Abstract
Modification of the plant N-glycosylation pathway towards human type structures is an important strategy to implement plants as expression systems for therapeutic proteins. Nevertheless, relatively little is known about the overall impact of non-plant glycosylation enzymes in stable transformed plants. Here, we analyzed transgenic lines (Nicotiana benthamiana and Arabidopsis thaliana) that stably express a modified version of human β1,4-galactosyltransferase (STGalT). While some transgenic plants grew normally, other lines exhibited a severe phenotype associated with stunted growth and developmental retardation. The severity of the phenotype correlated with both increased STGalT mRNA and protein levels but no differences were observed between N-glycosylation profiles of plants with and without the phenotype. In contrast to non-transgenic plants, all STGalT expressing plants synthesized significant amounts of incompletely processed (largely depleted of core fucose) N-glycans with up to 40% terminally galactosylated structures. While transgenic plants showed no differences in nucleotide sugar composition and cell wall monosaccharide content, alterations in the reactivity of cell wall carbohydrate epitopes associated with arabinogalactan-proteins and pectic homogalacturonan were detected in STGalT expressing plants. Notably, plants with phenotypic alterations showed increased levels of hydrogen peroxide, most probably a consequence of hypersensitive reactions. Our data demonstrate that unfavorable phenotypical modifications may occur upon stable in planta expression of non-native glycosyltransferases. Such important issues need to be taken into consideration in respect to stable glycan engineering in plants.
Keywords: β1,4-Galactosylation; Glyco-engineering; Nicotiana benthamiana; Transgenic plants; Developmental phenotype
Highlights
-
•
Introduction of β1,4-galactosylation into plants.
-
•
Over-expression of human β1,4-galactosyltransferase may cause a phenotype in plants.
-
•
Plant proteins are poor substrates for β1,4-galactosylation.
-
•
Human antibodies are efficiently β1,4-galactosylated in plants.
-
•
Alteration of cell wall composition caused by the overexpression of β1,4-galactosyltransferase.
1. Introduction
In recent years much effort has been devoted to engineering plants for the production of recombinant glycoproteins with optimized glycosylation. Accordingly, complex human glycoprotein therapeutics with defined N- and O-glycan moieties can be produced in plants (Strasser et al., 2014). These recent advances make whole plants useful alternatives to existing mammalian cell-culture based expression platforms. However, the impact of the introduced glycan modifications, the resulting alterations in Golgi protein organization and the concomitant excess/shortage of certain metabolites on overall plant physiology are less known. In particular, glyco-engineering leads to incorporation of mammalian-type sugar residues into endogenous plant glycoproteins, causes perturbations of nucleotide sugar flux and deposits additional proteins in the secretory pathway. The majority of the plant glyco-engineering approaches resulting in homogenous glycosylation rely on transient expression of heterologous glycosyltransferases and other proteins necessary for targeted glycosylation (Castilho et al., 2010). The transient expression technology is especially applicable to leaves from Nicotiana benthamiana plants and can be combined with powerful viral-based transient systems leading to high expression of glycoproteins with defined N-glycan structures (Jez et al., 2012). In commonly used protocols, all expression constructs are transferred simultaneously to plants by infiltration of leaves with a mixture of Agrobacteria and recombinant proteins are extracted 3–10 days post-infiltration. During this short time period the glyco-engineering procedure does not cause any severe morphological phenotype that would affect the quality and/or quantity of recombinant protein production. The stable integration of whole pathways into the plant genome and the putative effect on overall plant development and growth is poorly investigated. While no obvious phenotypical modifications were reported in transgenic plants that express human glycosyltransferases (e.g. β1,4-galactosyltransferase (GalT) and N-acetylglucosaminyltransferase III, IV and V) under standard growth conditions (Bakker et al., 2001; Rouwendal et al., 2007; Frey et al., 2009; Nagels et al., 2011, 2012) alterations on the plant development were observed upon stable expression of human Lewis fucosyltransferase and a nucleotide sugar transporter in transgenic Nicotiana tabacum (Joly et al., 2004; Khalil et al., 2010).
In previous studies human GalT was expressed in transgenic tobacco for several reasons: On the one hand this enzyme promotes the generation of complex N-glycans that terminate with β1,4-linked galactose, a widespread N-glycan structure in humans and the required acceptor substrate for subsequent sialylation (Castilho et al., 2010; Jez et al., 2013). On the other hand targeted to an early Golgi compartment the enzyme serves as powerful tool to eliminate plant specific core fucosylation (Bakker et al., 2006).
Here, we characterized plants (N. benthamiana and Arabidopsis thaliana) stably transformed with a chimeric human β1,4-galactosyltransferase that targets the enzyme to a late Golgi compartment.
2. Experimental procedures
2.1. Construction of binary vectors
The STGalT binary vector for N. benthamiana and A. thaliana transformation was described previously (Strasser et al., 2009). The binary vector used for transient expression of the monoclonal antibody (mAb) 4E10 (p4E10) was generated as described previously (Strasser et al., 2009).
2.2. Generation of transgenic plants
N. benthamiana wild type plants expressing STGalT (STGalT-WT) were described previously (Strasser et al., 2009). Selected STGalT-WT lines were crossed with the glycosylation mutant ΔXT/FT, lacking the plant-specific β1,2-xylose and core α1,3-fucose residues, leading to the generation of STGalTX-ΔXF plants (Strasser et al., 2009). Generation of STGalT transformants in a ΔXT/FT background was also done by direct transformation of the glycosylation mutant with the STGalT binary vector carrying an additional expression cassette for hygromycin resistance (STGalT-ΔXF). Putative transformed plantlets were selected on Kanamicyn-(STGalT-WT) or hygromycin-containing media (STGalT-ΔXF).
A. thaliana ecotype Columbia (Col-0) was transformed with the STGalT construct by floral dipping (Clough and Bent, 1998). Transformed seeds were selected on kanamycin-containing media.
For all transformation events the presence of the human GalT DNA was confirmed by PCR using gene-specific primers. Endogenous proteins of selected PCR-GalT positives were analyzed for the presence of galactose by Ricinus communis agglutinin I lectin blot as described earlier (Bakker et al., 2001).
Plants were cultivated in a growth chamber at a constant temperature of 24 °C, 60% relative humidity, and a 16 h light/8 h dark photoperiod.
2.3. Genomic PCR amplification (gPCR)
Genomic DNA was isolated from leaf material (∼8 mg) as described previously (Strasser et al., 2004b). The human GalT DNA was amplified by PCR using primers 5′-GGCAAAGCAGAACCCAAATGTGA-3′ and 5′-TCTTCTCCTCCCCAGCCCCAATAAT-3′. DNA extracts from ΔXT/FT plants and from ΔXT/FT plants transiently expressing STGalT served as negative and positive controls, respectively. For internal control the N. benthamiana catalase gene (NbCat) was amplified from all genomic DNA samples using specific primers (5′-CATTCGCGGTTTTGCTGTC-3′ and 5′-TGGTGGCGTGGCTATGATTTGTA-3′) (Strasser et al., 2004a).
2.4. Reverse transcription real-time PCR amplification (qPCR)
Total RNA was extracted from leaves of N. benthamiana using the SV Total RNA Isolation System (Promega). 500 ng of RNA were reverse transcribed at 42 °C using oligo(dT) primer and AMV reverse transcriptase (Promega). qPCR was performed with a C1000 Touch™ Thermal cycler (Biorad) with the iQ™ SYBR® GREEN Supermix (Biorad). N. benthamiana elongation factor1α gene (EF1α, accession number AY206004) was used as internal control. Specific primers were used to amplify a STGalT fragment (5′-CATGATCCGCCACTCAAGAGAC-3′ and 5′-GCTCGGTGTCCCGATGTCCACT-3′) and an EF1α fragment (5′-GCTGACTGTGCTGTCCTGATTATT-3′ and 5′-TCACGGGTCTGTCCATCCTTA-3′). Amplification occurred after an initial denaturation (7 min/95 °C) in 40 cycles (95° C/5 s–54° C/15 s–95° C/15 s). At the end of each run, a melting curve was recorded between 60 °C and 95 °C. To process the amplification data, the CFX Manager™ Software was used. The expression levels of hGalT were normalized in relation to ΔXT/FT plants. Values, resulting from 3 independent experiments, are given in mean ± standard error of the mean.
2.5. Preparation of total soluble protein (TSP) extracts
Leaf material (250 mg) was submerged in liquid nitrogen and ground in a swing mill (Retsch®, MM2000) for 2 min at amplitude 60. Two volumes (v/w) of 1 × PBS were added to the samples and incubated on ice for 10 min. Finally, the extracts were centrifuged (9000 g for 5 min at 4 °C) and the supernatant was stored at −20 °C for further analysis.
2.6. Immunoblot analysis of human GalT in transgenic N. benthamiana
Prior to separation by 12% SDS-PAGE, TSP extracts were mixed with reducing 4× Laemmli buffer and incubated for 5 min at 95 °C. Subsequently, the separated proteins were transferred to a nitrocellulose membrane and probed with protein-specific antibodies (monoclonal mouse anti-GalT, diluted 1:10,000 in TBS buffer containing 0.05% Tween-20). Detection was performed using HRP-conjugated secondary antibodies (anti-mouse IgG-peroxidase antibody diluted 1:10,000 in TBS-Tween). SuperSignal West Pico Chemiluminescent Substrate (Pierce, IL) was used as a substrate. Total soluble proteins from a ΔXT/FT plant transiently expressing STGalT were used as a positive control. The membrane was stained with Ponceau S (Sigma Aldrich) to visualize transferred proteins.
2.7. Histochemical detection of hydrogen peroxide (H2O2)
H2O2 was detected histochemicaly in leaves using 3,3-diaminobenzidine (DAB) as substrate (Weigel and Glazebrook, 2002). Leaves from about 30 day old plants were detached and dipped either in buffer (100 mM HEPES pH 6.8) or in buffer supplemented with DAB (1 mg/mL). The solutions were delivered to plant cells by vacuum infiltration and the leaves were incubated overnight in the growth chamber with constant light. The tissues were then decolorized with 96% ethanol for 30 min at 70 °C. This treatment decolorized the tissues, except for the brown colored product produced by DAB with H2O2. The leaves were mounted in water on microscope slides and visualized using a microscope.
2.8. Expression of mAb 4E10
Four to five week old plants were used for expression of 4E10-mAb by agroinfiltration (Strasser et al., 2009). Agrobacteria containing the binary construct were infiltrated at an OD600 of 0.3 (1.0 OD600 corresponds to 5 × 108 cells/mL). 3 days post-infiltration leaves were harvested and 4E10 was obtained by protein A based affinity purification as described previously (Strasser et al., 2009).
2.9. N-Glycan analysis
N-glycosylation profile of TSP extracts from N. benthamiana leaves was determined by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) of N-glycans released from peptic glycopeptides by peptide N-glycosidase A as described previously (Strasser et al., 2004b). The N-glycan composition of 4E10 was determined using reversed-phase liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS) of tryptic glycopeptides as described previously (Stadlmann et al., 2008). The glycoforms were identified by summed and deconvoluted spectra of the glycopeptide's elution range. Peaks were labeled according to the ProGlycAn system (www.proglycan.com), which basically lists the terminal sugar residues starting with those on the 6-arm.
2.10. Nucleotide sugar analysis
Leaf material (3 g) of N. benthamiana plants was prepared and analyzed via porous graphite carbon-electrospray ionization-mass spectrometry (PGC-ESI-MS) as described previously (Pabst et al., 2010).
2.11. Analysis of cell wall sugar composition by HPLC
250 mg of fresh leaf material was blended with 5 mL of 80% alcohol, homogenized with an Ultra Turrax for about 1 min and centrifuged at 9600 g for 10 min. The pellet was resuspend in 1 mL of 80% ethanol, centrifuged for 5 min at 16,800 g and washed twice with acetone. Finally 5 mg of the dried residue were hydrolyzed with 4 M trifluoroacetic acid at 100 °C for 3 h. The soluble phase was derivatized with 2-aminobenzoic acid (Anumula, 1994) and the fluorescent compounds were analyzed by reversed phase high performance liquid chromatography (HPLC) as described before (Stepan and Staudacher, 2011).
2.12. Dot blot analysis
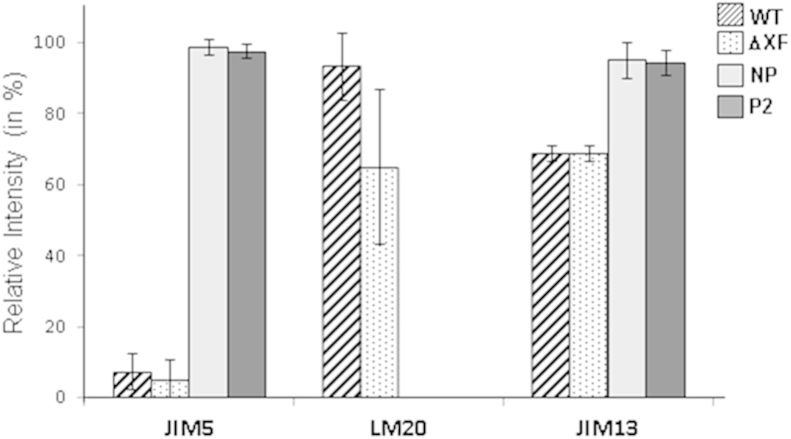
Cell wall fractions were isolated from 1 g of 4-week old N. benthamiana leaves as described previously (Sorensen and Willats, 2011). One μL of the pectin-containing fraction was spotted onto a nitrocellulose membrane and blocked overnight in PBS-Tween containing 3% BSA (blocking solution). Dot blots were analyzed using cell wall polymer-specific rat antibodies (JIM5 for un-esterified homogalacturonan, LM20 for esterified homogalacturonan and JIM13 for arabinogalactan proteins all diluted 1:30 with blocking solution). Detection was performed using HRP-conjugated secondary antibodies: goat polyclonal anti-rat IgG-peroxidase conjugated, Jackson Immuno Research for JIM5 and goat polyclonal anti-rat IgM-peroxidase conjugated, Jackson Immuno Research for LM20 and JIM13, all diluted 1:10,000 with blocking solution. Clarity™ Western enhanced Chemiluminescence reagents (Biorad) were used as substrates. Dot blot images captured with Gel Doc EQ were analyzed using Quantity One® (Biorad) software to measure relative dot intensities. To measure the relative intensity of the dots, the highest signal for each antibody was set to 100%.
3. Results
3.1. Phenotypical characterization of plants transformed with human β1,4-galactosyltransferase
In this study we analyzed plant lines that were transformed with a β1,4-galactosyltransferase (GalT) fusion construct targeting the enzyme to a trans-Golgi compartment. The native cytoplasmic tail, transmembrane domain and stem region (CTS) of human GalT was replaced by the rat α2,6-sialyltransferase CTS (STGalT) (Strasser et al., 2009). Four different types of transgenic STGalT expressing plants were generated (1) N. benthamiana wild type (STGalT-WT), (2) N. benthamiana ΔXT/FT glycosylation mutant (Strasser et al., 2008), either crossed to STGalT-WT (STGalTX-ΔXF) or directly transformed (STGalT-ΔXF) and (3) A. thaliana wild type plants (STGalT-Ath).
All plants were screened for the presence of the transgene by genomic PCR and subjected to lectin blotting using R. communis agglutinin (RCA) for detection of β1,4-galactosylated N-glycans (data not shown). Plants that were positive in both experiments were selected for further propagation. While progeny from some lines grew normally and were indistinguishable from non-transformed WT and ΔXT/FT plants (NP – normal phenotype), others exhibited morphological alterations, independently of their original transformation/genetic background.
From three independent transformation events, two STGalT-WT lines showed phenotype. One plant of STGalT-WT with and one without phenotype were crossed with the glycosylation mutant ΔXT/FT resulting in STGalTX-ΔXF lines with and without phenotype. 12 independent ΔXT/FT transformation events were analyzed, 8 were selected for propagation based on RCA results (STGalT-ΔXF) and 3 showed phenotype.
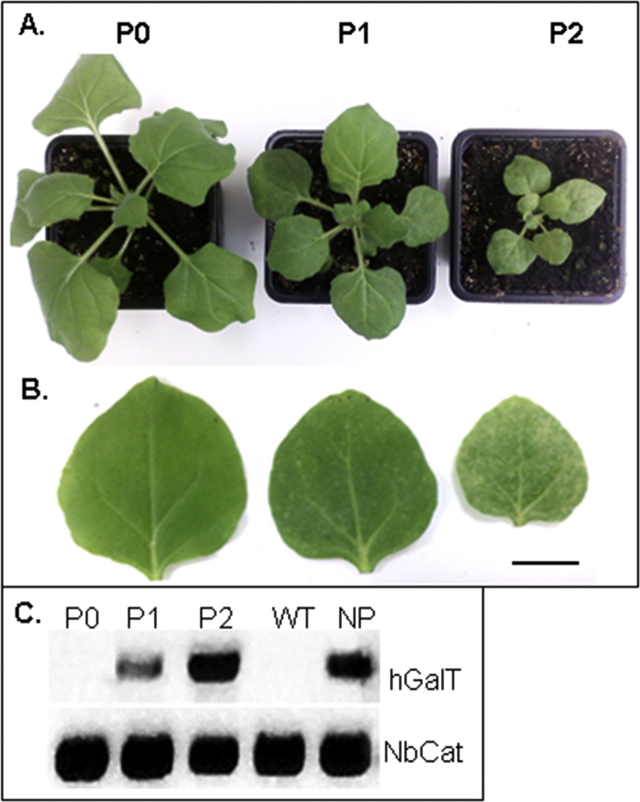
The phenotypic plants displayed stunted growth, morphologically abnormal leaves and a delayed development. Depending on the degree of morphological changes (no-low-high) offspring plants from STGalT-WT, STGalTX-ΔXF and STGalT-ΔXF were classified in three groups P0, P1, and P2 (Fig. 1A and B). Plants of the P0 type were virtually identical to WT or ΔXT/FT plants. P1 plants were generally smaller (stunted growth, reduced leaf surface area to about 25% of P0) and leaves were thicker than in control plants. In addition, leaves displayed dotted lesions indicating the onset of necrosis. These characteristics were more pronounced in plants grouped in P2. This group was extremely stunted in their growth, produced smaller (about 70% size reduction compared to P0), thicker leaves and flowering was significantly delayed (Fig. 1A and B).
Fig. 1.
Phenotype ofSTGalT transgenic plants. Five-week-old N. benthamiana transgenic lines can be separated into 3 groups (P0, P1, and P2) according to their morphological development (A) and leaf characteristics (B). P0 plants are identical to non-transformed plants. Figure shows a STGalT-WT line as an example, similar results are observed for some STGalTX-ΔXF and STGalT-ΔXF lines. (C) Genomic PCR of STGalT-WT plants show the presence of human GalT in P1, P2 and NP plants. P0 plants do not carry the human GalT gene and represent untransformed (WT) plants. DNA extracts from wild type plants (WT) served as negative control. N. benthamiana catalase gene (NbCat) served as an internal control for genomic DNA quality. Bar = 8 mm.
Further propagation of P1 plants showed segregation into the three phenotypes (P0:P1:P2 = 29%:48%:23%), while offspring from P0 and P2 gave rise only to the respective phenotype (P0 or P2). PCR analysis from genomic DNA revealed the presence of STGalT transgene in all P1 and P2 plants (Fig. 1C). P0 plants exhibiting no apparent phenotype gave no PCR signal indicating that they are in fact null segregants (without transgene) WT or ΔXT/FT plants. The segregation pattern of P1 and P2 indicates that these plants most probably represent hetero- and homozygous lines for the STGalT gene. Despite showing no obvious phenotype all tested RCA-positive NP plants carry the STGalT transgene (Fig. 1C).
STGalT A. thaliana transformants (STGalT-Ath) exhibited a pronounced phenotype (Supplementary Fig. S1). Compared to A. thaliana WT, STGalT-Ath plants display an extremely stunted and delayed growth, with small hairy leaves and truncated roots. When transferred to soil, the STGalT-Ath lines did not develop properly and propagation was not possible.
3.2. N-glycan profiling of N. benthamiana plants expressing STGalT
In a next step the glycosylation status of plants expressing the STGalT gene was investigated. To obtain a comprehensive picture and for comparison reasons we conducted a series of studies where a total of seven N. benthamiana lines were analyzed: WT, ΔXT/FT, STGalT in wild type background showing phenotype (STGalT-WT P1 and P2), STGalT in ΔXT/FT background with and without phenotype (P1, P2 and NP). The results obtained for STGalTX-ΔXF (obtained by crossing) and STGalT-ΔXF (obtained by direct transformation) were very similar therefore to simplify data P1, P2 phenotypes are represented by a STGalTX-ΔXF line while NP is represented by STGalT-ΔXF line.
From all lines the N-glycosylation status of three different target proteins was investigated: total soluble proteins (TSP), proteins secreted to the intercellular fluid (IF) and a recombinantly expressed protein (monoclonal antibody 4E10, IgG1 class) (Strasser et al., 2009).
TSP extracts were subjected to matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) analyses. N-glycan profiles of WT plants show three major structures, GnGnXF, MGnXF and MMXF (Table 1 and Supplementary Fig. S2). ΔXT/FT plants showed basically the same profile, with the exception that they almost completely lack xylose and fucose, the major glycan peaks were assigned to GnGn, MGn and MM (Table 1 and Supplementary Fig. S2). Some fucosylated oligosaccharides were present (GnGnF, 13%) due to an incomplete down regulation of core α1,3-fucosyltransferase (Strasser et al., 2008). TSP from plants that express STGalT showed a more heterogeneous glycosylation profile when compared to their non-transformed lines (WT or ΔXT/FT). In addition to the major glycoforms we observed many incompletely processed glycans with and without terminal galactose residues (Table 1 and Supplementary Fig. S2). These galactosylated structures account for about 40% in STGalT-WT (P1 and P2) plants and approximately 30% in STGalT-ΔXF plants (P1 and P2, NP). Although STGalT-WT lack the major glycoform observed in WT (GnGnXF), further elongated complex mono- or di-galactosylated structures (GnAXF or AAXF) were not detected. A striking difference between TSP glycosylation of WT and STGalT-WT (P1 and P2) was the significant reduction of core fucose. While in WT more or less all complex N-glycans were fucosylated (>90%), only about 20% carry this plant specific residue in STGalT-WT. Notably, no clear differences were observed between TSP N-glycans from STGalT-ΔXF P1, P2 and NP plants. However, the N-glycan profile differed from the non-transformed ΔXT/FT line. Both STGalT expressing plant lines (P2 and NP) carried large amounts of MM and MGn, but lacked GnGn, a dominant glycoform in ΔXT/FT (Table 1 and Supplementary Fig. S2). Instead, incompletely processed galactosylated structures (i.e. MA, Man4A and Man5A) were detected. Fully processed mono and di-galactosylated structures (GnA, AA) were found only at low levels or not detected at all on TSP.
Table 1.
Relative amounts (in %) of main N-glycan structures detected in total soluble proteins (TSP) and intercellular fluid (IF) of the different N. benthamiana lines. Analysis was performed in wild type (WT) plants and ΔXT/FT glycosylation mutant (ΔXF), which served as a control. The following STGalT expressing plants were analyzed: P2 of STGalT-WT and of STGalTX−-ΔXF and NP of STGalT-ΔXF. Other: refers to mainly oligomannosidic glycans. As N-glycan profiles of P1 plants did not differ from P2 they are not listed separately in this table. See also Fig. 2 and Supplementary Fig. S2.
| WT |
STGalT-WT (P2) |
ΔXF |
STGalTX−-ΔXF (P2) |
STGalT-ΔXF (NP) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSP | IF | TSP | IF | TSP | IF | TSP | IF | TSP | IF | |
| MM | 5.9 | 22 | 19 | 46.2 | 25 | 46.7 | 26.2 | 46.2 | ||
| MMX | 6.3 | 13.2 | 13.8 | 37.9 | ||||||
| MMXF | 17.8 | 27.5 | 7.9 | |||||||
| MGn | 5.3 | 16.7 | 24.3 | 29.5 | 29 | 26.5 | 26.2 | 29.8 | ||
| MGnX | 7.5 | |||||||||
| MGnXF | 19.2 | 16.8 | 5.5 | |||||||
| GnGn | 31.7 | 24.3 | ||||||||
| GnGnF | 13 | |||||||||
| GnGnXF | 45.6 | 42.5 | ||||||||
| MA | 5.7 | 17.5 | 9.8 | 17 | 9.5 | |||||
| MAX | 11 | |||||||||
| MAXF | 9 | |||||||||
| Man4A | 7 | 12.9 | 8.2 | 7.4 | 7.8 | 8 | ||||
| Man5A | 6 | 5.6 | 6.6 | |||||||
| ∑other | 11.1 | – | 15.4 | 10.5 | 12 | – | 14.7 | 9.6 | 16.2 | – |
Analysis of the N-glycosylation pattern of proteins present in the intercellular fluid (IF), which represent the secretome of a cell, revealed the presence of three major structures in WT plants, i.e. GnGnXF, MGnXF and MMXF. ΔXT/FT plants show basically the same profile, without xylose and core fucose (i.e. GnGn, MGn and MM) (Table 1 and Supplementary Fig. S2). The IF of STGalT-WT (P1, P2) plants displayed mostly incomplete processed and paucimannosidic structures, i.e. MM, MMX, MGn and Man4A (Table 1 and Supplementary Fig. S2). Interestingly and in contrast to WT they were virtually devoid of fucose residues and contain reduced amounts of xylose. Only one peak was assigned as galactosylated oligosaccharide (Man4A, 12.9%). IF derived N-glycan profiles from STGalT-ΔXF (P1, P2 and NP) were very similar. In line with the non-transformed ΔXT/FT line two major structures which account for 70–80 % of all structures, i.e. MM, MGn (Table 1 and Supplementary Fig. S2) were present. However, in contrast to ΔXT/FT no GnGn structures were detected and instead we found incompletely galactosylated structures (MA and Man4A). In total, the galactosylated N-glycans account for about 17%.
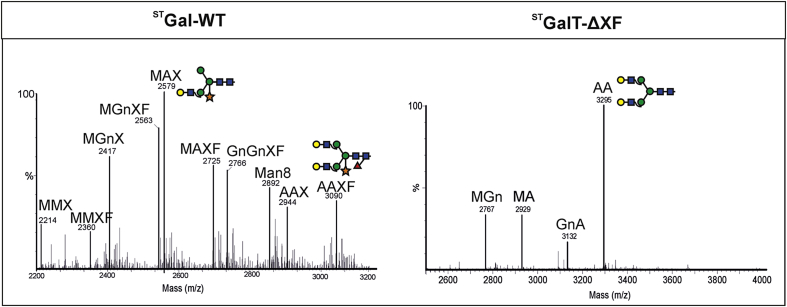
To monitor the N-glycosylation profile of a target protein that is heavily galactosylated in its native form, we transiently expressed the monoclonal antibody 4E10 (4E10-mAb) of the IgG1 class. IgG1 antibodies have a conserved N-glycosylation site in the Fc domain (Asn297) and serum IgG1 carries up to 80% of β1,4-galactosylated structures (Stadlmann et al., 2008). Liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS) based profiles of 4E10-mAb expressed in WT and ΔXT/FT displayed a single dominant glycan structure namely GnGnXF and GnGn, respectively (Strasser et al., 2009) (data not shown). In contrast, 4E10-mAb expressed in STGalT-WT (P1, P2) exhibited a largely heterogeneous glycosylation profile (Fig. 2). Up to 60% of the structures were incompletely processed and half thereof carried galactose residues (MAX, MAXF). In contrast to the glycosylation pattern observed in TSP and IF, 4E10-mAb also carried fully processed di-galactosylated structures (AAX, AAXF approx. 10%). Notably, while virtually all glycan species were xylosylated (>90%), only approximately 50% carried fucose residues. This was significantly more than in TSP and drastically different from the IF with virtually no fucose containing N-glycans. However, the amounts of fucose containing N-glycans were much less than in 4E10-mAb expressed in WT plants where virtually all N-glycans were fucosylated (GnGnXF). Note, the m/z ratio of peaks MAX/Man4GnX and Man4A/Man5Gn were identical. However, galactosidase digestion of such profiles clearly converted these peaks to a corresponding mass lacking the galactose residue, e.g. MAX → MGnX, MAXF → MGnXF etc. (Supplementary Fig. S3) indicating the presence of incompletely processed galactosylated oligosaccharides. 4E10-mAb produced in STGalT-ΔXF (P1, P2) plants (Fig. 2) exhibited a predominant glycoform, i.e. di-galactosylated AA structures and resembles the N-glycan profile of 4E10-mAb produced in STGalT-ΔXF NP plants (Strasser et al., 2009). In summary, the N-glycan profiles of STGalT-ΔXF P1, P2 and NP lines display the same glycoforms in TSP, IF and on mAb suggesting that the observed phenotype is not caused by major changes in overall N-glycosylation.
Fig. 2.
N-glycosylation profile of 4E10 mAb expressed inSTGalT-WT andSTGalT-ΔXF P2 plants. LC-ESI-MS of tryptic Fc glycopeptide for STGalT-WT (R/E293EQYNSTYR301) and for STGalT-ΔXF (K/288TKPREEQYNSTYR301 refers to incomplete trypsin digestion) are shown. Peaks were labeled in accordance with the ProGlycAn system (www.proglycan.com).
3.3. STGalT overexpression is a possible factor contributing to the plant phenotype
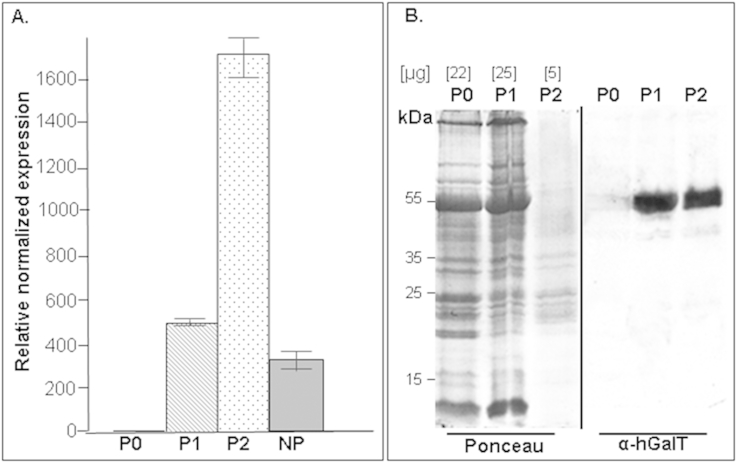
To elucidate factors possibly causing the abnormal plant phenotype, the expression level of STGalT mRNA was monitored by quantitative PCR (qPCR). mRNA was transcribed into cDNA and STGalT mRNA expression was normalized against the constitutively expressed elongation factor1α (EF1α). Different signal intensities of STGalT were obtained depending to the phenotype of the plants. While STGalT-ΔXF P1 and NP plants exhibited relatively low STGalT expression, STGalT-ΔXF P2 showed clearly increased STGalT mRNA levels (Fig. 3A). Expression of STGalT protein was monitored by immunoblotting using a human GalT-specific antibody. The mammalian glycosyltransferase was distinctly detected in total protein extracts of P1 and P2 plants (Fig. 3B). Staining of total proteins by Ponceau-S showed that despite significant lower levels of total proteins in P2, similar signal intensity was obtained by immunoblotting (Fig. 3B). This result points to an actually increased protein expression level of STGalT in P2 compared to P1, in line with qPCR results. Reduced total soluble protein levels of P2 plants are most probably a consequence of heavy leaf necrosis.
Fig. 3.
STGalT mRNA and protein expression. (A) RT-PCR analysis from STGalTX-ΔXF as a representative of a line exhibiting a phenotype (P0, P1 and P2) and from STGalT-ΔXF as a representative of a line without phenotype (NP). For each experiment data from four plants were collected. Experiment was done in triplicate. Bars show the relative amount of human GalT PCR product. (B) Determination of human STGalT expression by Western blot analysis using anti-human GalT antibodies (α-hGalT). Analysis was performed in total soluble protein extracts from STGalTX-ΔXF as a representative of a line showing phenotype (P0, P1 and P2). Ponceau S-staining shows the relative amount of proteins loaded on the gel. Molecular weights are shown in kilo Dalton (kDa).
Hydrogen peroxide (H2O2) is a form of reactive oxygen generated when plant tissues are stressed (Tripathy and Oelmuller, 2012). Accumulation of high levels of H2O2 can lead to oxidative damages or even be fatal to plant cells (Lorrain et al., 2003). At the cellular level, the accumulation of H2O2 can be detected when the leaf tissues are stained with 3,3-diaminobenzidine (DAB). To investigate the leaf lesions, particularly abundant in P2 plants, we performed a histochemical detection of H2O2 levels. Leaves from non-transgenic plants (ΔXT/FT) and from STGalT-ΔXF (NP and P2) were incubated with DAB to measure in situ production of H2O2 (Fig. 4). The tissue analysis showed a dramatic increase of H2O2 accumulation with distinct brown colorization in P2 plants compared to the other plants.
Fig. 4.
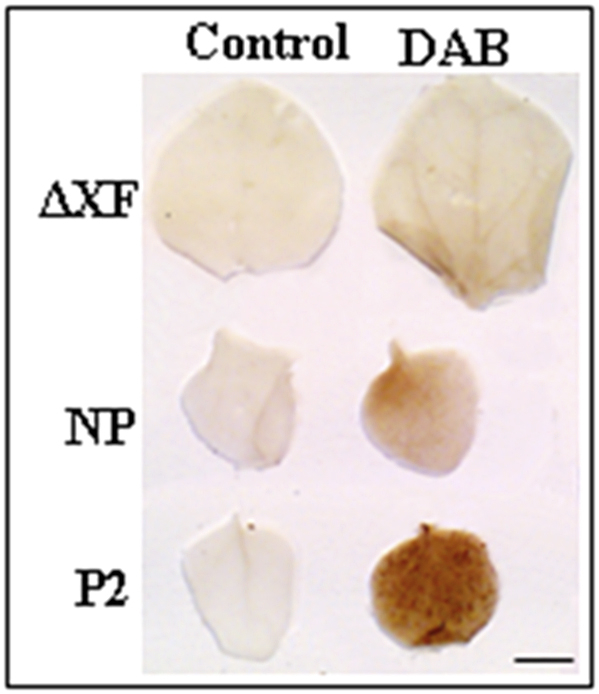
H2O2is produced inSTGalT-ΔXF P2 plants. Leaves of 30-day-old plants were analyzed for their cellular H2O2 content using 3,3-diaminobenzidine (DAB). Controls (leaves incubated without DAB) are shown alongside. DAB incubation of non-transgenic plants (ΔXF) and STGalT-ΔXF with normal phenotype (NP) show no significant differences to control samples. In contrast, STGalT-ΔXF P2 plants show accumulation of H2O2 when compared to control, seen as a brown precipitate after incubation with DAB. Bar = 6 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Alteration of cell wall composition in STGalT overexpressing N. benthamiana
Cell wall polysaccharides are involved in plant growth and in both biotic and abiotic stress resistance and cell wall carbohydrate biosynthesis or structure might be affected in STGalT plants. We hypothesized that the growth defects could be due to reduced availability of one or more nucleotide sugars required for polysaccharide biosynthesis. Quantification analysis of nucleotide sugars (including UDP-Gal, UDP-galacturonic acid, -glucuronic acid, -arabinopyranose, -arabinofuranose, -xylose, -rhamnose, -glucose, -sulfoquinovose, -N-acetylhexosamines, GDP-mannose, -gulose/-l-galactose, -fucose) from leaf material of different plant lines was carried out by porous graphitic carbon-electrospray ionization-mass spectrometry (PGC-ESI-MS) (Pabst et al., 2010). The results showed no significant differences between transgenic and non-transformed plants (Supplementary Fig. S4).
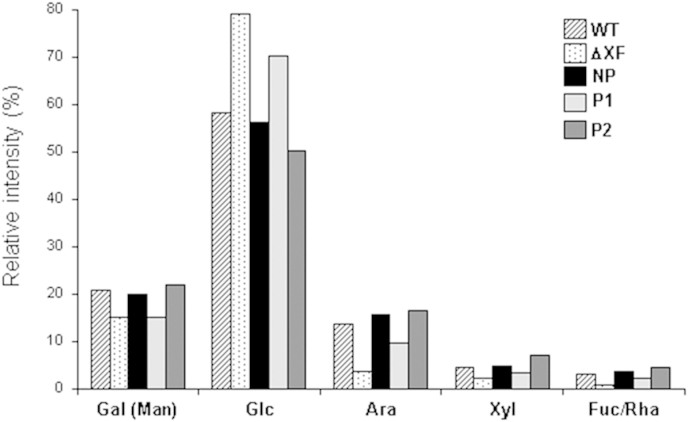
Next, we compared the cell wall composition of different transgenic and control lines regarding monosaccharide composition (galactose/mannose, glucose, arabinose, fucose/rhamnose and xylose). However, analysis via high performance liquid chromatography (HPLC) showed no obvious differences in their relative amounts (Fig. 5).
Fig. 5.
Analysis of cell wall monosaccharide composition by HPLC. The levels of several sugars (in %) in different plant lines were compared by high performance liquid chromatography (HPLC). The sum of peak intensities is set to 100%. Ara: arabinose; Fuc: fucose; Gal: galactose; Glc: glucose; Man: mannose; Rha: rhamnose; Xyl: xylose. Analysis was performed in P1, P2 and NP STGalT-ΔXF plants, in wild type (WT) and in ΔXT/FT (ΔXF) plants.
Finally we determined the epitope composition of homogalacturonans and arabinogalactans, polysaccharides made from a galactose precursor and major components of the plant primary cell wall. Isolated cell wall fractions from control (WT and ΔXT/FT) and from plants expressing STGalT (STGalT-ΔXF NP and P2), were analyzed by dot blot using polysaccharide specific antibodies (Fig. 6). The monoclonal JIM5 antibody detects homogalacturonan with a relatively low level of methyl-esterification (Clausen et al., 2003) while the LM20 antibody binds to highly methyl-esterified homogalacturonan (Verhertbruggen et al., 2009). STGalT expressing plants reacted much stronger with JIM5 than WT and ΔXT/FT did. By contrast, STGalT plants did not react with LM20 while WT and ΔXT/FT plants produced weak signals. Analysis with the arabinogalactan-specific antibody JIM13 (Yates et al., 1996) showed a moderately increased signal in STGalT plants compared to control plants. Our analysis did not reveal differences between STGalT plants showing the severe phenotype (P2) and plants with normal phenotype (NP). Nonetheless, the results indicate that overexpression of STGalT induces alterations in the structure of cell wall matrix polymers.
Fig. 6.
Analysis of cell wall polysaccharides by dot blotting. Pectin fractions of cell wall preparations of individual plants from wild type (WT), ΔXT/FT (ΔXF), STGalT-ΔXF NP and STGalTX-ΔXF P2 (4 plants each) were analyzed for reactivity towards partially un-esterified homogalacturonan (JIM5), highly esterified homogalacturonan-specific antibodies (LM20) and arabinogalactan-protein specific antibodies (JIM13). For each antibody most intensive signals were set to 100% and the histogram bars illustrate the average intensity of the reacting dots obtained for the 4 plants from each line. For each antibody.
4. Discussion
Here we analyzed different plant lines that stably express the human β1,4-galactosyltransferase (STGalT). In N. benthamiana as well as in A. thaliana some transgenic lines exhibited phenotypical alterations including a delay in development, stunted growth and massive reduction of biomass. These phenotypical alterations came as a surprise since previous studies reporting on the stable expression of different human GalT in tobacco, did not describe any obvious morphological changes, at least under standard growth conditions (Bakker et al., 2001, 2006). However, it cannot be excluded that plants with a phenotype were eliminated in very early selection stages of these studies. Severe growth and developmental phenotypes have been observed in the context of altered N-glycan processing in A. thaliana and other plant species (Strasser, 2014). For example, elimination of A. thaliana α-glucosidase I acting in an early N-glycan processing step caused defects in cell differentiation during embryo development (Boisson et al., 2001; Gillmor et al., 2002) or knockout of all three processing class I α-mannosidases resulted in a severe root growth phenotype and altered cell wall biosynthesis (Liebminger et al., 2009). By contrast, elimination of late-acting N-glycan processing enzymes did not seem to affect the growth or morphology of A. thaliana, N. benthamiana, tobacco or tomato at least under standard growth conditions (Wenderoth and von Schaewen, 2000; Strasser et al., 2004a, 2004b, 2008).
Interestingly, previous studies have shown that plants are largely amenable for N-glycan diversification obtained by the stable expression of human glycosylation proteins, as reported by the overexpression of human glycosyltransferases synthesizing bisected and multi-antennary N-glycans in planta (Rouwendal et al., 2007; Nagels et al., 2011, 2012; Frey et al., 2009). Also, overexpression of three proteins involved in the synthesis of the sugar nucleotide precursor CMP-sialic acid in A. thaliana, did not induce obvious phenotypical modifications (Castilho et al., 2008).
4.1. Which modifications cause the occurrence of the severe growth phenotype?
In our attempts to elucidate factors that are responsible for P1 and P2 phenotypes, we discovered that the expression level of STGalT is a major player. Our results showed that expression of STGalT at the level detected in P1 and NP plants is sufficient to efficiently galactosylate a reporter protein. A further increase of STGalT expression, as observed in P2 plants, does not lead to increased galactosylation of N-glycans, but directly or indirectly interferes with important plant physiological processes. Comprehensive N-glycosylation analyses of STGalT transgenic N. benthamiana plants revealed that protein N-glycosylation is not obviously correlated with the observed growth and developmental phenotype and hints at the interference with related processes. One possible explanation might be depletion of the donor nucleotide sugar UDP-Gal, which is the second most abundant UDP-sugar present in plants (next to UDP-Glc) (Rautengarten et al., 2014) and is used to build up cell wall polysaccharides, like arabinogalactans. In a previous study, it was reported that overexpression of the human UDP-Gal transporter in tobacco resulted in an increase of galactose incorporation into polysaccharide chains of arabinogalactan proteins and it was suggested that this may have been due to elevated UDP-Gal level in the Golgi apparatus (Khalil et al., 2010). However, in our study we did not observe a detectable alteration in nucleotide sugar pools.
Another study reported an altered gibberellin response in transgenic tobacco plants overexpressing human Lewis α1,4-fucosyltransferase (hFUT3). The vegetative growth of hFUT3 transgenic plants was delayed, the plants displayed significantly reduced height and shorter roots (Joly et al., 2004). Thus, it was speculated that hFUT3 expression may have affected enzymes involved in cell wall biosynthesis. By contrast, our data suggested that the overexpression of STGalT does not cause obvious changes in the overall cell wall sugar composition. Nevertheless it seems that the cell wall polysaccharide fine structure is changed. We showed alterations in the reactivity of un-esterified homogalacturonan as seen by a strong reaction to JIM5 antibodies in STGalT expressing plants. Similar results were described for korrigan, a dwarf mutant of A. thaliana that shows changes in pectin composition, namely an increased amount of homogalacturonans and reduced amounts of β1,4-galactans (His et al., 2001).
We also detect increased levels of H2O2 in P2 plants, most probably a consequence of hypersensitive reactions to leaf lesions and necrosis. Such phenomenon has been reported in mutants lacking the nucleotide sugar transporter GONST1 (Mortimer et al., 2013). Interestingly, gonst1 mutants had no reduction in glucomannan quantity and showed no detectable alterations in other cell wall polysaccharides. However, a class of glycosylated sphingolipids had reduced levels of mannosylation accompanied by a severe effect on plant growth and development (Mortimer et al., 2013). A. thaliana gonst1 mutants were severely dwarfed, developed spontaneous leaf lesions and a constitutive hypersensitive response with increased levels of H2O2. Such effects on glycosylated sphingolipids are possible in STGalT transgenic plants and in addition we cannot exclude that certain (low abundant) endogenous proteins differentially galactosylated in P1 and P2 plants exhibit altered functions causing the observed phenotype. These hypotheses need to be investigated in the future.
4.2. STGalT plants display unexpected N-glycan structures
N-glycan profiling revealed some unexpected results: (i) While total soluble proteins of STGalT expressing plants carry significant amounts of galactosylated N-glycans (30–40 %), in secreted proteins only about 13–17 % could be assigned to such structures. By contrast, 4E10-mAb expressed in STGalT-ΔXF showed up to 90% of galactosylated N-glycans with a major peak corresponding to di-galactosylated oligosaccharides (60%). Interestingly, di-galactosylated structures were virtually absent in TSP and in IF. (ii) STGalT transgenic plants generally produce much less fully processed complex N-glycans (GnGnXF, GnGn) than WT and ΔXT/FT. Instead they carry mainly incompletely processed structures (e.g. MGn and MA), which very likely results from insufficient processing by the endogenous N-acetylglucosaminyltransferase II. (iii) Core fucosylation is drastically decreased in STGalT-WT plants. It seems that STGalT expression or activity strongly interferes with this medial Golgi enzyme (Schoberer and Strasser, 2011). While the incomplete N-glycan processing is in line with previous reports on the expression of native human GalT and fusions thereof that target the enzyme to an early/medial Golgi compartment (Bakker et al., 2001, 2006; Schoberer et al., 2014) it was unexpected since we used a targeting signal that directs GalT to a trans Golgi compartment supposedly acting post fucosylation (Schoberer et al., 2010). By contrast, xylosylation was only slightly affected in STGalT-WT plants which might be explained by subtle differences in Golgi subcompartmentation of fucosylation and xylosylation. Using transiently expressed chimeric GalT fusions we recently observed that minor differences in Golgi targeting can significantly alter N-glycosylation profiles of recombinant proteins (Schoberer et al., 2014).
The activity of STGalT in a late stage of N-glycan processing might be the reason for significant amounts of galactosylated structures (30–40%, independently of the genetic background and phenotype) in TSP of STGalT expressing plants. In comparison, previous reports described only 10–15% galactosylation of total soluble proteins (Bakker et al., 2001). This emphasizes the importance of proper sub Golgi targeting of glycosyltransferases for efficient processing of N-glycans.
Two reasons could account for the fact that TSP, IF-derived proteins and a selected recombinant protein exhibit different amounts of galactosylation. (i) The attachment of this sugar residue might be a highly protein-specific event. While certain target proteins (e.g. IgGs) are efficiently di-galactosylated, others (e.g. plant specific) are not. We frequently observe this phenomenon when expressing different recombinant proteins in plants; some are more efficiently galactosylated than others, despite identical subcellular location. (ii) Some proteins can be more susceptible to plant β-galactosidases that remove terminal galactose and have mainly an extracellular activity (Stano et al., 2002).
Taken together, our data demonstrate that the stable expression of a heterologous N-glycan modifying enzyme in N. benthamiana plants may be accompanied by drastic effects on plant growth and development and unforeseen changes in overall N-glycosylation as a result of excessive overexpression of the foreign gene. Such issues should be considered when designing strategies for glycan-engineering in plants.
Contributions
AC, RS and HS: design and interpretation of all experiments.
JS; AC and PG: carried out experimental work.
MP, CG and FA: designed and carried out HPLC and mass spectrometry experiments.
JS and GS: design and interpretation of cell wall analysis and dot blot experiments.
AC, RS and HS: wrote the manuscript.
Acknowledgments
We thank Eric G. Berger for obtaining anti-GalT antibody and Koen Weterings (Bayer Crop Science) for transformation/regeneration of ΔXT/FT plants with STGalT. The work was supported by the Austrian Research Promotion Agency (Laura Bassi Centre of Expertise “Plant produced BioPharmaceuticals” Grant Nr. 822757) and the Austrian Science Fund (Grant Nrs. L575-B13, P21782-B12 and I1182-B22).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Anumula K.R. Quantitative determination of monosaccharides in glycoproteins by high-performance liquid chromatography with highly sensitive fluorescence detection. Anal. Biochem. 1994;220:275–283. doi: 10.1006/abio.1994.1338. [DOI] [PubMed] [Google Scholar]
- Bakker H., Bardor M., Molthoff J.W., Gomord V., Elbers I., Stevens L.H., Jordi W., Lommen A., Faye L., Lerouge P., Bosch D. Galactose-extended glycans of antibodies produced by transgenic plants. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2899–2904. doi: 10.1073/pnas.031419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker H., Rouwendal G.J., Karnoup A.S., Florack D.E., Stoopen G.M., Helsper J.P., van Ree R., van Die I., Bosch D. An antibody produced in tobacco expressing a hybrid beta-1,4-galactosyltransferase is essentially devoid of plant carbohydrate epitopes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7577–7582. doi: 10.1073/pnas.0600879103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson M., Gomord V., Audran C., Berger N., Dubreucq B., Granier F., Lerouge P., Faye L., Caboche M., Lepiniec L. Arabidopsis glucosidase I mutants reveal a critical role of N-glycan trimming in seed development. EMBO J. 2001;20:1010–1019. doi: 10.1093/emboj/20.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho A., Pabst M., Leonard R., Veit C., Altmann F., Mach L., Glossl J., Strasser R., Steinkellner H. Construction of a functional CMP-sialic acid biosynthesis pathway in Arabidopsis. Plant Physiol. 2008;147:331–339. doi: 10.1104/pp.108.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho A., Strasser R., Stadlmann J., Grass J., Jez J., Gattinger P., Kunert R., Quendler H., Pabst M., Leonard R., Altmann F., Steinkellner H. In planta protein sialylation through overexpression of the respective mammalian pathway. J. Biol. Chem. 2010;285:15923–15930. doi: 10.1074/jbc.M109.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M.H., Willats W.G., Knox J.P. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carb. Res. 2003;338:1797–1800. doi: 10.1016/s0008-6215(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Frey A.D., Karg S.R., Kallio P.T. Expression of rat beta(1,4.-N-acetylglucosaminyltransferase III in Nicotiana tabacum remodels the plant-specific N-glycosylation. Plant Biotechnol. J. 2009;7:33–48. doi: 10.1111/j.1467-7652.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Gillmor C.S., Poindexter P., Lorieau J., Palcic M.M., Somerville C. Alpha-glucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. J. Cell. Biol. 2002;156:1003–1013. doi: 10.1083/jcb.200111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- His I., Driouich A., Nicol F., Jauneau A., Hofte H. Altered pectin composition in primary cell walls of korrigan, a dwarf mutant of Arabidopsis deficient in a membrane-bound endo-1,4-beta-glucanase. Planta. 2001;212:348–358. doi: 10.1007/s004250000437. [DOI] [PubMed] [Google Scholar]
- Jez J., Antes B., Castilho A., Kainer M., Wiederkum S., Grass J., Ruker F., Woisetschlager M., Steinkellner H. Significant impact of single N-glycan residues on the biological activity of Fc-based antibody-like fragments. J. Biol. Chem. 2012;287:24313–24319. doi: 10.1074/jbc.M112.360701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jez J., Castilho A., Grass J., Vorauer-Uhl K., Sterovsky T., Altmann F., Steinkellner H. Expression of functionally active sialylated human erythropoietin in plants. Biotechnol. J. 2013;8:371–382. doi: 10.1002/biot.201200363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly C., Maftah A., Riou-Khamlichi C. Alteration of gibberellin response in transgenic tobacco plants which express a human Lewis fucosyltransferase. Plant Physiol. Biochem. 2004;42:629–637. doi: 10.1016/j.plaphy.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Khalil M.F., Kajiura H., Fujiyama K., Koike K., Ishida N., Tanaka N. The impact of the overexpression of human UDP-galactose transporter gene hUGT1 in tobacco plants. J. Biosci. Bioeng. 2010;109:159–169. doi: 10.1016/j.jbiosc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Liebminger E., Huttner S., Vavra U., Fischl R., Schoberer J., Grass J., Blaukopf C., Seifert G.J., Altmann F., Mach L., Strasser R. Class I alpha-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell. 2009;21:3850–3867. doi: 10.1105/tpc.109.072363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Vailleau F., Balague C., Roby D. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 2003;8:263–271. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- Mortimer J.C., Yu X., Albrecht S., Sicilia F., Huichalaf M., Ampuero D., Michaelson L.V., Murphy A.M., Matsunaga T., Kurz S., Stephens E., Baldwin T.C., Ishii T., Napier J.A., Weber A.P., Handford M.G., Dupree P. Abnormal glycosphingolipid mannosylation triggers salicylic acid-mediated responses in Arabidopsis. Plant Cell. 2013;25:1881–1894. doi: 10.1105/tpc.113.111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels B., Van Damme E.J., Pabst M., Callewaert N., Weterings K. Production of complex multiantennary N-glycans in Nicotiana benthamiana plants. Plant Physiol. 2011;155:1103–1112. doi: 10.1104/pp.110.168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels B., Van Damme E.J., Callewaert N., Weterings K. Introduction of tri-antennary N-glycans in Arabidopsis thaliana plants. Plant Sci. 2012;185–186:161–168. doi: 10.1016/j.plantsci.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Pabst M., Grass J., Fischl R., Leonard R., Jin C., Hinterkorner G., Borth N., Altmann F. Nucleotide and nucleotide sugar analysis by liquid chromatography-electrospray ionization-mass spectrometry on surface-conditioned porous graphitic carbon. Anal. Chem. 2010;82:9782–9788. doi: 10.1021/ac101975k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C., Ebert B., Moreno I., Temple H., Herter T., Link B., Donas-Cofre D., Moreno A., Saez-Aguayo S., Blanco F., Mortimer J.C., Schultink A., Reiter W.D., Dupree P., Pauly M., Heazlewood J.L., Scheller H.V., Orellana A. The Golgi localized bifunctional UDP-rhamnose/UDP-galactose transporter family of Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11563–11568. doi: 10.1073/pnas.1406073111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwendal G.J., Wuhrer M., Florack D.E., Koeleman C.A., Deelder A.M., Bakker H., Stoopen G.M., van Die I., Helsper J.P., Hokke C.H., Bosch D. Efficient introduction of a bisecting GlcNAc residue in tobacco N-glycans by expression of the gene encoding human N-acetylglucosaminyltransferase III. Glycobiology. 2007;17:334–344. doi: 10.1093/glycob/cwl078. [DOI] [PubMed] [Google Scholar]
- Schoberer J., Runions J., Steinkellner H., Strasser R., Hawes C., Osterrieder A. Sequential depletion and acquisition of proteins during Golgi stack disassembly and reformation. Traffic. 2010;11:1429–1444. doi: 10.1111/j.1600-0854.2010.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J., Strasser R. Sub-compartmental organization of Golgi-resident N-glycan processing enzymes in plants. Mol. Plant. 2011;4:220–228. doi: 10.1093/mp/ssq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J., Liebminger E., Vavra U., Veit C., Castilho A., Dicker M., Maresch D., Altmann F., Hawes C., Botchway S.W., Strasser R. The transmembrane domain of N-acetylglucosaminyltransferase I is the key determinant for its Golgi sub-compartmentation. Plant J. 2014;80:809–822. doi: 10.1111/tpj.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen I., Willats W.G. Screening and characterization of plant cell walls using carbohydrate microarrays. Meth. Mol. Biol. 2011;715:115–121. doi: 10.1007/978-1-61779-008-9_8. [DOI] [PubMed] [Google Scholar]
- Stadlmann J., Pabst M., Kolarich D., Kunert R., Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8:2858–2871. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- Stano J., Kovacs P., Micieta K., Neubert K., Tintemann H., Korenova M. Localization and measurement of extracellular plant galactosidases. Acta Histochem. 2002;104:441–444. doi: 10.1078/0065-1281-00665. [DOI] [PubMed] [Google Scholar]
- Stepan H., Staudacher E. Optimization of monosaccharide determination using anthranilic acid and 1-phenyl-3-methyl-5-pyrazolone for gastropod analysis. Anal. Biochem. 2011;418:24–29. doi: 10.1016/j.ab.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R. Biological significance of complex N-glycans in plants and their impact on plant physiology. Front. Plant Sci. 2014;5:363. doi: 10.3389/fpls.2014.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R., Altmann F., Glossl J., Steinkellner H. Unaltered complex N-glycan profiles in Nicotiana benthamiana despite drastic reduction of beta1,2-N-acetylglucosaminyltransferase I activity. Glycoconj. J. 2004;21:275–282. doi: 10.1023/B:GLYC.0000045099.29038.04. [DOI] [PubMed] [Google Scholar]
- Strasser R., Altmann F., Mach L., Glossl J., Steinkellner H. Generation of Arabidopsis thaliana plants with complex N-glycans lacking beta1,2-linked xylose and core alpha1,3-linked fucose. FEBS Lett. 2004;561:132–136. doi: 10.1016/S0014-5793(04)00150-4. [DOI] [PubMed] [Google Scholar]
- Strasser R., Stadlmann J., Schähs M., Stiegler G., Quendler H., Mach L., Glössl J., Weterings K., Pabst M., Steinkellner H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- Strasser R., Castilho A., Stadlmann J., Kunert R., Quendler H., Gattinger P., Jez J., Rademacher T., Altmann F., Mach L., Steinkellner H. Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous beta1,4-galactosylated N-glycan profile. J. Biol. Chem. 2009;284:20479–20485. doi: 10.1074/jbc.M109.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R., Altmann F., Steinkellner H. Controlled glycosylation of plant-produced recombinant proteins. Curr. Opin. Biotechnol. 2014;30C:95–100. doi: 10.1016/j.copbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Tripathy B.C., Oelmuller R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y., Marcus S.E., Haeger A., Ordaz-Ortiz J.J., Knox J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carb. Res. 2009;344:1858–1862. doi: 10.1016/j.carres.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Weigel D., Glazebrook J. How to transform arabidopsis. In: Weigel Detlef, Glazebrook Jane., editors. Arabidopsis: a Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2002. (Chapter 5) [Google Scholar]
- Wenderoth I., von Schaewen A. Isolation and characterization of plant N-acetyl glucosaminyltransferase I (GntI) cDNA sequences. Functional analyses in the Arabidopsis cgl mutant and in antisense plants. Plant Physiol. 2000;123:1097–1108. doi: 10.1104/pp.123.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates E.A., Valdor J.F., Haslam S.M., Morris H.R., Dell A., Mackie W., Knox J.P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology. 1996;6:131–139. doi: 10.1093/glycob/6.2.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.