Abstract
Spontaneously Hypertensive Rats (SHRs), a commonly-used animal model of ADHD, exhibit little habituation of the orienting response to repeated presentations of a non-reinforced visual stimulus. However, SHRs that have access to a running wheel for 5, 10, or 21 days exhibit robust habituation that is indistinguishable from normo-active rats. Two days of exercise, in comparison, was not sufficient to affect habituation. Here we tested the hypothesis that the effect of exercise on orienting behavior in SHRs is mediated by changes in noradrenergic function. In Experiment 1, we found that 5, 10, or 21 days of access to a running wheel, but not 2 days, significantly reduced levels of the norepinephrine transporter (NET) in medial prefrontal cortex. In Experiment 2, we tested for a causal relationship between changes in noradrenergic function and orienting behavior by blocking noradrenergic receptors during exercise. Rats that received propranolol (beta adrenergic/noradrenergic receptor blocker) during 10 days of exercise failed to exhibit an exercise-induced reduction in orienting behavior. The results inform a growing literature regarding the effects of exercise on behavior and the potential use of exercise as a treatment for mental disorders.
Keywords: habituation, norepinephrine transporter, prefrontal cortex, rat, ADHD
Introduction
Substantial research in humans and laboratory rodents has demonstrated that physical activity can enhance cognition and neural plasticity (Kramer & Erickson, 2007; Voss, Nagamatsu, Liu-Ambrose, & Kramer, 2011), suggesting that exercise could be used to alleviate cognitive dysfunction. To date, the vast majority of this research has focused on hippocampal-dependent learning and memory and associated changes in hippocampal plasticity. In rats, for example, exercise has been shown to improve spatial learning and contextual fear memory (Albeck, Sano, Prewitt, & Dalton, 2006; Baruch, Swain, & Helmstetter, 2004; Hopkins & Bucci, 2010a), an effect that is dependent on associated increases in hippocampal neurogenesis and brain-derived neurotrophic factor (BDNF) expression (Clark et al, 2008; Vaynman, Ying, & Gomez-Pinilla, 2004).
Exercise can also affect cognitive processes that depend on brain areas other than the hippocampus, including the prefrontal cortex and striatum (Colcombe et al, 2004; Voss et al., 2011b). For example, we have previously shown that voluntary wheel running enhances learning a striatal-dependent discrimination task (Eddy, Rifken, Toufexis, & Green, 2013; Eddy, Stansfield, & Green, 2014), facilitates habituation (Robinson, Hopkins, & Bucci, 2011; Robinson, Eggleston, & Bucci, 2012), and improves object recognition in both humans and rats (Hopkins & Bucci, 2010b; Hopkins, Nitecki, & Bucci, 2011; Hopkins, Davis, Vantieghem, Whalen, & Bucci, 2012). Moreover, exercise likely has broader effects on neural function beyond neurogenesis and BDNF expression. Indeed, neurogenesis does not occur outside the hippocampus (except for the subventricular zone) and exercise regimens that affect BDNF levels in hippocampus may not necessarily have the same effects in other regions. Yet, very few studies to date have investigated the neural mechanisms through which exercise affects non-hippocampal dependent processes, and even fewer have demonstrated causality (Eddy et al., 2014).
We addressed this by testing the hypothesis that changes in noradrenergic function mediate the effects of exercise on attentional orienting behavior in Spontaneously Hypertensive Rats (SHRs). The SHR strain is a commonly-used model of Attention-Deficit/Hyperactivity Disorder (ADHD), exhibiting the principal behavioral and cognitive characteristics of ADHD, including inattention and distractibility (Hopkins, Sharma, Evans, & Bucci, 2009; Kantak et al., 2008; Russell, 2007; Sagvolden, Russell, Aase, Johansen, & Farshbaf, 2005), as well as dys-regulated dopaminergic and noradrenergic (NE) function that may underlie ADHD (Arnsten, 2006; Biederman, 2005; Heal, Smith, Kulkarni, & Rowley, 2008; Russell, 2002). We have previously shown that compared to normo-active rats, SHRs exhibit excessive orienting behavior and little or no habituation when repeatedly presented with an irrelevant visual stimulus (Robinson et al., 2012), indicating that they are more prone to respond to distracting, irrelevant stimuli. Indeed, habituation of the orienting response is typically observed in normo-active rats when a stimulus is not followed by reinforcement, reflecting an adaptive decrease in attention to a behaviorally-irrelevant cue (Gallagher, Graham, & Holland, 1990; Kaye & Pearce, 1984). Access to a running wheel for 5, 10, or 21 days reduced hyper-orienting behavior in SHRs and resulted in habituation (Robinson & Bucci, 2014), while access to the wheel for only 2 days was without effect.
The present study expanded on these findings by testing the effects of 0, 2, 5, 10, or 21 days of exercise on norepinephrine transporter (NET) levels in the medial prefrontal cortex of SHRs (Experiment 1). In Experiment 2, we tested whether blocking noradrenergic receptors during exercise would eliminate the effects on orienting behavior. We focused on NET levels in PFC since prior research has demonstrated an important role for prefrontal NE in various aspects of attention (Arnsten, 2011; Dalley, McGaughy, O’Connell, Carindal, Levita, & Robbins, 2001; McGaughy, Ross, & Eichenbaum, 2008). In addition, prefrontal NE circuity has been shown to be dysfunctional in ADHD as well as SHRs (Biederman, 2005; Heal et al., 2008).
Materials and Methods
EXPERIMENT 1
Subjects
Sixty-four female SHRs were obtained from Harlan Laboratories (Indianapolis, IN) at 6–7 weeks of age. Rats were group housed (3–4/cage) and maintained on a 14/10 hr light-dark cycle throughout the experiment. After acclimating to the vivarium for 7 days, rats were assigned to one of eight groups: Rats in the exercise groups (EX) had access to a running wheel for 2, 5, 10, or 21 days (n=8/group). Rats in the corresponding non-exercising (NX) control groups (n=8/group) did not have access to a running wheel and remained in the home cage for a comparable amount of time (i.e., 2, 5, 10, or 21 days). All procedures were conducted in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Wheel running
Rats in the EX groups were provided with a stainless steel running wheel (35.6cm diameter, 4.8mm rods placed 1.6 cm apart; Med Associates, St. Albans, VT) that was accessible through an opening in the side of the home cage. An automatic counter mounted on the side of the apparatus monitored wheel rotations. The number of wheel rotations recorded each day was divided by the number of rats in the cage. The average daily distance run by an individual rat was then calculated by multiplying the number of rotations by the circumference of the wheel (1.12 m) to convert to meters.
Western blots
After the allotted days of access to the running wheels, rats in the EX groups (and the matched NX groups) were anesthetized with isofluorane and then euthanized via decapitation. Brains were removed and the medial prefrontal cortex (i.e., the prelimbic, infralimbic, medial orbital, and cingulate cortices; Paxinos & Watson 2007) was rapidly dissected on ice and frozen at −80°C. Tissue was bead-homogenized in homogenization buffer (10mM Tris-HCl, pH 7.6, 1 mM EDTA, 200 mM sucrose, HALT protease & phosphatase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA) using a FastPrep-24 (MP Biomedicals, Santa Ana, CA) for 30 sec at 6.5 m/s. The homogenates were centrifuged for 2 min at 1000×g and supernatants were centrifuged for 90 min at 16,100×g. Protein pellets were solubilized by sonication in Tris buffer (10mM Tris-HCl, pH 7.6, 1 mM EDTA, 0.5% Triton X-100, HALT protease & phosphatase inhibitor cocktail). Total protein concentrations were determined using the Coomassie Plus reagent (Pierce Biotechnology, Rockford, IL). Protein samples (33 µg) were resolved on 12% LDS-PAGE gels (Life Technologies, Carlsbad, CA) then transferred to Immobilon-FL PVDF membranes (Millipore, Billerica, MA), which were blocked and incubated with primary antibody for NET (Alpha Diagnostic International, Inc., San Antonio, TX) and sodium/potassium ATPase 1 (Cell Signaling Technology, Beverly, MA). Quantitative analyses were performed by measuring the relative fluorescent unit (RFU) ratio of NET immunoreactive bands normalized to sodium potassium ATPase immunoreactive bands in the same lane for each sample. RFU measurements were also normalized by region of interest (ROI) area. Analyses were carried out using a Licor Odyssey imager and application software version 3.0.30 (Licor Biosciences, Lincoln, NE). Each western blot run contained samples from one exercising and one non-exercising group of SHRs. Independent samples t-tests were run between each exercise group and its corresponding control group (α=0.05).
EXPERIMENT 2
Subjects
Sixty-four female SHRs were obtained at 6–7 weeks of age (Harlan) and maintained as described in Experiment 1. Rats were assigned to one of four groups: non-exercising SHRs that underwent a sham surgery (NX-SHAM), exercising SHRs that underwent a sham surgery (EX-SHAM), non-exercising SHRs that had a propranolol pellet implanted (NX-PROP), and exercising SHRs that had a propranolol pellet implanted (EX-PROP).
Apparatus
Exercise
Running wheels were the same as those used in Experiment 1.
Orienting behavior
Unconditioned orienting behavior was assessed in standard conditioning chambers (placed in sound-attenuating cubicles) as described previously (Robinson et al., 2012). Briefly, the chambers were dimly illuminated by a red house light (2.8W) that was located on the ceiling of the sound-attenuating cubicle to provide background lighting. The stimulus light (2.8-W bulb) was located on the center of the front wall of the chamber, 16 cm from the floor. Three pairs of photobeam sensors were mounted in the chamber 15 cm above the grid floor (i.e., just below the level of the stimulus light) and were used to detect rearing behavior. The sensors were evenly spaced along the wall so that a rearing response produced anywhere in the chamber would be detected by one of the sensors.
Procedures and data analysis
Pellet implantation
Rats were anesthetized with isofluorane and 1-cm rostral-caudal incision was made above the left scapula, 2 cm lateral of the midline. For rats in the PROP groups, a 25mg propranolol pellet (Innovative Research of America, Sarasota, FL) was inserted subcutaneously and the wound was closed with surgical staples. The pellet was designed to release propranolol (a β-adrenergic/noradrenergic receptor antagonist) continuously for up to 21 days. This method of drug delivery was chosen in order to maintain a constant concentration of propranolol no matter when the rats chose to run on the wheel during the light-dark cycle. It also eliminated the stress that would be induced by repeated daily injections. Rats in the SHAM groups were anesthetized and received an incision but no pellet was implanted.
Exercise
Twenty-four hours after surgery, rats in the EX-SHAM and EX-PROP groups were provided access to a running wheel for 10 days. The number of wheel rotations and distance ran was calculated as in Experiment 1.
Orienting procedure
The opening to the wheel was blocked off 2 hr prior to the orienting session to minimize the potential impact of exercise-related fatigue on orienting behavior. During the single 32-min session, rats received 12 non-reinforced presentations of the stimulus light (10-sec duration) during which the red house light was extinguished and the stimulus light flashed on/off at a frequency of 1Hz. The average inter-stimulus interval was 2.75 min.
Data analysis
Orienting was defined as rearing on the hind legs with both forepaws off the ground (Holland, 1977). During each presentation of the visual stimulus, breaks in the photobeams were monitored by a computer. The amount of time that photobeams were broken was summed for each trial, because previous studies indicate that it is unlikely that a rearing response would simultaneously break more than one photobeam (Keene & Bucci, 2007). Our prior studies demonstrated that non-exercising SHR rats exhibit little or no habituation of rearing behavior over the 12 trials, while exercising SHRs exhibit robust habituation like normo-active control rats (e.g., Robinson & Bucci, 2014; Robinson et al., 2012). Thus, the primary variable of interest was the change in the amount of rearing from the beginning to the end of the session. To assess this, the time spent rearing on the first two trials were averaged and compared to the average time spent rearing during the last two trials for each group. Follow up analyses were conducted to test for between-group differences in rearing during the first block of trials to determine if exercise or drug treatment had any non-specific effects on rearing behavior. Because the data violated normality, non-parametric measures (Wilcoxon T and Mann-Whitney U tests) were used to assess within- and between-group differences in rearing. To reduce the probability of obtaining a false positive result because of multiple pair-wise comparisons, a Bonferroni-corrected alpha level was set at 0.0125.
Results
EXPERIMENT 1
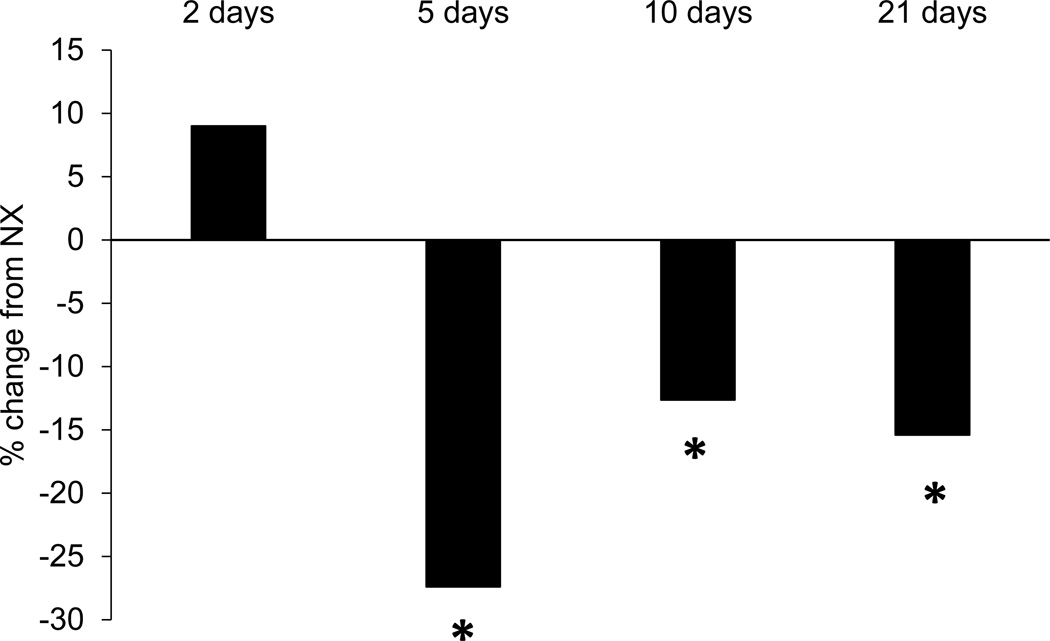
The average daily distance run by each rat in the exercise groups was 2.7 km for the 2-day group, 4.1 km for the 5-day group, 3.5km for the 10-day group, and 3.4 km for the 21-day group. Compared to NX rats, those that exercised for 5, 10, or 21 days exhibited a significant decrease in NET levels in medial prefrontal cortex (80 kDa band, which represents the mature, glycosylated form of the protein (Melikian, McDonald, Gu, Rudnick, Moore, & Blakely, 1994), as shown in Figure 1 [5–day EX, t(14)=2.5, p<0.03; 10-day EX, t(13)=2.2, p<0.05; 21-day EX, t(14)=4.7, p<0.001]. Rats in the 2-day EX group did not exhibit a decrease in NET (p>0.4). Data from one rat in the 10-day EX group was discarded because the NET levels were greater than 2 standard deviations from the group mean.
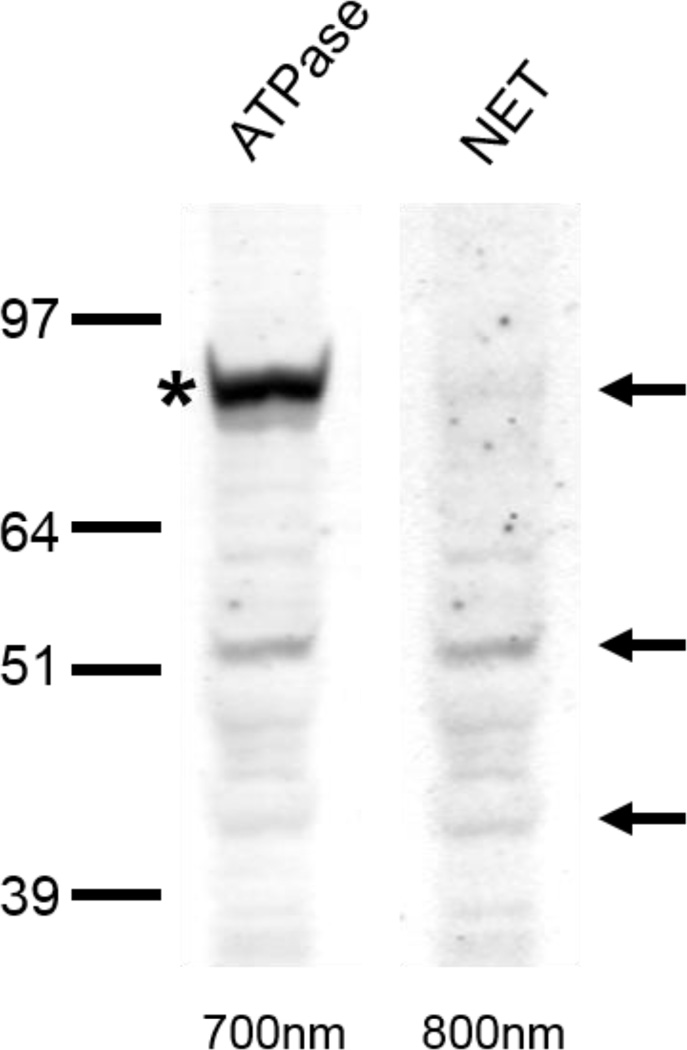
Figure 1.
A) Immunoreactivity of rat pre-frontal cortex to Na, K-ATPase and NET double-labeling. Crude membrane protein fractions were isolated from the pre-frontal cortex and subjected to LDS-PAGE and western blot. Arrows indicate the 80-, 54- and 46-kDa NET proteins. Asterisk indicates Na, K-ATPase. Due to secondary-secondary antibody interactions, NET bands are visible in both 700nm and 800nm Odyssey Imager channels. B) NET levels in the medial prefrontal cortex of rats that exercised as expressed as a percentage of the levels observed in non-exercising rats. Five, 10, or 21 days of exercise significantly reduced NET levels compared to non-exercising controls. *p<0.05.
EXPERIMENT 2
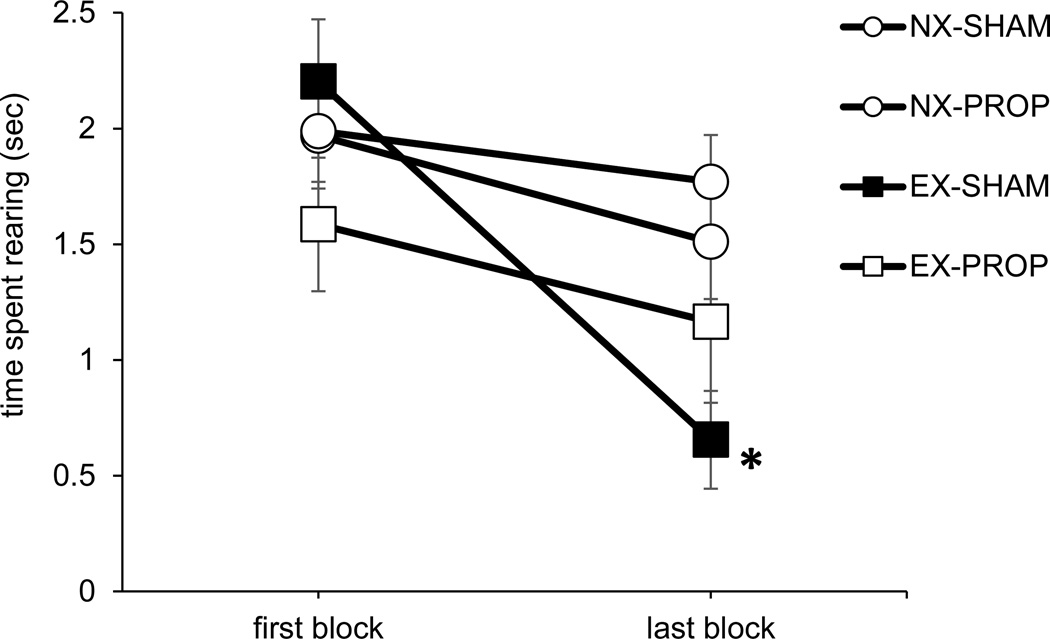
As illustrated in Figure 2, rats in the EX-SHAM group exhibited a significant decrease in rearing from the first two trials to the last two trials [T=1, p<0.001], while rats in the other groups did not exhibit a significant decrease across trials (ps>0.2). This replicates our prior findings with intact rats (Robinson & Bucci, 2014; Robinson et al., 2012) in that rearing behavior habituated in the group of sham-operated SHRs that had access to a running wheel (EX-SHAM) but it did not habituate in the sedentary sham-operated group (NX-SHAM). Moreover, we found that rats that received propranolol while they had access to the wheel (EX-PROP group) did not exhibit habituation of rearing behavior. Thus, blocking NE receptors prevented exercise-induced habituation of the orienting response to the light. Additional analyses did not reveal any group differences in rearing behavior during the first block of trials (ps > 0.2).
Figure 2.
Orienting behavior (rearing) during the first two trials (first block) and last two trials (last block). Rats in the EX-SHAM group exhibited a significant decrease in rearing behavior across trials (*p<0.001). Data are means ± SEM.
Discussion
In Experiment 1, voluntary wheel running reduced NET levels in the medial prefrontal cortex of SHRs, consistent with prior studies in normo-active rats in which NE levels in the brain were increased by exercise (Chaouloff, 1989; Dunn, Reigle, Youngstedt, Armstrong, & Dishman, 1996; Meeusen, Piacentini, & De Meirlier, 2001). Importantly, the reduction in NET was observed following 5, 10, or 21 days of access to the wheel, but not after 2 days. This pattern of results matches exactly our prior findings that 5, 10, or 21 days of exercise reduced excessive orienting behavior in SHRs, while 2 days of exercise was without effect (Robinson & Bucci, 2014; Robinson et al., 2012). This suggests that exercise-induced changes in attentional orienting behavior in SHRs may be mediated by changes in noradrenergic function in the medial prefrontal cortex.
The reduction in NET levels in exercising rats presumably resulted in less uptake of NE and an increase in synaptic NE concentration. Thus, to causally test the hypothesis that exercise affected orienting behavior through noradrenergic mechanisms, NE receptors were blocked with propranolol during exercise in Experiment 2 to attenuate the effects of additional NE in the synapse. Control rats that exercised but did not receive propranolol (EX-SHAM group) exhibited a decrease in orienting to the visual stimulus over trials (habituation), as observed previously (Hopkins et al, 2009; Robinson & Bucci, 2014; Robinson et al., 2011; 2012). In contrast, rats that received propranolol during the 10 days of exercise (EX-PROP) did not exhibit habituation of the orienting response, like rats in the non-exercising conditions. This suggests that ability of exercise to reduce excessive orienting behavior in SHRs is mediated at least in part through NE receptors. It is unlikely that the effects of propranolol were simply due to a general decrease in orienting behavior since rats in the NX-PROP group did not differ from those in the NX-SHAM group during the first block of trials.
To date, only a few other studies have investigated whether changes in noradrenergic function mediate the effects of exercise on behavior. For example, van Hoomissen, Holmes, Zellner, Poudevigne, & Dishman (2004) found that blocking noradrenergic function during exercise with slow-release propranolol pellets like the ones used here eliminated the increase in contextual fear memory produced by exercise. Similarly, it has been shown that nightly injections of propranolol reversed exercise-induced enhancements in spatial learning and memory (Ebrahami, Rashidy-Pour, Vafaei, & Akhavan, 2010). The present findings complement these studies by demonstrating that noradrenergic mechanisms may also mediate the effects of exercise on non-hippocampal dependent behavior. Nonetheless, future studies are necessary to determine if propranolol impacts exercise-induced changes in behavior via central or peripheral nervous system action. This is particularly important in studies using SHRs since they exhibit hypertension at older ages.
There are a variety of mechanisms through which blocking NE receptors could attenuate the effects of exercise on behavior. For example, NE receptors, like exercise, can modulate synaptic plasticity by facilitating long-term potentiation (LTP) and increasing BDNF expression (Furini et al., 2010; Winder et al., 1999; Dishman et al., 2006). Indeed, activation of NE receptors has been shown to generate long-lasting enhancements of synaptic strength that can facilitate plasticity (Connor, Wang, & Nguyen, 2011) through regulation of protein synthesis (Walling & Harley, 2004). It is possible that NE receptors mediate the effects of exercise on LTP and BDNF, thus blocking these receptors during exercise may prevent exercise-induced changes in neural plasticity. Consistent with this, propranolol has been shown to block an exercise-induced enhancement of BDNF expression in the hippocampus (Garcia, Chen, Garza, Cotman, & Russo-Neustadt, 2003; Ivy, Rodriguez, Garcia, Chen, & Russo-Neustadt, 2003; van Hoomissen et al., 2004). However, additional studies are needed to systematically test the interaction between noradrenergic function, exercise, and plasticity. Similarly, future studies are needed to determine if there are regional differences in exercise-induced changes in NE levels and NET expression within the subregions of medial prefrontal cortex, which was not possible in the present study because the entire medial prefrontal cortex needed to be harvested for the western blot assay.
The finding that noradrenergic mechanisms may mediate the effects of exercise on attentional orienting behavior in SHRs has several implications for ADHD. For instance, alterations in catecholaminergic function are thought to underlie the symptoms of ADHD, in at least some people diagnosed with the disorder (Arnsten, 2006; Biederman, 2005), and are also observed in SHRs (Heal et al., 2008; Russell, 2002). The present findings suggest that exercise may ameliorate dysfunctional NE neurotransmission and affect attentional function by decreasing the uptake of NE. Interestingly, the psychostimulants (e.g., methylphenidate) that are commonly used to treat ADHD also affect the uptake of NE (Kuczenski & Segal, 2001; Berridge et al., 2006). Although efficacious for the majority of patients, psychostimulants are ineffective in about 30% of cases (Biederman, Spencers, & Wilens, 2004). In addition to the potential for abuse, recent studies indicate that there may be long-term negative consequences of psychostimulant treatment, (Swanson et al., 2007; Volkow & Insel, 2003), warranting a search for adjunctive or replacement therapies for ADHD (Halperin & Healey, 2011). Exercise may be an ideal alternative treatment and recent studies have reported beneficial effects of an exercise intervention in children with ADHD (Chang, Liu, Yu, & Lee, 2012; Hoza et al., 2014; Pontifex, Saliba, Raine, Picchietti, & Hillman, 2013; Smith et al., 2013).
In summary, the present findings suggest that changes in noradrenergic function underlie the effects of exercise on attentional orienting behavior in SHRs, a commonly-used animal model of ADHD. It will be informative in future studies to carry out site-specific manipulations of noradrenergic receptors to determine whether the effects of exercise on NE are specific to areas such as prefrontal cortex. In addition, it will be important to determine if exercise affects the behavior of male rats through a similar mechanism. Here, we used female SHRs because they display more attentional impairments and hyper-social behavior than male SHRs (Hopkins et al., 2009), consistent with a growing evidence that females diagnosed with ADHD may be more cognitively impaired than males (Gershon, 2002; Nigg, Blaskey, Huang-Pollock, & Rappley, 2002; Spencer, Biederman, & Mick, 2007). However, the high incidence of ADHD in boys warrants an examination of the effects of exercise in males as well.
Acknowledgements
Research supported by a grant from the National Institute of Mental Health (R01MH082893) and by grants from the National Center for Research Resources (5P30RR032135-02) and the National Institute of General Medical Sciences (8 P30 GM103498-02) from the National Institutes of Health.
References
- Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behavioral Brain Research. 2006;168:345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biological Psychiatry. 2011;69:e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: A selective overview. Biological Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. The International Journal of Neuropsychopharmacology. 2004;7:77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiologica Scandinavica. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- Chang YK, Liu S, Yu HH, Lee YH. Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Archives of Clinical Neuropsychology. 2012;27(2):225–237. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor SA, Wang YT, Nguyen PV. Activation of β - adrenergic receptors facilitates heterosynaptic translation - dependent long - term potentiation. The Journal of Physiology. 2011;589(17):4321–4340. doi: 10.1113/jphysiol.2011.209379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. Journal of Neuroscience. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Zigmond MJ. Neurobiology of exercise. Obesity. 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Medicine & Science in Sports & Exercise. 1996;28(2):204–209. doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- Ebrahimi S, Rashidy-Pour A, Vafaei AA, Akhavan MM. Central β-adrenergic receptors play an important role in the enhancing effect of voluntary exercise on learning and memory in rat. Behavioural Brain Research. 2010;208(1):189–193. doi: 10.1016/j.bbr.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Eddy MC, Rifken KM, Toufexis DJ, Green JT. Gonadal hormones and voluntary exercise interact to improve discrimination ability in a set-shift task. Behavioral Neuroscience. 2013;127(5):744–754. doi: 10.1037/a0033728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy MC, Stansfield KJ, Green JT. Voluntary exercise improves performance of a discrimination task through effects on the striatal dopamine system. Learning & Memory. 2014;21(7):334–337. doi: 10.1101/lm.034462.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furini CR, Rossato JI, Bitencourt LL, Medina JH, Izquierdo I, Cammarota M. Beta-adrenergic receptors link NO/sGC/PKG signaling to BDNF expression during the consolidation of object recognition long-term memory. Hippocampus. 2010;20(5):672–683. doi: 10.1002/hipo.20656. [DOI] [PubMed] [Google Scholar]
- Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119(3):721–732. doi: 10.1016/s0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neuroscience & Biobehavioral Reviews. 2011;35(3):621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacology Biochemistry and Behavior. 2008;90:184–197. doi: 10.1016/j.pbb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. Interpreting the effects of exercise on fear conditioning: The influence of time of day. Behavioral Neuroscience. 2010a;24:868–872. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiology o Learning and Memory. 2010b;94(2):278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary physical exercise alters attentional orienting and social behavior in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2009;123(3):599. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: Differential effects on object recognition memory and BDNF expression. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Davis FC, Vantieghem MR, Whalen PJ, Bucci DJ. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience. 2012;215:59–68. doi: 10.1016/j.neuroscience.2012.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoza B, Smith AL, Shoulberg EK, Linnea KS, Dorsch TE, Blazo JA, Alerding CM, McCabe GP. A Randomized Trial Examining the Effects of Aerobic Physical Activity on Attention-Deficit/Hyperactivity Disorder Symptoms in Young Children. Journal of Abnormal Child Psychology. 2014 doi: 10.1007/s10802-014-9929-y. epub before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacology Biochemistry and Behavior. 2003;75(1):81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behavioral Neuroscience. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–348. doi: 10.1016/j.tics.2007.06.009. Neuropsychopharmacology, 33(5), 1028–1037. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. The Journal of Neuroscience. 2002;22(16):7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;53:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R, Piacentini MF, De Meirleir K. Brain microdialysis in exercise research. Sports Medicine. 2001;31(14):965–983. doi: 10.2165/00007256-200131140-00002. [DOI] [PubMed] [Google Scholar]
- Melikian HE, McDonald JK, Gu H, Rudnick G, Moore KR, Blakely RD. Human norepinephrine transporter: Biosynthetic studies using a site-directed polyclonal antibody. Journal of Biological Chemistry. 1994;269:12290–12297. [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological Executive Functions and DSM-IV ADHD Subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(1):59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego, CA: Academic Press; 2007. [Google Scholar]
- Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. The Journal of Pediatrics. 2013;162(3):543–551. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AM, Bucci DJ. Individual and combined effects of physical exercise and methylphenidate on orienting behavior and social interaction in spontaneously hypertensive rats. Behavioral Neuroscience. 2014;128:703–712. doi: 10.1037/bne0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AM, Eggleston RL, Bucci DJ. Physical exercise and catecholamine reuptake inhibitors affect orienting behavior and social interaction in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2012;126(6):762. doi: 10.1037/a0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AM, Hopkins ME, Bucci DJ. Effects of physical exercise on ADHD-like behavior in male and female adolescent spontaneously hypertensive rats. Developmental Psychobiology. 2011;53:383–390. doi: 10.1002/dev.20530. [DOI] [PubMed] [Google Scholar]
- Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder–the spontaneously hypertensive rat. Behavioural Brain Research. 2002;130:191–196. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. Journal of Neuroscience Methods. 2007;161:185–198. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Smith AL, Hoza B, Linnea K, McQuade JD, Tomb M, Vaughn AJ, Shoulberg EK, Hook H. Pilot physical activity intervention reduces severity of ADHD symptoms in young children. Journal of Attention Disorders. 2013;17:70–82. doi: 10.1177/1087054711417395. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. Journal of Pediatric Psychology. 2007;32(6):631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Volkow ND. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(8):1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of β-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behavioral Neuroscience. 2004;118(6):1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biological Psychiatry. 2003;54(12):1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. Journal Applied Physiology. 2011a;111(5):1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Chaddock L, Kim JS, Vanpatter M, Pontifex MB, Raine LB, Kramer AF. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011b;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling SG, Harley CW. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel β-adrenergic-and protein synthesis-dependent mammalian plasticity mechanism. The Journal of Neuroscience. 2004;24(3):598–604. doi: 10.1523/JNEUROSCI.4426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron. 1999;24(3):715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]