Abstract
Previous studies have shown an association between cervical cancer screening and racial/ethnic minority status, no usual source of care, and lower socioeconomic status. This study describes the demographics and health beliefs of women who report never being screened for cervical cancer by area of residence. Data from the 2010 Behavioral Risk Factor Surveillance System were used to study women aged 21–65 years who reported never being screened for cervical cancer. Multivariate logistic regression modeling was used to calculate predicted marginals to examine associations between never being screened and demographic characteristics and health belief model (HBM) constructs by metropolitan statistical area (MSA). After adjusting for all demographics and HBM constructs, prevalence of never being screened was higher for the following women: non-Hispanic Asians/Native Hawaiians/Pacific Islanders (16.5 %, 95 % CI = 13.7 %, 19.8 %) who live in MSAs; those with only a high school diploma who live in MSAs (5.5 %, 95 % CI = 4.7 %, 6.5 %); those living in non-MSAs who reported “fair or poor” general health (4.1 %, 95 % CI = 3.1 %, 5.4 %); and those living in either MSAs and non-MSAs unable to see a doctor within the past 12 months because of cost (MSA: 4.4 %, 95 % CI = 4.0 %, 4.8 %; non-MSA: 3.4 %, 95 % CI = 2.9 %, 3.9 %). The Affordable Care Act will expand access to insurance coverage for cervical cancer screening, without cost sharing for millions of women, essentially eliminating insurance costs as a barrier. Future interventions for women who have never been screened should focus on promoting the importance of screening and reaching non-Hispanic Asians/Native Hawaiians/Pacific Islanders who live in MSAs.
Keywords: Cervical cancer screening, Never screened, Health disparities, Metropolitan area, Health belief model
Introduction
In recent decades, the incidence of cervical cancer has declined because of the use of Pap tests [1, 2]. Pap tests can detect precancerous lesions that can be removed before they become cancer and detect cervical cancer early when treatment is more effective. Despite the reductions in incidence and death rates for cervical cancer, women who are members of some racial and ethnic minority groups and women without a usual source of health care continue to be diagnosed with and die of cervical cancer [3, 4]. Those at highest risk for the worst health outcomes are those who are never screened for cervical cancer.
Chen et al. [5] found that women who reported never being screened for cervical cancer were younger, single, of Hispanic ethnicity, uninsured, had less than a high school diploma, and low income (<$15,000 annual income). Studies that have examined characteristics of women who have never been screened for cervical cancer by area of residence have analyzed data from individual states or from urban versus rural counties and focused on specific racial and ethnic groups [6-11]. If researchers can identify the barriers that prevent women from accessing cervical cancer screening in specific areas (urban or rural), they may be able to better understand how access to resources can influence participation in cancer screenings [6-12]. Researchers also need to know which women live in urban and rural areas so they can identify who is in the most need of assistance and how best to reach them.
Researchers use the health belief model (HBM) to examine how health beliefs may influence people’s decisions about seeking cancer screening [13, 14]. The HBM identifies constructs that influence behavior and measures people’s willingness to engage in certain health behaviors [15]. For example, women may participate in cervical cancer screening if they believe they are at risk of health problems if they are not screened, recognize the benefits of cervical cancer screening, and have few barriers that impede on their ability to be screened. Women may also be more likely to participate in cervical cancer screening if they receive cues that encourage them to be screened [16].
Purpose
The purpose of this study is to describe the demographic characteristics, and health beliefs of women who have never received cervical cancer screenings by metropolitan area.
Methods
This study used data from the 2010 Behavioral Risk Factor Surveillance System (BRFSS), a cross-sectional, random-digit-dialed telephone survey that collects health-related information from non-institutionalized adults aged C18 years from the United States, including those in the territories of Guam, Puerto Rico, and the U.S. Virgin Islands. The response rate for 2010 was 54.6 % [17, 18]. Overall, 280,961 women in the United States and U.S. territories participated in the 2010 BRFSS survey [18].
This analysis excluded the following: (1) women from U.S. territories because data on area of residence was missing (n = 3,966), (2) women who did not need cervical cancer screening according to U.S. Preventive Services Task Force (USPSTF) [19] recommendations because of their age (n = 96,541), (3) women who reported having a hysterectomy (n = 46,035), and (4) women who had missing Pap test data (n = 195). This analysis included 134,224 female respondents aged 21–65 years (Fig. 1). The screening outcome for this study was whether women reported never being screened for cervical cancer with a Pap test versus ever being screened.
Fig. 1.
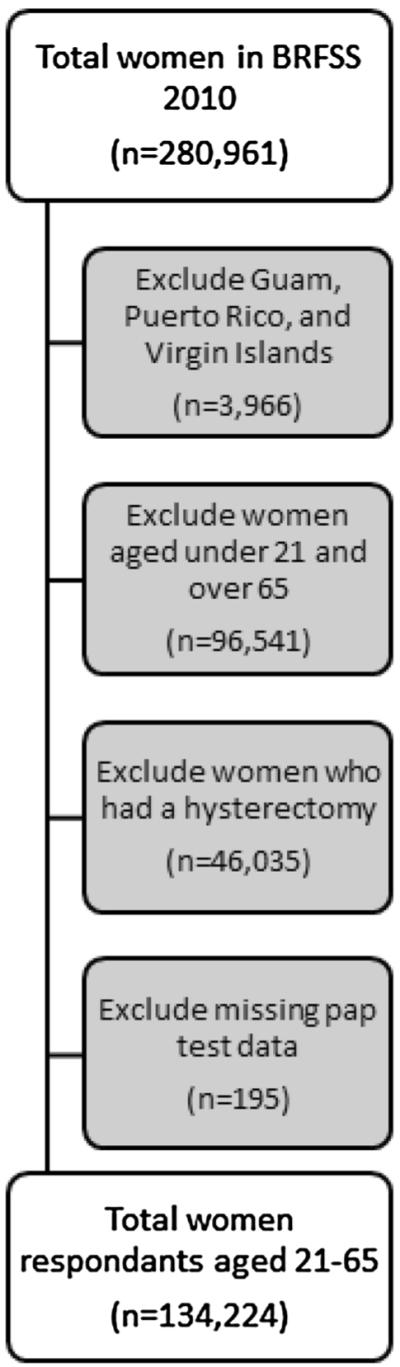
Study population as a subset of 2010 Behavioral Risk Factor Surveillance System data
To create a descriptive analysis, the data were stratified by whether respondents lived in a metropolitan statistical area (MSA) or non-MSA. An MSA is a geographical region with a relatively high population density at its core and close economic ties throughout the area. For this analysis, MSAs were defined as including the area (1) in the center city of an MSA and (2) outside the center city of an MSA but inside the center city. Non-MSAs were defined as including the area (1) inside a suburban county of the MSA, (2) in an MSA that has no center city, and (3) not in an MSA [7].
Demographic characteristics examined in this analysis included age (21–44; 45–65); race/ethnicity (white, non-Hispanic; black, non-Hispanic; Asian/Native Hawaiian/Pacific Islander, non-Hispanic; American Indian/Alaska Native, non-Hispanic; other race/multiracial, non-Hispanic; and Hispanic) and educational attainment (did not graduate high school, graduated high school, attended college or technical school, and graduated from college or technical school).
We used the HBM as a theoretical framework for analysis. Constructs examined included perceived susceptibility (a person’s belief that they can get an illness), perceived barriers (a person’s assessment of factors that prevents them from participating in health-promoting behaviors), cues to action (a person’s readiness to begin participation in a health behavior), and self-efficacy (a person’s ability to understand and engage in a health behavior on his or her own) [15]. These constructs were matched with the appropriate BRFSS survey variables [20]. Two HBM constructs, perceived severity and perceived benefits, could not be analyzed because no BRFSS survey questions addressed them.
Statistical Analysis
SAS version 9.2 with SAS-callable SUDAAN version 10.0.1 was used to account for the BRFSS’s complex sampling design. Descriptive analyses were stratified by MSA and non-MSA. Predicted marginals were used to assess associations between cervical cancer screening behavior (e.g., ever screened versus never screened) by demographic characteristics and HBM constructs. A logistic regression analysis was used to produce adjusted percentages (predicted marginals) to achieve a standardized weighted average for each level of the health variable of interest [20]. This method allows for comparison between the two cancer screening behaviors as if they had the same demographic and HBM characteristics. Separate models were created that used each health variable as the dependent variable and controlled for age, race/ethnicity, sex, and education level. Multinomial logistic regression was used if the categorical dependent variable of interest had more than two levels. p values were calculated by using the Wald F test (p < 0.05) [21].
Results
Table 1 shows that 4.4 % of women living in MSAs and 3.1 % of women living in non-MSAs had never been screened for cervical cancer. In both MSAs and non-MSAs, the majority of women were white, non-Hispanic (61.6 and 80.1 %, respectively) and aged 21–44 years (61.0 and 60.9 %, respectively). A higher proportion of women living in MSAs had graduated from college or technical school than women living in non-MSAs (43.8 and 37.1 %, respectively).
Table 1.
Demographic characteristics of women aged 21–65a years, by area of residencec, Behavioral Risk Factor Surveillance System (BRFSS), United States, 2010bd
| Characteristic | MSAb |
Non-MSAb |
||
|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | |
| Total | 74,266 | 100.0 | 59,958 | 100.0 |
| Screening status | ||||
| Ever screened | 72,158 | 95.6 (95.2–95.9) | 58,514 | 96.9 (96.5–97.2) |
| Never screened | 2,108 | 4.4 (4.1–4.8) | 1,444 | 3.1 (2.8–3.5) |
| Age group | ||||
| 21–44 | 31,413 | 61.0 (60.4–61.6) | 24,013 | 60.9 (60.1–61.5) |
| 45–65 | 42,986 | 39.0 (38.4–39.6) | 36,007 | 39.2 (38.5–39.9) |
| Race/ethnicity | ||||
| White, non-Hispanic | 52,767 | 61.6 (60.9–62.2) | 48,592 | 80.1 (79.4–80.8) |
| Black, non-Hispanic | 8,443 | 12.2 (11.8–12.6) | 4,011 | 8.2 (7.7–8.6) |
| Asian/Native Hawaiian/Pacific Islander, non-Hispanic | 2,263 | 4.9 (4.5–5.2) | 818 | 1.9 (1.7–2.2) |
| American Indian/Alaska Native, non-Hispanic | 587 | 0.7 (0.6–0.8) | 1,430 | 1.2 (1.0–1.3) |
| Other Race/Multiracial, non-Hispanic | 1,738 | 2.3 (2.1–2.5) | 1,297 | 1.9 (1.7–2.1) |
| Hispanic | 7,933 | 18.4 (17.8–19.0) | 3,417 | 6.7 (6.3–7.2) |
| Education | ||||
| Did not graduate high school | 4,995 | 9.0 (8.6–9.4) | 4,070 | 6.7 (6.2–7.1) |
| Graduated high school | 15,781 | 20.6 (20.1–21.1) | 16,910 | 27.1 (26.4–27.8) |
| Attended college or technical school | 20,069 | 26.5 (26.0–27.1) | 17,758 | 29.1 (28.4–29.8) |
| Graduated from college or technical school | 33,441 | 43.8 (43.2–44.5) | 21,237 | 37.1 (36.4–37.9) |
| Health belief model | ||||
| Perceived susceptibility | ||||
| Self-reported general health | ||||
| Excellent, very good, or good | 63,887 | 86.8 (86.4–87.3) | 51,051 | 87.4 (86.9–87.9) |
| Fair or poor | 10,317 | 13.2 (12.7–13.6) | 8,848 | 12.6 (12.1–13.1) |
| Are you a current smoker | ||||
| Yes | 62,095 | 85.2 (84.8–85.6) | 48,131 | 80.3 (79.6–80.9) |
| No | 11,961 | 14.8 (14.4–15.2) | 11,689 | 19.7 (19.1–20.4) |
| Perceived barriers | ||||
| Any health coverage | ||||
| Yes | 64,314 | 83.5 (83.0–84.0) | 49,964 | 82.2 (81.5–82.8) |
| No | 9,988 | 16.5 (16.0–17.0) | 9,969 | 17.8 (17.2–18.5) |
| Couldn’t see a doctor within past 12 months because of cost | ||||
| Yes | 12,053 | 18.9 (18.3–19.4) | 10,708 | 19.2 (18.5–19.8) |
| No | 62,205 | 81.1 (80.6–81.7) | 49,207 | 80.8 (80.2–81.5) |
| Cues to action | ||||
| Personal doctor/health care provider | ||||
| Yes, only one | 58,427 | 74.9 (74.3–75.4) | 46,745 | 77.8 (77.1–78.5) |
| More than one | 5,672 | 7.7 (7.4–8.0) | 4,818 | 7.2 (6.8–7.5) |
| No | 10,155 | 17.4 (16.9–18.0) | 8,350 | 15.0 (14.4–15.7) |
| Last routine checkup | ||||
| Within past year | 52,740 | 68.2 (67.6–68.8) | 40,248 | 67.0 (66.2–67.7) |
| Within past 2 years | 10,284 | 15.7 (15.2–16.1) | 8,297 | 14.5 (14.0–15.1) |
| Within past 5 years | 5,635 | 8.8 (8.4–9.2) | 5,077 | 9.0 (8.6–9.5) |
| 5 or more years ago | 4,561 | 6.4 (6.1–6.7) | 5,128 | 8.5 (8.0–8.9) |
| Never | 621 | 1.0 (0.9–1.1) | 650 | 1.0 (0.9–1.2) |
| Self-Efficacy | ||||
| Frequency of social and emotional support | ||||
| Always | 34,311 | 48.3 (47.7–49.0) | 28,869 | 50.7 (49.9–51.5) |
| Usually | 25,035 | 32.9 (32.4–33.5) | 19,649 | 32.5 (31.8–33.3) |
| Sometimes | 9,075 | 12.1 (11.7–12.5) | 6,824 | 10.9 (10.4–11.5) |
| Rarely | 2,239 | 3.0 (2.8–3.2) | 1,964 | 3.2 (2.9–3.5) |
| Never | 2,350 | 3.6 (3.4–3.9) | 1,762 | 2.6 (2.4–2.9) |
| Satisfaction with life | ||||
| Very satisfied/Satisfied | 68,649 | 94.5 (94.2–94.8) | 55,930 | 94.8 (94.4–95.2) |
| Dissatisfied/Very dissatisfied | 4,462 | 5.5 (5.2–5.8) | 3,204 | 5.2 (4.8–5.6) |
Based on the USPSTF recommendations for cervical cancer, screening takes place between ages 21–65. Women with incomplete pap test data (n = 195) are excluded from analyses. Additionally, women reporting hysterectomy and missing hysterectomy status (n = 46,035) are excluded from analyses
Metropolitan is defined as BRFSS MSCODE = 1 and 2; Non-metropolitan is defined as BRFSS MSCODE = 3,4, and 5
Excluding territories: Guam, Puerto Rico, and the Virgin Islands (n = 3,966 women 21–65 with no hysterectomy and complete pap test data)
Data are age-standardized to the 2010 BRFSS population (women aged 21–65)
Table 2 shows the prevalence of women by MSA and cervical cancer screening status after adjusting for all demographic characteristics and HBM constructs. More women who reported never being screened for cervical cancer lived in MSAs (4.1 %, 95 % CI = 3.8, 4.5) than in non-MSAs (2.9 %, 95 % CI = 2.6, 3.3). The prevalence of never being screened was highest among Asian/Native Hawaiian/Pacific Islander, non-Hispanics regardless of MSA status (MSAs: 16.5 %, 95 % CI = 13.7, 19.8; non-MSA: 10.0 %, 95 % CI = 6.7, 14.6). The women living in MSAs reported never being screened varied by education level (p = 0.000), whereas women living in non-MSAs did not (p = 0.6600).
Table 2.
Predicted marginals for womena who reported ever or never being screened for cervical cancer, by area of residencec, Behavioral Risk Factor Surveillance System (BRFSS), United States, 2010bd
| Characteristics | MSAc |
Non-MSAc |
||||
|---|---|---|---|---|---|---|
| Ever Screened | Never Screened |
Ever Screened | Never Screened |
|||
| % (95% CI) | % (95% CI) | p valuee | % (95% CI) | % (95% CI) | p valuee | |
| Total | 95.9 (95.5–96.2) | 4.1 (3.8–4.5) | 97.1 (96.7–97.4) | 2.9 (2.6–3.3) | ||
| Demographic characteristics | ||||||
| Age group | ||||||
| 21–44 | 94.9 (94.4–95.3) | 5.1 (4.7–5.6) | 0.0000 | 96.6 (96.1–97.0) | 3.4 (3.0–3.9) | 0.0000 |
| 45–65 | 97.8 (97.5–98.1) | 2.2 (1.9–2.5) | 98.0 (97.6–98.3) | 2.0 (1.7–2.4) | ||
| Race/ethnicity | 0.0000 | 0.0000 | ||||
| White, non-Hispanic | 97.2 (96.8–97.5) | 2.8 (2.5–3.2) | 97.6 (97.3–97.9) | 2.4 (2.1–2.7) | ||
| Black, non-Hispanic | 95.6 (94.6–96.5) | 4.4 (3.5–5.4) | 96.1 (95.1–97.0) | 3.9 (3.0–4.9) | ||
| Asian/Native Hawaiian/Pacific Islander, non-Hispanic |
83.5 (80.2–86.3) | 16.5 (13.7–19.8) | 90.0 (85.4–93.3) | 10.0 (6.7–14.6) | ||
| American Indian/Alaska Native, non-Hispanic |
94.7 (89.8–97.4) | 5.3 (2.6–10.2) | 96.6 (93.7–98.3) | 3.4 (1.7–6.3) | ||
| Other Race/Multiracial, non-Hispanic | 93.9 (90.9–96.0) | 6.1 (4.0–9.1) | 95.6 (92.9–97.3) | 4.4 (2.7–7.1) | ||
| Hispanic | 96.0 (95.1–96.7) | 4.0 (3.3–4.9) | 95.7 (93.6–97.1) | 4.3 (2.9–6.4) | ||
| Education | 0.0000 | 0.6600 | ||||
| Did not graduate high school | 95.7 (94.6–96.6) | 4.3 (3.4–5.4) | 96.8 (95.6–97.6) | 3.2 (2.4–4.4) | ||
| Graduated high school | 94.5 (93.5–95.3) | 5.5 (4.7–6.5) | 97.0 (96.4–97.6) | 3.0 (2.4–3.6) | ||
| Attended college or technical school | 95.6 (94.8–96.2) | 4.4 (3.8–5.2) | 96.9 (96.1–97.5) | 3.1 (2.5–3.9) | ||
| Graduated from college or technical school | 96.8 (96.3–97.2) | 3.2 (2.8–3.7) | 97.4 (96.7–98.0) | 2.6 (2.0–3.3) | ||
| Health belief model | ||||||
| Perceived Susceptibility | ||||||
| Self-reported general health | 0.0609 | 0.0091 | ||||
| Excellent, very good, or good | 95.8 (95.4–96.1) | 4.2 (3.9–4.6) | 97.3 (96.9–97.6) | 2.7 (2.4–3.1) | ||
| Fair or poor | 96.5 (95.8–97.1) | 3.5 (2.9–4.2) | 95.9 (94.6–96.9) | 4.1 (3.1–5.4) | ||
| Are you a current smoker | 0.0002 | 0.0740 | ||||
| Yes | 95.6 (95.3–96.0) | 4.4 (4.0–4.7) | 96.9 (96.5–97.3) | 3.1 (2.7–3.5) | ||
| No | 97.1 (96.5–97.7) | 2.9 (2.3–3.5) | 97.6 (96.9–98.1) | 2.4 (1.9–3.1) | ||
| Perceived barriers | ||||||
| Any health coverage | 0.0000 | 0.0000 | ||||
| Yes | 96.5 (96.1–96.8) | 3.5 (3.2–3.9) | 97.6 (97.3–97.9) | 2.4 (2.1–2.7) | ||
| No | 94.0 (92.9–94.9) | 6.0 (5.1–7.1) | 95.6 (94.5–96.5) | 4.4 (3.5–5.5) | ||
| Couldn’t see a doctor within past 12 months because of cost |
0.0151 | 0.0031 | ||||
| Yes | 96.6 (95.9–97.1) | 3.4 (2.9–4.1) | 97.9 (97.3–98.4) | 2.1 (1.6–2.7) | ||
| No | 95.6 (95.2–96.0) | 4.4 (4.0–4.8) | 96.6 (96.1–97.1) | 3.4 (2.9–3.9) | ||
| Cues to action | ||||||
| Personal doctor/health care provider | 0.0032 | 0.0004 | ||||
| Yes, only one | 96.3 (95.9–96.7) | 3.7 (3.3–4.1) | 97.4 (97.0–97.8) | 2.6 (2.2–3.0) | ||
| More than one | 96.3 (95.0–97.3) | 3.7 (2.7–5.0) | 98.1 (97.1–98.7) | 1.9 (1.3–2.9) | ||
| No | 94.8 (93.9–95.5) | 5.2 (4.5–6.1) | 95.9 (95.0–96.7) | 4.1 (3.3–5.0) | ||
| Last routine checkup | 0.0000 | 0.0000 | ||||
| Within past year | 96.8 (96.4–97.2) | 3.2 (2.8–3.6) | 98.0 (97.6–98.3) | 2.0 (1.7–2.4) | ||
| Within past 2 years | 95.4 (94.4–96.2) | 4.6 (3.8–5.6) | 97.2 (96.2–97.9) | 2.8 (2.1–3.8) | ||
| Within past 5 years | 94.1 (92.8–95.1) | 5.9 (4.9–7.2) | 95.4 (93.7–96.7) | 4.6 (3.3–6.3) | ||
| 5 or more years ago | 92.4 (90.7–93.8) | 7.6 (6.2–9.3) | 93.7 (92.0–95.1) | 6.3 (4.9–8.0) | ||
| Never | 92.2 (88.4–94.9) | 7.8 (5.1–11.6) | 93.5 (86.1–97.1) | 6.5 (2.9–13.9) | ||
| Self-efficacy | ||||||
| Frequency of social and emotional support | 0.0000 | 0.0001 | ||||
| Always | 96.1 (95.6–96.5) | 3.9 (3.5–4.4) | 97.1 (96.6–97.5) | 2.9 (2.5–3.4) | ||
| Usually | 96.3 (95.7–96.8) | 3.7 (3.2–4.3) | 97.8 (97.2–98.2) | 2.2 (1.8–2.8) | ||
| Sometimes | 95.6 (94.6–96.4) | 4.4 (3.6–5.4) | 96.6 (95.4–97.5) | 3.4 (2.5–4.6) | ||
| Rarely | 96.3 (94.4–97.6) | 3.7 (2.4–5.6) | 97.1 (95.7–98.1) | 2.9 (1.9–4.3) | ||
| Never | 92.0 (89.8–93.7) | 8.0 (6.3–10.2) | 93.1 (90.0–95.2) | 6.9 (4.8–10.0) | ||
| Satisfaction with life | 0.6482 | 0.7848 | ||||
| Very satisfied/Satisfied | 95.9 (95.6–96.2) | 4.1 (3.8–4.4) | 97.1 (96.7–97.4) | 2.9 (2.6–3.3) | ||
| Dissatisfied/Very dissatisfied | 95.6 (94.2–96.7) | 4.4 (3.3–5.8) | 96.9 (95.3–98.0) | 3.1 (2.0–4.7) | ||
Based on the USPSTF recommendations for cervical cancer, screening takes place between ages 21–65. Women with incomplete pap test data (n = 195) are excluded from analyses. Additionally, women reporting hysterectomy and missing hysterectomy status (n = 46,035) are excluded from analyses
Metropolitan is defined as BRFSS MSCODE = 1 and 2; Non-metropolitan is defined as BRFSS MSCODE = 3,4, and 5
Excluding territories: Guam, Puerto Rico, and the Virgin Islands (n = 3,966 women 21–65 with no hysterectomy and complete pap test data)
Data are age-standardized to the 2010 BRFSS population (women aged 21–65)
p values test difference within demographic and HBM construct groups
This study used two BRFSS variables to analyze the HBM construct of perceived susceptibility: general health and current smoking (Table 2). For women living in non-MSAs, a higher proportion who reported never being screened for cervical cancer reported “fair or poor” general health (4.1 %, 95 % CI = 3.1, 5.4), whereas women living in MSAs did not vary by health status. Among women living in MSAs who were current smokers, 4.4 % (95 % CI = 4.0, 4.7) reported never being screened.
For the HBM construct of perceived barriers, two variables were analyzed: health coverage and lack of access to a physician due to cost (Table 2). Regardless of the MSA status, more women who reported no health coverage (MSAs: 6.0 %, 95 % CI = 5.1, 7.1; non-MSAs: 4.4 %, 95 % CI = 3.5, 5.5) and costs prevented them from visiting a doctor within the past 12 months (MSAs: 4.4 %, 95 % CI = 4.0, 4.8; non-MSAs: 3.4 %, 95 % CI = 2.9, 3.9) also reported never being screened. The two HBM constructs analyzed for cues to action were: having a personal doctor or health care provider and last routine checkup. Regardless of MSA status, women were more likely to report never being screening if they reported not having a personal health care provider (MSAs: 5.2 %, 95 % CI = 4.5, 6.1; non-MSAs: 4.1 %, 95 % CI = 3.3, 5.0) and never having a routine checkup (MSAs: 7.8 %, 95 % CI = 5.1, 11.6; non-MSAs: 6.5 %, 95 % CI = 2.9, 13.9). Finally, for the HBM construct analyzed for self-efficacy: frequency of social and emotional support, more women living in MSAs who reported never receiving social and emotional support also reported never being screened for cervical cancer (8.0 %, 95 % CI = 6.3, 10.2).
Discussion
We found that the proportion of women who reported never being screened for cervical cancer varied by area of residence, demographic characteristics, and HBM constructs. Regardless of geographic location, some women report barriers accessing health care that may prohibit them from obtaining Pap tests for cervical cancer screening. Although other studies have reported similar results for women who have never been screened, they examined a single geographic region, which limited their ability to generalize their findings [4, 22]. Women living in MSAs who were from racial and ethnic minority populations, such as non-Hispanic Asian/Native Hawaiian/Pacific Islanders and those with a high school diploma had the highest proportion of those never screened.
Other studies have also shown that women who live in economically distressed urban areas with few resources are less likely to receive timely cervical cancer screening and more likely to be diagnosed with late-stage cancer [6, 23]. Coughlin et al. [8] found that low socioeconomic status (SES) (e.g., education, income) has a negative association on being up-to-date with cervical cancer screening among women living in MSAs. Although the authors focused solely on county SES characteristics of women living in MSAs who were never screened, their results for this population were similar to our study results. Other studies that used BRFSS data from earlier years have reported that women living in rural areas are less likely to receive Pap tests [7, 9, 11, 12]. We found that more women who reported never being screened lived in MSAs than non-MSAs. This finding suggests that a shift in educational or health care resources for cervical cancer screening might increase the likelihood of women being screening [24].
Non-Hispanic Asian/Native Hawaiian/Pacific Islander women have been previously identified as being at risk of not being screened for cervical cancer [9]. Regardless of MSA status, this study had similar findings for women in this population. A possible strategy for increasing the use of cancer screening is to better understand the culture of the people who are not participating in these screenings. Data for Asian populations are typically combined for analytic purposes, which can mask the diversity of health-seeking behaviors among Asians, Native Hawaiians, and Pacific Islanders [25–27]. Previous research has shown that Asian subpopulations have different belief systems and ideas that can influence their health care decisions and whether they receive preventive health services [26, 28–30]. Programs are needed that incorporate cultural awareness and seek out women where they live because place of residence affects women’s ability to seek and receive cervical cancer screening.
Women who report low educational attainment are also less likely to receive cervical cancer screening [8]. Educational attainment (as well as age and race/ethnicity) is associated with health literacy, which is defined as the ability to “obtain, process, and understand basic health information and services to make appropriate health decisions” [31, 32]. Women who are informed about and understand the importance of cervical cancer screening may be more inclined to receive regular Pap testing [33]. Educating women about cervical cancer screening and appropriate recommendations is often regarded as the responsibility of a health provider [34, 35]. However, for women from racial and ethnic minority groups who may not visit a doctor, social support has been shown to strongly influence self-efficacy, and it can ultimately affect a woman’s decision to be screened [36]. Because people with low educational attainment often have contact with others of similar educational attainment, appropriate health education is needed for entire populations and among established social networks [32]. Many beliefs shared within communities shape whether people seek health screenings [36].
A woman’s self-reported health status provides information about her perceived physical state [37]. In this study, self-report of “fair or poor” general health was associated with never receiving cervical cancer screening for women living in non-MSAs. Health status is particularly important in relation to cervical cancer because women may be unaware that the disease is asymptomatic in its early stages [38]. The findings of our study indicate that women who perceive their health to be poor may have lower perceived susceptibility to an illness. Jylha et al.’s review of self-reported health status literature explains that physical symptoms of an illness influences indication of “poor health” [37]. In particular with cervical cancer, if symptoms are not present, there is no indication any action needs to be taken. Our findings indicate that improved outreach or additional attention by public health officials and researchers may benefit women living in non-MSAs who report fair or poor health because they may not perceive themselves as being at risk of cervical cancer and therefore may not seek preventive screening.
Several limitations may have affected the interpretation of the findings of this study. First, the BRFSS is a self-reported questionnaire, which could lead to recall bias and social desirability effects [39]. Second, this study did not review medical records to confirm self-reported use of screening tests. Third, data were only collected from women with landline telephones [40]. The omission of cell phone users could lead to selection bias because this approach excludes younger women and those with lower socioeconomic status and less access to health care [40]. Despite these limitations, the BRFSS survey has been shown to be valid and reliable [17]. To our knowledge, this is the first study to use HBM constructs to examine the prevalence of cervical cancer screening. This study also used predicted marginal analysis to examine the effect of confounders, such as race/ethnicity, on screening prevalence.
Conclusions
The Affordable Care Act will help mediate one of the financial barriers faced by women, affordability of health services [41]. Specifically, cervical cancer screening as recommended by the USPSTF will be covered with no cost sharing for insured women [19, 41]. With this obstacle removed, the next step will be to educate women about the importance of cervical cancer screening. Efforts should focus on developing culturally appropriate interventions for racial/ethnic minority populations who live in urban areas and have the lowest educational attainment because they are less likely to be screened. The U.S. Department of Health and Human Services’ National Action Plan to Improve Health Literacy recognizes that barriers to health care are often exacerbated by limited health literacy [42]. The plan’s seven goals focus on community, policy, and provider engagement to make health information and health services more accessible through effective, culturally appropriate programs. Social support networks can also be used to share information about the importance of cervical cancer screening and increase health literacy in geographic areas that have limited access to health care [32, 36]. In addition, as researchers and public health continue to collect more information to better understand health behaviors and how they influence people’s decisions about whether to seek cervical cancer screening, they can incorporate this information into cervical cancer community outreach programs. These activities are important to make progress toward meeting the national screening objective in Healthy People 2020 and help to reduce the number of women diagnosed with and dying from cervical cancer [43, 44].
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarilyrepresent the official position of the Centers for Disease Control and Prevention.
References
- 1.Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes and Control. 2009;20(7):1129–1138. doi: 10.1007/s10552-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 2.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100(5):1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 3.Benard VB, Johnson CJ, Thompson TD, Roland KB, Lai SM, Cokkinides V, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(10):2910–2918. doi: 10.1002/cncr.23742. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberg NE, Hatcher J, Dignan MB, Shelton B, Wright S, Dollarhide KF. Faith moves mountains: An appalachian cervical cancer prevention program. American Journal of Health Behavior. 2009;33(6):627–638. doi: 10.5993/ajhb.33.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HY, Kessler CL, Mori N, Chauhan SP. Cervical cancer screening in the United States, 1993–2010: characteristics of women who are never screened. J Womens Health (Larchmt) 2012;21(11):1132–1138. doi: 10.1089/jwh.2011.3418. [DOI] [PubMed] [Google Scholar]
- 6.Barry J, Breen N. The importance of place of residence in predicting late-stage diagnosis of breast or cervical cancer. Health Place. 2005;11(1):15–29. doi: 10.1016/j.healthplace.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Bennett KJ, Probst JC, Bellinger JD. Receipt of cancer screening services: Surprising results for some rural minorities. The Journal of Rural Health. 2012;28(1):63–72. doi: 10.1111/j.1748-0361.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Coughlin SS, King J, Richards TB, Ekwueme DU. Cervical cancer screening among women in metropolitan areas of the United States by individual-level and area-based measures of socioeconomic status, 2000 to 2002. Cancer Epidemiology Biomarkers and Prevention. 2006;15(11):2154–2159. doi: 10.1158/1055-9965.EPI-05-0914. [DOI] [PubMed] [Google Scholar]
- 9.Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. Journal of Public Health Management and Practice. 2009;15(3):200–209. doi: 10.1097/PHH.0b013e3181a117da. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DE, Bolen J, Marcus S, Wells HE, Meissner H. Cancer screening estimates for U.S. metropolitan areas. American Journal of Preventive Medicine. 2003;24(4):301–309. doi: 10.1016/s0749-3797(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 11.Nuno T, Gerald JK, Harris R, Martinez ME, Estrada A, Garcia F. Comparison of breast and cervical cancer screening utilization among rural and urban Hispanic and American Indian women in the Southwestern United States. Cancer Causes and Control. 2012;23(8):1333–1341. doi: 10.1007/s10552-012-0012-0. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Social Science and Medicine. 2008;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Austin LT, Ahmad F, McNally MJ, Stewart DE. Breast and cervical cancer screening in Hispanic women: A literature review using the health belief model. Womens Health Issues. 2002;12(3):122–128. doi: 10.1016/s1049-3867(02)00132-9. [DOI] [PubMed] [Google Scholar]
- 14.Price-Haywood EG, Roth KG, Shelby K, Cooper LA. Cancer risk communication with low health literacy patients: A continuing medical education program. Journal of General Internal Medicine. 2010;25(Suppl 2):S126–S129. doi: 10.1007/s11606-009-1211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glanz K, Champion V, Strecher VJ. The health belief model. In: Viswanath K, editor. Health behavior and health education: theory, research, and practice. Jossey-Bass; San Francisco, CA: 2008. [Google Scholar]
- 16.Elder JP, Ayala GX, Harris S. Theories and intervention approaches to health-behavior change in primary care. American Journal of Preventive Medicine. 1999;17(4):275–284. doi: 10.1016/s0749-3797(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 17.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Sozialund Praventivmedizin. 2001;46(Suppl 1):S3–S42. [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services C Behavioral Risk Factor Surveillance System. 2010 May 2; 2012]; Available from: http://www.cdc.gov/brfss.
- 19.Moyer VA, United States Preventive Services Task Force Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. W312. [DOI] [PubMed] [Google Scholar]
- 20.Stanley SL, King JB, Thomas CC, Richardson LC. Factors associated with never being screened for colorectal cancer. Journal of Community Health. 2012;38(1):31–39. doi: 10.1007/s10900-012-9600-x. [DOI] [PubMed] [Google Scholar]
- 21.Gardner MJ, Altman DG. Confidence intervals rather than p values: Estimation rather than hypothesis testing. British Medical Journal. 1986;292:746–750. doi: 10.1136/bmj.292.6522.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatcher J, Studts CR, Dignan MB, Turner LM, Schoenberg NE. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. Journal of Health Care for the Poor and Underserved. 2011;22(1):176–193. doi: 10.1353/hpu.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schootman M, Jeffe DB, Baker EA, Walker MS. Effect of area poverty rate on cancer screening across US communities. Journal of Epidemiology and Community Health. 2006;60(3):202–207. doi: 10.1136/jech.2005.041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Cancer Screening–United States. 2010;61(3):41–55. 2012. MMWR. [Google Scholar]
- 25.Kagawa-Singer M, Pourat N. Asian American and Pacific Islander breast and cervical carcinoma screening rates and healthy people 2000 objectives. Cancer. 2000;89(3):696–705. doi: 10.1002/1097-0142(20000801)89:3<696::aid-cncr27>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes and Control. 2008;19(3):227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor VM, Jackson JC, Yasui Y, Nguyen TT, Woodall E, Acorda E, et al. Evaluation of a cervical cancer control intervention using lay health workers for Vietnamese American Women. American Journal of Public Health. 2010;100(10):1924–1929. doi: 10.2105/AJPH.2009.190348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo GJ, Le MN, Vong S, Lagman R, Lam AG. Cervical cancer screening: Attitudes and behaviors of young Asian American Women. Journal of Cancer Education. 2011;26(4):740–746. doi: 10.1007/s13187-011-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang CY, Ma GX, Tan Y. Overcoming barriers to cervical cancer screening among Asian American Women. North American Journal of Medicine and Science (Boston) 2011;4(2):77–83. doi: 10.7156/v4i2p077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JH, Sheppard VB, Schwartz MD, Liang WC, Mandelblatt JS. Disparities in cervical cancer screening between Asian American and non-Hispanic White women. Cancer Epidemiology, Biomarkers and Prevention. 2008;17(8):1968–1973. doi: 10.1158/1055-9965.EPI-08-0078. [DOI] [PubMed] [Google Scholar]
- 31.Selden C, Zorn M, Ratzan S, Parker RM. Services United State Department of Health and Human Services, editor. National Library of Medicine; Bethesda, MD: 2000. Health Literacy. [Google Scholar]
- 32.Paasche-Orlow MK, Wolf MS. The causal pathways linking health literacy to health outcomes. American Journal of Health Behavior. 2007;31:S19–S26. doi: 10.5555/ajhb.2007.31.supp.S19. [DOI] [PubMed] [Google Scholar]
- 33.Nelson W, Moser RP, Gaffey A, Waldron W. Adherence to cervical cancer screening guidelines for U.S. women aged 25–64: data from the 2005 Health Information National Trends Survey (HINTS) Journal of Women’s Health (Larchmt) 2009;18(11):1759–1768. doi: 10.1089/jwh.2009.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackerson K, Preston SD. A decision theory perspective on why women do or do not decide to have cancer screening: Systematic review. Journal of Advanced Nursing. 2009;65(6):1130–1140. doi: 10.1111/j.1365-2648.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez EA, Xie Y, Goldsteen K, Chalas E. Promoting knowledge of cancer prevention and screening in an underserved Hispanic women population: a culturally sensitive education program. Health Promotion Practice. 2011;12(5):689–695. doi: 10.1177/1524839910364370. [DOI] [PubMed] [Google Scholar]
- 36.Ashida S, Hadley DW, Goergen AF, Skapinsky KF, Devlin HC, Koehly LM. The importance of older family members in providing social resources and promoting cancer screening in families with a hereditary cancer syndrome. Gerontologist. 2011;51(6):833–842. doi: 10.1093/geront/gnr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jylha M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science and Medicine. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA: A Cancer Journal for Clinicians. 2002;52(3):134–149. doi: 10.3322/canjclin.52.3.134. [DOI] [PubMed] [Google Scholar]
- 39.Warnecke RB, Johnson TP, Chavez N, Sudman S, ORourke DP, Lacey L, et al. Improving question wording in surveys of culturally diverse populations. Annals of Epidemiology. 1997;7(5):334–342. doi: 10.1016/s1047-2797(97)00030-6. [DOI] [PubMed] [Google Scholar]
- 40.Blumberg SJ, Luke JV, Ganesh N, Davern ME, Boudreaux MH, Soderberg K. Wireless substitution: statelevel estimates from the National Health Interview Survey, January 2007-June 2010. National Center for Health Statistics. 2011;(39):1–26. 28. [PubMed] [Google Scholar]
- 41.Koh H, Sebelius KG. Promoting prevention through the Affordable Care Act. New England Journal of Medicine. 2010;363(14):1296–1299. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 42.Department of Health and Human Services . National action plan to improve health literacy. Washington, DC: 2010. ODPHP. [Google Scholar]
- 43.Department of Health and Human Services . Healthy People 2020. Washington; DC: 2011. [Google Scholar]
- 44.CDC . United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report. CDC and NCI; Atlanta, GA: 2013. [Google Scholar]


