Abstract
Background
Discordance between left- and right-sided filling pressures occurs in a subset of patients presenting with acute decompensated heart failure (ADHF). We hypothesized that a disproportionately increased right atrial pressure (RAP) relative to the pulmonary capillary wedge pressure (PCWP) would be associated with both renal dysfunction and mortality in ADHF.
Methods
A total of 367 patients admitted with ADHF with elevated intracardiac filling pressures were treated with intensive medical therapy guided by invasive hemodynamic monitoring. Baseline characteristics, hemodynamics, and renal function at admission were stratified by RAP/PCWP quartiles. The association of RAP/PCWP quartile with all-cause mortality after a median follow-up of 2.4 years was assessed in univariable and multivariable models, which included adjustment for the RAP.
Results
The median RAP/PCWP was 0.58 (interquartile range 0.43-0.75). Increasing RAP/PCWP was inversely associated with estimated glomerular filtration rate at baseline and with treatment (P < .0001) independently of RAP. High RAP/PCWP was associated with increased mortality (quartile 4 vs 1: hazard ratio [95% CI] 2.1 [1.3-3.5], P = .002). The association of RAP/PCWP with mortality persisted after adjustment for age, gender, mean arterial pressure, RAP, cardiac index, pulmonary vascular resistance, and estimated glomerular filtration rate (hazard ratio 2.4 [1.4-3.9], P = .007).
Conclusion
A disproportionate increase in right to left ventricular filling pressures is associated with renal dysfunction and mortality, independently of the right atrial pressure.
Right atrial pressure (RAP) is often coupled to pulmonary capillary wedge pressure (PCWP) in patients with heart failure, whether the left ventricular (LV) ejection fraction (LVEF) is reduced1,2 or preserved.3 Estimates of the jugular venous pressure (reflecting RAP) provide a useful bedside technique to estimate intracardiac filling pressures in patients with heart failure.4 However, the relationship between the RAP and PCWP (which can be expressed as the ratio between the RAP and PCWP)2,5 is complex, as a sizeable subset of patients has a discordant relationship between their right- and left-sided filling pressures.2,5,6
An emerging set of observations suggests that an increase in the RAP/PCWP ratio is associated with adverse outcomes in patients with heart failure. Specifically, an increase in the RAP/PCWP ratio was associated with renal impairment in 2 previous cohorts of patients with advanced heart failure2,5 and with echocardiographic evidence of right ventricular impairment.5 Furthermore, an increase in this ratio was associated with an increased short-term risk for the composite end point of death and heart failure hospitalization in the ESCAPE trial.5 However, the ESCAPE cohort was limited in size (n = 215) and was followed for only 6 months, perhaps explaining why the RAP/PCWP ratio was not associated with mortality. Herein, we examine the relationship between baseline and serial measurements of RAP/PCWP with renal function and mortality in a larger cohort of congested patients with advanced acute decompensated heart failure (ADHF) followed over a longer period (~2.5 years).
Methods
Study population
A retrospective data set containing adult advanced heart failure patients (age ≥18 years, n = 418) admitted to a dedicated heart failure intensive care unit (ICU) at the Cleveland Clinic Foundation Heart and Vascular Institute for invasive hemodynamically monitored management of ADHF over the past decade has been previously described.7 Patients were excluded if they had congenital heart disease, received renal replacement therapy, necessitated mechanical circulatory support, or had pulmonary arterial hypertension not related to left heart failure (World Health Organization groups 1, 3, 4, and 5).8 Because the goal of this analysis was to evaluate RAP/PCWP ratio in congested patients in this analysis, patients admitted with both RAP <10 mm Hg and PCWP <22 mm Hg were excluded from the analysis (n = 51) leaving 367 patients. Of these, demographic, clinical laboratory, B-type natriuretic peptide (BNP) levels (n = 209), hemodynamic, and transthoracic echocardiographic data (n = 265) were obtained chart review of the electronic medical record, EPIC (EPIC, Verona, WI).
Assessment of hemodynamics
Under fluoroscopic guidance, a pulmonary artery catheter (PAC) was placed into the internal jugular vein in either the cardiac catheterization laboratory or an adjacent procedure room to the ICU. All patients were supine with RAP, pulmonary pressures, and the mean PCWP assessed at end expiration at steady state with zeroing level to the phlebostatic axis. Assuming standard metabolic rates, cardiac output was calculated by the Fick principle by collecting a pulmonary arterial blood sample and measured arterial oxygen saturation with pulse oximetry. Systemic blood pressure was measured either noninvasively by a cuff sphygmomanometer or invasively via a peripheral arterial line. Every hour the nursing staff documented measured pressures in the chart. Cardiac output, however, was measured every 4 hours, and PCWP was measured twice daily and upon PAC removal.
Treatment in the heart failure ICU
Medical therapy for ADHF started promptly after PAC insertion at the clinicians' discretion, although all of them followed a structured hemodynamically guided drug titration protocol.9 While maintaining a mean arterial pressure (MAP) >65 mm Hg, intravenous nitroprusside and diuretics were prescribed to achieve hemodynamic goals, which included decreasing RAP to ≤8 mm Hg, PCWP ≤18 mm Hg, mean pulmonary pressure by 20%, and increasing cardiac index ≥2.2 L/min/m2.9 Inotrope use was based on clinical judgment.
Definitions
Renal function was measured on a daily basis via routine serum chemistries. However, to evaluate changes in hemodynamics with changes in renal function, only hemodynamic measures and laboratory data at the time of PAC placement and removal were used for the purpose of this study. The Modification of Diet in Renal Disease equation was used for the calculation of estimated glomerular filtration rate (eGFR).10 The lowest eGFR throughout the course of treatment for ADHF was considered the nadir estimate of renal function. The eGFR at time of PAC removal was considered the discharge estimate of renal function. Pulmonary vascular resistance (PVR) was calculated as the transpulmonary gradient divided by cardiac output. Transpulmonary gradient was calculated as the difference between mean pulmonary arterial pressure and the PCWP. Transmural gradient was calculated as the difference between mean PCWP and mean RAP.
Echocardiographic assessment
Echocardiographic data completed within the 30 days before admission to the ICU was included in this analysis. Biplane Simpson's method was used to measure LVEF. In the conventional parasternal long-axis and apical 4-chamber projections, mitral and tricuspid regurgitation were semiquantitatively graded by color flow Doppler: 1+ (jet area/left atrial area <10%); moderate, 2+ (jet area/left atrial area 10%-20%); moderate to severe, 3+ (jet area/left atrial area 20%-45%); or severe, 4+ (jet area/left atrial area >45%); right ventricular (RV) systolic dysfunction was visually assessed on scale of 0 to 4, with 0 corresponding to normal and 4 corresponding to severely hypokinetic RV wall motion.
Mortality and outcome assessment
The interval from the index right heart catheterization date till December 31, 2011, was defined as the interval of follow-up. Manual chart review for orthotopic heart transplant (OHT), LV assist device (LVAD) placement, and all-cause mortality was performed. Death status was confirmed by review of the Social Security Death Index.
Statistical methods
Continuous variables were expressed as either mean ± SD or median (interquartile range [IQR]) where appropriate. The analysis of variance (ANOVA) or Kruskal-Wallis test was used to compare parametric and nonparametric variables, respectively. Categorical variables were expressed as percentage (%) and analyzed via χ2 method or the Cochran-Armitage trend test. P < .05 was considered significant to reject the null hypothesis that there were no differences in renal function or survival between subjects with different RAP/PCWP ratios. Survival analyses were completed via the Kaplan-Meier method and log-rank analysis to compare survival curves across quartiles of RAP/PCWP. Patients were censored at the time of LVAD placement or OHT for the survival analysis. Cox proportional hazards models were used to compare time-to-event analysis to determine hazard ratios (HRs) and 95% CIs for mortality between the first and fourth quartiles of RAP/PCWP. Multivariable models adjusted for age, gender, MAP, RAP, cardiac index, PVR, and log-transformed eGFR. Additional adjustments were made for a subset with BNP values. Statistical analyses were performed using JMP Pro version 10 (SAS Institute, Inc, Cary, NC).
This study was approved by the Cleveland Clinic Institutional Review Board. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
Baseline characteristics
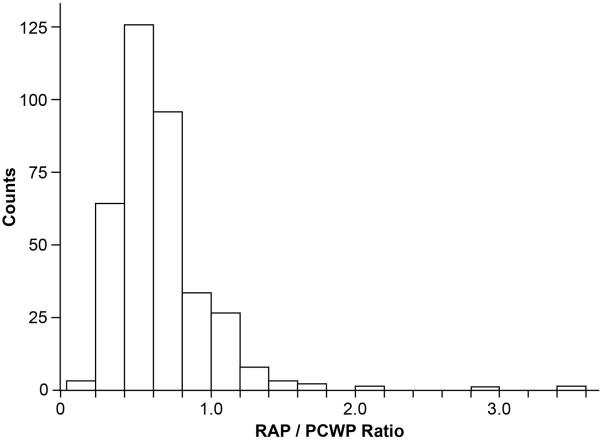
Baseline characteristics of the study cohort are described in Table I, which are representative of a patient population with advanced decompensated heart failure. Right atrial pressure/PCWP ratios were distributed with right skew (Figure 1). The median RAP/PCWP was 0.58 (0.43-0.75). Higher RAP/PCWP before treatment was inversely associated with vasodilator use (P = .006) but not associated with use of inotropes or loop diuretics.
Table I.
Patient demographics across RAP/PCWP ratio quartiles (n = 367)
| Q1 (<0.43) | Q2 (0.43-0.58) | Q3 (0.58-0.75) | Q4 (≥0.75) | P | |
|---|---|---|---|---|---|
| Age (y) | 58 ± 10 | 56 ± 14 | 58 ± 14 | 57 ± 12 | .65 |
| Male | 84.8% | 78.0% | 72.8% | 64.8% | .001* |
| ICM | 46.7% | 49.5% | 52.2% | 37.0% | .18 |
| LVEF(%) | 17 ± 8 | 17±7 | 19±10 | 27 ± 18 | <.0001 |
| LVEDD (cm) | 6.6 ± 1.1 | 6.6 ±1.1 | 6.3 ± 1.2 | 6.0 ± 1.4 | .02 |
| Diabetes mellitus II | 31.5% | 37.4% | 41.3% | 43.5% | .08* |
| ICD | 58.7% | 47.3% | 48.9% | 43.6% | .06* |
| CRT | 25.0% | 24.4% | 26.1% | 27.5% | .97 |
| β-Blocker | 74.7% | 60.0% | 78.6% | 65.2% | .03 |
| ACEI | 67.1% | 52.6% | 52.6% | 38.8% | .001* |
| ARB | 13.4% | 17.1% | 14.1% | 16.4% | .90 |
| Digoxin | 57.1% | 41.8% | 41.3% | 40.2% | .03 |
| Loop diuretic | 97.8% | 87.9% | 90.2% | 91.2% | .05 |
| Vasodilator (ICU) | 80.0% | 80.0% | 73.9% | 48.9% | .006 |
| Inotrope (ICU) | 59.8% | 48.4% | 45.7% | 59.3% | .11 |
| Loop diuretic (ICU) | 68.6% | 62.9% | 78.3% | 56.8% | .16 |
Continuous variables are expressed as mean ± SD or median (IQR). Missing values: LVEF and LVEDD: 102. Abbreviations: ICM, Ischemic cardiomyopathy; ICD, implanted cardiac defibrillator; CRT, cardiac resynchronization therapy; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P value determined by Cochran-Armitage trend test; other P values for continuous parametric variables calculated via ANOVA, for continuous nonparametric via Kruskal-Wallis test, and for categorical variables by χ2 method.
Figure 1.
Right atrial pressure/PCWP ratio distribution in study cohort (n = 367).
Baseline RAP/PCWP and hemodynamics
Baseline hemodynamics is shown stratified by quartile of RAP/PCWP (Table II). Increasing RAP/PCWP was associated with higher RAP, lower PCWP, and lower transmural gradient. Subjects with higher RAP/PCWP also had lower pulmonary pressures, but there was no association with PVR. Higher RAP/PCWP was associated with a higher cardiac index, lower right ventricular stroke work index, and a lower systemic vascular resistance (P = .03, P < .0001, and P < .0001, respectively).
Table II.
Hemodynamics and laboratory test results on admission across RAP/PCWP ratio quartiles (n = 367)
| Q1 (<0.43) | Q2 (0.43-0.58) | Q3 (0.58-0.75) | Q4 (≥0.75) | P | |
|---|---|---|---|---|---|
| MAP (mm Hg) | 85 (76-92) | 80 (73-90) | 81 (75-90) | 78 (69-90) | .053 |
| RAP (mm Hg) | 10 (8-12) | 15 (12-18) | 18 (15-21) | 22 (18-26) | <.0001 |
| Pulmonary systolic pressure (mm Hg) | 61 (52-72) | 60 (52-68) | 58 (49-70) | 49 (40-65) | <.0001 |
| Pulmonary diastolic pressure (mm Hg) | 30 (28-37) | 30 (27-35) | 30 (25-35) | 27 (22-33) | .0003 |
| Mean pulmonary pressure (mm Hg) | 42 (37-47) | 41 (35-46) | 41 (34-47) | 34 (28-43) | <.0001 |
| Pulmonary capillary wedge pressure (mm Hg) | 30 (26-33) | 29 (24-33) | 26 (23-31) | 23 (18-28) | <.0001 |
| Cardiac index (L/min/m2) | 1.7 ± 0.4 | 1.7 ± 0.5 | 1.8 ± 0.7 | 1.9 ± 0.7 | .03 |
| PCWP - RAP (mm Hg) | 20 (17-22) | 14 (11-16) | 9 (7-10) | 1 (-2to4) | <.0001 |
| Systemic vascular resistance (dyn · s/cm5) | 1673 (1414-2069) | 1571 (1264-1898) | 1382 (1115-1863) | 1278 (951-1640) | <.0001 |
| Pulmonary vascular resistance (Wood units) | 2.8 (1.9-5.0) | 3.3 (2.1-4.6) | 3.5 (2.2-5.5) | 3.3 (1.9-5.5) | .67 |
| Stroke volume (mL) | 42 (34-54) | 42 (34-54) | 44 (33-56) | 41 (31-55) | .99 |
| Right ventricular stroke work index (g · m/m2 per beat) | 9.2 (7.0-11.6) | 6.9 (5.8-8.8) | 6.7 (4.4-8.9) | 3.5 (2.7-7.3) | <.0001 |
| Brain natriuretic peptide (pg/mL) | 737 (344-1374) | 1237 (745-2568) | 889 (550-1473) | 972 (469-1797) | .06 |
| Sodium (mEq/L) | 137 ± 4 | 135 ± 6 | 136 ± 5 | 134 ± 14 | .09 |
| Creatinine (mg/dL) | 1.2 (1.0-1.7) | 1.3 (1.0-1.8) | 1.5 (1.1-1.9) | 1.6 (1.1-2.5) | .0004 |
| Blood urea nitrogen (g/dL) | 29 (22-39) | 33 (22-55) | 38 (22-66) | 45 (27-70) | .009 |
| Bilirubin (mg/dL) | 1.0 (0.5-1.4) | 1.2 (0.6-1.8) | 1.1 (0.6-1.3) | 1.2 (0.7-1.7) | .42 |
| ALT (IU/L) | 26 (19-44) | 38 (18-75) | 27 (16-45) | 22 (14-37) | .41 |
| AST (IU/L) | 24 (19-38) | 36 (25-51) | 30 (19-46) | 26 (19-46) | .23 |
P values for continuous variables calculated via ANOVA or Kruskal-Wallis test. Values are expressed as mean ± SD or median (IQR). Missing values: brain natriuretic peptide, 158; blood urea nitrogen, 105; bilirubin, 169; aspartate aminotransferase, 167; alanine aminotransferase, 167. Abbreviations: ALT, Alanine aminotransferase; AST, aspartate aminotransferase.
Changes in RAP/PCWP after vasoactive therapy
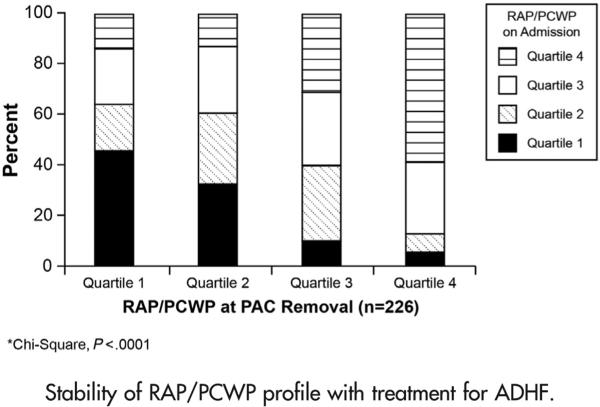
Among the 367 patients who had an initial PAC placed, there were 226 subjects who had documented hemodynamic measurements upon removal of the PAC. The overall median time with a PAC in place was 3 (2-5) days. Interestingly, few patients who had extreme values in RAP/PCWP classification (ie, high or low ends of the spectrum) moved to the opposite extreme of the spectrum despite aggressive treatment for ADHF. Specifically, 46% of the patients in quartile 1 and 59% of the patients in quartile 4 remained in their initial quartile despite treatment with diuretics and vasoactive drugs (P < .0001) (Figure 2). Admission RAP/PCWP was correlated with RAP/PCWP after treatment (Spearman ρ 0.5206, P < .0001). In contrast, although RAP/PCWP was inversely correlated with PCWP on admission (Spearman ρ −0.4093, P < .0001), RAP/PCWP was not correlated with PCWP at the time of PAC removal (Spearman ρ −0.0077, P = .9). In 65 patients, the RAP/ PCWP ratio decreased as a result of treatment. These patients had a disproportionately higher RAP/PCWP on admission than patients who did not reclassify to a lower RAP/PCWP quartile (RAP 19 [15-23] vs RAP 15 [11-20] mm Hg, P < .0001, and PCWP 25 [20-30] vs PCWP 28 [23-31] mm Hg, P = .02, respectively), yet there were no differences in demographics, admission cardiac index, PVR, LVEF, LV end-diastolic diameter (LVEDD), eGFR, or BNP (P > .05 for all).
Figure 2.
Stability of RAP/PCWP profile with treatment for ADHF.
Changes in RAP/PCWP and renal function and echocardiographic parameters
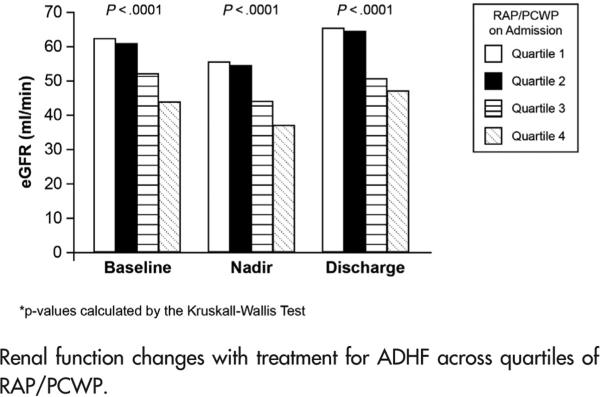
Increasing RAP/PCWP quartiles were associated with renal impairment on admission (Table II), during treatment, and at discharge (Figure 3, P < .0001 across quartiles, for baseline, nadir, and discharge eGFR). Although eGFR was inversely correlated with RAP as well (Spearman ρ −0.14, −0.21, and −0.19 for baseline, nadir, and discharge, P < .01 for all), the inverse correlations with eGFR were stronger for RAP/PCWP (Spearman ρ −0.25, −0.28, and −0.27 for baseline, nadir, and discharge, P < .0001 for all). In addition, after multivariable adjustment for RAP, log-transformed RAP/ PCWP was independently associated with baseline eGFR (β −14.4, P = .003).
Figure 3.
Renal function changes with treatment for ADHF across quartiles of RAP/PCWP.
Echocardiographic data were available in a subset (n = 265) of patients. Right atrial pressure/PCWP was positively correlated with LVEF (Spearman ρ 0.15, P = .02) and inversely correlated with both LVEDD and mitral regurgitation (Spearman ρ −0.16, P = .01 and Spearman ρ −0.16, P = .01, respectively). Although RAP/PCWP was positively correlated with tricuspid regurgitation (Spearman ρ 0.28, P < .0001), there was no correlation with echocardiographically evaluated right ventricular function (P = .54).
RAP/PCWP and survival
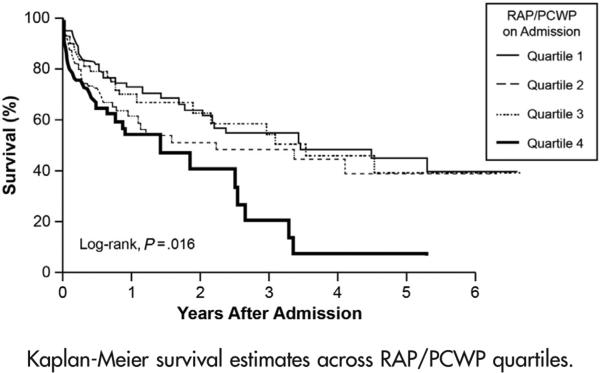
Of the 366 patients followed up for all-cause mortality, 134 (37%) had died at a median follow-up of 2.4 years after index admission to the ICU. There were 34 LVAD placements and 69 heart transplantations. Increasing quartiles of RAP/PCWP were associated with a significantly reduced survival (log-rank χ2 10.3, P = .016) (Figure 4). The highest RAP/PCWP quartile predicted a 2.1-fold increase in mortality (Table III) by the end of follow-up when compared to the lowest RAP/PCWP quartile (quartile 4 vs 1, HR 2.1, 95% CI 1.3-3.5, P = .002). In a sensitivity analysis (data not shown), there was a similar trend for LVAD- and OHT-free survival (HR 1.45 [95% CI 1.00-2.13], P = .051). After multivariable adjustment for age, gender, MAP, RAP, cardiac index, PVR, and log-transformed eGFR, the highest RAP/PCWP quartile was still independently associated with an increased risk of death (HR 2.2 [95% CI 1.1-4.5], P = .03). In a sensitivity analysis adjusting for the previous variables, but substituting log-transformed creatinine for log-transformed eGFR, the results were similar (quartile 4 vs 1, HR 2.2 [95% CI 1.1-4.4], P = .04).
Figure 4.
Kaplan-Meier survival estimates across RAP/PCWP quartiles.
Table III.
Cox proportional hazards model for mortality stratified according to RAP/PCWP
Hazard ratios calculated for quartile 1 versus quartile 4 of RAP/PCWP.
Adjusted for age, gender, MAP, RAP, cardiac index, PVR, and log-transformed eGFR.
Adjusted for the previous factors with BNP in a subset of patients (n = 209) with BNP measured.
In a subset of patients with available BNP data (n = 209) at the time of admission to the ICU, there was no correlation between BNP (Spearman ρ 0.0185, P = .8) and RAP/PCWP. However, higher RAP/PCWP was still independently associated with an increased risk for death when BNP was added to the multivariable adjustment mentioned previously (HR 2.7 [95% CI 1.05-6.9], P = .04).
Discussion
Our study substantially extends the current knowledge regarding the clinical consequences of a disproportionate elevation in right- to left-sided cardiac fillings pressures in patients with ADHF. We were able to demonstrate that a higher RAP/PCWP ratio during hemodynamically guided protocol-driven vasoactive therapy was associated with greater impairment of renal function at presentation, during treatment, and at discharge, independently of baseline RAP. In addition, we have demonstrated for the first time that a higher RAP/PCWP ratio was associated with increased mortality, independently of many other known clinical and hemodynamic prognostic markers in ADHF including RAP and BNP (in a subset). Overall, these findings add to our understanding of the hemodynamic pathophysiology of renal impairment and the prognostic implications of the relationship between right- and left-sided filling pressures in patients with ADHF.
Stability of RAP/PCWP profile with treatment
Right atrial pressure has good positive and negative predictive values to assess PCWP in most patients with heart failure.1 However, some patients experience discordant left- and right-sided pressures. Early work categorized these patients with arbitrary cut-off values of RAP ≥10 or <10 mm Hg and PCWP ≥22 or <22 mm Hg.1 Further studies in cohorts with advanced heart failure revealed that up to 25% to 33% observed discordant right-and left-sided filling pressures.2,6 Whether analyzed by pressure categories or as a continuous ratio, extremes of this relationship were largely stable before and after treatment in many patients with ADHF,2,5 which is consistent with our findings. Interestingly, those subjects who had a reduction in their RAP/PCWP quartile over time (approximately 25% of the cohort with follow-up hemodynamics) had higher right-sided filling pressures on admission; these data suggest that RAP/PCWP instability over time may represent corresponding changes in venous congestion. Overall, our data demonstrate that the balance between left- and right-sided filling pressures remained relatively consistent in a solid majority of cases despite the use of vasoactive drugs with specific hemodynamic goals guided by the PAC.
Relationship of RAP/PCWP with cardiac index and PVR
Our findings call into question the exact hemodynamic determinants driving this observed RAP-PCWP mismatch. In prior studies, increasing RAP/PCWP had been associated with reduced cardiac index and elevated PVR.2,5 In contrast, we found RAP/PCWP was directly associated with cardiac index and was not correlated with PVR (Table II). It is likely that differences among the cohorts may in part explain these disparate findings. In ESCAPE, the decision to use PAC to guide therapy was equipoise, whereas there was “intention-to-treat” under PAC in our study cohort. We have also selected congested patients with elevated filling pressures in our analysis. These logistical differences may explain why in ESCAPE, RAP/PCWP tertile was not associated with PCWP while in the current study, increasing RAP/PCWP quartile was associated with lower PCWP. Meanwhile, our data may imply that neither PVR nor cardiac index would likely be the primary determinants of the variability in or prognostic utility of RAP/PCWP in ADHF.
Relationship of RAP/PCWP and renal function
Consistent with previous reports,2,5 we demonstrate that increasing RAP/PCWP was associated with impaired renal function. Despite trends toward improvement in renal function with treatment in all RAP/PCWP quartiles, higher RAP/PCWP remained associated with lower estimated glomerular rate after treatment (Figure 3). Increased central venous pressure, a surrogate for RAP, has been linked with worsening renal function in a variety of cardiac diseases and contributed more to worsening renal function in ADHF than cardiac index.11,12 Our findings further expand the concept of “right-sided heart failure” beyond the simple representation of “backward congestion” at the level of venous return. In fact, higher RAP/PCWP points to an inability of venous and pulmonary circulations to provide adequate preload for the LV. The strikingly lower transmural gradient with increasing RAP/PCWP in our study (Table II) supports this hypothesis and suggests that the LV may be relatively underfilled resulting in worse renal function.
Such diminished venous circulatory reserve may be related to diastolic ventricular interaction. For example, canine models during acute volume loading revealed an interventricular interaction that may be related to a stiff constraining pericardium.13 As RV filling pressures increased with fluid overload, LV stroke volume failed to increase past a threshold, indicating pericardial constraint.13 A more extreme inverse phenomenon, possibly related to pericardial constraint, was seen in humans with heart failure as assessed by volume unloading with lower extremity suction.14 In that study, decreased RV filling led to higher LV stroke volume in those with severe chronic heart failure, but not normal subjects.14 We recognize that, in our study, higher RAP/PCWP was not associated with LV stroke volume and was associated with higher cardiac index in cross-sectional analyses but believe that the hypothesis that diastolic ventricular interaction might mediate the association of RAP/PCWP with impaired renal function remains a possibility, which warrants further exploration.
RAP/PCWP and mortality
In patients with symptomatic chronic heart failure, elevations in jugular venous pressure, a physical examination surrogate for RAP, were associated with worse outcomes.15 Similarly, an elevated PCWP has been shown to be a marker of increased risk.16-18 In the ESCAPE trial, the RAP/PCWP ratio was associated with the composite of death or hospitalization at 6 months in patients with ADHF Less is known about the long-term prognostic utility of the relative ratio of right- to left-sided ventricular filling pressures in heart failure. We now extend these findings by showing an independent association with mortality after longer term follow-up that persists despite adjustment for potential confounders including the RAP, BNP, and renal function. Such association may be partially explained by disproportionate RV dysfunction relative to LV dysfunction. Higher RAP/PCWP was related to increased right atria and RV area in ESCAPE5 and with lower right ventricular stroke work index in both ESCAPE and the current study. Echocardiographic and magnetic resonance imaging of impaired RV function correlate with adverse outcomes in chronic heart failure with reduced LVEF.19-21 However, the adverse prognostic utility of RAP/PCWP remained robust despite adjustment for RAP, suggesting that factors beyond RV dysfunction (eg, ventricular interaction) may be contributory.
Limitations
Our results must be interpreted in the context of several limitations in our study design. Because there were small changes in serum creatinine, we were likely underpowered to compare changes in renal function with treatment across groups. We cannot exclude the presence of selection bias for those undergoing evaluation and treatment of advanced ADHF at a tertiary care center. Because of the high severity of illness, this cohort is uniquely powered to compare mortality outcomes. Unlike ESCAPE,5 we found no stronger association of RAP/PCWP and mortality in those with a higher PCWP. There are 2 possible explanations: (1) our cohort was more congested in comparison to ESCAPE22 (PCWP 27 ± 7 and RAP 16 ± 6 mm Hg vs PCWP 25 ± 9 and RAP 14 ± 10 mm Hg, respectively), and (2) it is unclear to what extent that association was driven by hospitalizations, which were not included in our analysis. Yet, this cohort still has external validity because a majority of patients were taking evidence-based chronic heart failure therapies on admission. Because only 2 time points were analyzed, it is unknown whether intermediary transient hemodynamic changes correlate with renal function or outcomes. Furthermore, hemodynamics on subsequent admissions were not incorporated into the analysis limiting the ability to assess the long-term stability of RAP/PCWP. Correlations with RV function are limited by the inaccuracies of RV function assessment by echocardiography. The subjects' estimated metabolic rate was used in lieu of measured oxygen consumption to compute cardiac output by the Fick principle. However, any error introduced on this basis would be nondifferential across RAP/PCWP quartiles.
Conclusions
In patients with advanced ADHF, a higher RAP/PCWP was independently associated with both renal dysfunction and increased mortality. These results contribute to our understanding of cardiorenal interactions and further characterize a high-risk phenotype of heart failure.
Acknowledgments
Funding: Dr Tang is supported by National Institutes of Health grants R01HL103931 and P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06).
Footnotes
Conflict of interest: None declared.
References
- 1.Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18:1126–32. doi: 10.1016/s1053-2498(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant. 2012;31:67–72. doi: 10.1016/j.healun.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Drazner MH, Prasad A, Ayers C, et al. The relationship of right- and left-sided filling pressures in patients with heart failure and a preserved ejection fraction. Circ Heart Fail. 2010;3:202–6. doi: 10.1161/CIRCHEARTFAILURE.108.876649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drazner MH, Hellkamp AS, Leier CV, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1:170–7. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drazner MH, Velez-Martinez M, Ayers CR, et al. Relationship of right-to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail. 2013;6:264–70. doi: 10.1161/CIRCHEARTFAILURE.112.000204. [DOI] [PubMed] [Google Scholar]
- 6.Campbell P, Drazner MH, Kato M, et al. Mismatch of right- and left-sided filling pressures in chronic heart failure. J Card Fail. 2011;17:561–8. doi: 10.1016/j.cardfail.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail. 2013;15:433–40. doi: 10.1093/eurjhf/hfs209. [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Steimle AE, Stevenson LW, Chelimsky-Fallick C, et al. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation. 1997;96:1165–72. doi: 10.1161/01.cir.96.4.1165. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–8. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 12.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompen-sated heart failure. J Am Coll Cardiol. 2009;53:589–96. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Applegate RJ, Johnston WE, Vinten-Johansen J, et al. Restraining effect of intact pericardium during acute volume loading. Am J Physiol. 1992;262:H1725–33. doi: 10.1152/ajpheart.1992.262.6.H1725. [DOI] [PubMed] [Google Scholar]
- 14.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–4. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 15.Drazner MH, Rame JE, Stevenson LW, et al. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–81. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 16.Campana C, Gavazzi A, Berzuini C, et al. Predictors of prognosis in patients awaiting heart transplantation. J Heart Lung Transplant. 1993;12:756–65. [PubMed] [Google Scholar]
- 17.Keogh AM, Baron DW, Hickie JB. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am J Cardiol. 1990;65:903–8. doi: 10.1016/0002-9149(90)91434-8. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson LW, Tillisch JH, Hamilton M, et al. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66:1348–54. doi: 10.1016/0002-9149(90)91166-4. [DOI] [PubMed] [Google Scholar]
- 19.Kjaergaard J, Akkan D, Iversen KK, et al. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail. 2007;9:610–6. doi: 10.1016/j.ejheart.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Bourantas CV, Loh HP, Bragadeesh T, et al. Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. Eur J Heart Fail. 2011;13:52–60. doi: 10.1093/eurjhf/hfq161. [DOI] [PubMed] [Google Scholar]
- 21.Dini FL, Demmer RT, Simioniuc A, et al. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail. 2012;14:287–94. doi: 10.1093/eurjhf/hfr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–33. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]