as the costs of driving simulation technology come down. As driving simulation offers a scenario that more closely resembles an actual driving task, one would expect that driving simulation could provide a better means to assess risk of MVC in drivers with glaucoma.5
The purpose of the current study was to examine the association between measures of divided attention during driving simulation and history of recent MVC in patients with glaucoma and to compare this to conventional perimetric measures and divided attention measured using the UFOV.
Methods
This was a cross-sectional observational study of 153 subjects with glaucoma from the Diagnostic Innovations in Glaucoma Study (DIGS): functional impairment, conducted at the Visual Performance Laboratory of the Department of Ophthalmology, University of California San Diego (UCSD). Informed consent was obtained from all participants, and the UCSD institutional review board and human subjects committee prospectively approved all methods. All study methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects and the study was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act.
At each visit, subjects underwent comprehensive ophthalmologic examination including review of medical history, visual acuity, contrast sensitivity assessment using the Pelli-Robson contrast sensitivity chart (Precision Vision, La Salle, IL), slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, and SAP using the Swedish interactive threshold algorithm (SITA Standard 24-2; Carl Zeiss Meditec, Inc., Dublin, CA). Optic disc photographs were graded using a previously described method.20,21 Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented with a best-corrected visual acuity of less than 20/40, spherical refraction outside ±5.0 diopters (D) or cylinder correction outside 3.0 D, or any other ocular or systemic disease that could affect the optic nerve or the visual field. Glaucoma was defined by the presence of three or more consecutive abnormal SAP tests or evidence of progressive glaucomatous optic disc changes based on masked assessment of stereophotographs.
Standard Automated Perimetry
SAP was performed using the Humphrey Field Analyzer II (Carl Zeiss Meditec). All visual fields were evaluated by the UCSD Visual Field Assessment Center.22 Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors, were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease. An abnormal SAP test was defined as a visual field with a pattern standard deviation with P less than 0.05 and/or a Glaucoma Hemifield Test outside normal limits. Binocular SAP sensitivities were calculated from monocular SAP sensitivities using the binocular summation method described by Nelson-Quigg and colleagues.23
Useful Field of View (UFOV)
The UFOV was used to assess visual processing speed in milliseconds with and without conditions of divided attention. The test has been described in detail elsewhere.9,11,17 In brief, processing speed was initially evaluated by instructing the subject, using both eyes, to discriminate a foveal or central vision target (image of a car or truck) located in the center of a 17-inch touchscreen (subtending a 3° × 5° visual angle). Recognition of the target was registered by asking the patient to touch the screen to indicate which target was shown. During the test the presentation time was increased following an incorrect response and decreased following a correct response so that test results could be presented as the time needed to achieve a stable 75% accuracy for detection. The processing speed test was followed by a divided attention test, during which the same central discrimination task (image of a car or truck) was presented in addition to a concurrent peripheral localization task (an image of a car presented on one of eight radial spokes at a fixed eccentricity of approximately 11°). During this test the patient was asked to report on which spoke the outside object was located and the duration of presentation was increased or decreased depending on responses with the test result again presented as the time needed to achieve a stable 75% accuracy for detection. All subjects had prior experience of the UFOV test, having performed at least one test previously.
Driving Simulator
The ability to divide attention was assessed by measuring reaction times to stimuli presented during a divided attention protocol during simulated driving. The driving simulator, which has been described previously, consisted of a typical driving seat, a steering wheel, brake, and accelerator pedals, and a 40-inch screen (Fig. 1).10
Figure 1.
Driving simulator screen shot taken during the car following divided attention task. The divided attention stimulus is the gray symbol shown on the right hand side.
The driving simulator tested the ability to attend simultaneously to one of two central visual tasks of driving (adjusting speed while following another car that varies its speed or staying in a lane on a winding road) and to a peripheral visual task of perceiving a projected stimulus and responding by pushing a button on the steering wheel. The peripheral stimuli were presented at approximately 20° of visual angle in the upper right and upper left of the driving simulator screen and at three different contrasts (low, medium, and high). The contrast of the stimulus was altered using alpha blending techniques to achieve symbol transparencies of 0.1, 0.4, and 0.9. Therefore in the case of 0.1 symbol transparency, the symbol intensity and color that the driver perceived was 10% of the symbol intensity and color and 90% of the background intensity and color. The equivalent Michelson contrasts were 0.04, 0.14, and 0.27 for low-, medium-, and high-contrast stimuli, respectively. At maximum screen intensity the divided attention stimulus symbols were pure white, while the background was constant and consisted of a cloudy sky. There were an average of five stimuli presented at each contrast for each central driving task (a total of ∼15 per 3 minutes or ∼1 every 12 seconds) and stimuli stayed on the screen for a maximum of 3 and 6 seconds (uniform distribution) or until the driver responded. The next stimuli appeared between 3 and 6 seconds (again uniform distribution) after the driver responded or when the maximum display time had elapsed.
The main outcome measure of “reaction time” was defined as the time interval between appearance of the peripheral stimulus and the subject pressing the button, with a longer reaction time indicating worse performance. The mean reaction time for each central task (curve negotiation and car following) and contrast (low, medium, high) was calculated, giving a total of six sets of reaction times for each subject, and the false positive percentage, which was defined as the number of button presses occurring when no stimulus had been presented divided by the total number of stimuli presented, was calculated to assess speed-accuracy tradeoffs.10 False negative rate was calculated as the percentage of stimuli presented without the patient registering a response.
Reaction time was chosen as the outcome variable as difficulties with divided attention tasks seem to be related, at least in part, to a slowing of visual processing speed. Visual processing speed is common- ly studied in behavioral research by measuring reaction times.5,11,15,16,24 The use of reaction times has some limitations as the registration of a reaction requires a motor response (the act of pressing a button), in addition to lower and higher-order sensory functions. However, a large component of reaction time is the speed at which sensory data are carried to the brain, which depends on structural aspects of neural wiring and conduction.25 Reactions times are prolonged under more demanding conditions, such as with low contrast stimuli. However, if a stimulus is perceived, the motor response for a particular subject is likely to be constant regardless of contrast. Therefore, to minimize the possible confounding effect of motor response in reaction times, the difference in reaction times to the low and high contrast stimuli was calculated, with the aim of isolating the visual processing component.
Driving Tasks
Curve Negotiation
During the curve negotiation task, the driver was presented with a winding, three-lane road and was instructed to drive in the center lane. The velocity of the vehicle was constant such that the driver only had to operate the steering wheel. The vehicle speed was set at 15 m/s (54 km/h) for the first half of the test, increasing to 25 m/s (90 km/h) for the second half of the test.
As a subject might achieve fast reaction times by adopting a strategy in which the driving task is neglected, it was important to assess central driving task performance.26 This was measured using “curve coherence,” which was defined as the normalized cross-correlation function between the road curvature and the vehicle path curvature as a function of spatial shift. Curve coherence was calculated using the following equation, where n is the number of samples of the two signals and SD is the standard deviation of the signals, with a coherence of 1 indicating the two signals to be an exact match.
 |
Car Following
The second task was a car following task, during which the driver was instructed to drive down a straight road following a leading police car. The subject was instructed to follow the lead vehicle at a short distance, controlling the gas pedal and brake. The speed of the lead vehicle fluctuated according to a multisine function with frequencies chosen to achieve normal traffic speed fluctuations (0.028, 0.039, 0.061, 0.094, and 0.128 Hz).26,27 This yielded a SD in the acceleration profile of 1.4 m/s2 with three events with decelerations exceeding 3 m/s2 and three events with acceleration exceeding 3.0 m/s2. To facilitate a symmetric acceleration profile, the vehicle was boosted in its acceleration capabilities.
Central driving task performance was assessed using “speed coherence,” which is similar to the curve coherence measure calculated for the curve negotiation task. Speed coherence is a measure of the accuracy with which the driver can reproduce the lead vehicle speed fluctuations and was calculated using the speed cross correlation function, obtained according to the following equation26:
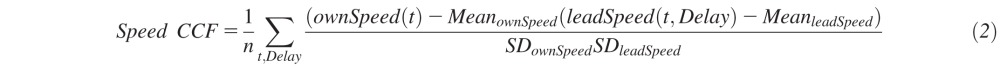 |
Where CCF is the cross correlation function, n is the number of samples of the two signals and SD is the standard deviation of the signals. Speed coherence was defined as the maximum correlation observed in the CCF; generally observed as some delay. The larger the coherence the better the driver was able to follow the lead car fluctuations, with a coherence of 1 indicating that the two speed signals match exactly.
To minimize the effect of unreliable tests and learning effect, all subjects underwent driving simulator training prior to test commencement. Training consisted of 2 minutes practice acceleration and deceleration, followed by 1 minute of each of the car following and curve negotiation tasks. All subjects also completed a short driving habits questionnaire to ascertain how many at fault accidents they had been involved in during the last 3 years, and average mileage driven per week. The Montreal Cognitive Assessment was also completed. This is a 30-point, 10-minute cognitive screening tool developed to detect mild cognitive impairment, which is similar to the Mini-Mental State Examination but has additional subtests focusing on aspects of attention relative to driving.28
Statistical Analysis
The ability of SAP, UFOV, and driving simulator parameters to distinguish those with and without a history of MVC was evaluated using receiver operating characteristic (ROC) curves with the area under the ROC curve (AUC) used to summarize the diagnostic accuracy of each parameter, where an AUC of 1.0 represents perfect discrimination and an AUC of 0.5 represents chance.29 ROC curves were adjusted for age differences between cases and controls using a previously described ROC regression technique30,31 and confidence intervals (CIs) were obtained using a bootstrap resampling procedure (n = 1000 resamples). The ability of each measure to predict history of MVC was also investigated using odds ratios (OR) followed by multivariate logistic regression controlling for potentially confounding factors including age and average distance driven per week. All statistical analyses were performed with commercially available software (Stata, version 13; StataCorp LP, College Station, TX). The α level (type I error) was set at 0.05.
Results
The study included 153 subjects with glaucoma with a mean (±SD) age of 67.2 ± 9.2 years. Eighteen of 153 subjects (11.8%) reported a recent history of MVC with the demographic and clinical characteristics of subjects summarized in Table 1.
Table 1.
Summary of Demographic and Clinical Characteristics (Mean ± SD [Median, Interquartile Range]) of Drivers With Glaucoma With and Without a Recent History of MVC
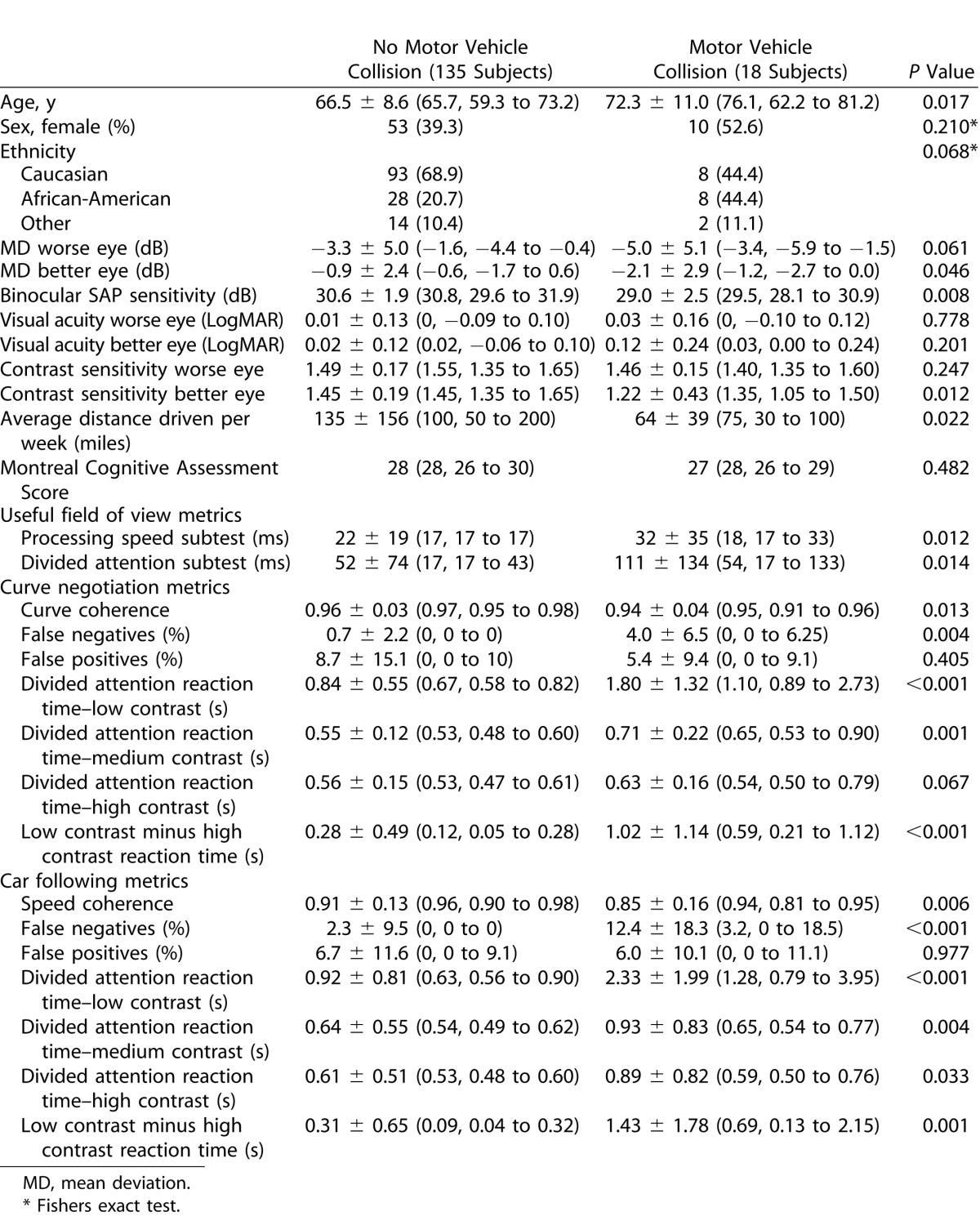
Drivers with a history of MVC were significant older than those without, however there was no difference in sex, ethnicity, or cognitive ability between groups. Those with a recent MVC had worse mean deviation (MD) in the better eye and binocular SAP sensitivity. Although visual acuity was similar between groups, contrast sensitivity in the better eye was worse in those reporting a recent MVC. Drivers reporting a MVC also tended to drive fewer miles than those not reporting a MVC (mean average mileages per week of 64 versus 135 miles respectively, P = 0.022).
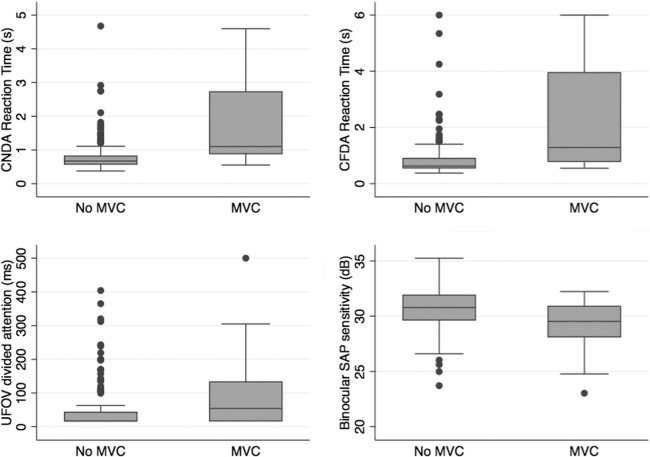
The MVC drivers had slower UFOV processing speeds with and without conditions of divided attention and had longer driving simulator divided attention reaction times for low-, medium-, and high-contrast stimuli for both driving tasks (Table 1 and Fig. 2). The greatest differences were for the low-contrast driving simulator divided attention tasks. For example, the average reaction time to the low-contrast divided attention stimulus during the curve negotiation time was 1.80 seconds in the MVC group compared with 0.84 in the no MVC group (P < 0.001) with corresponding values of 2.33 seconds and 0.92 seconds for the car following task (P < 0.001). The “motor-response corrected” driving simulator divided attention reaction times were also significantly longer in the MVC group (i.e., there was a greater difference between low- and high-contrast reaction times in the MVC group). Patients with a recent history of MVC also performed significantly worse on the central driving tasks (curve coherence [P = 0.013] and speed coherence [P = 0.006]) and had a higher rate of false negative responses to the divided attention stimuli than the no MVC group (Table 1). However, both groups had similar rates of false positives, indicating the differences in reaction times was unlikely to be due to speed-accuracy tradeoff.10
Figure 2.
Box plots showing the distribution of reaction times to the low contrast curve negotiation divided attention (CNDA) and car following divided attention (CFDA) driving simulator tasks in those with and without a history of MVC compared with UFOV divided attention task and binocular SAP sensitivity in the same subjects.
Table 2 shows the ORs from univariable logistic regression analyses and ORs adjusting for age, for each of the variables. Worse MD in the better or worse eyes were not significantly associated with increased odds of recent MVC (P = 0.059 and P = 0.193, respectively), however worse binocular SAP sensitivity was (P = 0.003), even after adjusting for age (P = 0.043). Visual acuity and contrast sensitivity in the better eye were also associated with MVC, however visual acuity became insignificant when age differences were accounted for.
Table 2.
ORs and Age-Adjusted AUCs for SAP, UFOV, and Driving Simulator Divided Attention Tasks, for Discriminating Drivers With Glaucoma With and Without a History of Recent MVC
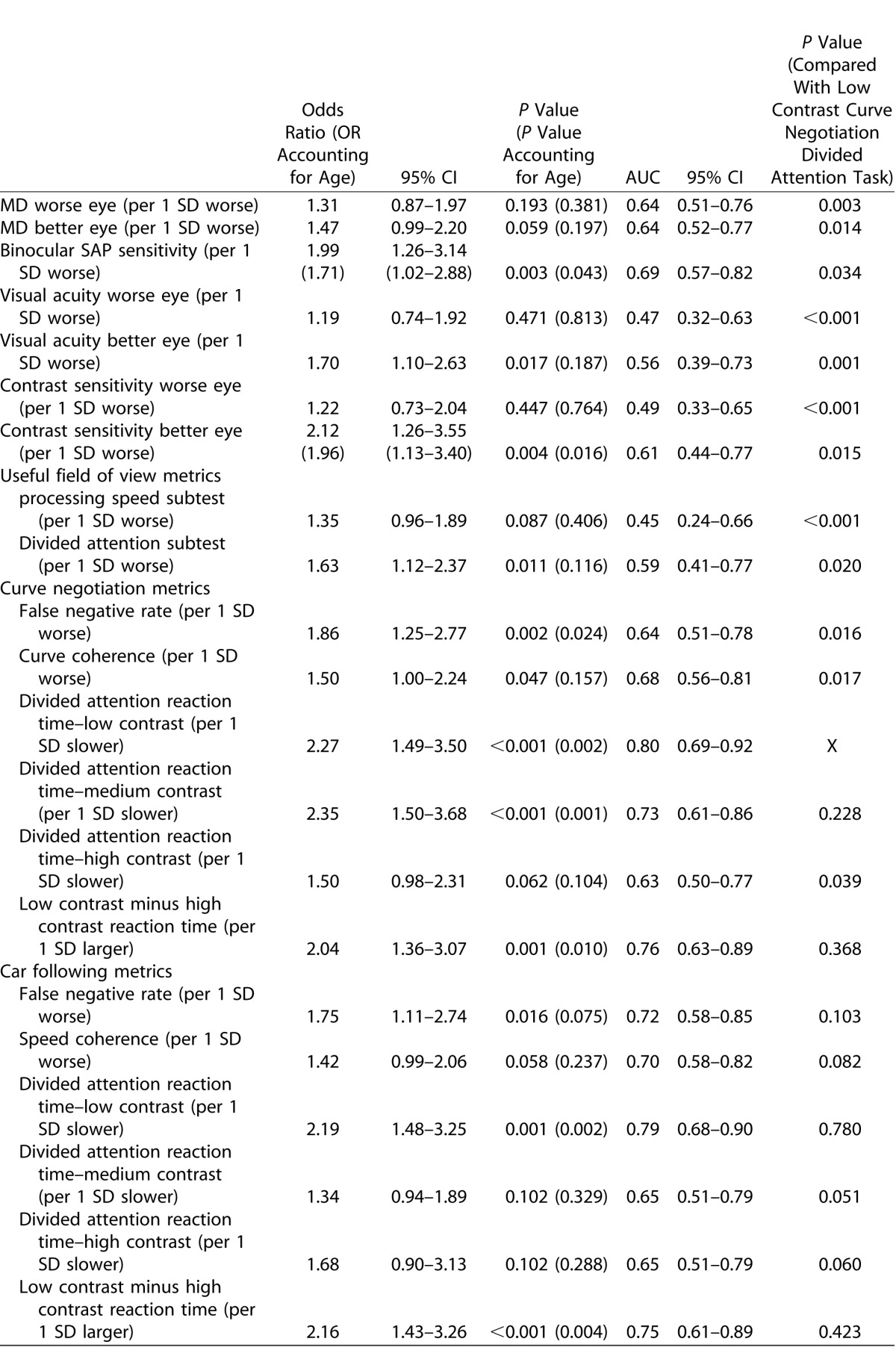
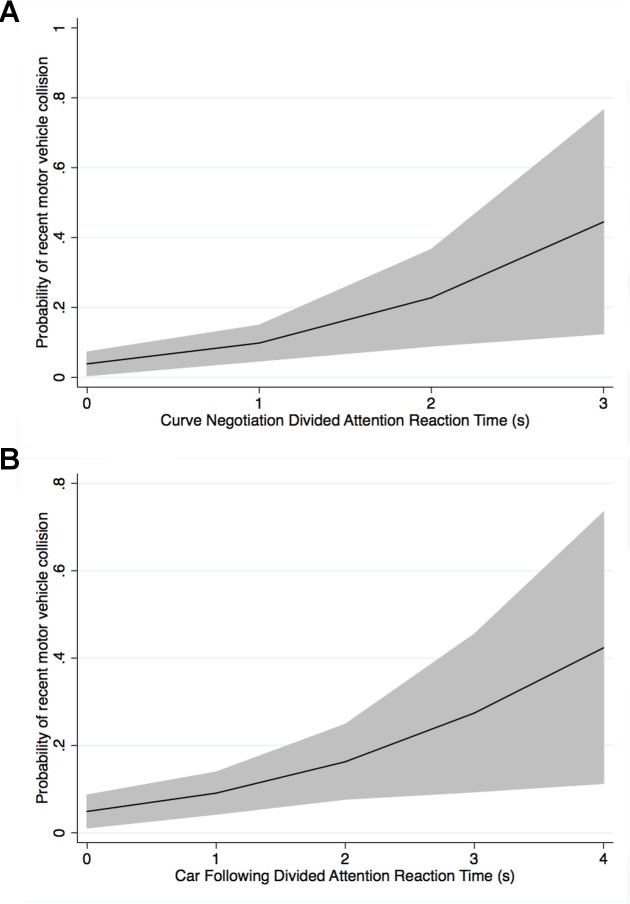
Worse performance on the UFOV divided attention test was associated with increased odds of MVC, but this association also diminished when differences in age between groups were accounted for. The variables with the strongest association with MVC were the reaction times to the low contrast-driving simulator divided attention tasks (P < 0.001 for curve negotiation and car following tasks), although the “motor-response corrected” driving simulator divided attention reaction times were also significantly associated with MVC (Table 2). Although higher driving simulator divided attention task false negative rates were also associated with increased odds of MVC for the curve negotiation and car following tasks, prolonged reaction time to the perceived divided attention stimuli was associated with higher odds. The relationship between reaction times to the low-contrast curve negotiation and car following driving simulator divided attention tasks, and predicted probability of MVC from the logistic regression adjusted for age is shown in Figure 3.
Figure 3.
Relationship between predicted probability of motor vehicle collision for drivers with glaucoma and reaction times to the driving simulator divided attention tasks for a patient at the sample mean age of 67.2 years (shaded areas represent the 95% confidence limits for the predicted probabilities).
Using ROC analysis, the parameter with the best ability to discriminate drivers with and without a history of MVC was reaction time to the curve negotiation divided attention task under low contrast (Table 2 and Fig. 4), which had an AUC of 0.80 (95% CI 0.69–0.92). This was very similar (P = 0.780) to the low-contrast divided attention task for the car following task (AUC = 0.79, 95% CI 0.68–0.90). Binocular SAP sensitivity and the UFOV divided attention tasks were significantly worse with AUCs of 0.69 and 0.59, respectively (P = 0.034 and P = 0.020, respectively for comparison with best driving task). The difference between low- and high-contrast reaction times also performed well with AUCs of 0.76 and 0.75 for curve negotiation and car following. The central driving task achieved AUCs of 0.68 for curve negotiation (curve coherence) and 0.70 for car following (speed coherence) and false negative rates to the driving simulator divided attention stimuli achieved AUCs of 0.64 and 0.72 for the curve negotiation and car following tasks respectively. MD, visual acuity, and contrast sensitivity in the better and worse eyes performed significantly worse than the best performing driving simulator tasks.
Figure 4.
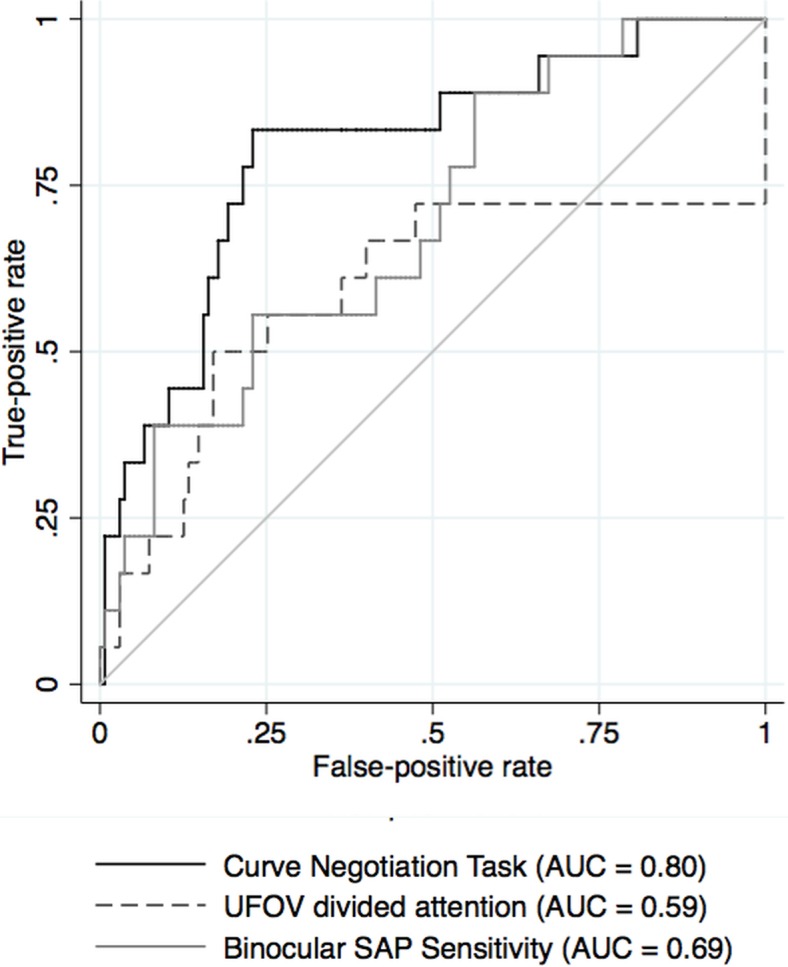
ROC curves showing the ability of reaction time to the low contrast curve negotiation divided attention driving simulator task, UFOV divided attention subtest, and binocular SAP sensitivity to differentiate drivers with glaucoma with and without a history of motor vehicle collisions.
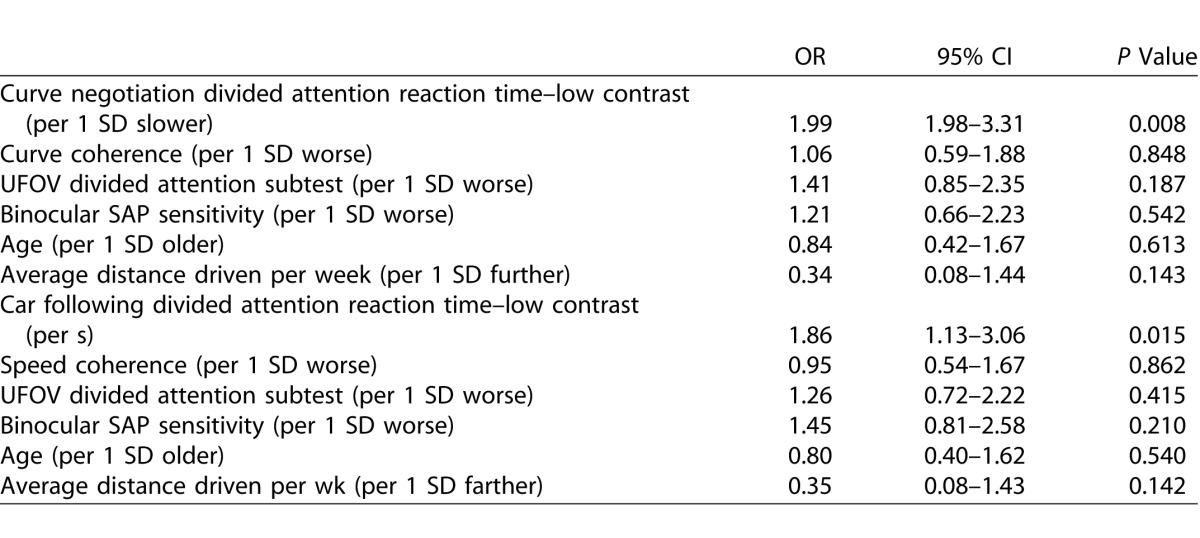
Table 3 shows the results of multivariable logistic regression models examining the relationship between reaction times to the low-contrast divided attention driving simulator tasks and odds of recent MVC. Longer driving simulator divided attention task reaction times were significantly associated with increased odds of MVC, even after accounting for performance on the central driving task, binocular SAP sensitivity, UFOV divided attention task, age, and average distance driven per week. Each 1 SD longer reaction time (∼0.75 s) on the low-contrast task during curve negotiation was associated with 99% higher odds of a MVC (OR = 1.99; 95% CI 1.98–3.31; P = 0.008). A similar result was seen for the low-contrast divided attention task in car following and both driving simulator divided attention tasks also remained significant when contrast sensitivity in the better eye was included in the models. The difference between low- and high-contrast driving simulator reaction times were also significant in the multivariable logistic regression models for both the curve negotiation (P = 0.041) and car following tasks (P = 0.013). Reaction times to the driving simulator divided attention tasks remained significant when false negative rates were included in the models.
Table 3.
Results of Multivariable Logistic Regression Analyses Examining the Odds of MVC Associated With Low Contrast Driving Simulator Divided Attention Task Reaction Times for the Curve Negotiation and Car Following Tasks, Controlling for Central Driving Task Performance (Curve Coherence or Speed Coherence), UFOV Divided Attention Task, Binocular SAP Sensitivity, Age, and Average Distance Driven Per Week
Discussion
The results of this study indicated that measures of ability to divide attention during simulated driving were more strongly associated with history of recent MVC in drivers with glaucoma than conventional functional measures such as visual acuity and SAP. The driving simulator assessment of divided attention also performed better than measurement of ability to divide attention using the UFOV test, with more demanding low-contrast stimuli performing particularly well.
Previous studies have shown drivers with glaucoma to be at increased risk of MVC compared with similarly aged drivers without glaucoma,8,10,32,33 which at least in part, seems to be due to impaired ability to divide attention or multi-task.6,10,34 As driving is a highly visual task, one might suppose that there would be good agreement between conventional measures of visual function and safe driving, however, the relationship is not strong.5,9 We found conventional measures of visual function to be only weakly associated with history of MVC.
In logistic regression analyses, worse visual acuity and contrast sensitivity in the better eye were associated with increased odds of recent MVC, however after accounting for age differences between groups, only contrast sensitivity in the better eye remained significant. Furthermore, in ROC analyses, neither visual acuity or contrast sensitivity were particularly good at differentiating drivers with and without a recent MVC, with AUCs of only 0.56 and 0.61 respectively, with 95% CIs for both crossing 0.5. Accounting for age, MD in the better and worse eyes was not associated with odds of MVC (P = 0.197 and P = 0.381, respectively). Binocular SAP sensitivity was associated with recent MVC (P = 0.043), however the ability of binocular SAP sensitivity to discriminate MVC and no MVC groups (AUC = 0.69) was significantly worse than the best performing reaction times to the driving simulator divided attention tasks.
Several previous studies have suggested impaired UFOV to be a useful marker of increased risk of MVC.9,11–13 For example, Owsley and colleagues12 found that drivers with a 40% or more reduction in UFOV have a 2.2 times greater risk of MVC compared with drivers with normal UFOV, and Ball and colleagues9 reported UFOV to have a sensitivity of 89% for 81% specificity in predicting older drivers with a history of MVC. Despite these promising results, some recent studies have suggested UFOV may not be as valuable a predictive tool as previously thought. A recent population-based study of 2000 older drivers found that after adjusting for potentially confounding variables, UFOV divided attention subtest was not significantly associated with rate of MVC.16 Hoffman and colleagues18 compared the ability of UFOV and simulated driving performance to predict a history of automobile accidents, however, neither UFOV or the chosen driving simulator variables were significant. We found drivers with glaucoma and a recent history of MVC had slower UFOV divided attention times compared with those with no MVC, with mean times of 111 milliseconds compared 52 milliseconds (P = 0.014), and slower UFOV divided attention times were predictive of increased risk of MVC (OR = 1.63, 95% CI 1.12–2.37, P = 0.011; Table 2). However, UFOV divided attention times also increased with age, and after including age in the logistic regression model, predictive ability diminished (OR = 1.39, 95% CI 0.92–2.11, P = 0.116).
In the present study, the best performing parameters were reaction times to divided attention tasks during simulated driving, particularly under low-contrast conditions, with AUCs of 0.80 for curve negotiation and 0.79 for car following, which was significantly better than SAP and UFOV. The multivariable logistic regression models also showed reaction times to low-contrast divided attention tasks provided additional value in predicting history of MVC, even after adjusting for potentially confounding variables including age and average distance driven per week, and accounting for binocular SAP sensitivity and performance on the central driving and UFOV divided attention tasks (Table 3). The “motor response corrected” reaction time, or the low-contrast minus high-contrast time, was also predictive of recent MVC for both curve negotiation and car following tasks, and remained so in the multivariable model, suggesting that the difference in reaction times between the MVC and no MVC groups was due to differences in visual processing under conditions of divided attention, rather than differences in motor responses.
Patients with glaucoma commonly report difficulties performing tasks under low-contrast conditions and are affected by contrast to a greater extent than healthy subjects.35 Reduced contrast sensitivity has also been shown to adversely affect driving performance, with reduced contrast sensitivity associated with an increased risk of MVC.35–37 We have previously shown that patients with glaucoma have reduced ability to divide attention compared with similarly aged controls, particularly when the test is performed at low contrast10 and the findings of the present study provide further evidence of the importance of contrast in performance-based tests. Although low-, medium-, and high-contrast divided attention tasks were all able to differentiate MVC and no MVC groups (Table 2), the largest AUCs were for the more demanding low-contrast tasks. Moreover, when reaction times to driving simulator tasks were included in the multivariable regression models accounting for confounding factors, only reaction times to low-contrast stimuli remained significant.
Reduced contrast sensitivity in the better eye was also associated with increased odds of MVC, however, the low-contrast divided attention simulated driving task performed better, most likely as it is better reflects the complexity of the driving task. Although the UFOV test includes a test of divided attention, a potential limitation of UFOV is that it is performed using a relatively high-contrast stimulus.36,38 It is possible that UFOV testing using varying contrast stimuli might have a stronger association with MVC and this would be an interesting subject for future study.
A further observation of interest is that drivers with a recent MVC performed worse on the central driving simulator task than the no MVC group, as indicated by worse curve and speed coherence (Table 1). It was therefore important to account for central task performance in the multivariable model, nevertheless, even accounting for central driving task performance, reaction times to the divided attention stimuli provided additional information. The MVC group also had higher false negative rates to the driving simulator divided attention stimuli, which is an expected result in those with more advanced disease as scotoma may prevent perception of a divided attention stimulus. However, false negative rates were less strongly associated with history of MVC compared with reaction times to the divided attention stimuli. Although we found cognitive ability was not significantly different between the MVC and no MVC group, this is likely to have been due to the overall good cognitive ability of participants. The effect of age on the ability to divide attention and risk of MVCs is also important. Elderly adults have previously been shown to have decreased ability to divide attention during simulated driving tasks, taking more time to perceive, analyze and make decisions regarding sudden road events than young drivers.39,40 Older age is also associated with slower processing speed using the UFOV test, with a recent population-based study showing that 44% of drivers aged 70 or over have slowed visual processing speed.41 Age-related decreases in cognitive ability may further impact driving ability and increase risk of MVCs. We found older age was associated with slower UFOV and driving simulator divided attention reaction times, however, even after accounting for age in the multivariable models, driving simulator divided attention reaction times were still predictive of history of MVC.
The study has some limitations. Due to the rarity of MVCs, there were a relatively small number of patients in the study who had experienced a collision. Furthermore, we relied on retrospective, self-reported history to ascertain occurrence of MVC and it is possible that there were some inaccuracies in patient recollection and reporting. An alternative approach would be to examine Department of Motor Vehicle (DMV) records, however MVCs may also be underreported to the DMV. Nevertheless, future studies should investigate whether driving simulator metrics are predictive of DMV-reported MVCs in glaucomatous subjects. Although driving simulators have been widely used to assess ability to divide attention and driving skills, it is possible that participants may show differences in behavior in real world driving, when the risks to safety are real. For example, patients with glaucoma may modify driving behavior by avoiding difficult conditions thus potentially reducing risk of MVCs.1,42 McGwin and colleagues42 found older persons with glaucoma had higher levels of avoidance of at night driving, driving in busy traffic, and driving during difficult weather conditions, with the result that they had similar odds of being involved in an at-fault MVC compared with similarly aged nonglaucomatous controls. Interestingly, we found drivers with glaucoma who had experienced a MVC actually drove fewer miles per week than those without a MVC. However, as this study was retrospective it is unclear whether these drivers modified driving behavior secondary to the MVC, or for other reasons.
It is also important to acknowledge that all subjects included in the study were current drivers. It would not be logical to include patients who no longer drive in a study using MVCs as its endpoint, however, as the decision to not drive may have been based on the results of conventional tests of visual function, there is the potential for bias toward the finding of poor association between conventional tests and risk of collision. However, one would expect that the better one replicates actual driving, and particularly driving events that provoke MVCs, the better one is likely to be able to predict collisions. It is possible that alternative simulated driving scenarios might perform even better than those evaluated in the current study. An on the road driving assessment might also perform better, and would address the issue of differences between simulated and real world driving, however, this type of assessment is expensive, time consuming and difficult to conduct with large numbers of subjects. It is also difficult to standardize test conditions during on road driving assessment. Furthermore, driving simulators have been validated by comparison to on-road assessment and there is strong correlation between the number of crashes during driving simulation and previous history of MVCs.43,44 It should also be emphasized that predicting MVCs is challenging, as causes of MVC are multifactorial and in the present study even the best performing parameter produced an AUC of only 0.80.45 Due to the small number of MVCs, the CIs for AUCs were wide, however, driving simulator divided attention metrics still performed significantly better than conventional metrics used for licensing such as visual acuity and standard perimetry, with the logistic regression analyses producing similar findings.
In conclusion, the results of this study demonstrated that ability to divide attention during driving simulation was strongly associated with history of MVCs in drivers with glaucoma. The UFOV divided attention test was also of value, however, it was not as useful as the more demanding driving simulator divided attention test, particularly when using a low contrast stimulus. Given the high individual and societal significance of MVCs, the present study underscores the need to develop better methods of risk assessment in drivers with glaucoma and other eye diseases, and provides evidence that predictive models that account for the ability to divide attention may provide a means to improve estimates of risk.
Acknowledgments
The study was registered at ClinicalTrials.gov with registration number NCT00221897.
Disclosure: Andrew J. Tatham, research suport from Heidelberg Engineering; Erwin R. Boer, none; Carolina P.B. Gracitelli, none; Peter N. Rosen, none; Felipe A. Medeiros, research support from Alcon Laboratories, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Allergan, Sensimed, Topcon, Reichert, National Eye Institute, consultant for Allergan, Carl Zeiss Meditec, Novartis
References
- 1. Van Landingham SW,, Hochberg C, Massof RW,, Chan E, Friedman DS,, Ramulu PY. Driving patterns in older adults with glaucoma. BMC Ophthalmol. 2013; 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ragland DR, Satariano WA,, MacLeod KE. Driving cessation and increased depressive symptoms. J Gerontol A Biol Sci Med Sci. 2005; 60: 399–403. [DOI] [PubMed] [Google Scholar]
- 3. Freeman EE, Gange SJ,, Muñoz B, West SK. Driving status and risk of entry into long-term care in older adults. Am J Public Health. 2006; 96: 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramulu PY, West SK,, Munoz B, Jampel HD,, Friedman DS. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009; 116: 1846–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medeiros FA, Weinreb RN, R Boer E, Rosen PN. Driving simulation as a performance-based test of visual impairment in glaucoma. J Glaucoma. 2012; 21: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haymes SA, Leblanc RP,, Nicolela MT, Chiasson LA,, Chauhan BC. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 7. Owsley C, McGwin G., Jr. Vision and driving. Vision Res. 2010; 50: 2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGwin G, Owsley C,, Ball K. Identifying crash involvement among older drivers: agreement between self-report and state records. Accid Anal Prev. 1998; 30: 781–791. [DOI] [PubMed] [Google Scholar]
- 9. Ball K, Owsley C,, Sloane ME, Roenker DL,, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993; 34: 3110–3123. [PubMed] [Google Scholar]
- 10. Tatham AJ, Boer ER,, Rosen PN, et al. Glaucomatous retinal nerve fiber layer thickness loss is associated with slower reaction times under a divided attention task. Am J Ophthalmol. 2014; 158: 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ball K,, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993; 64: 71–79. [PubMed] [Google Scholar]
- 12. Owsley C, Ball K,, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998; 279: 1083–1088. [DOI] [PubMed] [Google Scholar]
- 13. Rubin GS,, Ng ES, Bandeen-Roche K,, Keyl PM, Freeman EE,, West SK. A prospective population-based study of the role of visual impairment in motor vehicle crashes among older drivers: the SEE study. Invest Ophthalmol Vis Sci. 2007; 48: 1483–1491. [DOI] [PubMed] [Google Scholar]
- 14. Ball KK,, Roenker DL, Wadley VG,, et al. Can high-risk older drivers be identified through performance-based measures in a Department of Motor Vehicles setting? J Am Geriatr Soc. 2006; 54: 77–84. [DOI] [PubMed] [Google Scholar]
- 15. Potter MC, Faulconer BA. Time to understand pictures and words. Nature. 1975; 253: 437–438. [DOI] [PubMed] [Google Scholar]
- 16. Friedman C, McGwin G,, Ball KK, Owsley C. Association between higher order visual processing abilities and a history of motor vehicle collision involvement by drivers ages 70 and over. Invest Ophthalmol Vis Sci. 2013; 54: 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owsley C, Ball K,, Sloane ME, Roenker DL,, Bruni JR. Visual/cognitive correlates of vehicle accidents in older drivers. Psychol Aging. 1991; 6: 403–415. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman L, McDowd JM,, Atchley P, Dubinsky R. The role of visual attention in predicting driving impairment in older adults. Psychol Aging. 2005; 20: 610–622. [DOI] [PubMed] [Google Scholar]
- 19. McDowd JM, Hoffman L. Challenges in attention: measures, methods, and applications. : Hofer SM, Alwin DF, Handbook of Cognitive Aging: Interdisciplinary Perspectives. Thousand Oaks, CA: Sage Publications Inc.; 2008; 122–133. [Google Scholar]
- 20. Sample PA, Girkin CA,, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009; 127: 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medeiros FA,, Vizzeri G, Zangwill LM,, Alencar LM, Sample PA,, Weinreb RN. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology. 2008; 115: 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Racette L, Liebmann JM,, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010; 128: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson-Quigg JM,, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000; 41: 2212–2221. [PubMed] [Google Scholar]
- 24. O'Hare F, Rance G,, Crowston JG, McKendrick AM. Auditory and visual temporal processing disruption in open angle glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 6512–6518. [DOI] [PubMed] [Google Scholar]
- 25. Hultsch DF, MacDonald SW,, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002; 57: 101–115. [DOI] [PubMed] [Google Scholar]
- 26. Brookhuis K, De Waard D,, Mulder B. Measuring driving performance by car-following in traffic. Ergonomics. 1994; 37: 427–434. [Google Scholar]
- 27. Marsden G, McDonald M,, Brackstone M. Towards an understanding of adaptive cruise control. Transportation Research Part C: Emerging Technologies. 2001; 9: 33–51. [Google Scholar]
- 28. Nasreddine ZS, Phillips NA,, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 29. DeLong ER,, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44: 837–845. [PubMed] [Google Scholar]
- 30. Medeiros FA, Sample PA,, Zangwill LM, Liebmann JM,, Girkin CA, Weinreb RN. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006; 47: 2520–2527. [DOI] [PubMed] [Google Scholar]
- 31. Pepe MS. Three approaches to regression analysis of receiver operating characteristic curves for continuous test results. Biometrics. 1998; 54: 124–135. [PubMed] [Google Scholar]
- 32. McCloskey LW, Koepsell TD,, Wolf ME, Buchner DM. Motor vehicle collision injuries and sensory impairments of older drivers. Age Ageing. 1994; 23: 267–273. [DOI] [PubMed] [Google Scholar]
- 33. Owsley C, McGwin G,, Ball K. Vision impairment eye disease, and injurious motor vehicle crashes in the elderly. Ophthalmic Epidemiol. 1998; 5: 101–113. [DOI] [PubMed] [Google Scholar]
- 34. Crabb DP,, Fitzke FW, Hitchings RA,, Viswanathan AC. A practical approach to measuring the visual field component of fitness to drive. Br J Ophthalmol. 2004; 88: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richman J, Lorenzana LL,, Lankaranian D, et al. Relationships in glaucoma patients between standard vision tests, quality of life, and ability to perform daily activities. Ophthalmic Epidemiol. 2010; 17: 144–151. [DOI] [PubMed] [Google Scholar]
- 36. Owsley C,, McGwin G, Sloane ME,, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: relationship to visual function in older adults. Optom Vis Sci. 2001; 78: 350–359. [DOI] [PubMed] [Google Scholar]
- 37. Richman J, Lorenzana LL,, Lankaranian D, et al. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010; 128: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 38. Green KA,, McGwin G, Owsley C. Associations between visual, hearing, and dual sensory impairments and history of motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2013; 61: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDowd JM, Shaw RJ. Attention and Aging: A Functional Perspective. Mahwah: Lawrence Erlbaum Associates Publishers; 2000. [Google Scholar]
- 40. Chaparro A, Wood JM,, Carberry T. Effects of age and auditory and visual dual tasks on closed-road driving performance. Optom Vis Sci. 2005; 82: 747–754. [DOI] [PubMed] [Google Scholar]
- 41. Owsley C, McGwin G,, Searcey K. A population-based examination of the visual and ophthalmological characteristics of licensed drivers aged 70 and older. J Gerontol A Biol Sci Med Sci. 2013; 68: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGwin G, Mays A,, Joiner W, Decarlo DK,, McNeal S, Owsley C. Is glaucoma associated with motor vehicle collision involvement and driving avoidance? Invest Ophthalmol Vis Sci. 2004; 45: 3934–3939. [DOI] [PubMed] [Google Scholar]
- 43. Reimer B, D'Ambrosio LA,, Coughlin JE, Kafrissen ME,, Biederman J. Using self-reported data to assess the validity of driving simulation data. Behav Res Methods. 2006; 38: 314–324. [DOI] [PubMed] [Google Scholar]
- 44. Shechtman O, Classen S,, Awadzi K, Mann W. Comparison of driving errors between on-the-road and simulated driving assessment: a validation study. Traffic Inj Prev. 2009; 10: 379–385. [DOI] [PubMed] [Google Scholar]
- 45. Bowers AR, Anastasio RJ,, Sheldon SS,, et al. Can we improve clinical prediction of at-risk older drivers? Accid Anal Prev. 2013; 59: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]