Abstract
We show that chronic high fat diet (HFD) feeding affects the hypothalamus of male but not female mice. In our study we demonstrate that palmitic acid and sphingolipids accumulate in the central nervous system of HFD-fed males. Additionally, we show that HFD-feeding reduces proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) thus reducing estrogen receptor α (ERα) and driving hypothalamic inflammation in male but not female mice. Hypothalamic inflammation correlates with markers of metabolic dysregulation as indicated by dysregulation in glucose intolerance and myocardial function. Lastly, we demonstrate that there are blockages in mitophagy and lipophagy in hypothalamic tissues in males. Our data suggest there is a sexually dimorphic response to chronic HDF exposure, females; despite gaining the same amount of body weight following HFD-feeding, appear to be protected from the adverse metabolic effects of the HFD.
Keywords: Estrogen Receptor α, 17-β estradiol, Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1α, Palmitic Acid, Autophagy, Mitophagy, Lipophagy
Obesity and Inflammation
Obesity has become a major health problem worldwide, with the number of people who are either classified as overweight or obese people currently being higher than 2 billion[1]. Obesity is associated with and promotes an inflammatory state in a variety of tissues, namely liver, pancreas, adipose tissues, muscle and the central nervous system (CNS)[1]. An array of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6 are significantly increased in these tissues[2, 3]. The inflammatory state induced by excess consumption of nutrients/obesity is different when compared to ‘classical’ immune-mediated inflammatory pathways. In obesity, the inflammatory trigger is metabolic and emerges from metabolic cells, such as adipocytes or neurons. A host of signaling pathways in these cells, such as the kinases c-jun N-terminal kinase (JNK), inhibitor of κ kinase (IKK) and nuclear factor kappa B (NF-κB), have been shown to be activated upon nutrient overload. Further, specific activation of these pathways appears to be critical in inflammation-induced insulin resistance[1].
Within the CNS, the hypothalamus, a key regulatory site for mediating energy expenditure, food intake, and glucose homeostasis, becomes inflamed and insulin resistant following exposure to a high fat diet (HFD)[2, 3]. This condition promotes excessive feeding and body weight gain, leading to the onset of obesity and obesity-associated diseases.
Obesity affects both males and females; nonetheless, premenopausal females are generally protected from the development of obesity-associated metabolic complications when compared to males[4, 5]. Interestingly, this protection is lost in post-menopausal females and the incidence of obesity and obesity-associated diseases increases dramatically following menopause.
Inflammation is induced in the brain of male mice following HFD exposure
In our study, we analyzed the sexually dimorphic response to chronic exposure to HFD[6]. We exposed male and female wild-type C57BL/6 mice to a 42% HFD (TD.88137, Teklad Global Diets, Harlan, Indianapolis, USA), which was enriched in saturated fatty acids, for 4 weeks. As expected, mice became obese and, importantly the amount of body weight gained was not significantly different between the two sexes.
High levels of saturated fatty acids, and specifically palmitic acid (PA), have been shown to mediate insulin resistance in the hypothalamus[7]. We decided to evaluate, through mass spectrometry, the levels of this fatty acid in the CNS and we found it was significantly increased only in the brains of male mice fed on HFD. PA is also the precursor of sphingolipids (ceramides, glucosylceramides and sphingomyelins) and, consistently, these lipids were significantly increased in the hypothalamus of male animals fed with a HFD when compared to the females. Importantly, elevated levels of sphingolipids have previously been associated with reduced insulin signaling and increased inflammation within the CNS[8]. Indeed, higher levels of pro-inflammatory cytokines (Tumor Necrosis Factor α (TNFα), Interleukin 1β (IL1β) and IL6) were produced in the hypothalamus of HFD male but not female mice. Female animals fed the HFD, even though they significantly increased their body weight, did not show elevated levels of PA, sphingolipids nor pro-inflammatory cytokines in the CNS.
Importantly, hypothalamic inflammation in male mice was also associated with alterations in glucose homeostasis and reduced myocardial function, thus providing the physiological relevance of hypothalamic inflammation.
These results suggest that male and female mice differentially metabolize fatty acids acquired from the diet. The mechanism that underlies this difference is currently a topic of research in our laboratories.
ERαmodulates hypothalamic inflammation in neurons and astrocytes
As previously mentioned, our study showed that female mice, independent of body weight gain, are generally protected from the adverse metabolic effects of HFD. We hypothesized that estrogens/estrogen receptor α (ERα) might be responsible for these sexually dimorphic responses seen in mice following HFD-exposure.
Indeed, ERα knock-out mice are obese and glucose intolerant, confirming the importance of this protein in the control of body weight and glucose homeostasis[9]. Additionally, previous studies published by our laboratory and others, affirmed hypothalamic ERα is required to control food intake, body weight and glucose clearance[10, 11]. Further, men carrying deletion and/or point mutations in the gene coding for ERα are obese and glucose intolerant.
Importantly, the natural ligand for ERα, the steroid hormone 17-β estradiol (E2), has previously been shown to have an anti-inflammatory role in the brain[12].
Therefore, we sought to determine the effect of the HFD on hypothalamic ERα. Our results identified an inverse correlation between ERα and pro-inflammatory cytokine levels. In male mice following the HFD exposure, ERα transcript and protein levels were significantly decreased; this difference was not identified in females fed on the same diet.
To confirm the role of ERα in the modulation of inflammation we decided to use different in vitro models: the N43 hypothalamic cell line and primary neuronal cell cultures. Cells were treated with PA, the most abundant fatty acid in the diet used for our study, and which is increased in the brain of males following HFD exposure. PA treatment promoted inflammation in the cell line and primary neurons. Importantly, in primary neuronal cultures, following PA treatment, they show the sexually dimorphic response we identified in animals, specifically: neurons derived from male animals are significantly more inflamed than females following exposure to PA. Further, pre-treatment with E2 significantly inhibited the PA-induced inflammation only when ERα was expressed in the cells, suggesting that ERα was required for the E2 anti-inflammatory effect. To confirm the key role of ERα in the modulation of the inflammatory response we showed that viral overexpression of ERα was sufficient to significantly inhibit the pro-inflammatory effect of PA-treatment. These data suggest that ERα is necessary and sufficient to modulate the fatty acid-induced inflammatory response.
Up to now we had only considered neurons in our study, nonetheless, more than 50% of the cells in the CNS are non-neuronal. Microglia and astrocytes have both been shown to accumulate in the hypothalamus of animals during chronic HFD consumption[2]. To this end, our data showed that microglial cells in vivo (CX3CR1GFP/GFP mice) and in vitro (BV2 cells) did not express ERα. Consistently, in vitro, in BV2 cells, pretreatment with E2 did not inhibit the PA-induced inflammation, confirming that ERα is required to mediate the E2-mediated anti-inflammatory effect.
Conversely, astrocytes express ERα and PA treatment promoted activation and inflammation of primary astrocytic cell cultures together with loss of ERα. Further, E2 pretreatment inhibited PA-induced inflammation in astrocytes. Importantly, also in primary astrocytic culture, the response to PA was sexually dimorphic, with primary astrocytes from males being significantly more inflamed than females. Consistently, in vivo, astrogliosis was identified only in the hypothalamus of male mice following chronic HFD-exposure. These data suggest that astrocytes, together with neurons, are responsible for the sexually dimorphic responses we found in vivo.
PGC-1α regulates ERα in the hypothalamus
To identify a potential mechanism responsible for the HFD-induced decrease in ERα, we focused on the role of the transcriptional coactivator Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1 alpha (PGC-1α), a known factor in mediating metabolic pathways[13]. Previous studies showed that PGC-1α regulates ERα transcriptional activity in vitro[14, 15]. We confirmed that this occurs also in vivo, specifically in the hypothalamus where the two proteins are co-localized.
Chronic HFD feeding decreased PGC-1α in the hypothalamus of male mice leading to ERα loss and promotion of inflammation, whereas this did not occur in females. Therefore, our results suggest that both, PGC-1α and ERα are part of a hypothalamic-signaling network, involved in the inflammatory response to HFD.
Even though both, PGC-1α and ERα were decreased in HFD conditions, thus leading to hypothalamic inflammation, we determined that ERα is the critical player in the anti-inflammatory response. ERα overexpression, in the condition of PGC-1α depletion, was sufficient to significantly inhibit the PA induced inflammation. Nonetheless, ERα overexpression per se did not blunt PA induced inflammation thus suggesting that other proteins are involved in this pathway.
Autophagy is dysfunctional in the hypothalamus of HFD-fed male mice
Exposure of neurons and astrocytes to nutrient excess represents a significant stress for these cells. Thus, to limit cellular damage, cells stimulate adaptive mechanisms such as autophagy. Autophagy is a catabolic process involving the degradation of the cell`s own components[16]. A cytosolic cargo is sequestered within a double membrane vesicle called autophagosome and then delivered to lysosomes for degradation. The cargo can be formed by cytoplasmic proteins or organelles, such as mitochondria – mitophagy – or lipid droplets–lipophagy[16]. The autophagic process helps to maintain a balance between synthesis, degradation, and recycling of cellular components and therefore it is important for the maintenance of cellular function and growth. As previously mentioned, autophagy represents an important response to cellular stress, such as endoplasmic reticulum stress or oxidative stress, which are induced when eating a HFD[17]. However, when the cellular stressors continue over long periods, such as following chronic exposure to a HFD, an autophagy defect occurs, which inhibits the capacity of the cells to remove the damage. Diminished autophagy results in increased inflammation, obesity and obesity-associated diseases[16, 18].
Importantly, new data from our and other laboratories have shown that inhibition of the autophagic flux occurs in the hypothalamus following chronic HFD exposure (Figure 1)[19, 20]. We are currently focusing our studies on organelle-selective autophagy specifically, mitophagy and lipophagy, and how these processes are modified in HFD-obese mice. Following chronic HFD exposure, our data demonstrate the number of mitochondria degraded through the autophagic pathway is decreased in the hypothalamus (Figure 2 a, b), and again this occurs only in the males and not in the females. These findings suggest in males there is increased oxidative stress and accumulation of dysfunctional/toxic mitochondria, which may facilitate increased hypothalamic inflammation following chronic exposure to the HFD. The molecular mechanism that regulates this pathway is still unknown and is currently being studied in our laboratory.
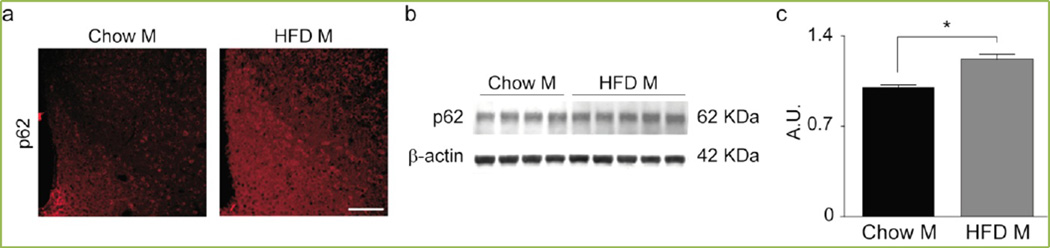
Figure 1. HFD inhibits the autophagic flux in the hypothalamus of male mice.
(a) Representative confocal images of the ARC of male mice exposed to chow or HFD for 16 weeks. Scale bar, 125 µm. (b–c) Representative immunoblot (b) and quantification (c) of p62 in the hypothalamus. Chow M, n = 4; HFD M, n = 5.Data are presented as mean ± SEM. *p < 0.05.
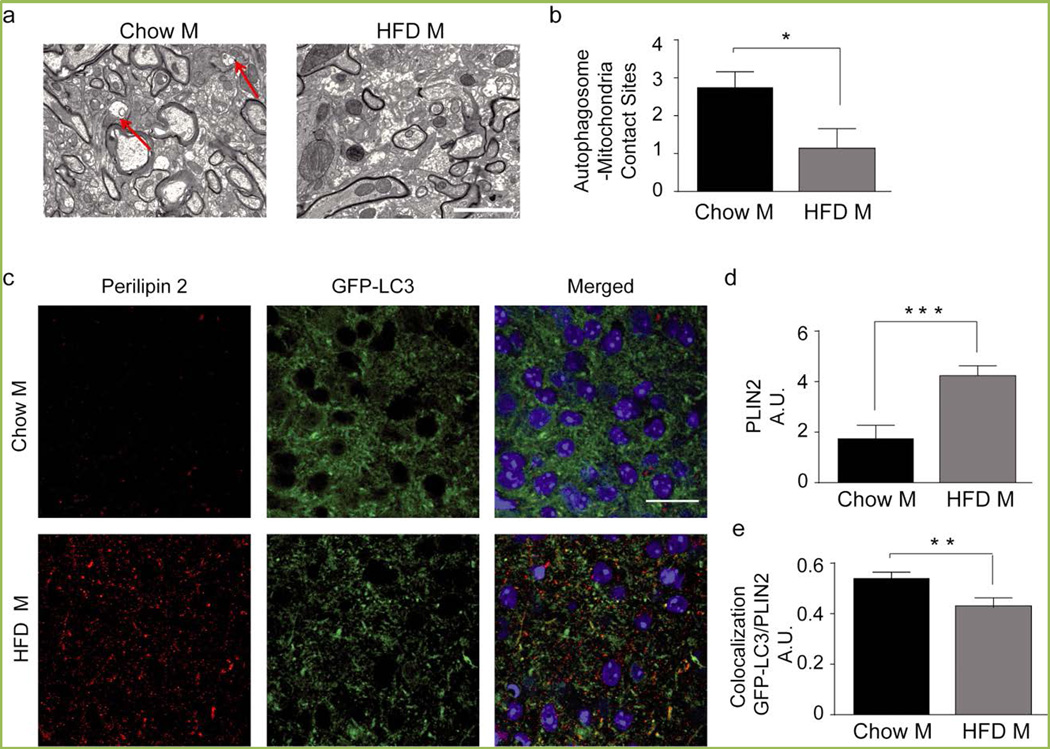
Figure 2. Chronic HFD leads to dysfunctional mitophagy and lipophagy in the hypothalamus of male mice.
(a) Representative electron microscopy images of the hypothalamic region of male mice fed chow or 42% HFD for 16 weeks. Red arrows indicate mitochondria enclosed in autophagic vesicles. Scale bar, 2000 nm. (b) Quantification of autophagosome-mitochondria contact sites. (c) Representative confocal images (c) and quantification (d) showing PLIN2immunoreactivity in the ARC of GFP-LC3 male mice following chow or HFD for 16 weeks. Scale bar, 25 µm. (e) Colocalization analysis of PLIN2 and autophagic puncta in the ARC.n=4/group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001.
The second organelle-specific form of autophagy that is a focus of our research is the lipophagic pathway. In the liver, long-term HFD exposure reduces lipophagy leading to lower β-oxidation rates and lipid accumulation in the cytoplasm of the hepatocytes[21]. Our data demonstrate that this also occurs in the hypothalamus of HFD exposed mice, as evidenced by perilipin-2 (PLIN2) staining, a protein expressed in the coat of the lipid droplets, which is significantly increased following HFD-feeding in male mice, suggesting that lipids are accumulated in this brain region (Figure 2 c, d). Interestingly, the fusion between lipid droplets and autophagosomes, as quantified by colocalization between PLIN2 and the marker of autophagosomes green fluorescent protein –microtubule-associated protein 1A/1B-light chain 3 (GFP-LC3), is reduced following HFD exposure, thus suggesting that the number of lipid droplets degraded by lipophagy is decreased in HFD-fed animals (Figure 2 c, e). Why lipid droplets are not delivered/recognized by autophagosomes in these conditions is still unknown and it is currently a topic of research in our laboratories.
Conclusion
Our data demonstrate how differently males and females respond when chronically fed a HFD. We have shown that PA and sphingolipids are increased in the hypothalamus of male mice and this promotes an inflammatory response, induced by a decrease in the PGC-1α/ERα pathway. From a physiological point of view, this inflammatory response correlates with metabolic dysfunction such as glucose intolerance and reduced myocardial activity, which characterize obesity-associated diseases (Figure 3).
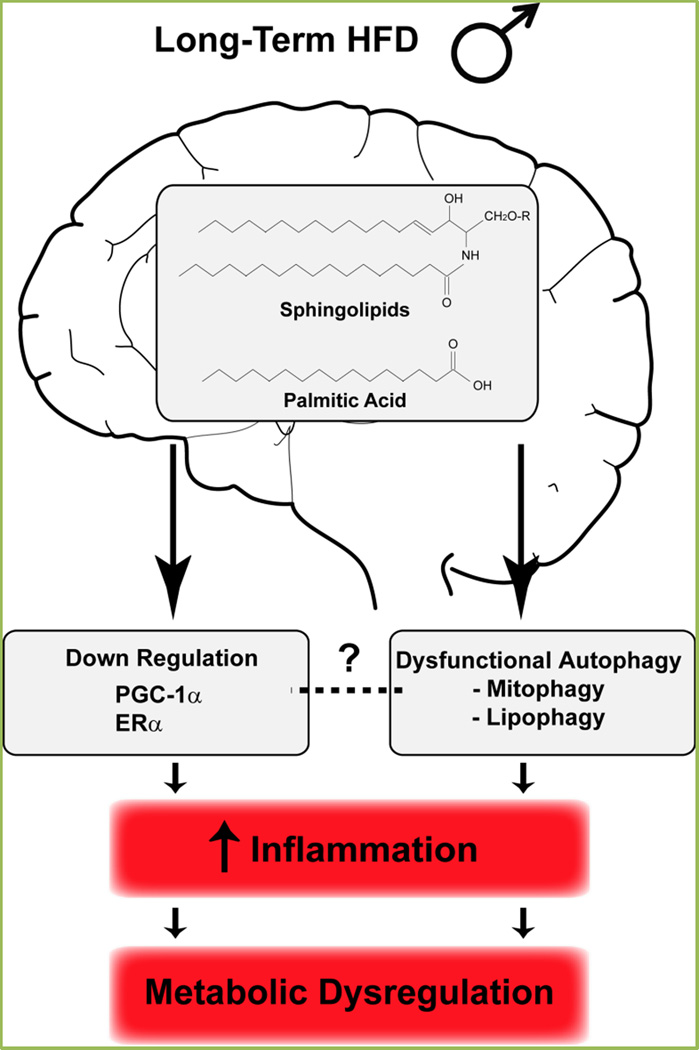
Figure 3. Long-term consumption of HFD leads to metabolic imbalance in male mice.
Our study demonstrates that the response to chronic HFD is sexually dimorphic. While females are generally protected from the adverse effects of HFD, males show hypothalamic inflammation, which correlates with signs of metabolic dysfunction, namely glucose intolerance and decreased cardiac activity. The molecular basis for this sexually dimorphic hypothalamic inflammation implicates the PGC-1α/ERα pathway. Our laboratories are currently evaluating the mechanisms and the role of dysregulated autophagy, which is seen in the hypothalamus of chronic fed males, leading to hypothalamic inflammation and obesity-associated diseases. See the main text for further details.
Recent studies, together with our data, have identified a dysregulation in the autophagic response in the hypothalamus following chronic HFD exposure. Whole cell autophagy and organelle-specific autophagy (mitophagy and lipophagy) appear to be reduced. Importantly, inhibited autophagic flux has been shown to enhance the inflammatory response driving to metabolic imbalance and further promoting obesity (Figure 3). Even though different mechanisms demonstrating the impact of autophagy on energy balance have been shown, the molecular mechanisms that underline the blockage in the autophagic flux following long-term HFD exposure have not been elucidated. Further, it is still unknown whether PGC-1α and ERα loss might affect hypothalamic autophagy.
The answers to these questions will lead to the development of new and effective sex-based treatments for the prevention of HFD/obesity-induced metabolic diseases.
Acknowledgments
DJC is supported by NIH/NIDDK P01 088761-01 and the Klarman Foundation for Eating Disorders. AC is supported by FONDECYT Regular grant 1140908 and Advanced Center for Chronic Diseases (ACCDiS) grant 15130011. CNR is supported by Clayton Foundation for Research and National Institutes of Health grant HL20948.
Footnotes
The authors declare that there are no conflicts of interests.
References
- 1.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 2.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 4.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiyama MG, Agellon LB. Sex differences in lipid metabolism and metabolic disease risk. Biochem Cell Biol. 2012;90:124–141. doi: 10.1139/o11-067. [DOI] [PubMed] [Google Scholar]
- 6.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, et al. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep. 2014;9:633–645. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 14.Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP. Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem. 2000;275:16302–16308. doi: 10.1074/jbc.M001364200. [DOI] [PubMed] [Google Scholar]
- 15.Bourdoncle A, Labesse G, Margueron R, Castet A, Cavailles V, Royer CA. The nuclear receptor coactivator PGC-1alpha exhibits modes of interaction with the estrogen receptor distinct from those of SRC-1. J Mol Biol. 2005;347:921–934. doi: 10.1016/j.jmb.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71:521–533. doi: 10.1017/S002966511200078X. [DOI] [PubMed] [Google Scholar]
- 18.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignacio-Souza LM, Bombassaro B, Pascoal LB, Portovedo MA, Razolli DS, Coope A, et al. Defective regulation of the ubiquitin/proteasome system in the hypothalamus of obese male mice. Endocrinology. 2014;155:2831–2844. doi: 10.1210/en.2014-1090. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]