Abstract
We previously demonstrated that transforming growth factor-β1 (TGF-β1), while having no effect alone, enhances nitric oxide (NO) production in primary, purified mouse astrocytes induced by lipopolysaccharide (LPS) plus interferon-γ (IFN-γ) by recruiting a latent population of astrocytes to respond, thereby enhancing the total number of cells that express Nos2. In this investigation, we evaluated the molecular signaling pathway by which this occurs. We found that purified murine primary astrocytes express mRNA for TGFβRII as well as the TGFβRI subunit ALK5, but not ALK1. Immunofluorescence microscopy confirmed the expression of TGFβRII and ALK5 protein in astrocytes. Consistent with ALK5 signaling, Smad3 accumulated in the nucleus of astrocytes as early as 30 min after TGF-β1 (3ng/ml) treatment and persisted up to 32 hr after TGF-β1 administration. Addition of ALK5 inhibitors prevented TGF-β1-mediated Smad3 nuclear accumulation and NO production when given prior to the Nos2 induction stimuli but not after. Finally, astrocyte cultures derived from Smad3 null mutant mice did not exhibit a TGF-β1-mediated increase in iNOS expression. Overall, this data suggests that ALK5 signaling and Smad3 nuclear accumulation is required for optimal enhancement of LPS plus IFNγ-induced NO production in astrocytes by TGF-β1.
Keywords: primary astrocytes, nitric oxide, LPS, IFNγ, ALK5, TGFβRI, heterogeneous
Introduction
Neuroinflammation occurs during the pathogenesis of several neurological diseases/disorders. One of the hallmarks of neuroinflammation is reactive gliosis, which is characterized by hypertrophy/hyperplasia of astrocytes and microglia (Hirsch et al. 2005; Malhotra et al. 1990; Ridet et al. 1997; Sofroniew 2009). Both microglia and astrocytes can modulate the inflammatory state by secreting either pro-inflammatory or anti-inflammatory mediators (Chung and Benveniste 1990; Lee et al. 1993; Meeuwsen et al. 2003; Romero et al. 1996). These mediators can act an autocrine and/or paracrine fashion to trigger the up- or down-regulation of other genes including the enzyme nitric oxide synthase-2 (NOS-2 or iNOS) (Hamby et al. 2008b; Romero et al. 1996).
Nos2 is the gene that encodes for the inducible isoform of NOS, the enzyme responsible for the catalytic conversion of L-arginine to the free radical nitric oxide (NO). Post-mortem brains of human patients who suffered from neurological diseases/disorders including Alzheimer's disease, Parkinson's disease, multiple sclerosis, cerebral ischemia and trauma (Fernandez-Vizarra et al. 2004; Forster et al. 1999; Katsuse et al. 2003; Liu et al. 2001; Luth et al. 2002; Sasaki et al. 2000; Wong et al. 2001) demonstrate iNOS immunoreactivity. Important to this study, immunoreactivity has been observed in astrocytes in post-mortem human brains from patients with neurodegenerative diseases (Katsuse et al. 2003; Liu et al. 2001; Luth et al. 2002; Wong et al. 2001), multiple sclerosis (Cross et al. 1998; Liu et al. 2001) and from those who suffered a acute neurological insults such as traumatic brain injury (Gahm et al. 2002; Luth et al. 2001). CNS tissue taken from mouse models of Alzheimer's diseases (Heneka et al. 2005), multiple sclerosis (Pozner et al. 2005; Tran et al. 1997) and traumatic brain injury (Luth et al. 2001; Wallace and Bisland 1994) also demonstrate marked astrocytic iNOS immunoreactivity. Notably, iNOS-derived NO products in these models have been demonstrated to be deleterious (Medeiros et al. 2007; Nathan et al. 2005; Wada et al. 1998)…
Although Nos2 is subject to regulation via several means, a potent regulator of its expression is the pleiotropic cytokine transforming growth factor-β1 (TGF-β1) (Nelson et al. 1991; Perrella et al. 1996; Perrella et al. 1994; Vodovotz and Bogdan 1994; Vodovotz et al. 1993). TGF-β1 belongs to the TGFβ superfamily. It signals by binding to TGFβRII which then heterodimerizes and transphosphorylates the TGFβ signaling receptor activin-like kinase (ALK) 5 or 1 – the expression of which is cell-type specific – initiating an intracellular serine/threonine kinase signaling cascade (de Caestecker 2004; Konig et al. 2005; Lux et al. 2006; Miyazawa et al. 2002) (Moustakas et al. 2001) (Attisano and Wrana 2002) Whereas ALK1 phosphorylates Smad1/5/8, ALK5 phosphorylates Smad2/3, each resulting in nuclear translocation of distinct signaling complexes producing disparate changes in gene expression (Miyazawa et al. 2002). Like iNOS, TGF-β1 is upregulated under neuropathological conditions (Finch et al. 1993; Flanders et al. 1998; Grammas and Ovase 2002; Huang et al. 1997; Krupinski et al. 1996; Krupinski et al. 1998; Lehrmann et al. 1998; Lehrmann et al. 1995; Logan et al. 1994; Morganti-Kossman et al. 1997; Morganti-Kossmann et al. 1999; Peress and Perillo 1995; Tanuma et al. 1997; Wang et al. 1995; Zetterberg et al. 2004). While it is traditionally thought of as having anti-inflammatory and neuroprotective functions (Buisson et al. 2003; Dhandapani and Brann 2003; Flanders et al. 1998; Kim et al. 2004), several recent studies reveal a pro-inflammatory role for TGF-β1 in the brain (Burton et al. 2002; Grammas and Ovase 2002; Lesne et al. 2003; Wyss-Coray et al. 1997a; Wyss-Coray et al. 1995; Wyss-Coray et al. 1997b). Recently, we've demonstrated that in a pure population of astrocytes – i.e., cultures devoid of microglia – that TGF-β1 potentiates NO production and iNOS expression induced by various pro-inflammatory stimuli (Hamby et al. 2006a; Hamby et al. 2008b). Interestingly, this enhancement occurs specifically by increasing the population of astrocytes that expressed the protein (Hamby et al. 2008a; Hamby et al. 2006a; Hamby et al. 2008b). Herein, we demonstrate that this TGF-β1-mediated enhancement in the pool of astrocytes expressing iNOS requires signaling of ALK5 and nuclear translocation of Smad3. Portions of this work have been published in abstract form (Hamby et al., 2007).
Materials and Methods
Primary Astrocyte Culture
Primary astrocytes were cultured from pooled cerebral cortices of CD1 pups (Charles River) or from single pups derived from Smad3 heterozygous [(+/-) × (+/-)] breeding pairs maintained congenic on a C57Bl/6 background (Ashcroft et al. 1999; Yang et al. 1999). Tail snips from individual pups were used to assess the genotype via PCR as described (Yang et al., 1999). An aseptic dissection of the cerebral cortices of postnatal day 1-3 mice was performed and cells mechanically and enzymatically dissociated prior to plating (Hamby et al. 2006a). Plating medium consisted of a media stock (MS) containing 10% fetal bovine serum (FBS; Hyclone), 10% iron-supplemented calf serum (CS; Hyclone), 10ng/ml epidermal growth factor (Invitrogen), 2mM L-glutamine (Mediatech), 50IU/ml penicillin and 50μg/ml streptomycin (Mediatech). MS was comprised of modified Eagle's medium (Earle's salt; Mediatech) supplemented with glucose and sodium bicarbonate to a final concentration of 25.7mM and 28.2mM, respectively. In most protocols, cells were plated at a density of 1-1.5 hemispheres/24-well plate or 1.2-1.6 hemispheres/6-well plate (both from Falcon Primaria, BD Biosciences). In experiments involving assessment of SMAD3 nuclear translocation, cells were first grown in T25 flasks, removed and then replated onto 8-well glass chamberslides (LabTek). Upon reaching confluence, astrocyte monolayers were treated with 8μM cytosine β-D-arabinofuranoside (Ara-C; Sigma-Aldrich) once for 5-6 days to eliminate the growth of any rapidly dividing cells such as microglia. Cultures were subsequently maintained in growth medium consisting of MS containing 10% CS, 2mM L-glutamine, 50IU/ml penicillin and 50μg/ml streptomycin. One day prior to experimentation, astrocyte cultures were treated with 75mM L-leucine methyl ester (LME; 1hr) to remove any residual microglia (Hamby et al. 2006a; Hamby et al. 2006b). Cells were grown, maintained, and stimulated at 37°C in a humidified atmosphere containing 6% CO2. All studies were performed on purified monolayers between 14-31 days in vitro (DIV).
Cytokine, Endotoxin and Drug Exposure
Cultures were treated with recombinant human TGF-β1 (R&D Systems; 3ng/ml) or its vehicle in DMEM supplemented with 5% CS, 2mM L-glutamine, 50IU/ml penicillin and 50μg/ml streptomycin. To induce Nos2, cells were stimulated with lipopolysaccharide (LPS; 0127:B8; 2μg/ml) plus recombinant mouse interferon-γ (IFNγ; R&D Systems; 3ng/ml). These concentrations were chosen as they provide a saturating response with respect to astrocytic NO production (Hamby et al., 2006a). In cultures derived from CD-1 mice, this combination of LPS plus IFNγ induces iNOS expression in roughly 5-10% of mouse pure primary astrocytes, which increases to 30-35% with TGF-β1 exposure (Hamby et al. 2008a; Hamby et al. 2006a). Similar results are found when IL-1β or TNF-α are used in lieu of LPS (Hamby et al., 2008a). In experiments assessing the effect of ALK5 activation, stock solutions of the ALK5 kinase inhibitors SB431542 or SB525334 (both from Tocris) were prepared in DMSO and diluted in incubation medium to their final concentration. All experimental conditions contained identical concentrations of DMSO, which never exceeded 0.15%.
NO Production
Production of nitric oxide (NO) was assessed indirectly by measurement of nitrite, an oxidative breakdown product of NO (Green et al. 1982; Hamby et al., 2006a,b). Nitrite accumulation was measured spectrophotometrically at 550nm in a microtiter plate reader (Thermolabs) and in most cases NO production was expressed as mean nitrite accumulation ± SEM. In the experiments involving single pup dissections, this normalization followed correction to the mean mg protein for each individual culture. This correction controlled for the variability in plating density between culture wells that unavoidably occurs with single pup dissections.
TGFβ Receptor mRNA Expression
mRNA expression was assessed via RT-PCR as previously described (Hamby et al. 2006b). cDNA samples (1μl) were amplified for 28 (ALK1, ALK5, TGFβRII) or 23 (β-actin) cycles in a Biorad iCycler using Taq DNA polymerase, PCR reagents (Invitrogen) and primers specific for either β-actin, ALK1, ALK5, or TGFβRII in a total reaction volume of 25μL. PCR amplimer pairs for analysis were as follows: ALK1, 5′-CTATGACATGGTACCCATGACC-3′ (sense) and 5′-ACACACTTTAGGCAGAG GAAGC-3′ (antisense); ALK5, 5′-ATCTTGTACCTTCTGATCCATCG-3′ (sense) and 5′-AGGAGCAGATATGAAGAGAGCAG-3′ (antisense); TGFβRII, 5′ACTTCACTT CCGGGTCATCATC-3′ (sense) and 5′-CATGAATATGGCCGAAGTGTTC-3′ (antisense); β-actin, 5′-GTGGGCCGCTCTAGGCACCAA-3′ (sense) and 5′-CTCTTTGATGTCACGCACGATTTC-3′ (antisense). PCR products were separated in a 2% agarose gel containing ethidium bromide (0.5μg/ml) and visualized with a UV transilluminator (UVP, Kodak). Ethidium bromide fluorescence was imaged using the Kodak Electrophoresis Documentation and Analysis System 120 and images processed using Adobe Photoshop.
TGFβ Receptors, Smad3 and iNOS Protein Analyses
Cultures were fixed with 4% paraformaldehyde (15 min) and then permeabilized using 0.25% Triton X-100 (PBS) for 7 min. Non-specific binding sites were then blocked via incubation with 10% normal goat serum (NGS) in PBS (25°C, 1 hr). TGF-β1 Receptors: A rabbit polyclonal TGFβRI (ALK5) antibody (8μg/ml, Santa Cruz Biotechnology) or TGFβRII antibody (2μg/ml, Santa Cruz Biotechnology) was added in PBS containing 5% NGS (4°C overnight) and the binding visualized with a Cy3-conjugated secondary antibody directed against rabbit IgG (7.5μg/ml; Jackson ImmunoResearch). DAPI (2μg/ml) was used to visualize nuclei. Images (40× magnification) were captured using a CRX digital camera (Digital Video Camera Co) mounted on an Olympus IX50 inverted microscope outfitted with epifluorescence and processed identically using Adobe Photoshop software. Smad3 and iNOS: For Smad3 single labeling, a rabbit polyclonal Smad3 antibody (2μg/ml, Santa Cruz Biotechnology) was added in PBS containing 5% NGS (4°C overnight) and its binding was visualized using either a Cy3-conjugated (7.5μg/ml; Jackson ImmunoResearch) or Alexa488-conjugated (10μg/ml; Molecular Probes) secondary antibody directed against rabbit IgG. For Smad3 and iNOS co-labeling experiments, a mouse monoclonal iNOS antibody (2.5μg/ml, BD Transduction Labs) and the aforementioned Smad3 antibody were added simultaneously in PBS containing 5% NGS (4°C overnight) and their binding visualized using Cy3-conjugated (7.5μg/ml; Jackson ImmunoResearch) and an Alexa488-conjugated (10μg/ml; Molecular Probes) secondary antibodies directed against mouse and rabbit IgG, respectively. Control experiments demonstrated that secondary antibodies showed no non-antigen cross-reactivity. DAPI (2μg/ml) was used to visualize nuclei. Images were captured using an AxioCam MR digital camera (Zeiss) mounted on an Axiovert 200 inverted microscope (Zeiss) and processed identically using Adobe Photoshop software.
Quantification of Smad3 nuclear accumulation
Images from five microscopic fields (40× magnification) were acquired. For each image, the total number of DAPI positive nuclei was automatically calculated using Scion NIH Image software while the number of cells exhibiting Smad3 nuclear accumulation was manually counted. The percentage of cells exhibiting Smad3 nuclear accumulation per image was calculated by dividing the number of cells exhibiting Smad3 nuclear accumulation by the total number of cells in each field (i.e., DAPI-labeled nuclei) followed by averaging the percentages from 5 fields/well. Data are expressed as the mean % cells with Smad3 nuclear accumulation + SEM. For statistical analyses, data were transformed prior to post-hoc analyses (Steel and Torrie 1980).
Statistical Analyses
All statistical analyses were performed using GraphPad Prism (Version 4.03, GraphPad Software, Inc.) as described in each figure legend. In all experiments, significance was assessed at p < 0.05.
Results
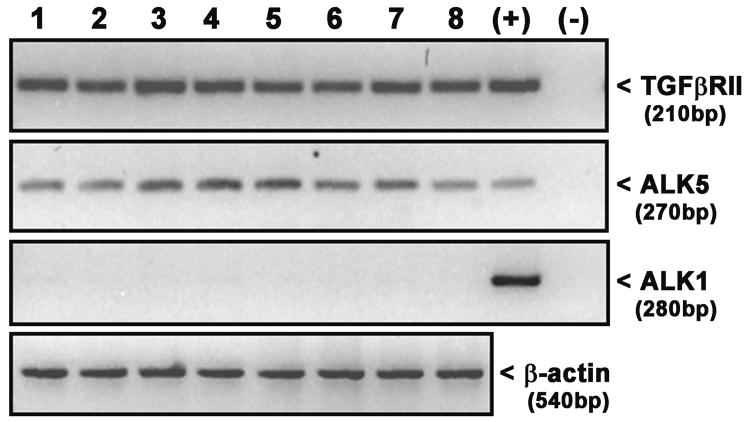
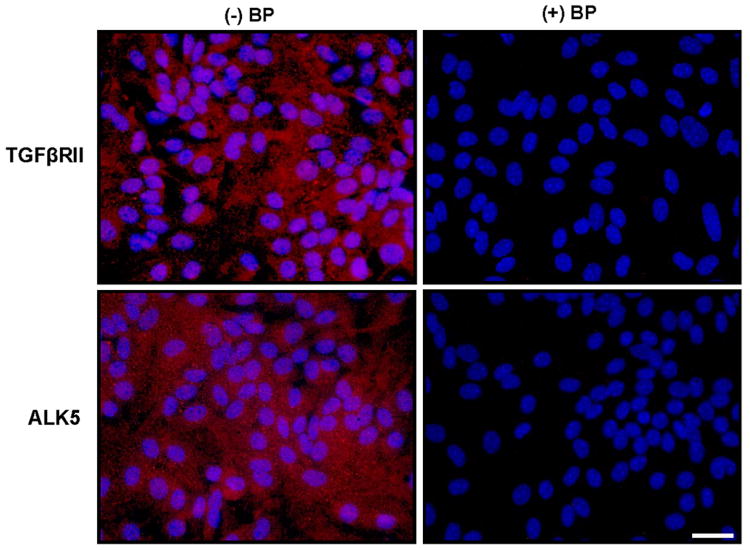
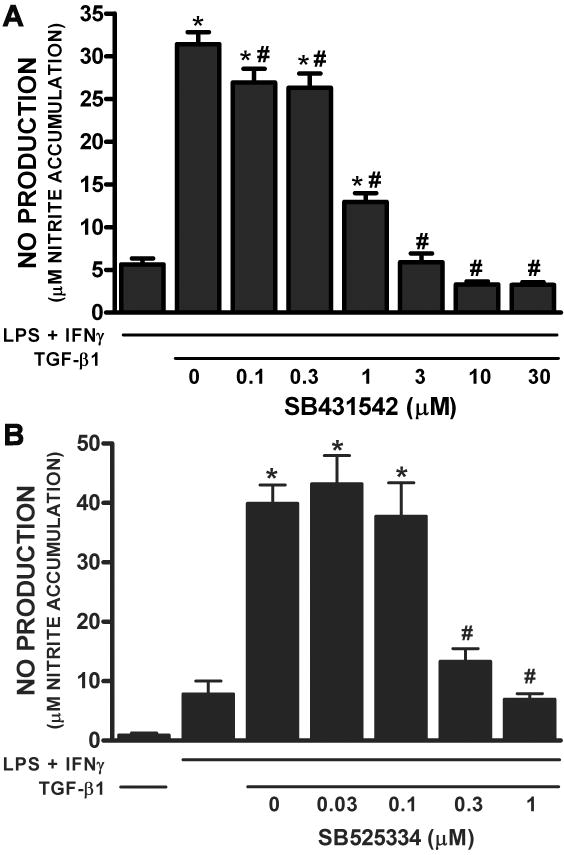
Astrocytes express mRNA for TGFβRII and the TGFβRI subunit ALK5 but not ALK1 as indicated via RT-PCR analyses (Fig. 1). Immunocytochemical analyses of TGFβRII and ALK5 expression reveals that astrocytes also homogeneously express both TGFβRII and ALK5 protein (∼100% of cells) staining (Fig. 2). No qualitative change in TGFβRII and ALK5 mRNA or protein expression levels was observed following a 24hr exposure to TGF-β1 (Fig. 1, data not shown, respectively). Consistent with its expression, the TGF-β1-mediated enhancement in LPS plus IFNγ-induced NO production required ALK5 signaling as evidenced by the concentration-dependent diminution in NO production that occurred in the presence of the ALK5 kinase inhibitors SB431542 and SB525334 (Figure 3).
Figure 1. Analysis of TGFβ receptor mRNA expression in murine primary astrocytes.
Astroctye cultures were exposed to TGF-β1 (3ng/ml) or its vehicle for 24 hr prior to addition of medium alone or that containing LPS + IFNγ (final concentration= 2μg/ml + 3ng/ml, respectively; 4hr). Thereafter, total RNA was isolated and TGFβRII, ALK5 and ALK1 mRNA expression was assessed via RT-PCR. Lanes 1,5, Basal; Lanes 2,6, TGF-β1; Lanes 3,7, LPS + IFNγ; Lanes 4,8, TGF-β1 + LPS +IFNγ. Positive control (+) used was mRNA isolated from brain microendothelial cultures (BMECs). Negative control (-) is H2O. β-actin mRNA expression was also assessed in astrocyte samples to demonstrate RNA integrity and approximate equal loading. Data from two separate experiments (lanes 1-4 and 5-8, respectively) from two separate dissections are shown.
Figure 2. Immunocytochemical assessment of TGFβ receptor expression in murine astrocytes.
Naïve astrocyte cultures were fixed and immunolabeled for TGFβRII (red) or ALK5 (red) in the presence [(+) BP; negative control] or absence [(-) BP] of their respective blocking peptides followed by DAPI counterstaining (blue) to illustrate the number of nuclei per field. A representative photomicrograph (40× magnification) is shown per treatment condition. Scale bar= 40μm.
Figure 3. Effect of ALK5 inhibition on astrocytic iNOS-derived NO production.
Primary astrocytes were treated with the indicated concentrations of either SB431542 (A) or SB525334 (B) for 1 hr prior to addition of medium containing vehicle or TGF-β1 (final = 3ng/ml). Twenty-four hr later, cultures were cultures were spiked with medium alone or that containing LPS plus IFNγ (final = 2μg/ml and 3ng/ml, respectively). (A) Twenty-four hr or (B) 29-32hr later, cell culture supernatants were collected and NO production (mean μM nitrite accumulation + SEM) was assessed. (A), n = 6 cultures from 2 separate dissections, (B) n=10-11 from 4 separate dissections. (*) indicates a significant increase over LPS plus IFNγ alone, whereas (#) indicates a significant diminution from control as determined by one-way ANOVA followed by the Student Newman Keul's post-hoc test. Significance was assessed at p<0.05.
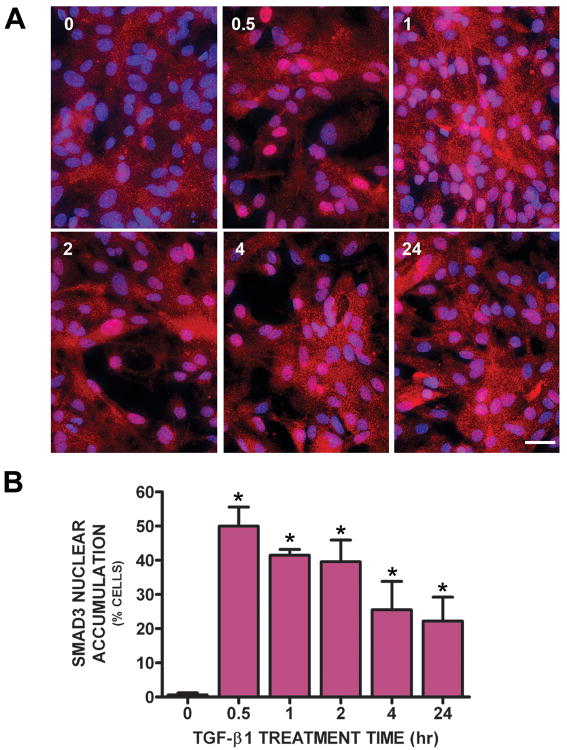
Signaling via ALK5 often, but not always, involves downstream Smads (Smad2/3) (Dai et al. 2003; Engel et al. 1999; Yu et al. 2002). To examine whether Smad3 was activated in primary astrocytes following TGF-β1 treatment, its nuclear accumulation was assessed immunocytochemically. Smad3 was excluded from astrocyte nuclei (i.e., was predominantly cytosolic) in vehicle-treated cultures (Figure 4A,B). However, 30min after TGF-β1 administration – the earliest time point evaluated – Smad3 accumulation was observed in approximately 50% of astrocyte nuclei (Fig. 4 A,B). Smad3 was still present in ∼20% of astrocytic nuclei 24 hr post-TGF-β1 addition (Fig. 4 A,B).
Figure 4. Immunocytochemical assessment of Smad3 nuclear translocation following TGF-β1 treatment.
(A) Astrocyte cultures were treated with either vehicle (0 hr) or 3ng/ml TGF-β1. After the indicated treatment times, cultures were fixed and immunolabeled for Smad3 (red) followed by DAPI counterstaining (blue) to illustrate the number of nuclei per field. A representative photomicrograph (40× magnification) is shown for each treatment condition. Scale bar = 40μm. (B) The percentage of cells exhibiting Smad3 nuclear accumulation per treatment condition was calculated (from a total of ∼100 cells present /field of view) and data plotted as mean % cells with Smad3 nuclear accumulation + SEM (n = 3-4 wells from 2 separate dissections). (*) indicates a significant increase in % of cells with Smad3 nuclear accumulation compared to control (0; non-TGF-β1 treated cells) as determined by one-way ANOVA followed by the Student Newman Keul's post-hoc test following appropriate transformation of the percentage data. Significance was assessed at p<0.05.
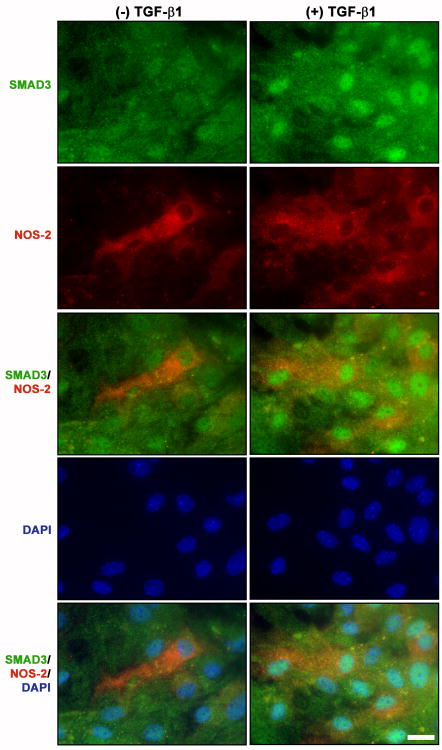
Given that nuclear Smad3 was evident at the time of iNOS induction, we next tested whether Smad3 nuclear accumulation correlated with astrocyte iNOS expression following TGF-β1 treatment. As expected, iNOS immunoreactivity resulting from exposure to LPS plus IFNγ alone occurred independently of Smad3 nuclear accumulation (Fig. 5; left panel). However, the majority of cells expressing iNOS also exhibited nuclear localization of Smad3 in cultures treated with LPS plus IFNγ in the presence of TGF-β1 (Fig. 5, right panel, arrows vs. arrowheads).
Figure 5. Immunocytochemical assessment of astrocytes exhibiting Smad3 nuclear accumulation and iNOS expression.
Cultures were treated with either vehicle [(-) TGF-β1] or TGF-β1 [(+) TGF-β1; 3ng/ml] for 24 hr prior to the addition of medium containing LPS plus IFNγ (final = 2μg/ml and 3ng/ml, respectively). Eight hr later, cultures were fixed and immunolabeled for SMAD3 (green) and iNOS (red) followed by DAPI counterstaining (blue) to illustrate the number of nuclei per field. Representative photomicrographs (63× magnification) from at least three experiments are shown for each treatment. Scale bar = 20μm.
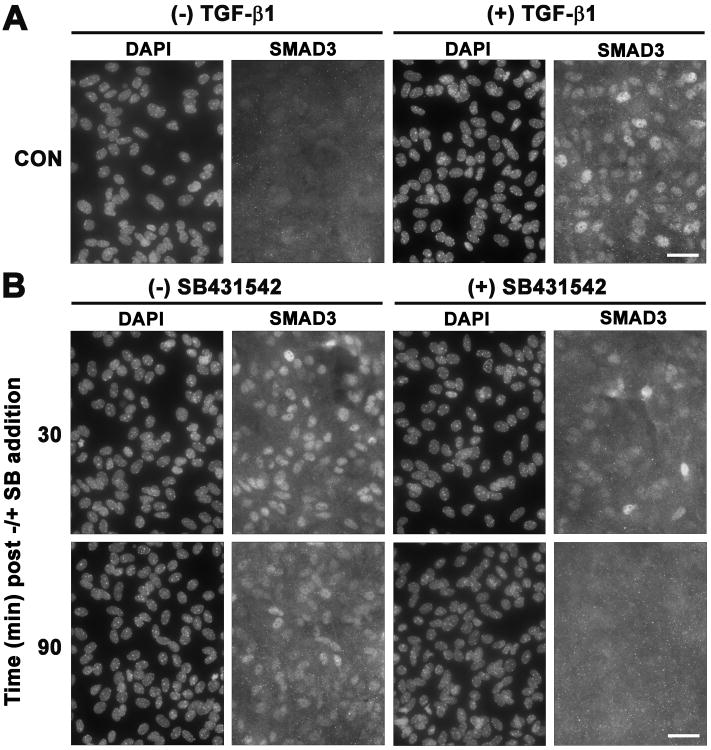
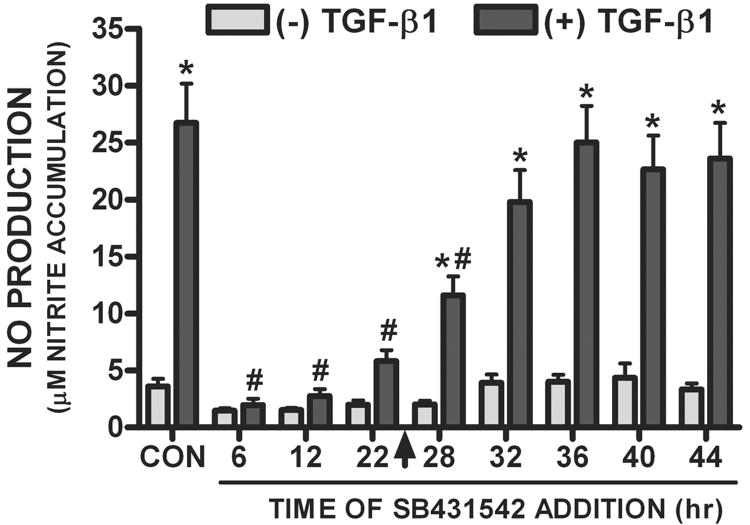
To determine the time-frame in which ALK5 signaling was required to facilitate Nos2 induction, SB431542 (20μM) was added at various times after TGF-β1 administration and Smad3 translocation and NO production were assessed. Addition of SB431542 (20μM) rapidly and completely reversed the TGF-β1-mediated nuclear translocation of Smad3 (Fig. 6), demonstrating its effectiveness in terminating ALK5 signaling. When ALK5 – hence Smad3 activation – was inhibited with SB431542 (20μM) at any time prior to LPS plus IFNγ addition (arrowhead), the TGF-β1-mediated enhancement in NO production was blocked (Fig. 7). However, the TGF-β1-mediated enhancement in astrocytic NO production persisted when the ALK5 inhibitor was added after (4-20hr) LPS plus IFNγ addition (Fig. 7). Thus, ALK5 signaling is required at the time of iNOS induction.
Figure 6. Time course of Smad3 nuclear translocation after SB431542 addition.
Astrocyte cultures were treated with either vehicle [(-) TGF-β1] or TGF-β1 [3ng/ml; (+) TGF-β1] for 60 min. Thereafter, cultures were either fixed (A) or exposed to vehicle [DMSO; (-) SB431542] or SB431542 [20μM; (+) SB431542] (B). Thirty or 90 min later, cultures were fixed (A,B), immunolabeled for Smad3 and counterstained for DAPI. Images from the same field of view per treatment group are shown. A representative photomicrograph (63× magnification) from at least three experiments is shown for each condition. Scale bar= 40μm.
Figure 7. Effect of SB431542 following TGF-β1 addition on NO production.
Cultures were exposed to either vehicle [(-) TGF-β1] or TGF-β1 [(+) TGF-β1; 3ng/ml] followed by the addition of SB431542 (20μM) at the indicated times. At 24 hr (arrow), medium containing LPS plus IFNγ (final = 2μg/ml and 3ng/ml, respectively) was added to cultures. Twenty-four hr later, culture supernatants were collected and NO production (mean μM nitrite accumulation + SEM) was assessed (n = 6 from 3 separate dissections). (*) indicates a significant increase due to TGF-β1, whereas (#) indicates a significant within group SB431542-mediated diminution as compared to CON. Significance, assessed at p<0.05, was determined by two-way ANOVA followed by the Bonferroni's post-hoc test.
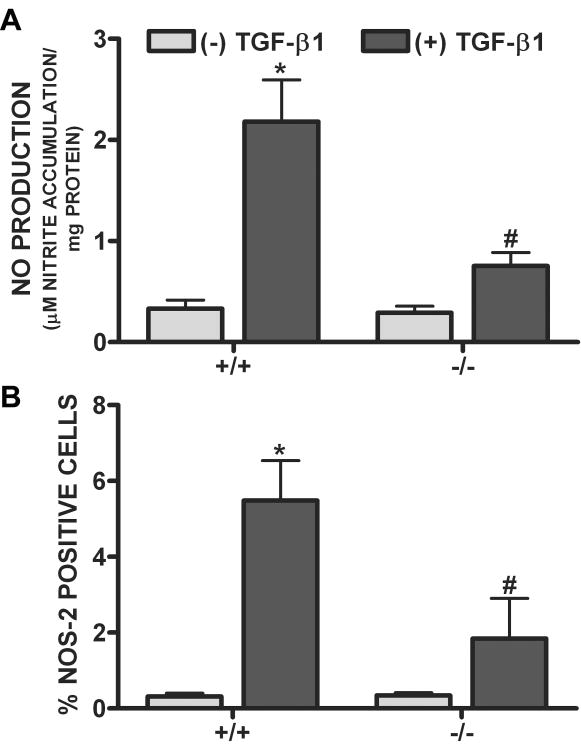
To specifically examine whether Smad3 was required for the TGF-β1-mediated enhancement in Nos2 induction, iNOS expression and NO production were examined and compared in astrocyte cultures derived from wild-type (+/+) and Smad3 null (-/-) mice (Fig. 8). While addition of LPS plus IFNγ led to comparable levels of iNOS expression and NO production in astrocyte cultures derived from both wildtype and Smad3 null mice, the TGF-β1-mediated enhancement was largely ablated in cultures lacking Smad3 (Fig. 8).
Figure 8. Effect of TGF-β1 in Smad3 null versus wildtype-derived astrocytes.
Astrocyte cultures derived from either Smad3 null (-/-) or wildtype (+/+) littermates were treated with vehicle [(-) TGF-β1] or TGF-β1 [(+) TGF-β1; 3ng/ml] for 24 hr prior to the addition of 2μg/ml LPS plus 3ng/ml IFNγ. (A) Twelve-14 hr later, culture supernatants were collected and NO production (mean μM nitrite accumulation/mg protein + SEM) was assessed (n = 15-18 from 3-4 separate dissections). (*) indicates a significant within group increase, whereas (#) indicates a significant between group diminution as determined by two-way ANOVA followed by the Bonferroni's post-hoc test. (B) Thereafter, cultures were fixed and immunolabeled for iNOS followed by DAPI counterstaining. The percentage of cells expressing iNOS per culture well was calculated and data plotted as mean % iNOS positive cells + SEM (n = 6 from 3 separate dissections). Following transformation of the percentage data, significance was assessed as in (A).
Discussion
The intracellular effectors and biological outcome of TGF-β1 signaling vary depending on the identity of TGFβRI, which can be either ALK5 or ALK1 (Miyazawa et al. 2002). When activated, ALK1 phosphorylates Smad1/5/8 whereas ALK5 phosphorylates Smad2/3. Herein, we find that the ability of TGF-β1 to enhance astrocytic Nos2 expression and NO production (Hamby et. al, 2006, 2008a,b) requires Smad3, the activation of which follows ALK5 signaling. Further, results support the notion that ALK5 signaling must occur prior to and at least concomitant with the induction stimuli in order for the TGF-β1-mediated enhancement of iNOS induction to occur.
Demonstration of expression of TGFβRII and ALK5, but not ALK1, mRNA in purified primary mouse astrocyte cultures (Fig. 1) suggests that the TGF-β1-mediated effect occurs via ALK5 signaling. This is in agreement with a previous report assessing TGFβRI and TGFβRII expression in astrocytes cultures derived from rat (Konig et al. 2005). The punctate staining pattern of TGFβRII protein found herein (Fig. 2) is also similar to that described in mouse astrocytes (Sousa et al. 2009). Likewise, the staining pattern for ALK5 protein expression in astrocytes (Fig. 2) agrees with the immunocytochemical assessments of ALK5 in other cell types (Riser et al. 1999). Finally, ALK5 inhibition, afforded by the addition of the pharmacological inhibitors SB431542 or SB525334 to astrocyte cultures (Grygielko et al. 2005; Hjelmeland et al. 2004; Laping et al. 2002), completely suppresses the TGF-β1-mediated enhancement in NO production (Fig. 3), confirming that TGF-β1 enhances iNOS expression through ALK5.
Consistent with signaling through ALK5, TGF-β1 treatment of astrocytes leads to activation and nuclear translocation of Smad3, a process blocked by inhibition of ALK5 kinase activity (Fig. 6). In astrocytes, the accumulation of Smad3 in the nucleus following TGF-β1 stimulation not only occurs fairly rapidly but is sustained (Fig. 4, 5). Although it is possible for Smad3 to remain in the nucleus for a long period of time, prolonged Smad activation in endothelial cells has previously been attributed to the continuous shuttling of Smads between the nucleus and cytoplasm in response to sustained receptor activation (Inman et al. 2002). Our results suggest that a similar mechanism may be occurring in astrocytes as astrocyte Smad3 nuclear accumulation induced by TGF-β1 rapidly dissipates following treatment with SB431542 (Fig. 6).
Notably, not all astrocytes exhibit Smad3 activation (i.e., nuclear localization) when stimulated with TGF-β1 (Fig 4). Likewise, the ability of TGF-β1 to up-regulate astrocytic iNOS expression in purified murine primary astrocyte cultures treated with LPS plus IFNγ is not uniform (Hamby et al. 2006a). Importantly, the percentage of cells that show nuclear translocation of Smad3 (Fig. 4) and iNOS expression (Hamby et al. 2006a) following stimulation with all three mediators is very similar, suggesting that differential Smad3 signaling could account for the heterogeneous enhancement of iNOS by TGF-β1. The correlation of Smad3 with iNOS in cells treated with TGF-β1, LPS and IFNγ support this notion (Fig. 5). Importantly, when Smad3 is depleted from the nucleus just prior to Nos2 induction, the TGF-β1-mediated enhancement in NO production is ablated (Fig. 7), suggesting that Smad3 signaling is required. In support, the ability of TGF-β1 to facilitate iNOS induction in Smad3 null-derived astrocytes was largely ablated (Fig. 8). Altogether, data indicate that Smad3 is required to be in the nucleus at the time of Nos2 induction in order for TGF-β1 to enhance the total number of cells that express iNOS in response to LPS plus IFNγ stimulation.
The Smad3 nuclear translocation found in TGF-β1-treated astrocytes herein is consistent with biochemical evidence showing that astrocytes derived from the Smad2/3 specific Smad binding element (SBE)-luciferase reporter mice display increased luciferase activity when treated with TGF-β1 (Lin et al. 2005). The present data extend these findings by demonstrating at the single cell level that only a fraction of astrocytes exhibit Smad3 nuclear accumulation following TGF-β1 treatment. The reason for the differential activation of Smad3 in the astrocyte population (Fig. 4) despite homogeneous expression of TGFβ receptors (Fig. 2) is presently not known. However, heterogeneous activation of Smad3 in cultured cells is not unprecedented and has been seen in TGF-β1 treated cultures of rat ovarian granulosa cells (Xu et al. 2002). Interestingly, heterogeneous I-Smad, Smad7 expression has also been shown to exist in other cell types including mouse endothelial and smooth muscle cells (Banas et al. 2007) and human fibroblasts (Ishida et al. 2006). Since, Smad7 competes with Smad2/3 for phosphorylation which can, through the recruitment of additional mediators, result in the inactivation of TGFβRI and even elicit TGFβ receptor degradation (Yan et al. 2009), it is possible that differential Smad7 expression/localization might account for inability of TGF-β1 to facilitate Smad3 nuclear accumulation and Nos2 induction in a subset of astrocytes. Future studies to understand the mechanism underlying heterogeneous Smad3 activation in astrocytes would be worthwhile.
The requirement for nuclear localization of Smad3 at the time of iNOS induction is consistent with a role for Smad3 in facilitating Nos2 transcription. However, the murine iNOS promoter does not have a canonical Smad-binding element (SBE), though Smad3 has been shown to bind to non-canonical elements including AP-1 (Zhang et al. 1998), which is present on the iNOS promoter (Xie et al. 1993). Additionally, Smad3 has been demonstrated to facilitate gene transcription without binding to DNA directly but, rather, via interaction with other transcription factors and co-activators in a transcriptional activation complex (Heldin et al. 2009). Notably, Smad3 has been shown to facilitate transcription through complexes with AP-1 and NFκB, both which are known to be involved in facilitating Nos2 transcription (Dhandapani et al. 2003; Zhang et al. 1998; Zhu et al. 2004).
In sum, we find that TGF-β1 modulates the induction of iNOS by inflammatory mediators in an ALK5/Smad3-dependent manner. These data additionally provide one explanation that accounts for the heterogeneous enhancement in LPS plus IFNγ-induced Nos2 expression that follows TGF-β1 treatment of astrocytes in vitro, namely, differential Smad3 activation. Additionally, results described herein may explain, in part, the reported heterogeneous expression of iNOS that occurs in astrocytes under neuroinflammatory conditions in vivo (Luth et al. 2001; Oleszak et al. 1998). Future studies are necessary to determine the exact mechanism for both the heterogeneous activation of Smad3 and its facilitation of Nos2 expression.
Acknowledgments
We'd like to thank Dr. Steve Clark (University of Connecticut Health Center) for generously providing us Smad3 heterozygous mice and Tracy Uliasz for her excellent technical assistance. This work was supported by grants from the NINDS: NS036812 and NS051445. MEH was supported via 5T32NS041224 during part of her training. Dr. Hamby's current address is UCLA Medical School, Neuroscience Research Building Suite 504, 635 Charles E. Young Dr. South, Los Angeles, CA 90095.
References
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1(5):260–6. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Banas MC, Parks WT, Hudkins KL, Banas B, Holdren M, Iyoda M, Wietecha TA, Kowalewska J, Liu G, Alpers CE. Localization of TGF-{beta} Signaling Intermediates Smad2, 3, 4, and 7 in Developing and Mature Human and Mouse Kidney. J Histochem Cytochem. 2007;55(3):275–285. doi: 10.1369/jhc.6A7083.2006. [DOI] [PubMed] [Google Scholar]
- Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, Vivien D. Transforming growth factor-beta and ischemic brain injury. Cell Mol Neurobiol. 2003;23(4-5):539–50. doi: 10.1023/A:1025072013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton T, Liang B, Dibrov A, Amara F. Transcriptional activation and increase in expression of Alzheimer's beta-amyloid precursor protein gene is mediated by TGF-beta in normal human astrocytes. Biochem Biophys Res Commun. 2002;295(3):702–12. doi: 10.1016/s0006-291x(02)00724-6. [DOI] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144(8):2999–3007. [PubMed] [Google Scholar]
- Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J Neuroimmunol. 1998;88(1-2):45–56. doi: 10.1016/s0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Dai C, Yang J, Liu Y. Transforming Growth Factor-Î21 Potentiates Renal Tubular Epithelial Cell Death by a Mechanism Independent of Smad Signaling. Journal of Biological Chemistry. 2003;278(14):12537–12545. doi: 10.1074/jbc.M300777200. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39(1):13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Hadman M, De Sevilla L, Wade MF, Mahesh VB, Brann DW. Astrocyte protection of neurons: role of transforming growth factor-beta signaling via a c-Jun-AP-1 protective pathway. J Biol Chem. 2003;278(44):43329–39. doi: 10.1074/jbc.M305835200. [DOI] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK Signaling in Transforming Growth Factor-Î2-mediated Transcription. Journal of Biological Chemistry. 1999;274(52):37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Encinas JM, Serrano J, Bentura ML, Munoz P, Martinez-Murillo R, Rodrigo J. Expression of nitric oxide system in clinically evaluated cases of Alzheimer's disease. Neurobiol Dis. 2004;15(2):287–305. doi: 10.1016/j.nbd.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM. TGF-beta 1 is an organizer of responses to neurodegeneration. J Cell Biochem. 1993;53(4):314–22. doi: 10.1002/jcb.240530408. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol. 1998;54(1):71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Forster C, Clark HB, Ross ME, Iadecola C. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathol (Berl) 1999;97(3):215–20. doi: 10.1007/s004010050977. [DOI] [PubMed] [Google Scholar]
- Gahm C, Holmin S, Mathiesen T. Nitric oxide synthase expression after human brain contusion. Neurosurgery. 2002;50(6):1319–26. doi: 10.1097/00006123-200206000-00024. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer's disease brain. Am J Pathol. 2002;160(5):1583–7. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Gragnolati AR, Hewett SJ, Hewett JA. TGFbeta1 and TNFalpha potentiate nitric oxide production in astrocyte cultures by recruiting distinct subpopulations of cells to express NOS-2. Neurochem Int. 2008a;52(6):962–71. doi: 10.1016/j.neuint.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006a;54(6):566–77. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 reduces the heterogeneity of astrocytic cyclooxygenase-2 and nitric oxide synthase-2 gene expression in a stimulus-independent manner. Prostaglandins Other Lipid Mediat. 2008b;85(3-4):115–24. doi: 10.1016/j.prostaglandins.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006b;150(1):128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Landström M, Moustakas A. Mechanism of TGF-[beta] signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Current Opinion in Cell Biology. 2009;21(2):166. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson's disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Hjelmeland MD, Hjelmeland AB, Sathornsumetee S, Reese ED, Herbstreith MH, Laping NJ, Friedman HS, Bigner DD, Wang XF, Rich JN. SB-431542, a small molecule transforming growth factor-Î2-receptor antagonist, inhibits human glioma cell line proliferation and motility. Molecular Cancer Therapeutics. 2004;3(6):737–745. [PubMed] [Google Scholar]
- Huang CC, Chang YC, Chow NH, Wang ST. Level of transforming growth factor beta 1 is elevated in cerebrospinal fluid of children with acute bacterial meningitis. J Neurol. 1997;244(10):634–8. doi: 10.1007/s004150050159. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Hill CS. Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell. 2002;10(2):283–94. doi: 10.1016/s1097-2765(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Ishida W, Mori Y, Lakos G, Sun L, Shan F, Bowes S, Josiah S, Lee WC, Singh J, Ling LE, et al. Intracellular TGF-[beta] Receptor Blockade Abrogates Smad-Dependent Fibroblast Activation In Vitro and In Vivo. J Invest Dermatol. 2006;126(8):1733. doi: 10.1038/sj.jid.5700303. [DOI] [PubMed] [Google Scholar]
- Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathology. 2003;23(1):9–15. doi: 10.1046/j.1440-1789.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172(11):7015–23. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- Konig HG, Kogel D, Rami A, Prehn JH. TGF-{beta}1 activates two distinct type I receptors in neurons: implications for neuronal NF-{kappa}B signaling. J Cell Biol. 2005;168(7):1077–86. doi: 10.1083/jcb.200407027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27(5):852–7. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Vodovotz Y, Li C, Slowik A, Beevers D, Flanders KC, Lip G, Kumar P, Szczudlik A. Inducible nitric oxide production and expression of transforming growth factor-beta1 in serum and CSF after cerebral ischaemic stroke in man. Nitric Oxide. 1998;2(6):442–53. doi: 10.1006/niox.1998.0204. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, et al. Inhibition of Transforming Growth Factor (TGF)-Î21–Induced Extracellular Matrix with a Novel Inhibitor of the TGF-Î2 Type I Receptor Kinase Activity: SB-431542. Molecular Pharmacology. 2002;62(1):58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993;46(1-2):19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24(4):437–48. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Kiefer R, Finsen B, Diemer NH, Zimmer J, Hartung HP. Cytokines in cerebral ischemia: expression of transforming growth factor beta-1 (TGF-beta 1) mRNA in the postischemic adult rat hippocampus. Exp Neurol. 1995;131(1):114–23. doi: 10.1016/0014-4886(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Lesne S, Docagne F, Gabriel C, Liot G, Lahiri DK, Buee L, Plawinski L, Delacourte A, MacKenzie ET, Buisson A, et al. Transforming growth factor-beta 1 potentiates amyloid-beta generation in astrocytes and in transgenic mice. J Biol Chem. 2003;278(20):18408–18. doi: 10.1074/jbc.M300819200. [DOI] [PubMed] [Google Scholar]
- Lin AH, Luo J, Mondshein LH, ten Dijke P, Vivien D, Contag CH, Wyss-Coray T. Global Analysis of Smad2/3-Dependent TGF-{beta} Signaling in Living Mice Reveals Prominent Tissue-Specific Responses to Injury. J Immunol. 2005;175(1):547–554. doi: 10.4049/jimmunol.175.1.547. [DOI] [PubMed] [Google Scholar]
- Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158(6):2057–66. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A. Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci. 1994;6(3):355–63. doi: 10.1111/j.1460-9568.1994.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Holzer M, Gartner U, Staufenbiel M, Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 2001;913(1):57–67. doi: 10.1016/s0006-8993(01)02758-5. [DOI] [PubMed] [Google Scholar]
- Luth HJ, Munch G, Arendt T. Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation. Brain Res. 2002;953(1-2):135–43. doi: 10.1016/s0006-8993(02)03280-8. [DOI] [PubMed] [Google Scholar]
- Malhotra SK, Shnitka TK, Elbrink J. Reactive astrocytes--a review. Cytobios. 1990;61(246-247):133–60. [PubMed] [Google Scholar]
- Medeiros R, Prediger RD, Passos GF, Pandolfo P, Duarte FS, Franco JL, Dafre AL, Di Giunta G, Figueiredo CP, Takahashi RN, et al. Connecting TNF-alpha signaling pathways to iNOS expression in a mouse model of Alzheimer's disease: relevance for the behavioral and synaptic deficits induced by amyloid beta protein. J Neurosci. 2007;27(20):5394–404. doi: 10.1523/JNEUROSCI.5047-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43(3):243–53. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7(12):1191–204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2(2):133–6. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, Kossmann T. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma. 1999;16(7):617–28. doi: 10.1089/neu.1999.16.617. [DOI] [PubMed] [Google Scholar]
- Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, et al. Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202(9):1163–9. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BJ, Ralph P, Green SJ, Nacy CA. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991;146(6):1849–57. [PubMed] [Google Scholar]
- Peress NS, Perillo E. Differential expression of TGF-beta 1, 2 and 3 isotypes in Alzheimer's disease: a comparative immunohistochemical study with cerebral infarction, aged human and mouse control brains. J Neuropathol Exp Neurol. 1995;54(6):802–11. doi: 10.1097/00005072-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Perrella MA, Patterson C, Tan L, Yet SF, Hsieh CM, Yoshizumi M, Lee ME. Suppression of interleukin-1beta-induced nitric-oxide synthase promoter/enhancer activity by transforming growth factor-beta1 in vascular smooth muscle cells. Evidence for mechanisms other than NF-kappaB. J Biol Chem. 1996;271(23):13776–80. doi: 10.1074/jbc.271.23.13776. [DOI] [PubMed] [Google Scholar]
- Perrella MA, Yoshizumi M, Fen Z, Tsai JC, Hsieh CM, Kourembanas S, Lee ME. Transforming growth factor-beta 1, but not dexamethasone, down-regulates nitric-oxide synthase mRNA after its induction by interleukin-1 beta in rat smooth muscle cells. J Biol Chem. 1994;269(20):14595–600. [PubMed] [Google Scholar]
- Pozner RG, Berria MI, Negrotto S, Schattner M, Gomez RM. Differential astrocyte response to Theiler's murine encephalomyelitis virus infection. Intervirology. 2005;48(5):279–84. doi: 10.1159/000085095. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–7. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Riser BL, Ladson-Wofford S, Sharba A, Cortes P, Drake K, Guerin CJ, Yee J, Choi ME, Segarini PR, Narins RG. TGF-[bgr] receptor expression and binding in rat mesangial cells: Modulation by glucose and cyclic mechanical strain. Kidney Int. 1999;56(2):428. doi: 10.1046/j.1523-1755.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- Romero LI, Tatro JB, Field JA, Reichlin S. Roles of IL-1 and TNF-alpha in endotoxin-induced activation of nitric oxide synthase in cultured rat brain cells. Am J Physiol. 1996;270(2 Pt 2):R326–32. doi: 10.1152/ajpregu.1996.270.2.R326. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shibata N, Komori T, Iwata M. iNOS and nitrotyrosine immunoreactivity in amyotrophic lateral sclerosis. Neurosci Lett. 2000;291(1):44–8. doi: 10.1016/s0304-3940(00)01370-7. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences. 2009;32(12):638. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VDO, Almeida JC, Eller CM, Gomes FVCA. Characterization of Tgf-Î21 Type Ii Receptor Expression in Cultured Cortical Astrocytes. Vitro Cellular & Developmental Biology - Animal. 2009;42(7):171. doi: 10.1290/0602013.1. [DOI] [PubMed] [Google Scholar]
- Steel R, Torrie J. Principles and Procedures of Statistics: A Biometrical Approach. New York: McGraw-Hill Book Co; 1980. p. 633. [Google Scholar]
- Tanuma N, Kojima T, Shin T, Aikawa Y, Kohji T, Ishihara Y, Matsumoto Y. Competitive PCR quantification of pro- and anti-inflammatory cytokine mRNA in the central nervous system during autoimmune encephalomyelitis. J Neuroimmunol. 1997;73(1-2):197–206. doi: 10.1016/s0165-5728(96)00199-3. [DOI] [PubMed] [Google Scholar]
- Tran EH, Hardin-Pouzet H, Verge G, Owens T. Astrocytes and microglia express inducible nitric oxide synthase in mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;74(1-2):121–9. doi: 10.1016/s0165-5728(96)00215-9. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Bogdan C. Control of nitric oxide synthase expression by transforming growth factor-beta: implications for homeostasis. Prog Growth Factor Res. 1994;5(4):341–51. doi: 10.1016/0955-2235(94)00004-5. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993;178(2):605–13. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Kraydieh S, Busto R, Dietrich WD. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998;43(6):1427–36. doi: 10.1097/00006123-199812000-00096. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Bisland SK. NADPH-diaphorase activity in activated astrocytes represents inducible nitric oxide synthase. Neuroscience. 1994;59(4):905–19. doi: 10.1016/0306-4522(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, White RF, Barone FC, Feuerstein GZ. Transforming growth factor-beta 1 exhibits delayed gene expression following focal cerebral ischemia. Brain Res Bull. 1995;36(6):607–9. doi: 10.1016/0361-9230(94)00243-t. [DOI] [PubMed] [Google Scholar]
- Wong A, Luth HJ, Deuther-Conrad W, Dukic-Stefanovic S, Gasic-Milenkovic J, Arendt T, Munch G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer's disease. Brain Res. 2001;920(1-2):32–40. doi: 10.1016/s0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Borrow P, Brooker MJ, Mucke L. Astroglial overproduction of TGF-beta 1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol. 1997a;77(1):45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol. 1995;147(1):53–67. [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer's disease. Nature. 1997b;389(6651):603–6. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177(6):1779–84. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Oakley J, McGee EA. Stage-Specific Expression of Smad2 and Smad3 During Folliculogenesis. Biology of Reproduction. 2002;66(6):1571–1578. doi: 10.1095/biolreprod66.6.1571. [DOI] [PubMed] [Google Scholar]
- Yan X, Liu Z, Chen Y. Regulation of TGF-beta signaling by Smad7. Acta Biochim Biophys Sin (Shanghai) 2009;41(4):263–72. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. Embo J. 1999;18(5):1280–91. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. TGF-[beta] receptor-activated p38 MAP kinase mediates Smad-independent TGF-[beta] responses. EMBO J. 2002;21(14):3749. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Andreasen N, Blennow K. Increased cerebrospinal fluid levels of transforming growth factor-beta1 in Alzheimer's disease. Neurosci Lett. 2004;367(2):194–6. doi: 10.1016/j.neulet.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394(6696):909–13. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Culmsee C, Klumpp S, Krieglstein J. Neuroprotection by transforming growth factor-beta1 involves activation of nuclear factor-kappaB through phosphatidylinositol-3-OH kinase/Akt and mitogen-activated protein kinase-extracellular-signal regulated kinase1,2 signaling pathways. Neuroscience. 2004;123(4):897–906. doi: 10.1016/j.neuroscience.2003.10.037. [DOI] [PubMed] [Google Scholar]