Abstract
Background
Catheter ablation for atrial fibrillation is used increasingly in older patients, yet the risks and benefits are not completely understood. With such uncertainty, local medical opinion may influence catheter ablation use.
Methods
In a 100% sample of Medicare beneficiaries 65 years or older who underwent catheter ablation for atrial fibrillation between January 1, 2007, and December 31, 2009, we investigated variation in use by hospital referral region (HRR) for 20,176 catheter ablation procedures.
Results
Across 274 HRRs, median age was 71.2 years (interquartile range, 70.5-71.8), a median of 98% of patients were white, and a median of 39% of patients were women. The median age-standardized prevalence of atrial fibrillation was 77.1 (69.4-84.2) per 1000 beneficiaries; the median rate of catheter ablation was 3.5 (2.4-4.9) per 1000 beneficiaries. We found no significant associations between the rate of catheter ablation and prevalence of atrial fibrillation (P = 0.99), end-of-life Medicare expenditures per capita (P = 0.09), or concentration of cardiologists (P = 0.45), but a slight association with Medicare expenditures per capita (linear regression estimate, 0.016; 95% CI, 0.001-0.031; P = 0.04). Examined HRR characteristics explained only 2% of the variation in HRR-level rates of catheter ablation (model R2 = 0.016).
Conclusion
The rate of catheter ablation for atrial fibrillation in older patients was low, varied substantially by region, and was not associated with the prevalence of atrial fibrillation, the availability of cardiologists, or end-of-life resource use, and was only slightly associated with overall Medicare expenditures per capita.
Introduction
Atrial fibrillation impairs patients’ functional status and quality of life and places a substantial financial burden on health care systems.1,2 Guidelines recommend catheter ablation for symptomatic, drug-refractory atrial fibrillation.3,4 Randomized trials of catheter ablation have primarily enrolled younger white men,5 but the procedure is used increasingly among Medicare beneficiaries. Among these older individuals, catheter ablation seems to be safe, yet the risks and benefits are not as well defined, particularly in patients with comorbid conditions.6 In the face of such uncertainty, local medical opinion may determine the extent to which catheter ablation is used in older patients.7
Geographic variation in medical care is a well-known phenomenon. Variation in Medicare expenditures has been investigated, and regions of above-average spending have been localized repeatedly to the southeastern United States, as well as to major metropolitan areas. Examples derived from the Dartmouth Atlas of Healthcare (www.dartmouthatlas.org) include overall spending,8 end-of-life expenditures,8 and drug treatments.9 Medical procedures including lower-extremity amputation10 and surgical carotid artery revascularization11 show similar patterns of geographic variation. More recently, geographic variation in medical services has been linked to provider and hospital characteristics and provider preferences.12
Catheter ablation for atrial fibrillation is an elective procedure, and although it is a promising therapeutic option, alternatives exist.3 We hypothesized that significant geographic variation exists in the use of catheter ablation among Medicare beneficiaries and that this variation is associated with physician, patient, and health system factors. To address our hypothesis, we analyzed data from the US Centers for Medicare & Medicaid Services across regional health care markets.
Methods
We obtained a 100% sample of claims data from the US Centers for Medicare & Medicaid Services for patients 65 years or older who underwent catheter ablation for atrial fibrillation. All included patients were enrolled in fee-for-service Medicare and were living in the United States at the time of the procedure. As previously reported,6 we identified and included patients from inpatient, outpatient, carrier, and denominator files who underwent intracardiac catheter ablation (Current Procedural Terminology [CPT] code 93651) for atrial fibrillation as the primary diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 427.31) between January 1, 2007, and December 31, 2009. We included repeat procedures performed on separate days in the analysis. To improve specificity and avoid including procedures not performed for atrial fibrillation, we excluded patients who underwent atrioventricular node ablation (CPT code 93650) or had anomalous atrioventricular excitation or Wolff-Parkinson-White syndrome (ICD-9-CM diagnosis code 426.7) or paroxysmal supraventricular tachycardia (427.0). For our main analyses, we also excluded 320 ablations performed in patients older than 85 years to avoid a highly skewed age distribution and to better reflect the population considered eligible for ablations in standard practice. We included the oldest age group in a secondary analysis, presented in the Appendix.
To assess geographic variation, we investigated the use of catheter ablation for atrial fibrillation by hospital referral region (HRR), 306 regional health care markets defined by the presence of at least 1 hospital that performs major cardiovascular and neurosurgical procedures. We defined the HRRs according to the Dartmouth Atlas of Healthcare and linked patients to HRRs using zip codes of residence and HRR crosswalk files.7 We present procedure rates for 274 HRRs with more than 10 procedures only. To explore patient migration patterns, we used map coordinates to identify pairs of HRRs with common borders. We linked service location zip codes to HRRs to identify catheter ablations performed within the HRR of residence. To address potential referrals into the HRR, we also assessed catheter ablations performed in either an HRR bordering the HRR of residence or a more distant HRR.
We defined the underlying prevalence of atrial fibrillation (ie, the rate of those who were diagnosed with atrial fibrillation at a certain point in time) in each HRR using claims data for a nationally representative 5% sample of Medicare beneficiaries in 2008. Among beneficiaries aged 65 to 85 years as of July 1, 2008, and enrolled in fee-for-service Medicare for the calendar year, we determined the prevalence of atrial fibrillation on the basis of at least 1 inpatient claim or at least 2 outpatient claims for atrial fibrillation (ICD-9-CM code 427.31), consistent with previous studies.6,13 The institutional review board of the Duke University Health System approved the study.
Statistical Analysis
Within each HRR, we calculated mean age and frequency distributions by sex and race for patients undergoing catheter ablation. Medians, interquartile ranges, and ranges were calculated across HRRs. We calculated age-adjusted rates of the prevalence of atrial fibrillation per 1000 beneficiaries by HRR using direct standardization to the Medicare population in 2008 for the following age groups: 65 to 69 years, 70 to 74 years, 75 to 79 years, and 80 to 85 years. For our secondary analysis, we included all patients 80 years and older in the oldest age group. We calculated the age-adjusted rates of catheter ablation for atrial fibrillation per 1000 Medicare beneficiaries overall and per 1000 beneficiaries with prevalent atrial fibrillation by dividing the average number of procedures per year between 2007 and 2009 by the respective number of beneficiaries in 2008.
For each HRR, we present the ratio of the age-adjusted HRR-level prevalence of atrial fibrillation to the national average prevalence of atrial fibrillation, and the ratio of the HRR-level rate of catheter ablation for atrial fibrillation to the national average rate of catheter ablation for atrial fibrillation. In a robust multivariable linear regression model14 that used HRR as the unit of analysis, we examined associations between the age-adjusted rate of catheter ablation for atrial fibrillation and the underlying age-adjusted prevalence of atrial fibrillation, mean Medicare expenditures per enrollee, number of cardiologists per 100,000 beneficiaries, and mean Medicare end-of-life spending per decedent, a surrogate for general health care utilization. We derived this information from the Dartmouth Atlas of Healthcare.8
We conducted a sensitivity analysis to account for the geographic distribution of electrophysiologists. We searched the Medicare 5% carrier claims in 2009 for implantable cardioverter defibrillator (ICD) procedures (HCPCS code 33249), a procedure most commonly performed by electrophysiologists. We estimated the number of electrophysiologists per 100,000 Medicare beneficiaries in each HRR based on the number of distinct physician National Provider Identifier codes found on the ICD procedure claims.
The CHA2DS2-VASc algorithm is an established tool to assess the risk of stroke in patients with atrial fibrillation.15 A CHA2DS2-VASc score of 2 or higher is indicative of patients with several comorbid conditions, and for most patients with a CHA2DS2-VASc score of 2 or higher, oral anticoagulation is recommended.15 We conducted a post-hoc analysis to investigate variability across HRRs in the proportion of patients suitable for oral anticoagulation (CHA2DS2-VASc ≥ 2) and of higher-risk patients (CHA2DS2-VASc ≥ 3) receiving catheter ablation for atrial fibrillation using patient-level data from a prior analysis.6 We used the Spearman rank correlation to test whether the HRR-level proportion of patients with CHA2DS2-VASc ≥ 2 was correlated with the HRR-level catheter ablation rate for atrial fibrillation per 1000 beneficiaries with atrial fibrillation. We considered results significant at a 2-sided level of P = .05. We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses.
Results
Of 306 HRRs in total, 274 were eligible for the catheter ablation analysis after exclusions. Across the eligible HRRs, a total of 20,176 catheter ablations were performed in Medicare fee-for-service patients between 2007 and 2009. Across HRRs, the median age of patients undergoing catheter ablation for atrial fibrillation was 71.2 years (interquartile range, 70.5-71.8) (Table 1). A median of 39% (interquartile range, 33.3%-45.5%) were women, and a median of less than 1% (interquartile range, 0%-1.9%) were black. Beneficiaries in the oldest age category studied, 80 to 85 years, accounted for a median of 6.3% of catheter ablations overall, but this proportion ranged from more than 20% in 6 regions to less than 1% in 39 regions.
Table 1.
Characteristics of the Study Population Within Hospital Referral Regions
| Characteristic* | All Medicare Beneficiaries | Medicare Beneficiaries Undergoing Catheter Ablation† | ||
|---|---|---|---|---|
| Median (IQR) | Range | Median (IQR) | Range | |
| Age, y | 73.5 (73.3-73.9) | 72.4-74.8 | 71.2 (70.5-71.8) | 68.6-74.4 |
| Age group, % | ||||
| 65-69 y | 31.4 (29.6-32.9) | 25.3-38.5 | 44.5 (38.4-50.0) | 15.4-73.7 |
| 70-74 y | 26.4 (25.6-27.0) | 22.6-29.1 | 31.1 (26.7-36.7) | 9.1-56.0 |
| 75-79 y | 22.0 (21.4-22.8) | 18.7-24.7 | 17.2 (12.9-21.0) | 0.0-41.2 |
| 80-85 y | 20.1 (18.8-22.2) | 14.6-28.3 | 6.3 (3.4-9.4) | 0.0-25.0 |
| Women, % | 56.0 (55.0-57.0) | 50.2-61.3 | 39.4 (33.3-45.5) | 6.7-75.9 |
| Race, % | ||||
| Black | 3.6 (1.1-8.7) | 0.1-38.7 | 0.0 (0.0-1.9) | 0.0-26.3 |
| White | 92.4 (85.0-96.5) | 28.1-99.3 | 98.0 (95.5-100.0) | 72.2-100.0 |
| Other | 2.0 (1.0-4.5) | 0.4-71.1 | 0.0 (0.0-2.6) | 0.0-27.8 |
Abbreviation: IQR, interquartile range.
Patient characteristics were calculated as means or frequencies within hospital referral regions. Medians and interquartile ranges were calculated across hospital referral regions.
Analysis of patients who underwent catheter ablation was restricted to the 274 hospital referral regions with more than 10 procedures.
Based on the 5% nationally representative Medicare data, the median age-standardized prevalence of atrial fibrillation across HRRs was 77.1 (interquartile range, 69.4-84.2) per 1000 beneficiaries. Based on the 100% claims data for catheter ablations for atrial fibrillation, the median age-standardized rate of catheter ablation was 0.3 (interquartile range, 0.2–0.4) per 1000 Medicare beneficiaries. The median age-standardized rate of catheter ablation was 3.5 (interquartile range, 2.4-4.9) per 1000 beneficiaries with prevalent atrial fibrillation (Table 2).
Table 2.
Adjusted Rates of Catheter Ablation for Atrial Fibrillation
| Catheter Ablation for Atrial Fibrillation* | Median (IQR) | Range |
|---|---|---|
| Adjusted rate per 1000 Medicare beneficiaries | 0.3 (0.2-0.4) | 0.1-4.2 |
| Adjusted rate per 1000 Medicare beneficiaries with prevalent atrial fibrillation | 3.5 (2.4-4.9) | 0.8-67.1 |
| Ratio of the HRR-level rate per 1000 beneficiaries to the U.S. average (0.302) | 0.9 (0.6-1.2) | 0.2-13.9 |
| Ratio of the HRR-level rate per 1000 beneficiaries with prevalent atrial fibrillation to the U.S. average (3.94) | 0.9 (0.6-1.3) | 0.2-17.0 |
Abbreviations: HRR, hospital referral region; IQR, interquartile range.
Rates were adjusted for age. The analysis was restricted to the 274 hospital referral regions with more than 10 catheter ablation procedures.
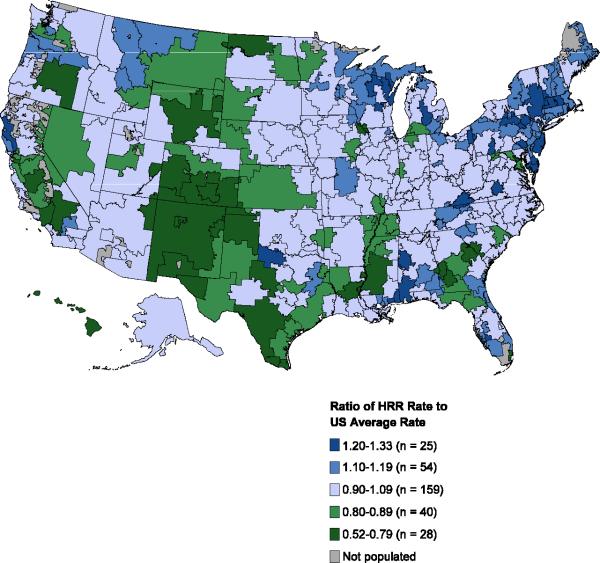
Geographic variation by HRR is plotted in Figure 1. Panel A shows the ratio of the age-adjusted HRR-level prevalence of atrial fibrillation to the US average prevalence of atrial fibrillation per 1000 beneficiaries. Variation ranged from 0.5 to 1.3 (median, 1.0; interquartile range, 0.9-1.1), suggesting no major variation in the age-adjusted prevalence of atrial fibrillation across the United States. Panel B depicts the ratio of the HRR-level rate of catheter ablation per 1000 beneficiaries with prevalent atrial fibrillation to the US average rate of catheter ablation per 1000 beneficiaries with prevalent atrial fibrillation. The ratio varied substantially from 0.2 to 17.0 (median, 0.9; interquartile range, 0.6-1.3), implying geographic variation in the use of catheter ablation for atrial fibrillation across the United States, irrespective of the local underlying prevalence of atrial fibrillation. Similarly, the ratio of the HRR-level rate of catheter ablation for atrial fibrillation to the US average per 1000 beneficiaries (with or without a diagnosis of atrial fibrillation) varied substantially from 0.2 to 13.9 (median. 0.9; interquartile range, 0.6-1.2).
Figure 1.
Geographic Variations in the Prevalence of Atrial Fibrillation and the Rate of Catheter Ablation in the United States
Panel A shows the ratio of the HRR-level prevalence of atrial fibrillation to the average US prevalence of atrial fibrillation per 1000 Medicare beneficiaries.
Panel B shows the ratio of the HRR-level rate of catheter ablation to the average US rate of catheter ablation per 1000 Medicare beneficiaries with prevalent atrial fibrillation.
Note: Only HRRs with more than 10 procedures are shown in Panel B (n = 274/306).
Of the HRRs studied, the lowest catheter ablation rate was 0.8 per 1000 beneficiaries with prevalent atrial fibrillation, and the highest rate was 67.1 per 1000 beneficiaries with prevalent atrial fibrillation. Figure 1 highlights areas of higher and lower rates of catheter ablation. Overall, about 63% of catheter ablations (12,740/20,176) were performed in the patient's HRR of residence, and 85% (17,141/20,176) were performed in either the HRR of residence or a neighboring HRR. Among catheter ablations in the HRR with the highest ablation rate, 85% were performed in patients who resided in the HRR or in a neighboring HRR.
In multivariable linear regression models (Table 3), 2% of the variation in HRR-level rates of catheter ablation was explained by the HRR characteristics we examined (model R2 = 0.016). There were no significant associations with the underlying prevalence of atrial fibrillation (linear regression estimate, 0.000; 95% CI, –0.002 to 0.002; P = .99), average end-of-life Medicare expenditures (–0.006; 95% CI, –0.013 to 0.001; P = .09), or the concentration of cardiologists (–0.004; 95% CI, –0.015 to 0.007; P = .45). We observed a slight association between catheter ablation and average Medicare expenditures (0.016; 95% CI, 0.001 to 0.031; P = .04). In other words, for each additional $1000 in average Medicare spending, there was an associated 0.016 increase in the rate of catheter ablation for atrial fibrillation per 1000 Medicare beneficiaries. Based on our sensitivity analysis using providers implanting ICDs as a surrogate for electrophysiologists, we found no significant association between the HRR-level rate of catheter ablations for atrial fibrillation and the density of electrophysiologists (0.0007; 95% CI, -0.0033 to 0.0048; P = .72).
Table 3.
Multivariable Model of Associations Between Age-Adjusted Rates of Catheter Ablation for Atrial Fibrillation per 1000 Beneficiaries and Hospital Referral Region Descriptive Factors*
| Variable | Adjusted Estimate (95% CI) | P Value |
|---|---|---|
| Adjusted prevalence of atrial fibrillation | –0.000 (–0.002 to 0.002) | 0.99 |
| Average Medicare expenditure per $1000 | 0.016 (0.001 to 0.031) | 0.04 |
| Average end-of-life inpatient expenditure per $1000 | –0.006 (–0.013 to 0.001) | 0.09 |
| Cardiologists per 100,000 Medicare beneficiaries | –0.004 (–0.015 to 0.007) | 0.45 |
Multivariable model includes all variables listed. The analysis was restricted to the 274 hospital referral regions with at least 10 catheter ablation procedures.
In a post-hoc analysis, the median proportion of patients with CHA2DS2-VASc ≥ 2 and of patients with CHA2DS2-VASc ≥ 3 among the 274 eligible HRRs was 0.95 (interquartile range, 0.92-0.98) and 0.78 (interquartile range, 0.72-0.84), respectively. The proportions of patients with CHA2DS2-VASc ≥ 2 and CHA2DS2-VASc ≥ 3 were not correlated with the rate of catheter ablation at the HRR level (Figure 2, P = 0.21 and P = 0.87, respectively).
Figure 2.
HRR-Level Age-Adjusted Rate of Catheter Ablation for Atrial Fibrillation Among Medicare Beneficiaries With CHA2DS2-VASc ≥ 2 (Panel A) and ≥ CHA2DS2-VASc ≥ 3 (Panel B)
In secondary analyses, we included all age groups, including those patients aged 85 years and older. The results of these secondary analyses were very similar to our main findings and are shown in Supplemental Tables 1 and 3.
Discussion
We observed substantial geographic variation in the use of catheter ablation for atrial fibrillation in Medicare fee-for-service beneficiaries. The variation in ablations did not correspond to the underlying prevalence of atrial fibrillation. Although the age-adjusted prevalence of atrial fibrillation was greatest in the major metropolitan areas of the East and West Coasts, catheter ablation rates were higher in areas where the prevalence of atrial fibrillation was relatively low. HRR-level data regarding the geographic distribution of electrophysiologists who perform catheter ablation were not available for the analysis. However, an analysis of the Heart Rhythm Society Electrophysiology Workforce Study suggests that there is limited overlap between states with more electrophysiologists per capita and HRRs where we observed above-average rates of catheter ablation.16 Even after exclusion of procedures among patients older than 85 years (n = 320 ablations, 1.6% of the total procedures among fee-for-service Medicare beneficiaries 65 years or older), we observed relatively limited use of catheter ablation in older patients with atrial fibrillation.
The reasons for the absence of an association between the prevalence of atrial fibrillation and the use of catheter ablation are unclear but may reflect provider preferences and uncertainty about safety, effectiveness, and net clinical benefit of catheter ablation in older adults. Relevant insights from clinical trials of catheter ablation are limited, because most investigations enrolled younger patients.3 Yet, nationwide data suggest that the complication rate after catheter ablation in Medicare beneficiaries is low.6 However, apart from single-center experiences, referring providers and catheter ablation providers are left without generalizable real-world estimates of effectiveness in older patients. In addition, geographic variation in catheter ablation for atrial fibrillation may be associated with geographic variation in the training of electrophysiologists performing the procedure. High rates of catheter ablation may correspond to the availability of highly skilled interventional electrophysiologists. Yet, using providers who implanted ICDs as a surrogate for electrophysiologists, we found no significant association with the rate of catheter ablation.
Patient preferences also may play an important role in the variation we observed.17,18 Some older patients with atrial fibrillation may prefer less invasive management. Others might choose catheter ablation, expecting to maintain an active lifestyle free of atrial fibrillation symptoms or in an attempt to avoid further medical therapy.
We observed no association between Medicare end-of-life expenditures,19 density of cardiovascular specialists, or electrophysiologists and catheter ablation rates, but did find a small association of average overall Medicare spending with catheter ablation rates. The latter is consistent with prior analyses suggesting that patients in higher-spending regions receive more care.19 However, all examined factors combined explained only 2% of the geographic variation observed in the rate of catheter ablation for atrial fibrillation.
Recent analyses have highlighted significant geographic variation in Medicare services, the majority of which is attributable to post-acute care and inpatient care.12 Geographic variations in catheter ablation do not follow previously observed patterns of variation in health care interventions. The Dartmouth Atlas investigated geographic variations in hospitalization, medical discharges, Medicare reimbursement, and quality of care.7 In all analyses, the southeastern United States accounted for a high proportion of Medicare costs as a result of more frequent use of resources. Catheter ablation appears to be an exception to these patterns, with several referral regions distributed across the country demonstrating higher rates of catheter ablation rather than a concentration in one region of the country. Use of implantable cardioverter-defibrillators, another procedure predominantly performed by electrophysiologists, has also exhibited significant geographic variation.20 However, the patterns indicated low utilization in areas of low population density and thus were different from those in the present analysis of catheter ablation for atrial fibrillation. The comparison of catheter ablation with the use of implantable cardioverter-defibrillators does have limitations, as the latter has been shown to improve all-cause mortality and is generally more available than catheter ablation. A recent study of Medicare-related health care spending reported significant variation in spending across local hospital service areas that form HRRs, rather than across the HRRs themselves.21 One center-based hypothesis could thus be that tertiary referral centers experienced in the treatment of older patients potentially affected by more comorbid conditions might be more willing to perform catheter ablation in such patients. As the overall use of catheter ablation was modest (ie, 3.5 ablations per 1000 beneficiaries with prevalent atrial fibrillation), we were limited in our ability to investigate variation by single catheter ablation centers or providers.
A natural question that arises is, “Are these rates too low or too high?” Overall, the absolute rates that we observed were low. However, our analysis cannot answer whether the observed rates reflect underuse or overuse of catheter ablation. Consistent with current guideline recommendations, determinations about appropriate use require knowledge of prior treatment and symptoms. Ablation indication data are not present in Medicare claims data. The goal of our analysis was to describe overall variation in the utilization of an important therapy for patients with medically refractory atrial fibrillation rather than to explore quality of care.
Although not the focus of our analysis, we observed that white men appear to undergo catheter ablation for atrial fibrillation more commonly than women and ethnic/racial minorities. Atrial fibrillation also most frequently occurs in white men, a fact that might, in part, explain the differential racial distribution. Ablation of atrial fibrillation is primarily indicated to control symptoms of the arrhythmia. As claims data lack information on symptoms, it remains speculative if differences in the burden of symptoms might also contribute to the unbalanced sex distribution. Future work will need to investigate these circumstances.
Our analysis was limited by our inability to directly account for the geographic distribution of electrophysiologists who perform catheter ablations and by the absence of data on patient and physician preferences. Our approach of using providers who implanted ICDs as a surrogate for electrophysiologists might not have identified interventionalists performing catheter ablations sufficiently. The overall low use of catheter ablation in elderly patients in general further prevented center-based analyses. We also cannot rule out misclassification of atrial flutter as atrial fibrillation, though we required that claims contain codes specific to atrial fibrillation. In previous work, we excluded beneficiaries with a diagnosis of atrial flutter within 6 months before catheter ablation and did not identify relevant differences in the results.6 We acknowledge that ablation rates vary by atrial fibrillation pattern (ie, paroxysmal, persistent, permanent), which cannot be distinguished using administrative data and current ICD-9-CM codes. However, we are unaware of data to suggest that atrial fibrillation patterns vary by region. Finally, our analysis includes data from 2007 through 2009 only; patterns of variation may change over time as the procedure becomes more widely used. Yet, the important procedural principles of atrial fibrillation ablation were established before our study period and remain the foundation of ablation procedures today.22
In conclusion, there was significant geographic variation in the use of catheter ablation that did not correspond to the underlying prevalence of atrial fibrillation or stroke risk profile and only minimally mirrors previously recognized patterns of health care resource use. Although explanations for this phenomenon remain speculative, uncertainty about the efficacy and safety of catheter ablation in older patients, physician preferences, and patient preferences are likely contributors. Prospective data on the safety and effectiveness of catheter ablation in older patients and women are needed, particularly given the relatively limited use in older patients. Until such data become available, it remains unclear whether the observed geographic variation represents appropriate use. With the emerging focus on patient-centered and preference-sensitive care, understanding how patient and physician preferences influence patterns of care is of paramount importance.
Acknowledgments
Funding/Support
This work was supported by grant R01HL102214 from the National Heart, Lung, and Blood Institute. Dr Benjamin was supported in part by National Heart, Lung, and Blood Institute grants R01HL092577 and RC1HL101056. The National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Contributions: Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Disclosures: Dr Piccini reported serving as a consultant for Janssen Pharmaceuticals, BMS/Pfizer, and Medtronic; and receiving grant funding from ARCA biopharma, GE Healthcare, and Janssen Pharmaceuticals. Dr Curtis reported receiving grant funding from GlaxoSmithKline, Johnson & Johnson, and GE Healthcare. Drs Piccini and Curtis have made available online detailed listings for financial disclosures (https://www.dcri.org/about-us/conflict-of-interest). No other disclosures were reported.
References
- 1.Rienstra M, Lubitz SA, Mahida S, et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation. 2012;125:2933–43. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–96.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 5.Piccini JP, Lopes RD, Kong MH, et al. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009;2:626–33. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 6.Piccini JP, Sinner MF, Greiner MA, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–7. doi: 10.1161/CIRCULATIONAHA.112.109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennberg JE, Fisher ES, Skinner JS. Geography and the debate over Medicare reform. Health Aff (Millwood) 2002;(Suppl Web Exclusives):W96–W114. doi: 10.1377/hlthaff.w2.96. [DOI] [PubMed] [Google Scholar]
- 8. [May 17, 2013];The Dartmouth Atlas of Healthcare. http://www.dartmouthatlas.org.
- 9.Zhang Y, Baicker K, Newhouse JP. Geographic variation in Medicare drug spending. N Engl J Med. 2010;363:405–9. doi: 10.1056/NEJMp1004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000-2008. J Am Coll Cardiol. 2012;60:2230–6. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel MR, Greiner MA, DiMartino LD, et al. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003-2006. Arch Intern Med. 2010;170:1218–25. doi: 10.1001/archinternmed.2010.194. [DOI] [PubMed] [Google Scholar]
- 12.Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368:1465–8. doi: 10.1056/NEJMp1302981. [DOI] [PubMed] [Google Scholar]
- 13.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rousseeuw PJ, Yohai V. Robust regression by means of s estimators. In: Franke J, Härdle W, Martin RD, editors. Lecture notes in statistics - robust and nonlinear time series analysis. Springer; New York: 1984. pp. 256–74. [Google Scholar]
- 15.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deering TF, Clair WK, Delaughter MC, et al. A Heart Rhythm Society Electrophysiology Workforce study: current survey analysis of physician workforce trends. Heart Rhythm. 2010;7:1346–55. doi: 10.1016/j.hrthm.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee S, Wennberg JP, Barry MJ, et al. Improving Patient Decision-Making in Health Care: A 2011 Dartmouth Atlas Report Highlighting Minnesota. Dartmouth Institute for Health Policy and Clinical Practice; Lebanon, NH: 2011. [PubMed] [Google Scholar]
- 18.Anthony DL, Herndon MB, Gallagher PM, et al. How much do patients’ preferences contribute to resource use? Health Aff (Millwood) 2009;28:864–73. doi: 10.1377/hlthaff.28.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AJ, Polsky D, Yang F, et al. Geographic variation in implantable cardioverterdefibrillator use and heart failure survival. Med Care. 2012;50:10–7. doi: 10.1097/MLR.0b013e3182293510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Baik SH, Fendrick AM, et al. Comparing local and regional variation in health care spending. N Engl J Med. 2012;367:1724–31. doi: 10.1056/NEJMsa1203980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg BA, Hammill BG, Daubert JP, et al. Periprocedural imaging and outcomes after catheter ablation of atrial fibrillation. Heart. 2014;100:1871–7. doi: 10.1136/heartjnl-2014-306067. [DOI] [PMC free article] [PubMed] [Google Scholar]