Abstract
The human sense of fairness is an evolutionary puzzle. To study this, we can look to other species, in which this can be translated empirically into responses to reward distribution. Passive and active protest against receiving less than a partner for the same task is widespread in species that cooperate outside kinship and mating bonds. There is less evidence that nonhuman species seek to equalize outcomes to their own detriment, yet the latter has been documented in our closest relatives, the apes. This reaction probably reflects an attempt to forestall partner dissatisfaction with obtained outcomes and its negative impact on future cooperation. We hypothesize that it is the evolution of this response that allowed the development of a complete sense of fairness in humans, which aims not at equality for its own sake but for the sake of continued cooperation.
Cooperation could not have evolved without mechanisms to ensure the sharing of payoffs. For an individual to cooperate with an unrelated partner to achieve goals that it cannot achieve alone or to exchange favors over time requires an ability to compare payoffs with investments. Given the ample evidence for mutualistic cooperation and reciprocal altruism (1, 2) in humans as well as other species (hereafter, animals), we therefore expect well-developed capacities for payoff evaluation in species that flexibly cooperate with individually known partners. We also expect negative reactions to excessive payoff imbalances, because such imbalances undermine cooperation among nonrelatives, which requires proportionality between effort and gain so that gains among parties jointly contributing to a given enterprise are shared.
Along with the human sense of fairness and justice, responses to inequity have enjoyed a long history of scholarship in philosophy, law, economics, and psychology. Yet the evolution of these responses and possible parallels in other species have only recently come into focus. Even though “contrast effects,” which describe how animals respond to unanticipated individual reward outcomes, have been known for nearly a century (3), the first study to measure reactions to interindividual outcome contrasts was published only in 2003 (4). In this study, brown capuchin monkeys (Cebus apella) became agitated and refused to perform a task for which a companion received superior rewards [see (5) for a video]. The monkeys’ protest was not due to the mere sight of unavailable superior rewards, because they showed it only if these rewards actually went to their partner. If superior rewards were merely visible, they were mostly ignored (4, 6). Since this early study, inequity responses have been explored in a number of species and found to be most pronounced in animals that cooperate outside of the bonds of mating and kinship.
We propose that sensitivity to (in)equity offers several evolutionary benefits. First, animals need to recognize when they receive less than a partner, because this tells them that the benefits of cooperation may be in danger. By protesting against this situation, they show a response known as inequity aversion (IA). Evidence indicates that this behavior is widespread in cooperative species under many circumstances. As the reliance on cooperation increases, individuals also benefit from sensitivity to receiving more than another, which risks undermining cooperative partnerships. This behavior is likely taxonomically restricted, because it requires prediction of the partner's reaction to getting less and its effect on the relationship. It also requires restraint to refrain from an immediately advantageous outcome. The pressure for increased cooperation combined with advanced cognitive abilities and emotional control allowed humans to evolve a complete sense of fairness. Here, we review the literature on IA in humans and other animals within an evolutionary framework of cooperation, social reciprocity, and conflict resolution. Our main conclusion is that the sense of fairness did not evolve for the sake of fairness per se but in order to reap the benefits of continued cooperation.
Responses to inequity
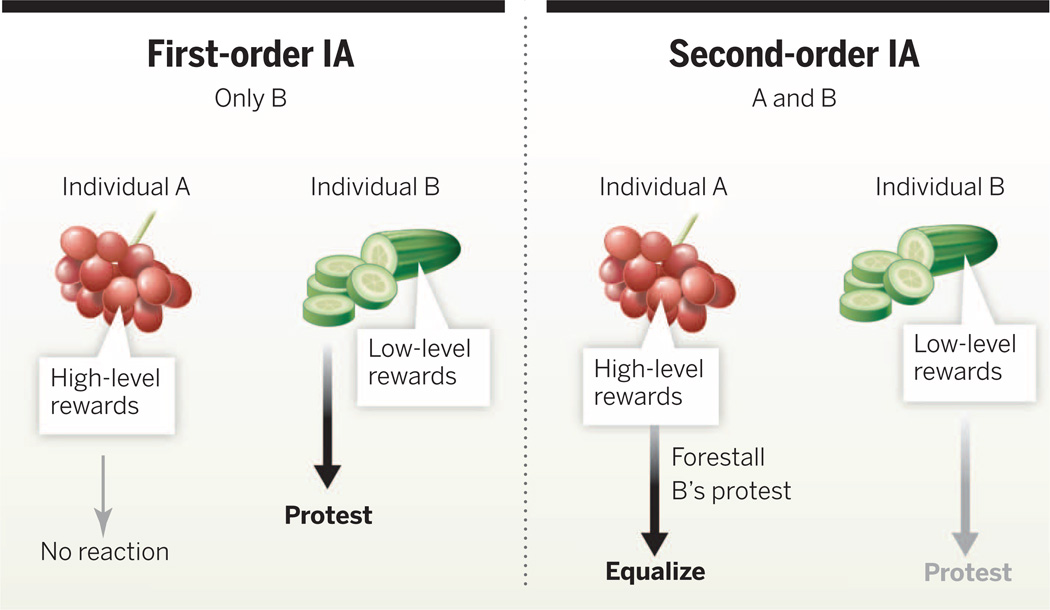
IA has been defined as a negative reaction to unequal outcomes (7). It is further subdivided into “disadvantageous IA,” or reactions to inequity to the detriment of the actor, and “advantageous IA,” also known as overcompensation, or reactions to inequity that benefits the actor (7). Responses to the first kind of IA offer a clear advantage if they help increase one’s own share. Not surprisingly, human studies indicate that disadvantageous IA emerges earlier (8) and is more pronounced than advantageous IA (9). Young children may even pay a cost to maintain their advantage (10), although advantageous IA may have a more explicitly social focus (11). Disadvantageous IA is also the most common type in animals (see below). However, responses to overcompensation are also expected, as they, too, provide a long-term benefit (12). We have called disadvantageous IA “first-order inequity aversion” to indicate that it is the primary reaction, using “second-order inequity aversion” for the less common and less pronounced advantageous IA (Fig. 1) (13).
Fig. 1. A diagram of the relationship between first-order IA and second-order IA.
Individual A received high-level rewards, and individual B received low-level rewards. Individuals who recognize when they receive less than another may react against this situation so as to maintain beneficial outcomes of cooperation, for instance, by finding a new cooperative partner. As reliance on cooperation increases, individuals also benefit from recognizing when they receive more, as this allows them to forestall first-order IA reactions in their partners and there by maintain a successful cooperative relationship. Second-order IA requires advanced cognition and emotional control and thus far has only been seen in chimpanzees and humans. This is the foundation of the full-blown human sense of fairness.
The connection between IA and fairness is not straightforward. The hallmark of the human sense of fairness is the idea of impartiality; that is, human fairness or justice is based on the idea of appropriate outcomes applied to everyone within the community, not just a few individuals, and, in particular, not just oneself. Thus, outcomes are judged against a standard, or an ideal. There is variation in this ideal across cultures or situations, but there is consistency within a given context. This complete sense of fairness likely requires abstraction at the community level as well as language (to establish a consistent set of ideals), both of which capacities may be restricted to our species (14). Community concern is not wholly absent in other primates, however, and neither is impartiality, such as when policing males break up fights (15).
Inasmuch as social ideals escape measurement, a sense of fairness is impossible to prove or disprove in animals. Reactions to inequity, on the other hand, are open to empirical investigation by creating situations in which one individual receives more or less than another. These reactions typically manifest as a rejection of a received reward or an unwillingness to participate in the interaction (Fig. 2A). In most experiments, subjects must complete a simple task to receive a reward (Table 1). To control for the social aspect of the interaction, these experiments often combine with ones on contrast effects that measure how subjects respond to a lesser reward after having just received a better reward (contrast) or another lesser one (control). It has been found that the mere visibility of better rewards is not the issue, because primates reliably perform tasks for lesser rewards regardless of whether or not better ones are immediately in front of them (6, 16). Experiments on IA have shown that there is substantial variation among species in this response, even within the primates; some species respond more strongly to contrast effects (17), others more strongly to disadvantageous inequity (4, 16); some respond to both (18), and some seem indifferent to either condition (19, 20).
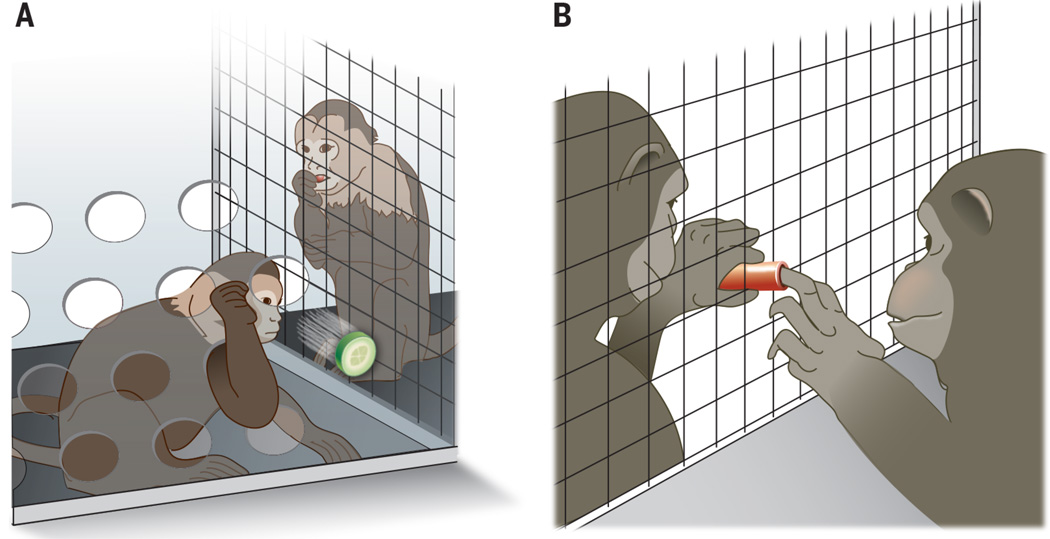
Fig. 2. Subjects’ responses in the standard inequity task and the UG.
(A) Capuchin monkeys during the original “monkeys reject unequal pay” experiment (4). The monkey on the left is rejecting the lesser reward, a cucumber slice, after viewing the partner receive a more preferred grape for the same amount of work. See video (5). (B) Chimpanzees during the UG (81). The chimpanzee who has just made the token choice (right) hands the token to her partner, who needs to accept and hand it over to the experimenter in order for both of them to receive the rewards corresponding with the token choice.
Table 1. Publications since 2003 on IA in a variety of nonhuman species.
The studies are divided into those using an effortful task and those that merely fed unequal foods. Tasks include exchange, in which the subject returns a token to the experimenter and subsequently receives a food reward; pulling, in which subjects must pull in a tray to bring themselves and/or others food rewards; target, in which the subject most hold on to a token for a specified period of time to receive a food reward; and none, in which the subject (and/or partner) receives food for “free,” without completing a task. The “shake paw” and “sit on command” tasks were specific to domestic dogs (because they are in their behavioral repertoire already).The UG requires a proposer to choose one of two reward divisions, which then must be accepted by the responder, who can either accept the proposal, in which case both subjects get the food as proposed, or reject it, in which case neither subject gets anything. The table also notes the nature of the task and whether individuals were sitting near each other or not.
| Species | Task | Side-by-side and near |
Evidence of | Contrast effect |
Other effects | |
|---|---|---|---|---|---|---|
| 1st-order IA | 2nd-order IA | |||||
| Food rewards for task | ||||||
| Chimpanzees | Exchange (22) | Yes | Yes | – | No | Social tie |
| Exchange (16) | Yes | Yes | Yes | Yes | Rank, sex, task | |
| Exchange (92) | Yes | Yes | – | No | Sex | |
| Exchange (23) | No | No | – | – | ||
| UG (79) | No | – | No | – | ||
| UG (80) | No | No | No | – | ||
| UG (81) | Yes | – | Yes | – | ||
| Bonobo | Exchange (23) | No | Maybe | – | – | |
| UG (80) | No | No | No | – | ||
| Orangutan | Exchange (23) | No | No | – | – | |
| Exchange (19) | Yes | No | No | No | ||
| Long-tailed macaque | Pulling (31) | Yes | Yes | Rank, social tie | ||
| Rhesus macaque | Target (18) | Yes | Yes | No | Yes | Ontogeny |
| Capuchin monkey | Exchange (4) | Yes | Yes | – | No | |
| Exchange (6) | Yes | Yes | – | No | Task, effort | |
| Pulling (27) | Yes | Yes | – | No | ||
| Pulling (28) | No | Yes | Yes | – | Rank, visual access | |
| Exchange (30) | Yes | No | – | No | ||
| Exchange (93) | No | No | – | – | ||
| Squirrel monkey | Exchange (17) | Yes | No | No | Yes | Sex, task |
| Target (20) | Yes | No | No | Yes | Sex | |
| Owl monkey | Target (20) | Yes | No | No | No | |
| Common marmoset | Target (20) | Yes | No | No | No | |
| Tamarin | Exchange (74) | Yes | No | No | Yes | Task |
| Domestic dog | Shake paw (33) | Yes | Yes | – | No | |
| Various (34) | Yes | Yes | – | – | ||
| Sit on command (35) | Yes | Yes/No | No | – | Age, ownership history, training history | |
| Crow | Exchange (36) | Yes | Yes | – | – | Effort |
| Raven | Exchange (36) | Yes | Yes | – | – | Effort |
| Feeding without task | ||||||
| Chimpanzee | None (32) | No | No | – | No | Rank |
| Bonobo | None (32) | No | No | – | No | |
| Gorilla | None (32) | No | No | – | No | |
| Capuchin monkey | None (94) | Yes | No | – | Yes | |
| None (95) | Yes | No | – | – | ||
| None [(93), study 2] | Yes | No | – | – | ||
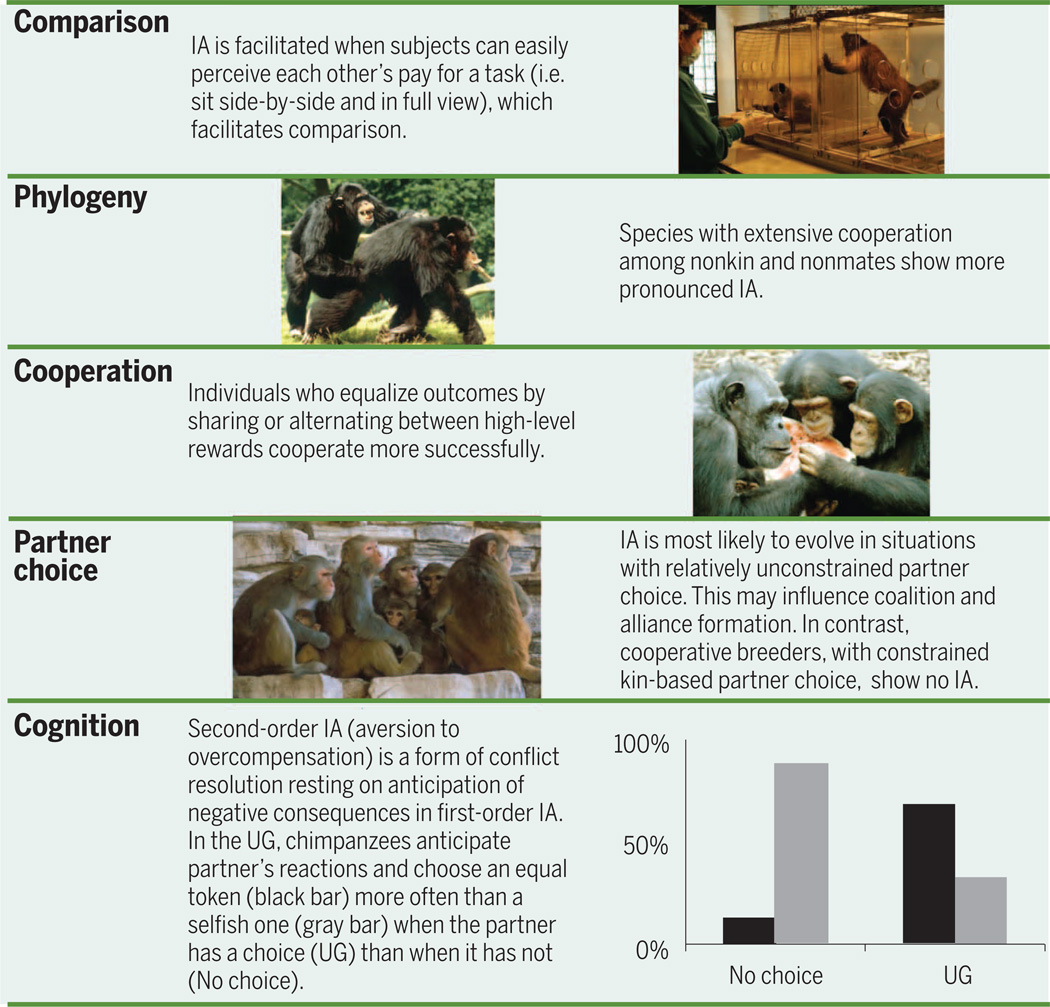
There are also important individual differences in response that hint at the situations in which inequity responses provide an advantage. For instance, merely feeding unequal foods fails to generate the same reaction; hence, an effortful task is essential (6, 16, 20) (Table 1), even though the nature of the task may be irrelevant (20). A second methodological issue emerges when we consider all reported studies regardless of species. Animals tested with an effortful task respond to inequity almost exclusively when seated closely side-by-side, compared with tests in which they sat far apart or across from each other, in which few IA responses were observed (Table 1). This suggests that physical proximity may be integral to IA outcomes, possibly because of the relationship between proximity and cooperation and the way proximity facilitates information gathering about the partner (21). Finally, individual differences have been found in some species, notably chimpanzees, who show substantial variation even within the same experiment (16, 22, 23). Responses also seem influenced by dominance rank, sex, and relationship quality. This is the case in humans as well, where factors such as relationship quality (24), personality (25), and the scale of competition (26) influence responses to unfair outcomes. Additional work to determine the influence of these and other factors on animal IA responses will provide additional nuance in our understanding of the evolution of IA (Table 1).
First-order IA has been documented in controlled experiments in capuchin monkeys [(4, 6, 27–29), but see also (30)], macaques (18, 31), chimpanzees [(16, 22), but see also (32, 23)], dogs (33–35), and crows (36), and it has been implied in rodents (37). These animals refuse lesser rewards if a partner receives better ones and/or stop performing after multiple exposures to such outcomes. At first sight, this response is counterintuitive, as it reduces absolute outcome (the subject passes up a less-preferred, but still beneficial, reward) while increasing inequity (the partner still receives the preferred reward versus the other receiving nothing). If the goal of IA is to minimize current inequity (7), these animals show the wrong response.
New lines of evidence, however, have led to a reassessment of this evaluation. First, humans, too, respond in this way. The workhorse of inequity studies has been the ultimatum game (UG), in which one individual, the proposer, must decide how to divide a set sum of money. The second individual, the responder, then must decide whether to accept this division—inwhich case both individuals receive the money as allocated—or refuse it, in which case neither party receives anything (38). Decades of research demonstrate that, while there is variation among cultures (39), human proposers tend to make higher offers than the minimum required and responders tend to reject offers that are skewed (40), showing that humans, too, meet the first criterion, turning down net positive outcomes.
In most situations of unfairness, we have no recourse, however. How do humans respond when a refusal punishes only themselves? The impunity game (IG) is a related game for which a refusal by the responder still allows the proposer their allocated sum, whereas the responder receives nothing. This situation is similar to most inequity tasks applied to animals, in which subjects have the option to refuse but their refusal does not alter the other’s outcome (41). Recent studies show refusals at about half the levels seen in the UG (42), bringing the human reaction close to that of animals refusing poorer rewards even if doing so decreases absolute gains and increases inequity.
The game context cannot include all possible outcomes that exist in natural social interactions, however. In the standard inequity task, refusals only hurt the actor, whereas in a natural social context, protest against inequity may lead to the actor either receiving a larger share or seeking out a better partner to work with. Despite the short-term costs, rejection of inequity may produce long-term gains by signaling to the partner that a relationship is about to end or by leading the actor to exit the relationship and replace it with a better one.
First-order IA and cooperation
The evolution of cooperation requires that its benefits reach all contributing parties in roughly similar amounts. Natural selection works on every individual’s relative advantage compared with others; hence, gaining an absolute benefit is insufficient. If individuals were satisfied with any absolute benefit, they might still face negative fitness consequences if they were doing less well than competing others. It makes sense, therefore, to compare one’s gains with those of others (43). Additionally, individuals must base decisions to cooperate on the entire history of interaction with a particular partner, not just any single interaction. Reciprocity requires a long-term evaluation of effort versus payoff balance.
The above perspective applies only to species with extensive cooperation outside of kinship relationships. The absence of flexible partner choice in the hymenoptera, for example, eliminates the need to compare efforts with payoffs. Our closest relatives, bonobos and chimpanzees, on the other hand, frequently cooperate with nonkin. Chimpanzees hunt together (44), form political coalitions and other reciprocal relations (45), collectively defend territories (46) and mates (47), and actively share food [e.g., (48)]. DNA collected in the field shows that most long-term male-male partnerships lack kinship ties (49). Bonobos show the same pattern. Females frequently share food and maintain a cooperative network that allows them to dominate males despite the fact that females are the migratory sex, hence largely unrelated within each community (50). In captive settings, bonobos even share food with outsiders (51).
Experimental studies of cooperation in primates began in 1936 with an experiment on cooperatively pulling chimpanzees (52). Since then, mutualistic cooperation has been demonstrated experimentally in most of the great apes, many monkey species, and also in nonprimates, including elephants, hyenas, and birds (53). Thus, we might expect that members of these species are sensitive to their own outcomes relative to those of a social partner. This would be in line with early work on IA in economics, which linked responses to inequity and cooperation (7). Individuals who perceive unequal outcomes may use this information to cease cooperation and find a new partner. If outcomes are sufficiently unequal, by chance alone cooperating with other partners will likely result in better outcomes (43). Research in other species supports a connection with cooperation in three different ways: (i) responses to inequity in the context of cooperation, (ii) phylogenetic comparisons, and (iii) responses in species facing partner-choice restrictions.
Reward distribution in cooperation experiments
Capuchin monkeys have been widely tested on the classical barpull paradigm in which two individuals work together (52). They produce mutual food rewards and appear to grasp the need for a partner (54). However, when individuals cooperate for unequal rewards, their behavior becomes more contingent upon their partner’s, reflecting sensitivity to reward distribution. These monkeys show “payment for labor” in that they share more easily with partners who have helped them obtain food than with partners who did not. Conversely, partners quit helping if rewards are not shared (55). This sensitivity to payoffs is not limited to situations in which rewards are preassigned by the experimenter. It extends to those in which the monkeys themselves decide the reward division. Monkeys are less likely to pull for clumped rewards that their partner can monopolize than for distributed rewards that are easily divided. They make this distinction on the very first trial, indicating that it is not a conditioning effect, and the distinction varies with the level of tolerance between both partners (56).
Moreover, although these monkeys cooperate to the same degree for distributed rewards that are either equal or unequal, partnerships that alternated each individual’s access to a preferred reward when rewards were unequal were almost three times as likely to cooperate successfully (57). The reluctance to cooperate with a monopolizing partner suggests that it is not inequity per se but the way partner attitude combines with inequity that impedes cooperation. This is reminiscent of children’s focus on partiality over inequity (58) and moreover has implications for human cooperation, whereby individuals are not likely to forget the past and cooperate just because the payoff structure is now in their favor. In these experiments, monkeys did not respond with refusal to an isolated instance of inequity but required multiple instances before cooperation broke down (different thresholds for ceasing cooperation may be one cause of the individual variation in these responses). Even if rewards even out over time, in any given interaction one individual will usually do better than another. The monkeys appeared to integrate outcomes over multiple trials, allowing for cooperation in a wide range of situations.
Chimpanzees, too, are sensitive to reward distribution. They cooperate more successfully with a partner who, in other contexts, shares more tolerantly (59). Given a choice between potential partners, they prefer partners with whom they have a tolerant relationship (60). When goals conflict, such as when two individuals have the option to cooperate for equal (5 versus 5 rewards) or unequal (10 versus 1) payoffs, chimpanzees still manage to obtain food on the majority of trials. Even though dominant individuals prefer the possibility of 10 rewards, on almost half the trials the pair negotiate to work for the equal division (61). On the other hand, given a choice, chimpanzees prefer to work alone rather than collaborate (62) and, unlike capuchin monkeys (55), may not share more with a helper than a nonhelper (63). The latter result needs further testing, however, given indications that wild chimpanzees that contributed to a group hunt are given preferential access to the resulting meat (44).
Phylogeny: Cooperative versus noncooperative species
Another way to explore the interplay between cooperation and inequity is to look across species. Pronounced first-order IA has been observed in chimpanzees and brown capuchin monkeys (4, 6, 16, 22, 27, 28), two species that are highly cooperative—for example, they hunt in groups for prey that is hard to capture by a single hunter (48, 64). Moreover, chimpanzees seem attentive to their partner’s rewards, even if they are inferior to their own (16), and both species behave prosocially in at least some experimental tests [(65–67), but see (68, 69)], thus having the potential for second-order IA. Beyond these two primates, recent evidence indicates that bonobos (23) and several macaque species (Macaca spp.) (18, 31) also respond negatively to getting a reward inferior to that of a partner. These primates, too, are highly cooperative. There are observations of group hunting in bonobos (70) and, although macaques do not show such behavior, they have an extensive alliance network among both kin and nonkin (71).
On the other hand, primates less likely to cooperate with nonkin, including orangutans (Pongo spp.) (19, 23) and squirrel monkeys (Saimiri spp.) (17, 20), have thus far failed to show IA. Neither taxonomic relations among the primates nor brain size, relative brain size, or social organization predict the known distribution of IA as well, it appears, as does the tendency to cooperate with individuals who are neither kin nor mates (41). Beyond the primates, IA has also been documented in domestic dogs (Canis lupus familiaris) (33, 34), a species derived from a long line of cooperative hunters (72). Like monkeys, dogs are sensitive only to whether their outcomes are wanting as compared with those of others (35). Corvids are cooperative birds (73), and some species have shown IA in experiments. They may be more sensitive to inequities in effort than in reward, however (36).
Future research is needed to determine the degree to which the hypothesis of coevolution of IA and cooperation (41) extends beyond these species. For instance, do other animals with frequent nonkin cooperation, such as elephants, cetaceans, and noncanine social carnivores, also respond negatively to situations of inequity? We also need more research on noncooperative species. For example, a comparison between domestic cats and dogs may be useful, where we would predict cats (solitary hunters) to be less sensitive to reward distribution than dogs.
Constrained partner choice
Not all cooperative animals can easily find new partners. For example, the Callithrichidae (marmosets and tamarins) are cooperative breeders, a social system in which both parents and adult offspring are essential for offspring care. For obvious reasons, the cost of partner switching is high. Of the two callithrichid species tested on IA, neither responded negatively to receiving a lesser reward than their social partner (20, 74). Even though not classified as cooperative breeders, owl monkeys (Aotus spp.), too, show pair-bonding and dual parental care and also fail to respond to inequity (20).
Even without cooperative breeding, in species with relationships developed over many years of play, grooming, mutual support, and other services, responses to inequity should wear off since replacement of long-term partners becomes too costly. There is indeed evidence that IA is less pronounced in well-established human friendships compared with relationships among acquaintances and colleagues (24), and the same has been reported for chimpanzees. A group of captive chimpanzees that grew up and lived together in the same space for more than 30 years showed far less IA than a similarly housed group of chimpanzees with a much shorter history (22).
Future research is needed to explore the degree to which both relationship quality and the costs of partner switching influence responses to inequity. One might predict, for instance, that if the evolution of IA requires cooperation under relatively unconstrained partner choice, hunting parties may be a prime example. Hunting parties change composition from one occasion to the next, whereas long-term friendships and pair-bonding may not be as conducive to pronounced IA. In the laboratory, we might anticipate that individuals show different responses in newly formed partnerships as compared with longer-term ones, particularly in the case of biparental care or cooperatively breeding species in which long-term relationships have produced offspring. For species for whom the costs of partner switching are too high, we may expect to see other partner-control mechanisms, such as punishment, play a greater role (75). Understanding the situations in which partner choice influences inequity responses will be critical for understanding the formation of coalitions and alliances (76).
Second-order inequity aversion
Until recently, second-order IA was unreported for nonhuman animals. Its explanation is more complex than that of first-order IA, which simply requires that one individual responds to an unequal outcome to avoid being taken advantage of. For second-order IA, in contrast, the advantages are less obvious, because this reaction occurs when the actor enjoys an advantage. Apart from humans, evidence for second-order IA is thus far restricted to chimpanzees. The first sign came from a study in which the apes reacted negatively not only to a lesser reward but also when they received a better one. In other words, subjects responded to any inequity, not just the disadvantageous kind (16). Subsequently, chimpanzees were tested on the UG, considered the gold standard of the human sense of fairness (see “Responses to inequity” above). In most cultures, humans typically offer a 50/50 split (77, 78). In contrast, one UG study on chimpanzees found them to share the smallest possible amount with their partner [(79); see also (80)]. However, because the methodology of this experiment deviated substantially from the typical human UG, Proctor et al. (Fig. 2B) (81) applied a more intuitive UG for both apes and 3- to 5-year-old human children.
Proposers were presented with a choice of two differently colored tokens that could be exchanged for food. The tokens represented equal versus unequal reward divisions, and the partner needed to agree and participate in the exchange (Fig. 2B), an element similar to the typical human UG. Token choices in this situation were compared with choices when the partner’s agreement was not needed. Similar to humans in the UG, the chimpanzees more often split the rewards equally if they needed their partner than if they did not. Because children behaved similarly in this token-exchange game, the study suggests shared patterns of proactive decision-making in relation to fair outcomes in both species (81).
Even though neither the apes nor the children in this study actively refused offers, behavioral protest did occur. Subjects occasionally reacted to selfish offers by spitting water at the other or hitting the mesh partition (apes) or saying “you got more than me” (children). Acceptance of offers despite behavioral protest is typical of young children (82). Strategic choices in the UG may be tied to emotional control rather than to social preferences, knowledge of norms, or perspective-taking abilities. In one study, 85% of the younger children claimed to reject unfair offers, but only 12.5% of them actually did. Only after 7 years of age do children resist the temptation of rewards and begin to refuse low offers for strategic reasons (83).
Reasons to refuse unfair offers in the UG are obvious enough. Refusals punish the actor, which may lead to better outcomes in the future. The individual making the offer, on the other hand, may anticipate negative reactions and strive for an equitable outcome to forestall them. This would amount to anticipatory conflict resolution, which may be the main rationale for second-order IA if those who divide the rewards try to eliminate reasons for frustration in their partners (Fig. 1). The better the anticipatory capacities of a species, the better it will be able to avoid first-order IA in others by showing second-order IA. Planning ahead has been demonstrated in apes in relation to tool use (84), as has anticipatory conflict resolution. Captive bonobos and chimpanzees show a grooming and play peak right before feeding time and engage in high levels of appeasing and sociosexual body contact upon food arrival (85, 86). These primates thus anticipate competition and actively seek to reduce it. Second-order IA in chimpanzees may serve the same goal. Given the need to anticipate the partner’s reactions as well as forgo short-term positive outcomes to gain long-term ones, individuals must have some emotional control. Although there are no studies linking self-control and IA in other species, in human children self-control is a limiting factor. Perhaps not surprisingly, the species with strong IA responses also delay gratification in experimental tests [e.g., (87, 88)].
Finally, second-order IA may directly benefit an individual by enhancing its reputation, which may increase that individual’s long-term access to beneficial relationships (12). Humans are much more likely to donate in a public goods game when they are recognizable (89) and cooperate more when they have the feeling of being watched (90), indicating that being nice only occurs when positive fitness gains are expected from a second-order IA reaction. To what degree this explanation may apply to species other than our own is as yet unclear, although there is evidence that apes pay attention to the generosity of others without necessarily having directly experienced it (91).
The evolution of fairness
Not only are signs of first-order IA evident in several cooperative species, in the form of a negative reaction to disadvantageous unequal outcomes, but also our closest relatives, the anthropoid apes, show evidence of second-order IA, an essential component of human fairness because it seeks to equalize outcomes. Thus, humans and other species seem to share basic reactions to inequity, which serve the need for sustained cooperation (Table 2). Humans’ unprecedented brain enlargement allows for greater understanding of the benefits of self-control in the context of resource division. Additionally, the development of language enabled communication about third parties, which may have enhanced the role of reputation building. Despite these differences, many of the basic emotional reactions and calculations underlying our sense of fairness seem rooted in our primate background. We suggest that future research more explicitly investigate what we consider the key variables underlying IA, including dependence on cooperation, anticipation of the way resource division affects relationships, and the freedom to choose and change partners, as well as the relative roles of first- and second-order IA. A cross-species investigation with a standardized paradigm may further illuminate the factors involved and help verify or falsify the model proposed.
Table 2.
Studies on a variety of animals thus far have indicated five domains that help explain the variation in IA.
 |
ACKNOWLEDGMENTS
S.F.B. was funded by NSF CAREER grant SES 0847351 and NSF SES 1123897.
REFERENCES AND NOTES
- 1.Dugatkin LA. Cooperation Among Animals: An Evolutionary Perspective. New York: Oxford University Press; 1997. Oxford Series in Ecology and Evolution. [Google Scholar]
- 2.Kappeler PM, Van Schaik CP. Cooperation in Primates and Humans: Mechanisms and Evolution. Springer; 2005. [Google Scholar]
- 3.Tinklepaugh OL. An experimental study of representative factors in monkeys. J. Comp. Psychol. 1928;8:197–236. doi: 10.1037/h0075798. [Google Scholar]
- 4.Brosnan SF, De Waal FBM. Monkeys reject unequal pay. Nature. 2003;425:297–299. doi: 10.1038/nature01963. doi: 10.1038/nature01963; pmid: 13679918. [DOI] [PubMed] [Google Scholar]
- 5.Two monkeys were paid unequally: Excerpt from Frans de Waal's TED Talk. 2013 www.youtube.com/watch?v=meiU6TxysCg. [Google Scholar]
- 6.van Wolkenten M, Brosnan SF, de Waal FBM. Inequity responses of monkeys modified by effort. Proc. Natl. Acad. Sci. U.S.A. 2007;104:18854–18859. doi: 10.1073/pnas.0707182104. doi: 10.1073/pnas.0707182104; pmid: 18000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Q. J. Econ. 1999;114:817–868. doi: 10.1162/003355399556151. [Google Scholar]
- 8.Blake PR, McAuliffe K. “I had so much it didn’t seem fair”: Eight-year-olds reject two forms of inequity. Cognition. 2011;120:215–224. doi: 10.1016/j.cognition.2011.04.006. doi: 10.1016/j.cognition.2011.04.006; pmid: 21616483. [DOI] [PubMed] [Google Scholar]
- 9.Loewenstein GF, Thompson L, Bazerman MH. Social utility and decision making in interpersonal contexts . Pers. Soc. Psychol. 1989;57:426–441. doi: 10.1037/0022-3514.57.3.426. [Google Scholar]
- 10.Sheskin M, Bloom P, Wynn K. Anti-equality: Social comparison in young children. Cognition. 2014;130:152–156. doi: 10.1016/j.cognition.2013.10.008. doi: 10.1016/j.cognition.2013.10.008; pmid: 24291266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAuliffe K, Blake PR, Kim G, Wrangham RW, Warneken F. Social influences on inequity aversion in children. PLOS ONE. 2013;8:e80966. doi: 10.1371/journal.pone.0080966. doi: 10.1371/journal.pone.0080966; pmid: 24312509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank RH. In: Evolution and the Capacity for Commitment. Nesse RM, editor. New York: Russell Sage Foundation; 2001. pp. 57–76. [Google Scholar]
- 13.Brosnan SF, de Waal FBM. Fairness in animals: Where to from here? Soc. Justice Res. 2012;25:336–351. doi: 10.1007/s11211-012-0165-8. [Google Scholar]
- 14.de Waal F. The Bonobo and the Atheist: In Search of Humanism Among the Primates. New York: W.W. Norton; 2013. [Google Scholar]
- 15.de Waal FBM. In: Coalitions and Alliances in Humans and Other Animals. Harcourt AH, de Waal FBM, editors. Oxford: Oxford Univ. Press; 1992. pp. 233–258. [Google Scholar]
- 16.Brosnan SF, Talbot C, Ahlgren M, Lambeth SP, Schapiro SJ. Mechanisms underlying responses to inequitable outcomes in chimpanzees, Pan troglodytes. Anim. Behav. 2010;79:1229–1237. doi: 10.1016/j.anbehav.2010.02.019. doi: 10.1016/j.anbehav.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talbot CF, Freeman HD, Williams LE, Brosnan SF. Squirrel monkeys’ response to inequitable outcomes indicates a behavioural convergence within the primates. Biol. Lett. 2011;7:680–682. doi: 10.1098/rsbl.2011.0211. doi: 10.1098/rsbl.2011.0211; pmid: 21508022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopper LM, Lambeth SP, Schapiro SJ, Bernacky BJ, Brosnan SF. The ontogeny of social comparisons in rhesus macaques (Macaca mulatta) J. Primatol. 2013;2:109. [Google Scholar]
- 19.Brosnan SF, Flemming TE, Talbot C, Mayo L, Stoinski TS. Orangutans (Pongo pygmaeus) do not form expectations based on their partner’s outcomes. Folia Primatol. 2011;82:56–70. doi: 10.1159/000328142. doi: 10.1159/000328142; pmid: 21625145. [DOI] [PubMed] [Google Scholar]
- 20.Freeman HD, et al. Different responses to reward comparisons by three primate species. PLOS ONE. 2013;8:e76297. doi: 10.1371/journal.pone.0076297. doi: 10.1371/journal.pone.0076297; pmid: 24130767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommer R. Small group ecology. Psychol. Bull. 1967;67:145–152. doi: 10.1037/h0024201. doi: 10.1037/h0024201; pmid: 5343340. [DOI] [PubMed] [Google Scholar]
- 22.Brosnan SF, Schiff HC, de Waal FBM. Tolerance for inequity may increase with social closeness in chimpanzees. Proc. Biol. Sci. 2005;272:253–258. doi: 10.1098/rspb.2004.2947. doi: 10.1098/rspb.2004.2947; pmid: 15705549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bräuer J, Call J, Tomasello M. Are apes inequity averse? New data on the token-exchange paradigm. Am. J. Primatol. 2009;71:175–181. doi: 10.1002/ajp.20639. doi: 10.1002/ajp.20639; pmid: 19021260. [DOI] [PubMed] [Google Scholar]
- 24.Clark MS, Grote NK. In: Handbook of Psychology: Personality and Social Psychology. Millon T, Lerner MJ, Weiner IB, editors. Vol. 5. New York: Wiley; 2003. pp. 447–461. [Google Scholar]
- 25.Brandstätter H, Königstein M. Personality influences on ultimatum bargaining decisions. Eur. J. Pers. 2001;15:S53–S70. doi: 10.1002/per.424. [Google Scholar]
- 26.Barclay P, Stoller B. Local competition sparks concerns for fairness in the ultimatum game. Biol. Lett. 2014;10:20140213–20140213. doi: 10.1098/rsbl.2014.0213. doi: 10.1098/rsbl.2014.0213; pmid: 24850897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher GE. Attending to the outcome of others: Disadvantageous inequity aversion in male capuchin monkeys (Cebus apella) Am. J. Primatol. 2008;70:901–905. doi: 10.1002/ajp.20576. doi: 10.1002/ajp.20576; pmid: 18521838. [DOI] [PubMed] [Google Scholar]
- 28.Takimoto A, Kuroshima H, Fujita K. Capuchin monkeys (Cebus apella) are sensitive to others’ reward: An experimental analysis of food-choice for conspecifics. Anim. Cogn. 2010;13:249–261. doi: 10.1007/s10071-009-0262-8. doi: 10.1007/s10071-009-0262-8; pmid: 19609580. [DOI] [PubMed] [Google Scholar]
- 29.Takimoto A, Fujita K. I acknowledge your help: Capuchin monkeys’ sensitivity to others’ labor. Anim. Cogn. 2011;14:715–725. doi: 10.1007/s10071-011-0406-5. doi: 10.1007/s10071-011-0406-5; pmid: 21519900. [DOI] [PubMed] [Google Scholar]
- 30.Silberberg A, Crescimbene L, Addessi E, Anderson JR, Visalberghi E. Does inequity aversion depend on a frustration effect? A test with capuchin monkeys (Cebus apella) Anim. Cogn. 2009;12:505–509. doi: 10.1007/s10071-009-0211-6. doi: 10.1007/s10071-009-0211-6; pmid: 19184138. [DOI] [PubMed] [Google Scholar]
- 31.Massen JJM, Van Den Berg LM, Spruijt BM, Sterck EHM. Inequity aversion in relation to effort and relationship quality in long-tailed Macaques (Macaca fascicularis) Am. J. Primatol. 2012;74:145–156. doi: 10.1002/ajp.21014. doi: 10.1002/ajp.21014; pmid: 22038902. [DOI] [PubMed] [Google Scholar]
- 32.Bräuer J, Call J, Tomasello M. Are apes really inequity averse? Proc. Biol. Sci. 2006;273:3123–3128. doi: 10.1098/rspb.2006.3693. doi: 10.1098/rspb.2006.3693; pmid: 17015338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Range F, Horn L, Viranyi Z, Huber L. The absence of reward induces inequity aversion in dogs. Proc. Natl. Acad. Sci. U.S.A. 2009;106:340–345. doi: 10.1073/pnas.0810957105. doi: 10.1073/pnas.0810957105: pmid: 19064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Range F, Leitner K, Virányi Z. The influence of the relationship and motivation on inequity aversion in dogs. Soc. Justice Res. 2012;25:170–194. doi: 10.1007/s11211-012-0155-x. [Google Scholar]
- 35.Horowitz A. Fair is fine, but more is better: Limits to inequity aversion in the domestic dog. Soc. Justice Res. 2012;25:195–212. doi: 10.1007/s11211-012-0158-7. [Google Scholar]
- 36.Wascher CAF, Bugnyar T. Behavioral responses to inequity in reward distribution and working effort in crows and ravens. PLOS ONE. 2013;8:e56885. doi: 10.1371/journal.pone.0056885. doi: 10.1371/journal.pone.0056885; pmid: 23437262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heidary F, et al. Food inequality negatively impacts cardiac health in rabbits. PLOS ONE. 2008;3:e3705. doi: 10.1371/journal.pone.0003705. doi: 10.1371/journal.pone.0003705; pmid: 19002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Güth W, Schmittberger R, Schwartze B. An experimental analysis of ultimatum bargaining. J. Econ. Behav. Organ. 1982;3:367–388. doi: 10.1016/0167-2681(82)90011-7. [Google Scholar]
- 39.Henrich J, Fehr E, Bowles S, Boyd R, Camerer C. Foundations of Human Sociality: Economic Experiments and Ethnographic Evidence from Fifteen Small-Scale Societies. Oxford: Oxford Univ. Press; 2004. [Google Scholar]
- 40.Camerer C. Behavioral Game Theory: Experiments in Strategic Interaction. Princeton, NJ: Russell Sage Foundation, Princeton Univ. Press; 2003. [Google Scholar]
- 41.Brosnan SF. A hypothesis of the co-evolution of cooperation and responses to inequity. Front. Decis. Neurosci. 2011;5:43. doi: 10.3389/fnins.2011.00043. pmid: 21519380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamagishi T, et al. The private rejection of unfair offers and emotional commitment. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11520–11523. doi: 10.1073/pnas.0900636106. doi: 10.1073/pnas.0900636106; pmid: 19564602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brosnan SF. Nonhuman species’ reactions to inequity and their implications for fairness. Soc. Justice Res. 2006;19:153–185. doi: 10.1007/s11211-006-0002-z. [Google Scholar]
- 44.Boesch C. Cooperative hunting in wild chimpanzees. Anim. Behav. 1994;48:653–667. doi: 10.1006/anbe.1994.1285. [Google Scholar]
- 45.Mitani JC. In: Cooperation in Primates and Humans: Evolution and Mechanisms. Kapeller P, van Schaik CP, editors. Berlin: Springer; 2006. pp. 101–113. [Google Scholar]
- 46.Wilson ML, Hauser MD, Wrangham RW. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav. 2001;61:1203–1216. doi: 10.1006/anbe.2000.1706. [Google Scholar]
- 47.Watts DP. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park. Uganda. Behav. Ecol. Sociobiol. 1998;44:43–55. doi: 10.1007/s002650050513. [Google Scholar]
- 48.Boesch C, Boesch H. Hunting behavior of wild chimpanzees in the Taï National Park. Am. J. Phys. Anthropol. 1989;78:547–573. doi: 10.1002/ajpa.1330780410. doi: 10.1002/ajpa.1330780410; pmid: 2540662. [DOI] [PubMed] [Google Scholar]
- 49.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. doi: 10.1073/pnas.0611449104; pmid: 17456600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuichi T. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 2011;20:131–142. doi: 10.1002/evan.20308. doi: 10.1002/evan.20308; pmid: 22038769. [DOI] [PubMed] [Google Scholar]
- 51.Tan J, Hare B. Bonobos share with strangers. PLOS ONE. 2013;8:e51922. doi: 10.1371/journal.pone.0051922. doi: 10.1371/journal.pone.0051922; pmid: 23300956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nissen HW, Crawford MP. A preliminary study of food-sharing behavior in young chimpanzees. J. Comp. Psychol. 1936;22:383–419. doi: 10.1037/h0062234. [Google Scholar]
- 53.Brosnan SF, Bshary R. Cooperation and deception: From evolution to mechanisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:2593–2598. doi: 10.1098/rstb.2010.0155. doi: 10.1098/rstb.2010.0155; pmid: 20679104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendres KA, de Waal FBM. Capuchins do cooperate: The advantage of an intuitive task. Anim. Behav. 2000;60:523–529. doi: 10.1006/anbe.2000.1512. doi: 10.1006/anbe.2000.1512; pmid: 11032655. [DOI] [PubMed] [Google Scholar]
- 55.de Waal FBM, Berger ML. Payment for labour in monkeys. Nature. 2000;404:563. doi: 10.1038/35007138. doi: 10.1038/35007138; pmid: 10766228. [DOI] [PubMed] [Google Scholar]
- 56.de Waal FB, Davis JM. Capuchin cognitive ecology: Cooperation based on projected returns. Neuropsychologia. 2003;41:221–228. doi: 10.1016/s0028-3932(02)00152-5. doi: 10.1016/S0028-3932(02)00152-5; pmid: 12459220. [DOI] [PubMed] [Google Scholar]
- 57.Brosnan SF, Freeman C, De Waal FBM. Partner’s behavior, not reward distribution, determines success in an unequal cooperative task in capuchin monkeys. Am. J. Primatol. 2006;68:713–724. doi: 10.1002/ajp.20261. doi: 10.1002/ajp.20261; pmid: 16786518. [DOI] [PubMed] [Google Scholar]
- 58.Shaw A, Olson K. Fairness as partiality aversion: The development of procedural justice. J. Exp. Child Psychol. 2014;119:40–53. doi: 10.1016/j.jecp.2013.10.007. doi: 10.1016/j.jecp.2013.10.007; pmid: 24291349. [DOI] [PubMed] [Google Scholar]
- 59.Melis AP, Hare B, Tomasello M. Engineering cooperation in chimpanzees: Tolerance constraints on cooperation. Anim. Behav. 2006;72:275–286. doi: 10.1016/j.anbehav.2005.09.018. [Google Scholar]
- 60.Melis AP, Hare B, Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006;311:1297–1300. doi: 10.1126/science.1123007. doi: 10.1126/science.1123007; pmid: 16513985. [DOI] [PubMed] [Google Scholar]
- 61.Melis AP, Hare B, Tomasello M. Chimpanzees coordinate in a negotiation game. Evol. Hum. Behav. 2009;30:381–392. doi: 10.1016/j.evolhumbehav.2009.05.003. [Google Scholar]
- 62.Bullinger AF, Melis AP, Tomasello M. Chimpanzees, Pan troglodytes, prefer individual over collaborative strategies towards goals. Anim. Behav. 2011;82:1135–1141. doi: 10.1016/j.anbehav.2011.08.008. [Google Scholar]
- 63.Melis AP, Schneider A-C, Tomasello M. Chimpanzees, Pan troglodytes, share food in the same way after collaborative and individual food acquisition. Anim. Behav. 2011 doi: 10.1016/j.anbehav.2011.05.024. [Google Scholar]
- 64.Rose LM. Vertebrate predation and food-sharing in Cebus and Pan. Int. J. Primatol. 1997;18:727–765. doi: 10.1023/A:1026343812980. [Google Scholar]
- 65.Horner V, Carter JD, Suchak M, de Waal FBM. Spontaneous prosocial choice by chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13847–13851. doi: 10.1073/pnas.1111088108. doi: 10.1073/pnas.1111088108; pmid: 21825175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lakshminarayanan VR, Santos LR. Capuchin monkeys are sensitive to others’ welfare. Curr. Biol. 2008;18:R999–R1000. doi: 10.1016/j.cub.2008.08.057. doi: 10.1016/j.cub.2008.08.057; pmid: 19000809. [DOI] [PubMed] [Google Scholar]
- 67.de Waal FBM, Leimgruber K, Greenberg AR. Giving is self-rewarding for monkeys. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13685–13689. doi: 10.1073/pnas.0807060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silk JB, et al. Chimpanzees are indifferent to the welfare of unrelated group members. Nature. 2005;437:1357–1359. doi: 10.1038/nature04243. doi: 10.1038/nature04243; pmid: 16251965. [DOI] [PubMed] [Google Scholar]
- 69.Drayton L, Santos L. Insights into intraspecies variation in primate prosocial behavior: Capuchins (Cebus apella) fail to show prosociality on a touchscreen task. Behav. Sci. 2014;4:87–101. doi: 10.3390/bs4020087. doi: 10.3390/bs4020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Surbeck M, Hohmann G. Primate hunting by bonobos at LuiKotale, Salonga National Park. Curr. Biol. 2008;18:R906–R907. doi: 10.1016/j.cub.2008.08.040. doi: 10.1016/j.cub.2008.08.040; pmid: 18957233. [DOI] [PubMed] [Google Scholar]
- 71.Chapais B, Berman CM. Kinship and Behavior in Primates. Oxford: Oxford University Press; 2004. [Google Scholar]
- 72.Gácsi M, McGreevy P, Kara E, Miklósi A. Effects of selection for cooperation and attention in dogs. Behav. Brain Funct. 2009;5:31. doi: 10.1186/1744-9081-5-31. doi: 10.1186/1744-9081-5-31; pmid: 19630939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seed AM, Clayton NS, Emery NJ. Cooperative problem solving in rooks (Corvus frugilegus) Proc. Biol. Sci. 2008;275:1421–1429. doi: 10.1098/rspb.2008.0111. doi: 10.1098/rspb.2008.0111; pmid: 18364318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neiworth JJ, Johnson ET, Whillock K, Greenberg J, Brown V. Is a sense of inequity an ancestral primate trait? Testing social inequity in cotton top tamarins (Saguinus oedipus) J. Comp. Psychol. 2009;123:10–17. doi: 10.1037/a0012662. doi: 10.1037/a0012662; pmid: 19236140. [DOI] [PubMed] [Google Scholar]
- 75.Raihani NJ, Thornton A, Bshary R. Punishment and cooperation in nature. Trends Ecol. Evol. 2012;27:288–295. doi: 10.1016/j.tree.2011.12.004. doi: 10.1016/j.tree.2011.12.004; pmid: 22284810. [DOI] [PubMed] [Google Scholar]
- 76.Mesterton-Gibbons M, Gavrilets S, Gravner J, Akçay E. Models of coalition or alliance formation. J. Theor. Biol. 2011;274:187–204. doi: 10.1016/j.jtbi.2010.12.031. doi: 10.1016/j.jtbi.2010.12.031; pmid: 21195717. [DOI] [PubMed] [Google Scholar]
- 77.Henrich J, et al. In search of Homo Economicus: Behavioral experiments in 15 small-scale societies. Am. Econ. Rev. 2001;91:73–78. doi: 10.1257/aer.91.2.73. [Google Scholar]
- 78.Camerer CF, Loewenstein G. In: Advances in Behavioral Economics. Camerer CF, Loewenstein G, Rabin M, editors. Princeton, N. J.: Princeton Univ. Press; 2004. pp. 3–52. [Google Scholar]
- 79.Jensen K, Call J, Tomasello M. Chimpanzees are rational maximizers in an ultimatum game. Science. 2007;318:107–109. doi: 10.1126/science.1145850. doi: 10.1126/science.1145850; pmid: 17916736. [DOI] [PubMed] [Google Scholar]
- 80.Kaiser I, Jensen K, Call J, Tomasello M. Theft in an ultimatum game: Chimpanzees and bonobos are insensitive to unfairness. Biol. Lett. 2012;8:942–945. doi: 10.1098/rsbl.2012.0519. doi: 10.1098/rsbl.2012.0519; pmid: 22896269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proctor D, Williamson RA, de Waal FBM, Brosnan SF. Chimpanzees play the ultimatum game. Proc. Natl. Acad. Sci. U.S.A. 2013;110:2070–2075. doi: 10.1073/pnas.1220806110. doi: 10.1073/pnas.1220806110; pmid: 23319633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LoBue V, Nishida T, Chiong C, DeLoache JS, Haidt J. When getting something good is bad: Even three-year-olds react to inequality. Soc. Dev. 2011;20:154–170. doi: 10.1111/j.1467-9507.2009.00560.x. [Google Scholar]
- 83.Steinbeis N, Bernhardt BC, Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron. 2012;73:1040–1051. doi: 10.1016/j.neuron.2011.12.027. doi: 10.1016/j.neuron.2011.12.027; pmid: 22405212. [DOI] [PubMed] [Google Scholar]
- 84.Osvath M, Osvath H. Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: Self-control and pre-experience in the face of future tool use. Anim. Cogn. 2008;11:661–674. doi: 10.1007/s10071-008-0157-0. doi: 10.1007/s10071-008-0157-0; pmid: 18553113. [DOI] [PubMed] [Google Scholar]
- 85.de Waal FBM. Topics in Primatology, Vol. 1, Human Origins. Tokyo: University of Tokyo Press; 1992. pp. 37–50. [Google Scholar]
- 86.Koyama N, Dunbar R. Anticipation of conflict by chimpanzees. Primates. 1996;37:79–86. doi: 10.1007/BF02382923. [Google Scholar]
- 87.Evans TA, Beran MJ. Delay of gratification and delay maintenance by rhesus macaques (Macaca mulatta) J. Gen. Psychol. 2007;134:199–216. doi: 10.3200/GENP.134.2.199-216. doi: 10.3200/GENP.134.2.199-216; pmid: 17503695. [DOI] [PubMed] [Google Scholar]
- 88.Evans TA, Westergaard GC. Self-control and tool use in tufted capuchin monkeys (Cebus apella) J. Comp. Psychol. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. doi: 10.1037/0735-7036.120.2.163; pmid: 16719595. [DOI] [PubMed] [Google Scholar]
- 89.Semmann D, Krambeck H-J, Milinski M. Strategic investment in reputation. Behav. Ecol. Sociobiol. 2004;56:248–252. doi: 10.1007/s00265-004-0782-9. [Google Scholar]
- 90.Bateson M, Nettle D, Roberts G. Cues of being watched enhance cooperation in a real-world setting. Biol. Lett. 2006;2:412–414. doi: 10.1098/rsbl.2006.0509. doi: 10.1098/rsbl.2006.0509; pmid: 17148417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Subiaul F, Vonk J, Okamoto-Barth S, Barth J. Do chimpanzees learn reputation by observation? Evidence from direct and indirect experience with generous and selfish strangers. Anim. Cogn. 2008;11:611–623. doi: 10.1007/s10071-008-0151-6. doi: 10.1007/s10071-008-0151-6; pmid: 18357476. [DOI] [PubMed] [Google Scholar]
- 92.Hopper LM, Lambeth SP, Schapiro SJ, Brosnan SF. Social comparison mediates chimpanzees’ responses to loss, not frustration. Anim. Cogn. 2014 doi: 10.1007/s10071-014-0765-9. in press. doi: 10.1007/s10071-014-0765-9; pmid: 24880642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fontenot MB, Watson SL, Roberts KA, Miller RW. Effects of food preferences on token exchange and behavioural responses to inequality in tufted capuchin monkeys, Cebus apella. Anim. Behav. 2007;74:487–496. doi: 10.1016/j.anbehav.2007.01.015. [Google Scholar]
- 94.Roma PG, Silberberg A, Ruggiero AM, Suomi SJ. Capuchin monkeys, inequity aversion, and the frustration effect. J. Comp. Psychol. 2006;120:67–73. doi: 10.1037/0735-7036.120.1.67. doi: 10.1037/0735-7036.120.1.67; pmid: 16551166. [DOI] [PubMed] [Google Scholar]
- 95.Dindo M, De Waal FBM. Partner effects on food consumption in brown capuchin monkeys. Am. J. Primatol. 2007;69:448–456. doi: 10.1002/ajp.20362. doi: 10.1002/ajp.20362; pmid: 17146793. [DOI] [PubMed] [Google Scholar]