Abstract
Objectives
In mammals, cochlear hair cell loss is irreversible and may result in a permanent sensorineural hearing loss. Secondary to this hair cell loss, a progressive loss of spiral ganglion neurons (SGNs) is presented. In this study, we have investigated the effects of neural-induced human mesenchymal stem cells (NI-hMSCs) from human bone marrow on sensory neuronal regeneration from neomycin treated deafened guinea pig cochleae.
Methods
HMSCs were isolated from the bone marrow which was obtained from the mastoid process during mastoidectomy for ear surgery. Following neural induction with basic fibroblast growth factor and forskolin, we studied the several neural marker and performed electrophysiological analysis. NI-hMSCs were transplanted into the neomycin treated deafened guinea pig cochlea. Engraftment of NI-hMSCs was evaluated immunohistologically at 8 weeks after transplantation.
Results
Following neural differentiation, hMSCs expressed high levels of neural markers, ionic channel markers, which are important in neural function, and tetrodotoxin-sensitive voltage-dependent sodium currents. After transplantation into the scala tympani of damaged cochlea, NI-hMSCs-injected animals exhibited a significant increase in the number of SGNs compared to Hanks balanced salt solution-injected animals. Transplanted NI-hMSCs were found within the perilymphatic space, the organ of Corti, along the cochlear nerve fibers, and in the spiral ganglion. Furthermore, the grafted NI-hMSCs migrated into the spiral ganglion where they expressed the neuron-specific marker, NeuN.
Conclusion
The results show the potential of NI-hMSCs to give rise to replace the lost cochlear cells in hearing loss mammals.
Keywords: Hair Cells, Auditory; Mesenchymal Stem Cells; Hearing Loss; Cell Differentiation; Transplantation
INTRODUCTION
The human cochlea contains only 15,000-20,000 sensory hair cells which are constantly threatened and decreased by insults such as exposure to excessive noise, ototoxic drugs, infections and the general process of aging. Although some regeneration of vestibular hair cells has been reported in the mammalian inner ear, sensory hair cells of the organ of Corti do not regenerate [1,2]. Moreover, the loss of cochlear hair cells results in the secondary degeneration of spiral ganglion neurons (SGNs), most likely through loss of trophic factors that enhance SGNs survival [3,4]. Also SGNs are not capable of postembryonic mitosis to produce new neurons, thus aggravating the hearing impairment and reducing the possibilities for rehabilitation [5]. Therapeutic approaches to sensorineural hearing loss include genetic manipulation, such as Atoh1 gene insertion [6] and retinoblastoma gene deletion [7], to induce new hair cell production, and stem cell transplantation to replace the lost or damaged hair cells and SGNs [8,9].
Among the various types of stem cells, bone marrow-derived mesenchymal stem cells (MSCs) are one of the most promising candidates for cell replacement therapy. MSCs exhibit marked self-renewal capacity and the ability to differentiate not only into osteoblasts, chondrocytes, adipocytes, myocytes, but also into neurons in vitro and in vivo [10,11]. The biggest advantage of using MSCs over other cell types is the ability to use them in autologous transplantation [12].
Stem cells have been introduced to the search for new therapeutic strategies for mammalian cochlear cell regeneration in the past several years. Previous studies have suggested that inner ear neurons or hair cell-like cells could be generated in vivo or in vitro from embryonic stem cells [13], from neural stem cells [14], from umbilical cord blood stem cells [15], from induced pluripotent stem cells [16], and from adult inner-ear stem cells [17]. Primary MSCs have also been proven to survive in the inner ear up to a few weeks; however, none till date have reported the regeneration or replacement of hair cell-like phenotypes after MSCs transplantation. Furthermore, none have shown whether neural differentiated MSCs can serve as a source for cell replacement therapy in injured cochleae. We demonstrated transdifferentiation of bone marrow-derived hMSCs into functional neural cells using basic fibroblast growth factor (bFGF) and forskolin followed previous study [18], and investigated that the neural-induced human MSCs (NI-hMSCs) have the potential to replace the lost SGNs and the damaged cells within the organ of Corti in deaf guinea pigs.
MATERIALS AND METHODS
Preparation of hMSCs and in vitro neural differentiation
In this study, we isolated and characterized human tissue-derived stem cells and finally differentiated into neuronal cells for clinical application in the future. Bone marrow was obtained from the mastoid process of healthy 29- to 51-year-old donors during mastoidectomy for ear surgery. Informed consent was obtained from ten donors according to Guideline of the Ethics Committee of the Chonnam National University Medical School (Institutional Review Board No. I-2009-03-016). The morphological features of the hMSCs were the same as those previously described [10,19]. Fluorescence activated cell sorting (FACS) analysis was performed as described previously [18]. Briefly, hMSCs were harvested in trypsin containing ethylenediaminetetraacetic acid (EDTA) (HyClone, Logan, UT, USA), washed twice with phosphate-buffered saline (PBS; Amresco Inc., Solon, OH, USA) and stained on ice according to the recommendation of the manufacture with the monoclonal antibodies (BD Biosciences PharMingen, Heidelberg, Germany), including PE-CD13, FITC-CD14, FITC-CD34, PE-CD44, FITC-CD45, PE-CD90, and PE-CD166. A least 10,000 events were collected and analyzed with flow cytometry. The MSCs we used in the present study were from passages four to ten.
To induce neural differentiation, hMSCs were grown in Dulbecco's modified eagle medium containing 1% fetal bovine serum and supplementary 100-ng/mL bFGF (Invitrogen, Carlsbad, CA, USA) for seven days. After then, the cells were incubated in the presence of 10 µM forskolin (Sigma Chemical Co., St. Louis, MO, USA). Over the next seven days the cells were subjected to immunocytochemical, electrophysiological, and reverse transcription-polymerase chain reaction (RT-PCR) analyses, and also used for transplantation into deafened guinea pig cochleae.
RT-PCR analysis
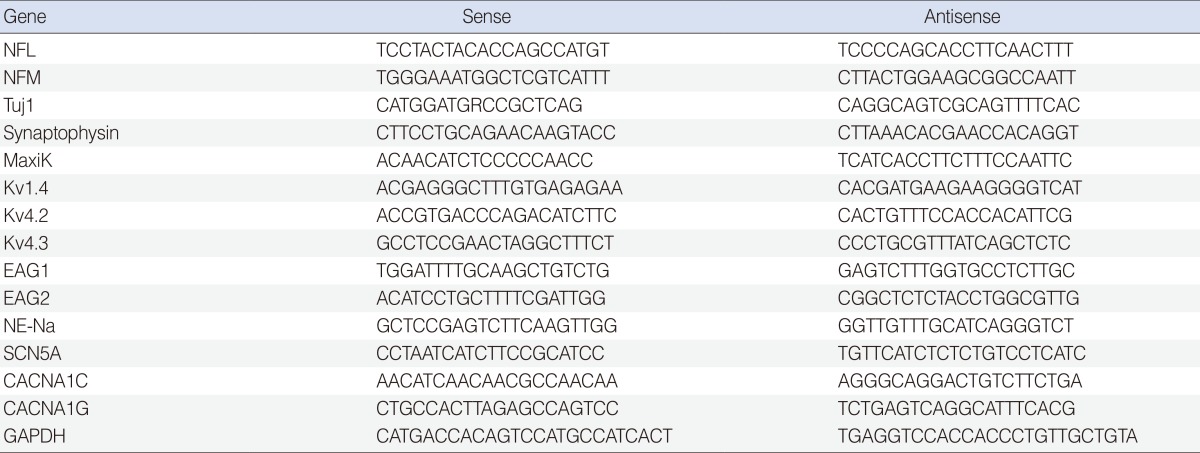
RT-PCR analysis was also performed as previously described [18]. The forward and reverse PCR oligonucleotide primers chosen to amplify the cDNA are listed in Table 1.
Table 1. Sequence of polymerase chain reaction primers.
Immunocytochemistry
Immunochemical determination of cell type specific markers in hMSCs and NI-hMSCs were performed as previously described [18]. Cell type-specific markers used were β-tubulin III (Tuj1, 1:500), neurofilament-L (NF-L, 1:300), neurofilament-M (NF-M, 1:300), neurofilament-H (NF-H, 1:300), glial fibrillary acidic protein (GFAP, 1:300), microtubule-associated protein (MAP2, 1:300), and neuronal nuclei (NeuN, 1:200). These primary antibodies were purchased from Chemicon (Chemicon, Temecula, CA, USA). Nuclei were stained with hematoxylin for cell counting. To perform quantitative analysis, the numbers of positive cells was counted on each acquired image by Image J 1.42 (National Institutes of Health, Bethesda, MD, USA), and the ratio to the number of nuclei was analyzed for each antigen.
Electrophysiology
The cells grown on cover slips for 2 weeks were placed in a recording chamber on the stage of an inverted microscope (Eclipse TE 2000-S, Nikon, Tokyo, Japan), and voltage-dependent ionic currents and resting membrane potentials were recorded using the whole-cell patch clamp technique [18].
Animal models and NI-hMSCs transplantation
All animal protocols were reviewed and approved by the Institutional Animal Care Committee of Research Institute of Medical Science at Chonnam National University. Forty adult female guinea pigs (body weight 250-400 g) in total were used to obtain the final data shown in this study: Hanks balanced salt solution (HBSS, n=20) and NI-hMSCs in HBSS (n=20). Each animal was sacrificed at 8 weeks after transplantation to verify the survival of grafted stem cells.
In order to destroy the sensory epithelium, guinea pigs were deafened by an injection of 10% neomycin (500 µL in PBS) through the right tympanic membrane under deep anesthesia with an intraperitoneal injection of sodium pentothal (45 mg/kg of body weight) [20]. The ototoxic drug treatment was accomplished 7 days before NI-hMSCs transplantation. One week after deafening procedure, we performed auditory brainstem response test for all injured guinea pigs and excluded the animals from this experiment unless they displayed no response to 90 dB or less prior to transplantation (data not shown).
To transplant the NI-hMSCs into the cochlea, the right bulla was exposed and opened to visualize the basal cochlea. A small hole was made into the scala tympani at the most basal cochlear turn using a 1-mm diamond drill. HBSS containing NI-hMSC aggregates (1×105 cells in 5 µL) or HBSS alone were then injected into the right scala tympani through the hole using a Hamilton syringe and an infusion pump (at the speed of 2 µL/min). Finally, the hole was plugged with connective tissue and fibrin based adherent agents (Greenplast, Green-Cross, Seoul, Korea), and the incision was approximated with sutures [19].
Fluorescent immunohistochemistry
After anesthesia (as described above), the cochleae were carefully removed, trimmed, fixed in 10% neutral buffered formalin (NBF) overnight, and decalcified for two weeks in 10% EDTA in NBF. The fixed, decalcified cochleae embedded in paraffin wax prior to being sliced into 5-µm-thick serial paramodiolar sections. Every fifth following section was chosen for analysis to ensure a separation of 25 µm between the sections. A total five sections per cochlea were analyzed. After blocking in 1% normal goat serum, the sections were incubated with primary antibodies against human nuclei (1:200) and MAP2 (1:300) at 4℃. Then, the sections were incubated with secondary antibodies were used to visualize primary antibodies. 4', 6-diamidino-2-phenylindole were used to label nuclei. All primary and secondary antibodies were purchased from Chemicon.
Cell counting and statistics
For the quantification of the number of SGNs, midmodiolar sections were stained with hematoxylin and eosin. The numbers of transplant-derived cells, identified by positive human nuclear immunofluorescence, were also counted in the NI-hMSCs-injected cochlea. To establish the turn number, we followed the method as described by Parker et al. [14]. Briefly, the hook region was identified and the wide basal turn was labeled turn 1.0. The SGNs, which were identified by their morphology, were counted by superimposing a 100×100-µm box on the spiral ganglion (SG) image and counting cells within each box. All values are expressed as mean±standard error of the mean. The one-way analysis of variance test (Bonferroni post hoc comparison) was used to analyze the differences between groups, with P<0.05 being considered significant.
RESULTS
Characterization of isolated bone marrow-derived hMSCs
After approximately 4 weeks in culture, human MSCs isolated from bone marrow became more uniform and grew in a monolayer with typical fibroblast-like morphology, consistent with previous reports [10,18]. The proliferation rate of hMSCs remained consistent at least up to the 15th passage.
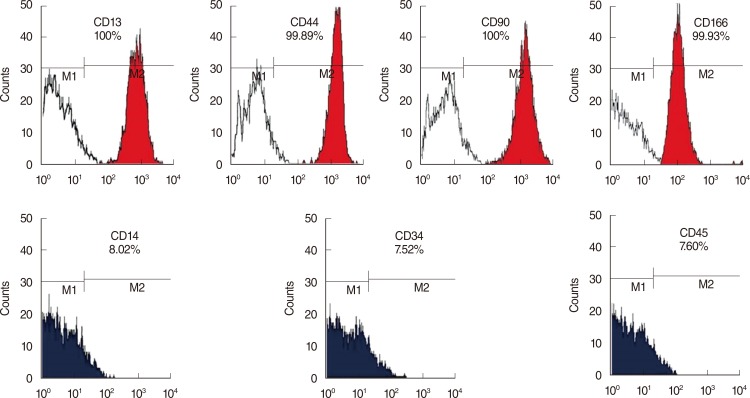
To elucidate the types of the hMSCs, FACS analysis was performed with various cell surface markers, including MSC-specific cell type markers and or hematopoietic stem cell-specific markers. As shown in Fig. 1, the bone marrow-derived hMSCs expressed more than 95% MSC-specific markers such as CD13, CD44 (endoglin), CD90 (Thy-1), or CD166, but did not express markers for hematopoietic stem cells, including CD14, CD34, and CD45. These cell surface profiles demonstrated that the stem cells isolated from human bone marrow were MSCs phenotypically.
Fig. 1. Characterization of bone marrow-derived human mesenchymal stem cells (hMSCs). Primary hMSCs expressed MSC specific markers including CD13 (+), CD44 (+), CD90 (+), and CD166 (+), but did not express hematopoietic stem cell markers including CD14 (-), CD34 (-), and CD45 (-).
Functional neural differentiation of hMSCs
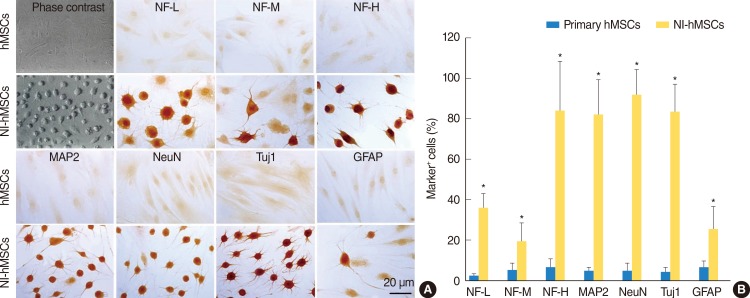
In the presence of neural induction supplements, the majority of NI-hMSCs exhibited bipolar or multipolar morphologies with branched processes (Fig. 2A). In primary undifferentiated hMSCs, the immunoreactivities for astrocyte marker (GFAP) and neuronal markers (NF-L, NF-M, NF-H, MAP2, NeuN, and Tuj1) were very low or undetectable when grown in medium in the absence of bFGF and forskolin supplements. However, supplementation with bFGF and forskolin considerably increased the proportion of the hMSCs expressing these neuronal or glial cell type specific markers (Fig. 2). Following terminal differentiation with bFGF and forskolin, a large number of neuronal markers-positive neurons were found in cultures as compared with GFAP-positive astrocytes (Fig. 2B).
Fig. 2. In vitro differentiation of human mesenchymal stem cells (hMSCs). hMSCs were induced to differentiate into neural cells in the presence of basic fibroblast growth factor and forskolin for two weeks. (A) Immunocytochemistry revealed that the expressions of neurofilament-L (NF-L), neurofilament-M (NF-M), neurofilament-H (NF-H), microtubule-associated protein (MAP2), neuronal nuclei (NeuN), β-tubulin III (Tuj1), and glial fibrillary acidic protein (GFAP) in neural-induced hMSCs (NI-hMSCs) were increased than those in primary hMSCs. (B) Immunocytochemical data depicted the high ratio of NI-hMSCs expressing above neural markers. The number of positive cells was counted and the ratio to the number of nuclei was analyzed for each antigen (n=8, *P<0.05 compared with primary hMSCs).
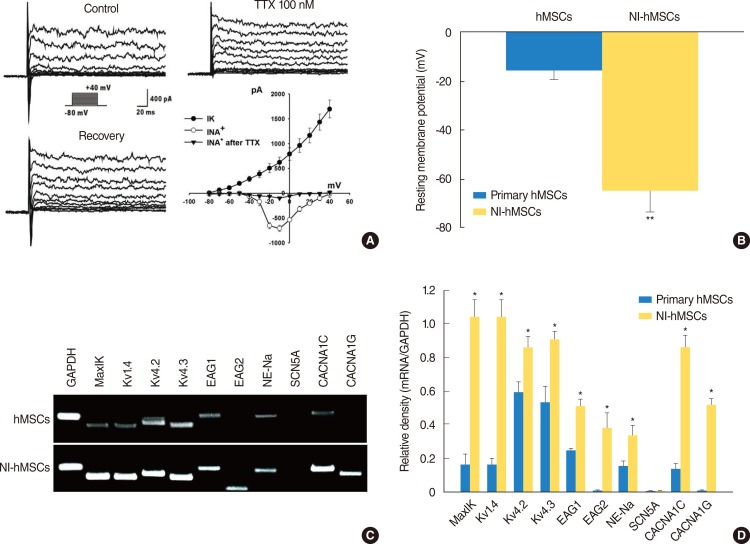
To assess the in vitro differentiation of the hMSCs into functional neurons, we evaluated the electrophysiological properties of hMSCs before and after neural induction using the patch clamp technique in whole-cell configuration. Primary hMSCs grown in the regular media in the absence of bFGF and forskolin were quiescent, exhibiting virtually no sodium current (data not shown) which is responsible for both initiation and propagation of action potentials of the neurons throughout the nervous system. However after differentiation with bFGF and forskolin, more than 66% of the NI-hMSCs expressed -710.0±61.96 pA of voltage-dependent sodium currents (Fig. 3A). Furthermore, the sodium currents were blocked by 100-nM tetrodotoxin (TTX). Concomitant with sodium current induction, the hMSCs grown with the differentiation factors showed sustained outward currents (Fig. 3A), and these currents showed a voltage-dependence and kinetics characteristics for delayed rectifier potassium currents. Whole-cell patch clamp recordings under current-clamp mode were performed to measure resting membrane potentials of primary MSCs and NI-hMSCs. The resting membrane potential of NI-hMSC (-64.58±8.46 mV, n=24) was recorded more negatively than that of control hMSCs (-16±3.27 mV, n=24; P<0.01) (Fig. 3B).
Fig. 3. Electrophysiological features of the neural-induced human mesenchymal stem cells (NI-hMSCs). (A) hMSCs demonstrated neuronal characteristics after neural differentiation under voltage-clamp recording. The holding potential was -80 mV and depolarizing steps were applied from -80 mV to +40 mV in 10 mV increments. Large voltage-dependent sodium currents were activated evidently from a depolarizing step of -30 mV and blocked reversibly by tetrodotoxin (TTX) 100 nM. In addition, the hMSCs grown with basic fibroblast growth factor (bFGF) and forskolin showed sustained outward potassium currents as well. Peak current-voltage relationship was plotted against the voltages and demonstrated the voltage-dependence of potassium currents (IK) and sodium currents (INa) (n=16). (B) Under current clamp condition, the resting membrane potential of NI-hMSCs was recorded more negatively than that of control hMSCs grown without bFGF and forskolin (n=16, **P<0.01 compare with primary hMSCs). (C) Expression of molecular markers for ion channel subunits was increased after neural differentiation in hMSCs. The mRNA expression of human large-conductance, voltage- and calcium-dependent K+ channel marker, MaxiK; voltage-dependent K+ channel marker, Kv1.4, Kv4.2, and Kv4.3; either-à-go-go K+ channel marker, Eag1 and Eag2; tetrodotoxin-sensitive Na+ channel marker, NE-Na; voltage-dependent L-type Ca2+ channel, alpha 1C subunit marker, CACNA1C; and voltage-dependent T-type Ca2+ channel, alpha 1G subunit marker, CACNA1G were increased in NI-hMSCs, however, that of TTX-insensitive sodium channel marker, SCN5A were not detected. Reverse transcription-polymerase chain reaction assay was repeated five times independently from different cells. The representative data are shown. (D) The intensity of each gene was normalized to GAPDH and these results were repeated at least three times (*P<0.05 compared with primary hMSCs).
In addition to electrophysiological study, we also investigated the mRNA expression for ion channels related to outward and inward currents in primary and NI-hMSCs with RT-PCR using the specific primers shown in Table 1. The mRNA expression levels of MaxiK (responsible for human large-conductance, voltage-and calcium-dependent K+ channel), Kv1.4, Kv4.2, and Kv4.3 (responsible for human voltage-dependent K+ channel), Eag1 and Eag2 (responsible for human either-à-go-go K+ channel), NE-Na (responsible for TTX-sensitive Na+ channel), CACNA1C (responsible for human voltage-dependent L-type Ca2+ channel, alpha 1C subunit), and CACNA1G (responsible for human voltage-dependent T-type Ca2+ channel, alpha 1G subunit) in NI-hMSCs were significantly higher than the primary hMSCs (P<0.05) (Fig. 3C, D). However, the mRNA expression of TTX-insensitive sodium channel SCN5A was detected neither in hMSCs nor in NI-hMSCs. The relative densities of the specific mRNA to the GAPDH are shown in Fig. 3D, and the data provide the molecular basis for the functional ionic currents observed in NI-hMSCs. These results indicate that the hMSCs acquired neuronal cell fate and thereby expressed neuron-specific phenotypes after being terminally differentiated in vitro.
Survival and localization of transplanted NI-hMSCs in the SG and in the organ of Corti
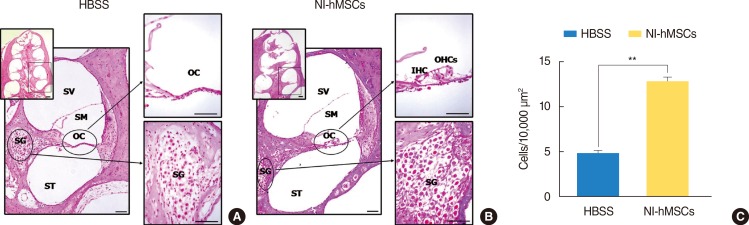
In HBSS alone-injection group (Fig. 4A), the organ of Corti showed a severe loss of sensory hair cells from the basal to third turn, and the spiral ganglia displayed a neuronal degeneration and decreased number of cell bodies (4.79±0.36/10,000 µm2) in a cochlea. Basal turn of cochlea was magnified as a box. In NI-hMSCs-injection group, however, the architecture of the organ of Corti is relatively well preserved (Fig. 4B) and the number of cell bodies (12.70±0.49/10,000 µm2) in the spiral ganglia was increased profoundly 8 weeks after stem cell transplantation (n=8; P<0.01) (Fig. 4C).
Fig. 4. Hematoxylin and eosin staining at eight weeks after neural-induced human mesenchymal stem cells (NI-hMSCs) transplantation. (A, B) All turns of cochlea were studied and basal one was magnified as a box, individually. (A) In a Hanks balanced salt solution (HBSS) alone-injected animal, severe loss of sensory hair cells in the organ of Corti and the degeneration of spiral ganglion neurons were observed. (B) However, cochlea of a NI-hMSCs-injected guinea pig demonstrated relatively preserved organ of Corti and spiral ganglion. (C) Quantification of spiral ganglion cell counts demonstrated that cochleae of NI-hMSCs-injected animals exhibited a significant increase in the number of cell body compared to HBSS alone-injected animals (n=8, **P<0.01). Normal animals (n=10) were used as a control. OC, organ of Corti; SG, spiral ganglion; SM, scala media; ST, scala tympani; SV, scala vestibule; IHC, inner hair cell; OHC, outer hair cell. Scale bars indicate 50 µm.
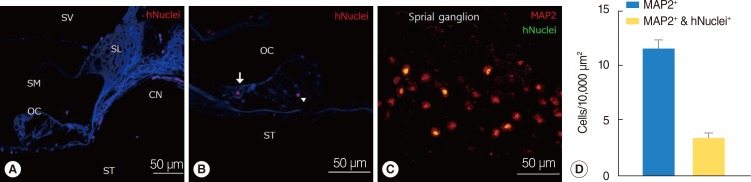
We used human-specific antinuclei antibody to specifically identify NI-hMSCs in the cochleae of transplanted guinea pig inner ear (Fig. 5). The engrafted hMSCs were found in all transplanted guinea pig cochleae. At 8 weeks after transplantation, cells that were immunoreactive for antihuman nuclei antibody were found in multiple regions in the cochlea including the scala tympani, the scala vestibuli, the scala media, and the SG (Fig. 5A-C). As shown in Fig. 5A, the grafted NI-hMSCs were also observed in the spiral limbus, the osseous spiral lamina and surprisingly along the cochlear nerve fibers projecting to the SG. Within the organ of Corti, transplanted stem cells were identified including in the inner hair cell layer and in the supporting cell layer (Fig. 5B).
Fig. 5. Localization and in vivo differentiation of transplanted human mesenchymal stem cells (hMSCs). (A-D) Immunohistochemical study was performed eight weeks after neural-induced hMSCs (NI-hMSCs) transplantation into 10% neomycin-treated guinea pig inner ear. (A) Transplanted hMSCs stained with human nuclei (hNuclei; red) were found along the cochlear nerve fibers close to the organ of Corti. (B) Human nuclei expressing transplant derived-hMSCs (red) were located in the inner hair cell layer (arrow) and supporting cell layer (arrowhead). (C) NI-hMSCs (green) migrated into the damaged spiral ganglion were stained with microtubule-associated protein (MAP2) (red), and merged as yellow. These results depict that NI-hMSCs have the capacity not only to survive and localize in the inner ear, but also to regenerate or replace the damaged cochlear cell types. (D) Quantification of spiral ganglion neuron (MAP2+) and transplant-derived cell (hNuclei+) counts indicated that transplanted hMSCs were transdifferentiated into mature neurons in the spiral ganglion. CN, cochlear nerve fibers; OC, organ of Corti; SG, spiral ganglion; SL, spiral limbus; SM, scala media; ST, scala tympani; SV, scala vestibule.
To further evaluate the types of the transplanted NI-hMSCs, tissue type specific markers were tested in the transplanted cochleae. In the SG, 11.58±0.86 cells were labeled with neuronal marker MAP2 at eight weeks after transplantation, which shown SGNs coming from both guinea pigs and NI-hMSCs. NI-hMSCs, which were double stained with anti-MAP2 and antihuman nuclei antibody, were counted 3.41±0.41 cells per 10,000 µm2 in the inner ear (Fig. 5C, D). The transplanted hMSCs were also found in every turns of the cochlea, implicating a spread within the fluid compartments.
DISCUSSION
This study presents the novel demonstration of a therapeutic approach leading to substantial recovery of cochlear cells using neural differentiated MSCs in deaf mammals. It has been reported that the neural induction of MSCs is required for better treatment in animal models of neurological dysfunction such as Parkinson disease, stroke, and brain ischemia [10,12]. Neural differentiation has been achieved with different experimental protocols using chemical agents [12] or cocultures with astroglial cells [21]. Our results have shown that bFGF and forskolin induces the differentiation of bone marrow-derived hMSCs into functional neural cells which had the morphological and phenotypical characteristics. Similar to neuronal cells derived from other MSCs or embryonic stem (ES) cells [22], in vitro-transdifferentiated hMSCs exhibited immunocytochemical and electrophysiological properties of neuronal cells. NI-hMSCs were identified to generate TTX-sensitive voltage-dependent sodium current, a hallmark of mature neurons and crucial for signal transmission in the nervous system, and to exhibit about -64 mV of resting membrane potential, indicating that the hMSCs also have functional characteristics of neurons. It has been known that undifferentiated hMSCs express the TTX-sensitive sodium channel gene (NE-Na), potassium channel genes (MaxiK, Kv1.4, Kv4.2, Kv4.3, Eag1) and calcium channel gene (CACNA1C) [23]. Our study provides the evidence that the level of these ion channel mRNA expressions is increased after neural induction, further demonstrating differentiation of hMSCs toward neuronal cells. In addition, we identified novel information that two functional ion channel genes (Eag2 and CACNA1G) were expressed in hMSCs after neural differentiation (Fig. 3).
Also, we transplanted NI-hMSCs into deaf guinea pig cochleae and proved that hMSCs survived up to eight weeks and spread to the scala media, scala vestibule, organ of Corti, SG and along the nerve fibers. As shown in Fig. 5, transplanted NI-hMSCs were predominantly located within the SG and a basal membrane.
There are few previous reports of experiments on the transplanting MSCs into the cochlea [24,25,26]. These studies used the primary undifferentiated MSCs and showed that primary MSCs can survive in normal or damaged inner ear. However, the distribution of transplant-derived MSCs was different from the experiments. In detail, engrafted MSCs were localized mostly in the perilymphatic space of cochleae in normal mice [24]. However, in the animal of damaged cochlea, stem cells were found mainly in the modiolus and lateral wall [25,26]. The present study is consistent with these studies in terms of migration and distribution of transplanted stem cells since engrafted NI-hMSCs were localized predominantly around SG, organ of Corti, and cochlear nerve fiber in the damaged cochlea. After transplantation into the scala tympani, the NI-hMSCs probably spread through the scala tympani toward the apical turn of cochlea, and then after passing through the helicotrema, the cells move through the scala vestibule toward the basal turn. In addition, although bone marrow-derived MSCs were shown to survive, previous studies did not report the survival of MSCs more than 4 weeks. The decreased survival rate of stem cell transplantation at later stage is supposed to relate with lack of nutrition or local growth factor in the cochlea [27]. In the present study, however, the survival cells were found in all animals at 8 weeks.
In this study, the number of SGNs was increased in NI-hMSCs injection group compare to HBSS alone group (Fig. 4), and the grafted hMSCs also were positively stained for MAP2, a mature neuronal marker, in SG (Fig. 5C). These results suggest that the hMSCs differentiate into neuron and replace the lost or damaged neuronal cells in the SG. It is known that damaged cells and tissues of the host brain are capable of releasing molecules that stimulate production of neurotrophic factors in transplanted MSCs [28]. Previous studies have also shown that neural stem cells migrated to the site of lesion and differentiated into pyramidal cortical neurons and glia, effectively replacing the targeted cells and reversing neuronal loss [29]. Our results suggest that the differentiation of transplanted MSCs could be influenced by the cues or signals in the damaged tissue.
Taken together, this study provides the evidence of advantages of NI-hMSCs in the development of new strategies for treating sensorineural hearing loss. In addition, since presence of some hair cells and cochlear nerve enhances cochlear implant function, regeneration of SGNs and replacement of hair cells via NI-hMSC transplantation would also improve the therapeutic effect of cochlear implantation. These results could enhance further research about the development of a stem cell based therapy for hearing impairment.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0023147) and by a grant (CRI10072-1) of Chonnam National University Hospital Research Institute of Clinical Medicine.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Holley MC. Keynote review: the auditory system, hearing loss and potential targets for drug development. Drug Discov Today. 2005 Oct;10(19):1269–1282. doi: 10.1016/S1359-6446(05)03595-6. [DOI] [PubMed] [Google Scholar]
- 2.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993 Mar;259(5101):1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 3.Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995 Oct;39(5):799–807. [PubMed] [Google Scholar]
- 4.Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006 Aug;169(2):528–543. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000 Jun;97(13):7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005 Mar;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 7.Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005 Feb;307(5712):1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2003 Nov;100(23):13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oshima K, Heller S. Sound from silence. Nat Med. 2005 Mar;11(3):249–250. doi: 10.1038/nm0305-249. [DOI] [PubMed] [Google Scholar]
- 10.Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, et al. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008 Sep;26(9):2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- 11.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000 Nov;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 12.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004 Jun;113(12):1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012 Oct;490(7419):278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, et al. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007 Oct;232(1-2):29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revoltella RP, Papini S, Rosellini A, Michelini M, Franceschini V, Ciorba A, et al. Cochlear repair by transplantation of human cord blood CD133+ cells to nod-scid mice made deaf with kanamycin and noise. Cell Transplant. 2008;17(6):665–678. doi: 10.3727/096368908786092685. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura K, Nakagawa T, Ono K, Ogita H, Sakamoto T, Yamamoto N, et al. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009 Sep;20(14):1250–1254. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003 Oct;9(10):1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 18.Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010 Apr;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YB, Cho HH, Jang S, Jeong HS, Park JS. Transplantation of neural differentiated human mesenchymal stem cells into the cochlea of an auditory-neuropathy guinea pig model. J Korean Med Sci. 2011 Apr;26(4):492–498. doi: 10.3346/jkms.2011.26.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13(1):119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc Natl Acad Sci U S A. 2003 Sep;100(Suppl 1):11854–11860. doi: 10.1073/pnas.1834196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galie M, Sbarbati A, et al. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008 Oct;17(5):909–916. doi: 10.1089/scd.2007.0197. [DOI] [PubMed] [Google Scholar]
- 23.Li GR, Deng XL, Sun H, Chung SS, Tse HF, Lau CP. Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells. 2006 Jun;24(6):1519–1528. doi: 10.1634/stemcells.2005-0307. [DOI] [PubMed] [Google Scholar]
- 24.Sharif S, Nakagawa T, Ohno T, Matsumoto M, Kita T, Riazuddin S, et al. The potential use of bone marrow stromal cells for cochlear cell therapy. Neuroreport. 2007 Mar;18(4):351–354. doi: 10.1097/WNR.0b013e3280287a9a. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007 Jul;171(1):214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007 Sep;117(9):1629–1635. doi: 10.1097/MLG.0b013e31806bf282. [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005 Jan;302(1):40–47. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002 Dec;22(4):275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004 Aug;101(32):11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]