Abstract
This study investigated menstrual cycle phase differences in heart rate (HR) and RR interval variability (RRV) in 49 healthy, premenopausal, eumenorrheic women (age 30.2 ± 6.2). HR and RRV were computed from ambulatory 24-hour ECG, collected for up to 6 days, with at least one day each during early-to-mid follicular and mid-luteal menstrual phases. Phase effects on HR and RRV were assessed using linear mixed effects models with a random intercept to account for the correlation of observations within each subject as well as intrasubject variation. During follicular phase monitoring, women had significantly lower average HR (−2.33 bpm), and higher SDRR, rMSSD, high frequency (0.04–0.15 Hz) and low frequency (0.15–.40 Hz) RRV than during the luteal phase. These results provide strong support for the influence of menstrual phase on cardiac autonomic regulation in premenopausal women.
Epidemiological studies show that the risk of coronary heart disease is much lower in premenopausal women compared with age-matched men (Kannel & Wilson, 1995) but this risk differential disappears in postmenopausal women compared with age-matched men (Colditz et al., 1987). A body of research suggests that hormonal differences between the pre- and postmenopausal setting contribute to this cardioprotection for premenopausal women.
Reproductive hormones, primarily estrogen, appear to modulate cardiovascular function through a number of mechanisms, including stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) and sympatho-adrenomedullary (SAM) systems (Goldstein, Shapiro, Chicz-DeMet, & Guthrie, 1999; Morgan & Pfaff, 2002). After the menopause, hormone replacement therapy (HRT) can attenuate women’s increased risk. For example, HRT decreases blood pressure and increases arterial compliance (da Costa et al., 2004), and improves baroreflex sensitivity. In addition, HRT has been shown to enhance autonomic regulation of the heart both in women who experienced natural menopause and those who had hysterectomy with oophorectomy (Chao et al., 2005; Farag et al., 2002; Liu, Kuo, & Yang, 2003; Mercuro et al., 2000). Similarly, exogenous estrogen increases parasympathetic cardiac autonomic regulation in animals (Saleh, Connell, & Saleh, 2000).
Reduced autonomic regulation of the heart is one of many well established risk factors for heart disease, predicting heart disease development in initially healthy participants in community studies (Liao et al., 1997; Tsuji et al., 1996); atherosclerosis progression (Huikuri et al., 1999); and adverse events occurrence in post-myocardial infarction (Bigger, 1992; Kleiger, Miller, Bigger, Moss, & the Multicenter Post-Infarction Research Group, 1987) and heart failure (la Cour, Avlund, & Schultz-Larsen, 2006) patients. In these studies, cardiac autonomic regulation was measured noninvasively using indices of RR interval variability (RRV). Time and frequency domain measures of high frequency RRV are widely regarded to reflect cardiac parasympathetic modulation. Lower frequency or more global indices also reflect parasympathetic modulation and under certain conditions, also may reflect the contribution of the sympathetic nervous system (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996).
These converging lines of evidence suggest that cardiac autonomic regulation might vary within the menstrual cycle. Although hormonal changes across menstrual phases are not as great as the change in hormonal levels between the pre- and postmenopausal eras of a woman’s life, the physiological mechanisms of menstrual phase variation in cardiovascular function could help elucidate mechanisms linking menopause to increased cardiac risk. Studies examining the impact of menstrual cycle phase on cardiac autonomic regulation have produced inconsistent results. Princi et al. reported greater levels of high frequency (HF) RRV during the luteal phase (Princi et al., 2005). Using 24-hour continuous ECG recordings, Vallejo et al. also found evidence of greater cardiac parasympathetic modulation during the luteal phase (Vallejo, Marquez, Borja-Aburto, Cardenas, & Hermosillo, 2005), but Nakagawa et al. found no phase differences in RRV (Nakagawa et al., 2006). Others have reported greater HF-RRV in the follicular, rather than luteal phase (Saeki, Atogami, Takahashi, & Yoshizawa, 1997; Sato, Miyake, Akatsu, & Kumashiro, 1995). Further, Sato et al. (Sato et al., 1995) found greater low frequency/high frequency ratio (LF/HF) during the luteal than follicular phase due to both greater increase in the LF and greater decrease in the HF components. Finally, some studies report no menstrual phase differences in RRV (Leicht, Hirning, & Allen, 2003; Yildirir, Kabakci, Akgul, Tokgozoglu, & Oto, 2002). Discrepancies across these studies may have been due to relatively small samples (n=6, 10, 10, and 20 in Princi, Saeki, Leicht, and Sato respectively) and, in all but one exception (Vallejo et al., 2005), the computation of RRV from brief (5–20 min) ECG recording intervals.
Addressing the limitations of previous studies, the objective of this study was to examine whether there is reliable evidence for a menstrual cycle phase influence on RRV. The protocol improves on earlier studies in several ways. We report on a larger sample of women than in most prior studies. Using a within-in subjects design, RRV was assessed using 24-hour recordings for up to 6 days, with at least one day in both the early to mid-follicular and mid-luteal menstrual phases for each participant. Order of menstrual phase monitoring was counterbalanced to control for confounding effects of protocol timing. Ambulatory ECG monitoring was used in an attempt to sample a broad range of situations in everyday life.
METHODS
Participants
Participants were 49 women ages 19 to 44 (30.2 ± 6.2) who were recruited to participate in the Heart Matters study, a study of the cardiac autonomic consequences of negative interpersonal interactions and conflict. Participants were recruited through flyers posted around Columbia University Medical Center and the University of California at Irvine. Exclusion criteria included: BMI > 30 kg/m2; diagnosed cardiovascular disease or hypertension; taking any medication to control BP; active psychiatric disease; diabetes; drug or alcohol abuse; or any other condition or medication likely to influence the autonomic nervous system. Further, women were excluded if they reported any of the following: postmenopausal, either naturally or through hysterectomy; pregnant; taking oral contraceptives; tubal ligations; history of menstrual irregularities (e.g., variable cycle length or frequency); menstrual cycle-related disorders (e.g., premenstrual syndrome, dysmenorrhea); or menstrual cycle length < 26 or > 32 days. Information on eligibility criteria was based on participants’ reports during a screening interview. All participants provided written informed consent. The study was approved by the institutional review boards of the New York State Psychiatric Institute and the University of California at Irvine.
Procedure
Participants were monitored for cardiac autonomic control using continuous ECG monitoring. Our aim was to collect five analyzable days of data, with approximately 24 hours each day, from each participant. Women were monitored in both the early-to-mid follicular and mid-luteal phases of their menstrual cycle. To counterbalance for phase order effects, participants were randomly assigned to begin the ECG monitoring days in one phase, with subsequent monitoring in the other phase. Participants were randomly assigned to complete either two or three days of data in each phase.
After signing informed consent, participants were randomized and began completing daily menstrual symptoms ratings as the first phase of the study. The daily ratings were used to confirm menstrual cycle length, estimate when to schedule test days within each woman’s cycle, and to exclude from participation women whose daily self-reports indicated chronic mood disturbance or menstrual cycle disorders. Next, the ECG monitoring phase of the study began. The menstrual phase scheduling protocol is described below and in Figure 1. Scheduling of ECG monitoring days was arranged for participants’ convenience within the constraints of the protocol; however, consecutive monitoring days were limited to a maximum of two at a time.
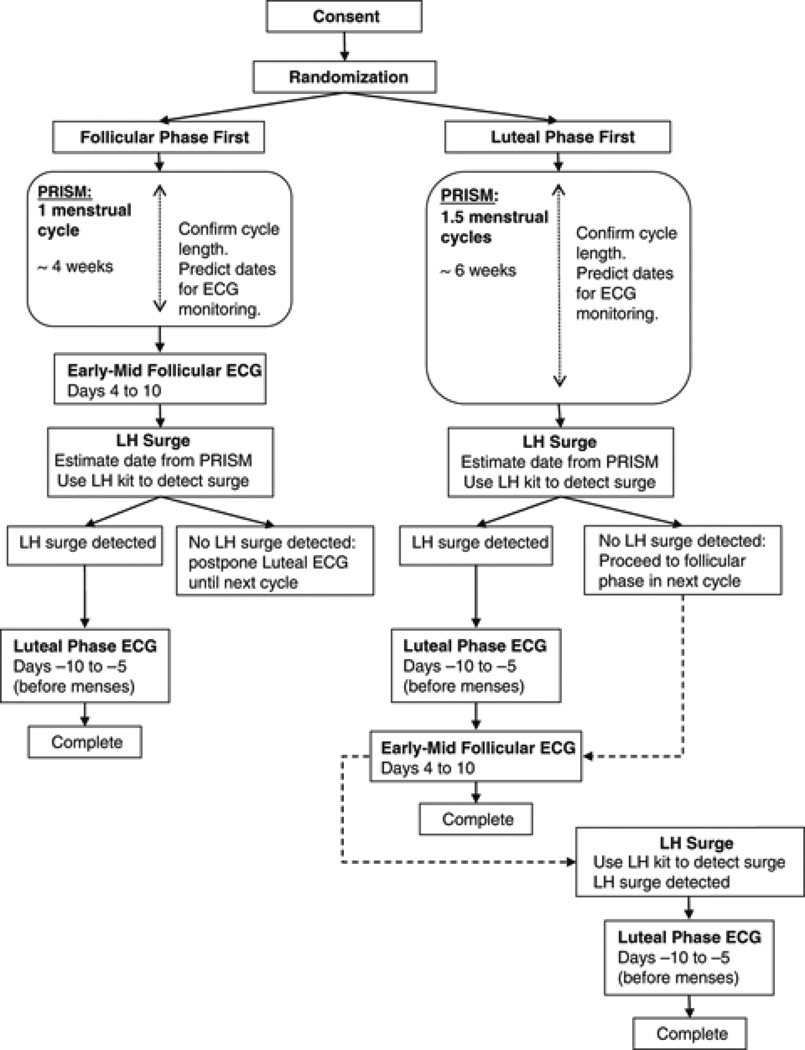
Figure. 1.
Study protocol: Menstrual phase tracking and timing of ECG monitoring.
Monitoring in follicular phase first
Women randomized to begin ECG monitoring in the follicular phase kept a daily log of menstrual cycle events and symptoms ratings through one full menstrual cycle, usually about 4 to 5 weeks. Menses onset (Day 1 of menstrual cycle) was reported on the daily logs and via telephone to study staff. After the second Day 1 was reported, follicular phase ECG monitoring occurred between Days 4 to 10 of that cycle. Luteal phase monitoring followed during that same cycle.
Monitoring in luteal phase first
Women randomized to begin ECG monitoring in the luteal phase completed the same daily logs, but they did so for approximately six weeks i.e. one full menstrual cycle and through their next cycle’s follicular phase. During the luteal phase of that second cycle, ECG monitoring occurred. Cycle length from the daily logs was used to predict the luteinizing hormone (LH) surge date. Beginning two days prior to the estimated LH surge date, women used an ovulation test kit at home for up to seven days to detect their LH surge. Participants reported LH test results to study staff via telephone. Luteal phase ECG monitoring took place between three days after the LH surge until no later than five days before the next predicted Day 1. Follicular phase monitoring occurred during Days 4 to 10 in the cycle immediately following luteal phase monitoring.
Protocol exceptions
Women in both groups did not undergo luteal phase monitoring during an anovulatory cycle i.e. the home tests did not confirm an LH surge. Women randomized to the follicular phase first who did not ovulate in that same cycle completed luteal phase monitoring in the next cycle during which LH surge was detected. Women randomized to the luteal phase first who did not ovulate began ECG monitoring immediately afterward in their next follicular phase, i.e. phase-monitoring order was switched to avoid prolonging their time in the study. They continued in that same cycle, pending LH surge confirmation, with luteal phase monitoring. In rare cases, participants were allowed to switch from the randomly assigned phase order if scheduling constraints prevented feasible adherence to this difficult protocol. In addition, participants were asked to complete extra monitoring days when equipment failure or noncompliance produced excessive missing data in the ECG or other key variables for the parent study.
Measures
Menstrual cycle characteristics
The Prospective Record of the Impact and Severity of Menstrual Symptoms calendar (PRISM) (Reid, 1985) was completed every day as described above. The PRISM probes dates and ratings of menstrual cycle bleeding and menstrual discomfort; psychological states; physical symptoms; daily exercise; life events and general physical discomfort. In this study it was used to confirm women’s menstrual cycle length before beginning ECG monitoring.
Ovulation and luteal phase timing
The Clearblue® Easy Read Ovulation Test (Inverness Medical Innovations, Inc. 2005; US patents 5,656,503; 5,622,871; 5,602,040) is a seven-day, over-the-counter, home-use ovulation kit. It detects the LH surge from a urine sample. The kit was used in this study to confirm that ovulation had occurred through a positive LH surge, based on the kit’s specifications. Luteal phase procedures were timed based on the test’s results.
Instrumentation
Continuous ECG waveforms, sampled at 200 Hz, were recorded with a three-lead configuration using a LifeShirt® ambulatory monitor (VivoMetrics, Ventura CA). ECG data were stored on a flashcard in the LifeShirt recorder, then uploaded to a network server for storage and analysis.
On the first monitoring day, study staff met with participants between 8:00am and 11:00am. Staff trained participants to apply the LifeShirt device to themselves and begin and end a recording session. In most cases, participants applied the LifeShirt and ran the sessions from home on all subsequent monitoring days. Participants were instructed to wear the device from early morning, beginning no later than 11:00am until at least through their waking time the next day. They wore the device while sleeping. For feasibility reasons, we allowed participants to remove and reapply or shutdown and restart the device during the 24-hour period as needed for bathing. Participants were asked to refrain from aerobic or other strenuous exercise during scheduled monitoring days.
RR Interval Variability
The digitized ECG waveforms were exported from the Vivologic software provided with the LifeShirt system and submitted to custom-written event detection software to produce an RR interval series. Errors in the software’s automated detection of R-waves were corrected by visual inspection. Ectopic beats were corrected by interpolation.
The RR interval series were used to compute five outcome variables: heart rate (HR); the standard deviation (SDRR); the root mean squared successive difference (rMSSD); spectral power in the low (LF: 0.04–0.15 Hz) and high (HF: 0.15–0.50 Hz) frequency bands. The spectra of these series were calculated on 300-second epochs using an interval method for computing Fourier transforms similar to that described by DeBoer, Karamaker, and Strackee (deBoer, Karemaker, & Strackee, 1984).
Prior to computing Fourier transforms, the mean of the RR interval series was subtracted from each value in the series and the series then was filtered using a Hanning window (Harris, 1978) and the power, i.e., variance (in msec2), over the LF and HF bands was summed. Estimates of spectral power were adjusted to account for attenuation produced by this filter (Harris, 1978).
Data Reduction and Analysis
For each monitoring day, HR and the four RRV indices from all valid epochs were averaged to generate mean HR and RRV for that day, consistent with recognized standards (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Only epochs containing at least 240 sec of valid data were used. Monitoring days with at least 12 total hours of valid data were included in analyses, per standard practice in research and clinical Holter monitoring (Bigger, J. T., Jr. et al., 1992). Participants with fewer than 5 monitoring days of data, due to poor data quality or attrition from the study, but with a minimum of one valid monitoring day from each menstrual phase were included in analyses. Data from the first 53 women who enrolled in the Heart Matters study, which is ongoing, were analyzed. Of these, data from 49 met these criteria and were included in the results reported here.
Distributions of all outcomes except HR were positively skewed, as they are routinely in most samples. As a result, a natural logarithmic (ln) transformation was computed for SDRR, rMSSD, LF-HRV and HF-HRV to correct the skew. All results are reported in natural log units for these variables.
Effects of menstrual phase on HR and RRV were determined by mixed effects models. Menstrual phase (follicular, luteal) was the primary predictor variable. Subject effects were treated as random. Phase order group was included as a fixed effect, as well as the group by phase interaction term, to assess possible order effects. If either the group effect or interaction effect were significant, it would suggest that the phase order of ECG monitoring influenced the outcomes. Separate mixed effects models were analyzed for HR and each of the RRV variables as outcomes using the following equation:
where b represents the random subject effect, β represents a fixed effect, and ε represents the error term. The alpha level was set at 0.05.
RESULTS
Participant characteristics, number of completed monitoring days and data on counterbalancing order of menstrual phase monitoring appear in Table 1.
Table 1.
Participant Characteristics
| Women (N = 49) | |
|---|---|
| Age (years) | 30.6 ± 6.3 |
| Weight (lbs) | 128.9 ± 20.3 |
| Height (in.) | 64.3 ± 3.3 |
| BMI (kg/m2) | 21.9 ± 2.9 |
| Protocol order | |
| Follicular first | 31 (63.3%) |
| Luteal first | 18 (36.7%) |
Note: Demographics values are shown as mean ± SD. Protocol order values are shown as n (%) of participants.
Over 81% of women completed five monitoring days (Table 2). Three women completed a sixth ECG day because of missing data in other variables for the parent study. Because the extra day’s ECG data were valid, they were retained in our data set. The randomly assigned phase order was changed for two women. One woman switched from monitoring first in the luteal to follicular phase due to an anovulatory menstrual cycle. The other woman was switched from monitoring first in the follicular to luteal phase because of scheduling constraints. The proportion of women in each phase order was not significantly different (χ2=3.449, p=0.0633). Even so, the numbers per group were quite different (31 follicular first, 18 luteal first). Consequently, we decided to control for phase order in the analyses.
Table 2.
Distribution of ECG Monitoring Days Completed by Participants
| Number of days | Follicular phase monitoring days | Luteal phase monitoring days | Total monitoring days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequencya | Person-daysb | Percentc | Frequency | Person-Days | Percent | Frequency | Person-Days | Percent | |
| 1 | 2 | 2 | 1.8% | 2 | 2 | 1.6% | 0 | 0 | 0.0% |
| 2 | 32 | 64 | 56.6% | 22 | 44 | 36.1% | 2 | 4 | 1.7% |
| 3 | 13 | 39 | 34.5% | 24 | 72 | 59.0% | 0 | 0 | 0.0% |
| 4 | 2 | 8 | 7.1% | 1 | 4 | 3.3% | 7 | 28 | 11.9% |
| 5 | 37 | 185 | 78.7% | ||||||
| 6 | 3 | 18 | 7.7% | ||||||
| Totalsd | 49 | 113 (48.1%) | 100% | 49 | 122 (51.9%) | 100% | 49 | 235 | 100% |
| Days per person (Mean (SD)) | 2.3 (0.6) | 2.5 (0.6) | 4.8 (0.7) | ||||||
Frequency is the number of persons completing a given number of monitoring days.
Person-days = Number of days × Frequency.
Percent = (number of person-days/total person-days) × 100. These values represent the percentage of monitoring days contributed by participants who completed a given number of monitoring days, for example, women completing a total of two follicular phase days contributed 65.3% of all follicular phase days.
Totals are sums of above columns, except for person-days. Total values in person-days column are Person-days Sum (Percent of total person-days by phase).
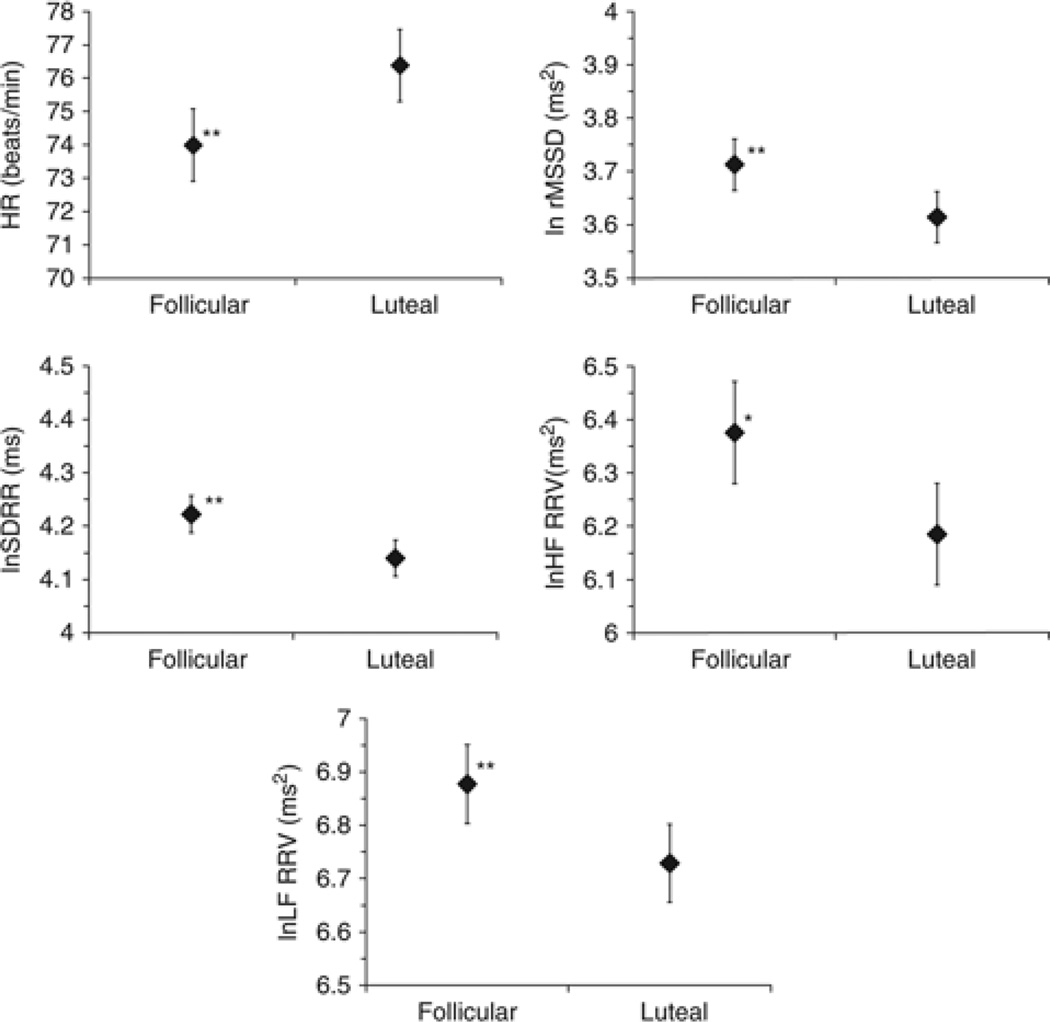
Results of the mixed model analyses are presented in Table 3. When HR was the outcome variable, the main effect of phase was significant (F(1,184) = 20.27, p <.0001). Average HR was 2.33 bpm lower in the follicular phase compared to luteal phase (t(184) = −2.84, p = .0058).
Table 3.
Mixed Model Results for All Outcome Variables
| Outcome | Coefficients | |||
|---|---|---|---|---|
| Intercept | Phase order group | Menstrual phase | Phase Order × Menstrual Phase | |
| HR (bpm) | 76.52 (1.48)*** | −0.28 (1.86) | −2.33 (0.83)** | 0.12 (1.06) |
| lnHF (ms2) | 6.24 (0.15)*** | −0.12 (0.19) | 0.19 (0.07)* | 0.01 (0.09) |
| lnLF (ms2) | 6.68 (0.12)*** | 0.10 (0.15) | 0.14 (0.05)** | 0.01 (0.06) |
| lnRMSSD (ms2) | 3.65 (0.08)*** | −0.08 (0.10) | 0.10 (0.04)** | 0.001 (0.05) |
| lnSDRR (ms) | 4.14 (0.05)*** | −0.007 (0.07) | 0.08 (0.02)** | 0.006 (0.03) |
Note: Tabled values are coefficient estimate (std. error) from the mixed model analysis results. Coefficients (std. errors) were produced for the menstrual phase effect with luteal phase as the referent group. A negative coefficient indicates the mean value of the outcome was lower in the follicular than luteal phase. Degrees of freedom for all outcomes are 47 for the intercept and 184 for phase.
HR = heart rate; lnHF = natural log of high frequency RRV power; lnLF = natural log of low frequency RRV power; lnRMSSD = natural log of root mean squared successive difference; lnSDRR = natural log of the standard deviation of R-R intervals.
p<.05,
p<.01,
p<.001.
In these women, lnSDRR was 0.08 units greater in the follicular compared to the luteal phase on average (t(184) = 3.20, p = .002). For lnrMSSD and lnHF-RRV power, the main effect of phase was significant (lnrMSSD: F(1,184) = 16.72, p <.0001; lnHF: F(1,184) = 16.63, p <.0001), with lnrMSSD on average 0.10 units higher in the follicular than in the luteal phase (t(1844) = 2.59, p = .01) and lnHF power 0.19 units higher on average in the follicular than in the luteal phase (t(184) = 2.52, p = .01) Finally, lnLF power in women was on average 0.14 units greater in the follicular compared to the luteal phase (t(184) = 3.00, p = .003).
Neither the effect of phase order group nor the phase order group by phase interaction term was significant for any of the five models tested. Figure 2 contains graphs of the menstrual phase main effects for all five outcomes; for simplicity, the nonsignificant phase order main and interaction effects are not included in the graphs.
Figure. 2.
Menstrual phase differences in HR and RRV outcomes. HR=heart rate; ln=natural logarithim; SDRR=standard deviation of RR interval series; LF=low frequency (0.04–0.15 Hz); HF=high frequency (0.15–0.50 Hz); RRV=RR interval variability. *p<.05, **p<.01.
DISCUSSION
The results of this study demonstrate menstrual phase differences in cardiac autonomic regulation, measured as RRV. In healthy, premenopausal women, all indices of RRV were greater and HR was lower i.e. parasympathetic tone was greater, during the early to mid-follicular phase of the menstrual cycle compared to the mid-luteal phase. The body of published research examining menstrual phase effects on cardiac autonomic regulation is relatively small, and the findings have varied substantially. In most studies, the disparate findings might be attributable to methodological limitations, primarily small samples, short ECG recording periods, and single ECG recordings during each phase of the menstrual cycle (Leicht et al., 2003; Princi et al., 2005; Saeki et al., 1997; Sato et al., 1995; Yildirir et al., 2002).
In contrast, we studied 49 women, a larger sample than in prior studies. We estimated RRV in both phases for all participants, using an average of 2.4 days in follicular phase and 2.5 days in luteal phase. We were successful in collecting these repeated measurements; less than 2% of the data from each phase were from single-day recordings. Finally, we used 24-hr ambulatory ECG monitoring. Although RRV estimates derived from brief and 24-hour ECG recordings show substantial concordance (Perkiomaki et al., 2001; Sloan et al., 1994), longer recordings provide more stable estimates. Hormonal fluctuations both within and between menstrual phases follow a relatively slow course. If hormonal fluctuations contribute to phase effects on RRV, longer ECG recordings may be important for capturing their average impact within each phase.
We know of only two other studies that used 24-hour continuous ECG recordings. Vallejo et al. (Vallejo et al., 2005) studied healthy women ages 21 to 35 during menses (days 1–5; i.e. early follicular phase), late follicular and luteal phases. They report greater rMSSD, suggesting greater parasympathetic modulation, during early follicular than both late follicular and luteal phases. They also found, however, that menstrual phase was associated with rMSSD only for women younger than 29.5 years. Comparisons of these results with ours are limited because Vallejo et al. assessed menstrual phase differences using a between-subjects, rather than within-subjects design with small groups of women studied during early follicular (n=7), late follicular (n=8) or luteal (n=15) phases. Each woman was monitored only for one day, further limiting the reliability of their RRV measures. Nakagawa et al. (Nakagawa et al., 2006) collected one 24-hour ECG recording in both the mid-follicular (days 7–12) and late luteal (days 18–26) phases in a small sample of 11 healthy women. They found no phase differences in RRV, but they report only time domain measures of RRV. Evidence from psychophysiological studies of neuroendocrine responses across different menstrual cycle phases lends support to our finding that parasympathetic tone is greater during the follicular phase. Studies employing within-subject designs have found that, compared to the follicular phase, women tested during the luteal phase of their cycles demonstrate higher norepinephrine (Girdler et al., 1998; Goldstein et al., 1999; Nakagawa et al., 2006) and higher heart rate (Stoney, Langer, & Gelling, 1986).
Our data also contribute to the increasing recognition that RRV across the frequency spectrum reflects cardiac parasympathetic modulation (Taylor, Carr, Myers, & Eckberg, 1998). Some have suggested that LF power represents cardiac sympathetic regulation (Friedman & Thayer, 1998; Pagani et al., 1986) and evidence suggests that at least under certain conditions, the sympathetic nervous system may contribute to LF power (Pomeranz et al., 1985). Our finding that all indices of RRV are greater during the follicular phase of the menstrual cycle suggests, however, that during ambulatory monitoring, it is the parasympathetic nervous system that contributes more heavily to RRV.
Limitations
While our data provide strong support generally for menstrual phase differences in RRV, there are limitations in this study. The data reported here represent secondary analyses from a study designed and powered to address very different hypotheses than the questions addressed in this report. Further, this report includes only approximately the first half of women who enrolled in the parent study, which is ongoing. As a result, the findings reported here should be viewed as preliminary.
In the parent study, we attempted to collect data from five monitoring days representing both the luteal and follicular phases of the menstrual cycle to control for menstrual phase effects. This demanding protocol, however, was difficult for many participants. As a result, 18.4% did not complete a full five days of monitoring. It would be preferable to obtain equal numbers and similar timing of ECG days during each menstrual phase. A related limitation is that because of the demanding nature of the protocol, not all participants wore the ambulatory monitor for a full 24 hours on each monitoring day. Following standards established in studies in cardiology, we excluded monitoring days that yielded less than 12 hours of data (Bigger, Fleiss, Rolnitzky, & Steinman, 1992; Bigger, J. T., Jr. et al., 1992). It is conceivable that this approach may have produced some bias in sampling the ECG over the monitoring days but it is unclear how it could affect menstrual cycle differences in RRV.
Menstrual phase was not determined using serum hormone levels. Given that participants were young, healthy women with regular cycles, however, the use of daily logs to confirm cycle length and LH surge testing lends confidence to the phase timing used (Ghazeeri, Vongprachanh, & Kutteh, 2000; Nielsen, Barton, Hatasaka, & Stanford, 2001). The findings cannot be attributed to specific hormones or other mechanisms; however, given prior evidence, the different hormonal settings in the early to mid-follicular and mid-luteal phases likely contribute to the observed phase differences.
Clinical significance
Few studies present data to assess the clinical impact of menstrual cycle-based autonomic variation but in those that do, findings are consistent with an increased risk of clinical cardiac events during periods of reduced cardiac autonomic modulation. Rosano et al. reported that women with regular menses and paroxysmal supraventricular tachycardia (PSVT) showed greater incidence of arrhythmia during the luteal phase of the menstrual cycle compared with the follicular phase (Rosano et al., 1996). Women with menstrual cycle dependent PSVT undergoing electrophysiologic studies had inducible arrhythmias during the luteal but not during the follicular phase of the menstrual cycle (Myerburg et al., 1999). Because cardiac vagal modulation has anti-arrhythmic effects, these data are consistent with our findings of decreased cardiac autonomic activity in the luteal phase.
Conclusions
A small body of research has investigated differences in autonomic regulation of the heart during different phases of the menstrual cycle. Results of these studies have been mixed. Some studies report differences in autonomic regulation across menstrual phases, but others find no differences by phase. This study used a within-subjects design, 24-hr monitoring and at least one day in each menstrual phase for all participants. We found evidence that in healthy premenopausal women, heart rate is lower and RRV higher during the early to mid-follicular than during the mid-luteal phase. These results suggest that premenopausal women’s hormonal status contributes to cardiac autonomic regulation. Future work should investigate the specific hormonal conditions associated with these phase differences.
Acknowledgments
This study was supported by 5 R01 HL072057 (RP Sloan, PI) from the National Heart Lung and Blood Institute, Inverness Medical, Inc., and the Nathaniel Wharton Fund.
References
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. Stability over time of heart period variability in patients with previous myocardial infarction and ventricular arrhythmias. The American Journal of Cardiology. 1992;69(8):718–723. doi: 10.1016/0002-9149(92)90493-i. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. The American Journal of Cardiology. 1992;69(9):891–898. doi: 10.1016/0002-9149(92)90788-z. [DOI] [PubMed] [Google Scholar]
- Chao HT, Kuo CD, Su YJ, Chuang SS, Fang YJ, Ho LT. Short-term effect of transdermal estrogen on autonomic nervous modulation in postmenopausal women. Fertility and Sterility. 2005;84(5):1477–1483. doi: 10.1016/j.fertnstert.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. The New England Journal of Medicine. 1987;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- da Costa LIS, de Oliveira MA, Rubim VSM, Wajngarten M, Aldrighi JM, Rosano GM, Neto CD, Gebara OCE. Effects of hormone replacement therapy or raloxifene on ambulatory blood pressure and arterial stiffness in treated hypertensive postmenopausal women. The American Journal of Cardiology. 2004;94(11):1453–1456. doi: 10.1016/j.amjcard.2004.07.153. [DOI] [PubMed] [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events, particularly for heart rate variability spectra. IEEE Transactions in Biomedical Engineering, BME-31. 1984:384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- Farag NH, Nelesen RA, Parry BL, Loredo JS, Dimsdale JE, Mills PJ. Autonomic and cardiovascular function in postmenopausal women: The effects of estrogen versus combination therapy. American Journal of Obstetrics and Gynecology. 2002;186(5):954–961. doi: 10.1067/mob.2002.122248. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. Journal of Psychosomatic Research. 1998;44(1):133–151. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- Ghazeeri G, Vongprachanh P, Kutteh W. The predictive value of five different urinary LH kits in detecting the LH surge in regularly menstruating women. Int J Fertil Womens Med. 2000;45(5):321–326. [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Research. 1998;81(2):163–178. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Shapiro D, Chicz-DeMet A, Guthrie D. Ambulatory blood pressure, heart rate, and neuroendocrine responses in women nurses during work and off work days. Psychosomatic Medicine. 1999;61(3):387–396. doi: 10.1097/00006842-199905000-00020. [DOI] [PubMed] [Google Scholar]
- Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proceedings of the IEEE. 1978;66:51–83. [Google Scholar]
- Huikuri HV, Jokinen V, Syvanne M, Nieminen MS, Airaksinen KE, Ikaheimo MJ, Koistinen JM, Kauma H, Kesaniemi AY, Majahalme S, Niemela KO, Frick H. Heart Rate Variability and Progression of Coronary Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Archives of Internal Medicine. 1995;155(1):57–61. [PubMed] [Google Scholar]
- Kleiger RE, Miller JP, Bigger JT, Moss AJ the Multicenter Post-Infarction Research Group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. American Journal of Cardiology. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- la Cour P, Avlund K, Schultz-Larsen K. Religion and survival in a secular region. A twenty year follow-up of 734 Danish adults born in 1914. Social Science and Medicine. 2006;62(1):157–164. doi: 10.1016/j.socscimed.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Leicht AS, Hirning DA, Allen GD. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Experimental Physiology. 2003;88(3):441–446. doi: 10.1113/eph8802535. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kuo TBJ, Yang CCH. Effects of estrogen on gender-related autonomic differences in humans. American Journal of Physiology - Heart and Circulatory Physiology. 2003;285(5):H2188–H2193. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Rosano GMC. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. The American Journal of Cardiology. 2000;85(6):787–789. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behavioural Brain Research. 2002;132(1):85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Myerburg RJ, Cox MM, Interian A, Jr, Mitrani R, Girgis I, Dylewski J, Castellanos A. Cycling of inducibility of paroxysmal supraventricular tachycardia in women and its implications for timing of electrophysiologic procedures. The American Journal of Cardiology. 1999;83(7):1049–1054. doi: 10.1016/s0002-9149(99)00013-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Ooie T, Takahashi N, Taniguchi Y, Anan F, Yonemochi H, Saikawa T. Influence of Menstrual Cycle on QT Interval Dynamics. Pacing and Clinical Electrophysiology. 2006;29(6):607–613. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Barton SD, Hatasaka HH, Stanford JB. Comparison of several one-step home urinary luteinizing hormone detection test kits to OvuQuick(R) Fertility and Sterility. 2001;76(2):384–387. doi: 10.1016/s0015-0282(01)01881-7. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Perkiomaki JS, Zareba W, Kalaria VG, Couderc J, Huikuri HV, Moss AJ. Comparability of nonlinear measures of heart rate variability between long- and short-term electrocardiographic recordings. American Journal of Cardiology. 2001;87(7):905–908. doi: 10.1016/s0002-9149(00)01537-x. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Princi T, Parco S, Accardo A, Radillo O, De Seta F, Guaschino S. Parametric evaluation of heart rate variability during the menstrual cycle in young women. Biomedical Sciences Instrumentation. 2005;41:340–345. [PubMed] [Google Scholar]
- Reid RL. Premenstrual syndrome. In: Leventhal JM, Hoffman JJ, Keith LG, Taylor PJ, editors. Obstetrics, gynecology, and fertility. Chicago: Year Blood Medical Publishers, Inc.; 1985. [Google Scholar]
- Rosano GMC, Leonardo F, Rosano GMC, De Luca F, Sarrel PM, Beale CM, Collins P. Cyclical variation in paroxysmal supraventricular tachycardia in women. The Lancet. 1996;347(9004):786–788. doi: 10.1016/s0140-6736(96)90867-3. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Atogami F, Takahashi K, Yoshizawa T. Reflex control of autonomic function induced by posture change during the menstrual cycle. Journal of the Autonomic Nervous System. 1997;66(1–2):69–74. doi: 10.1016/s0165-1838(97)00067-2. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ, Saleh MC. Acute injection of 17[beta]-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Autonomic Neuroscience. 2000;84(1–2):78–88. doi: 10.1016/s1566-0702(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Sato N, Miyake S, Akatsu Ji, Kumashiro M. Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosomatic Medicine. 1995;57:331–335. doi: 10.1097/00006842-199507000-00004. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Myers MM, Bigger JT, Steinman RC, Gorman JM. Brief interval HPV by different methods of analysis correlates highly with 24-hour analyses in normals. Biological Psychology. 1994;38:133–142. doi: 10.1016/0301-0511(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Stoney CM, Langer AW, Gelling PD. The Effects of Menstrual Cycle Phase on Cardiovascular and Pulmonary Responses to Behavioral and Exercise Stress. Psychophysiology. 1986;23(4):393–402. doi: 10.1111/j.1469-8986.1986.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Vallejo M, Marquez MF, Borja-Aburto VH, Cardenas M, Hermosillo AG. Age body mass index, and menstrual cycle influence young women's heart rate variability. Clinical Autonomic Research. 2005;15(4):292–298. doi: 10.1007/s10286-005-0272-9. [DOI] [PubMed] [Google Scholar]
- Yildirir A, Kabakci G, Akgul E, Tokgozoglu L, Oto A. Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Annals of Noninvasive Electrocardiology. 2002;7(1):60–63. doi: 10.1111/j.1542-474X.2001.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]