Abstract
Despite many studies on the age-related positivity effect and its role in visual attention, discrepancies remain regarding whether one’s full attention is required for age-related differences to emerge. The present study took a new approach to this question by varying the contextual demands of emotion processing. This was done by adding perceptual distractions, such as visual and auditory noise, that could disrupt attentional control. Younger and older participants viewed pairs of happy–neutral and fearful–neutral faces while their eye movements were recorded. Facial stimuli were shown either without noise, embedded in a background of visual noise (low, medium, or high), or with simultaneous auditory babble. Older adults showed positive gaze preferences, looking toward happy faces and away from fearful faces; however, their gaze preferences tended to be influenced by the level of visual noise. Specifically, the tendency to look away from fearful faces was not present in conditions with low and medium levels of visual noise, but was present where there were high levels of visual noise. It is important to note, however, that in the high-visual-noise condition, external cues were present to facilitate the processing of emotional information. In addition, older adults’ positive gaze preferences disappeared or were reduced when they first viewed emotional faces within a distracting context. The current results indicate that positive gaze preferences may be less likely to occur in distracting contexts that disrupt control of visual attention.
Keywords: aging, emotion, positive gaze, attentional control, eye tracking
It has been widely observed that older adults, compared to younger adults, are more attuned to positive than negative information (for reviews, see Reed & Carstensen, 2012; Reed, Chan, & Mikels, 2014). This phenomenon is called the positivity effect (Carstensen & Mikels, 2005; Mather & Carstensen, 2003), and it has been documented in relation to attention and memory tasks across a wide range of stimuli, including emotional faces and images, word lists, advertising slogans, and health-related messages (for reviews, see Kryla-Lighthall & Mather, 2009; Isaacowitz & Noh, 2011; Reed & Carstensen, 2012, but cf. Grühn, Smith, & Baltes, 2005).
A number of studies using eye tracking to assess real-time visual attention have demonstrated age-related positive looking (gaze) preferences (Isaacowitz & Choi, 2011; Isaacowitz, Wadlinger, Goren, & Wilson, 2006a, 2006b; Nikitin & Freund, 2011). For example, Isaacowitz et al. (2006a, 2006b) presented emotional and non-emotional (neutral) synthetic faces to younger and older adults and assessed their gaze preference patterns by comparing the proportion of fixation on positive and negative versus neutral faces. Results from these two studies revealed that older adults showed a looking preference toward positive (happy) faces and away from negative faces (angry and sad). Younger adults, on the other hand, showed no preference or a slight preference for looking at negative (fearful) faces. In a similar study with real faces expressing emotions of anger, happiness, and neutrality, age was negatively related to gaze preference for angry faces: older adults looked less frequently and for shorter durations at angry faces than younger adults (Nikitin & Freund, 2011). Complementary evidence for such an age difference in gaze preferences has also been reported by researchers utilizing mood-disrupting images. Older adults spent less time looking at the most negative areas of images than younger adults (Isaacowitz & Choi, 2012; Noh, Lohani, & Isaacowitz, 2011). This effect persisted even after participants had been instructed to focus on the negative regions of the stimuli (Isaacowitz & Choi, 2011). These positive gaze preferences seem strongest when emotional goals become salient to older adults. However, in a few available studies examining downstream effects of gaze on mood, older adults displayed positive gaze preferences, especially when in a bad mood, while younger adults showed mood-congruent gaze patterns (Isaacowitz, Toner, Goren, & Wilson, 2008; Noh et al., 2011).
The main conceptual framework used to explain such positivity biases in cognition has been socioemotional selectivity theory (SST; Carstensen, 2006; Carstensen, Isaacowitz, & Charles, 1999). According to SST, the perspective of a more limited future leads older adults and others facing similar time constraints to prioritize goals related to emotional experiences over other goals that might produce delayed benefits at the expense of current affective experience. Consequently, older adults are more motivated to pursue goals related to emotional satisfaction, leading them to pay more attention to positive relative to negative information (e.g., Mather & Carstensen, 2005; Reed & Carstensen, 2012). Because this positivity effect reflects emotional goals and implementing such goals requires sufficient cognitive control, SST posits that the positivity effect depends upon the availability of cognitive resources (e.g., Knight et al., 2007; Kryla-Lighthall & Mather, 2009; Reed & Carstensen, 2012). Support for this prediction came from Mather and Knight (2005), who found that a positivity effect in a task assessing memory for emotional pictures only emerged for older adults with better scores on tests of attentional control. In a follow-up study, when Mather and Knight (2005) manipulated attention during picture encoding, the positivity effect found in a full-attention condition was reversed when older adults were forced to divide their attention with a simultaneous tone-detection task. With divided attention, older adults were more likely to recall negative pictures than younger adults.
This study assessed the role of attentional control play in age-related positive gaze preferences. A few studies have examined whether the full control of visual attention is necessary for age-related differences and a positivity effect to emerge, however, results have been mixed. It remains unclear whether disrupting control of visual attention will interfere with the positive gaze preferences typically observed in older adults (Allard & Isaacowitz, 2008; Allard, Wadlinger, & Isaacowitz, 2010; Knight et al., 2007).
Age-related Positive Gaze Preferences and Attentional Control
Two previous eye-tracking studies have examined the role of attentional control in the positivity effect by assessing how age-related positive gaze preferences are affected by dual-task constraints. Knight et al. (2007) found a similar pattern to that of Mather and Knight (2005, Experiment 2) regarding visual attention. Older adults showed a higher proportion of fixations directed toward positive than negative stimuli when each was paired with neutral stimuli in the full attention condition. This pattern was reversed in the divided-attention condition, in that older adults were more likely to fixate on negative than positive stimuli. Based on these findings, Knight et al. (2007) concluded that older adults’ positive gaze patterns are more likely to reflect goal-directed processing in the context of full attention and that use of gaze “as a tool of motivation requires cognitive effort” (p. 712). In contrast, Allard and Isaacowitz (2008) found no evidence of the link between attentional control and positive gaze preferences. Unlike Mather and Knight (2005, Experiment 2) and Knight et al. (2007), who assessed dual-task performance using a between-subjects design, Allard and Isaacowitz (2008) utilized a within-subjects design to examine how an individual fixates on emotional stimuli without distraction. This allowed for comparison of the data with regard to how the same individual fixates when his or her attention is divided. Allard and Isaacowitz’s (2008) participants were presented with positive, negative, and neutral images, and fixation percentage was assessed by measuring the amount of time one’s gaze was fixated on the central emotional part(s) of each image relative to any other part of the image. Older adults demonstrated greater fixation on positive and neutral images compared to negative images, regardless of whether emotional images were presented in the full- or divided-attention conditions, the latter of which involved performing a simultaneous auditory lexical decision-making task.
At first glance, the discrepant results between these studies appear to challenge the notion that full control of visual attention—in other words, an environment free from distraction—is required for the positivity effect to emerge. However, such inconsistent findings could be due, at least in part, to methodological differences. One difference may be the different assessments of dual-task performance and the different measurements of visual fixation. For example, in the study by Knight et al. (2007), the secondary task was presented for a longer duration than in the study by Allard and Isaacowitz (2008) (6 s versus 2 s). Therefore, the reversal of the positivity effect observed by Knight et al. (2007) could be due to the greater demand for attentional control processes incurred by the secondary task for older adults (they were less able to direct their attention in a goal-consistent manner). However, the fact that some older adults displayed the positivity effect in Allard and Isaacowitz’s (2008) study suggests that displaying positive gaze preferences may not require the full control of visual attention. Indeed, in another study in which participants were asked to view emotional stimuli, there was no evidence that increased pupil dilation (a psychophysiological measure of attentional control) was associated with positive gaze preferences in older adults (Allard, Wadlinger, & Isaacowitz, 2010).
Taken together, the existing literature seems to suggest that older adults may exhibit positive gaze preferences with certain forms of attentional distractions. However, in the aforementioned studies, the role of attentional control in positive gaze preferences was assessed within an implicit regulatory context (i.e., viewing emotional material naturally without explicit instructions for deliberate emotion regulation). Consequently, one may argue that it is not clear whether positive gaze preferences actually reflect more positive emotional regulation goals for older adults. Although older adults are especially likely to utilize positive gaze as a regulatory tool when they are motivated to improve their bad mood (Isaacowitz et al., 2008; Noh et al., 2011), the positivity effect and later mood outcomes are not always causally linked (Isaacowitz, 2012; Isaacowitz & Blanchard-Fields, 2012). Despite this caveat, literature from the fields of aging and emotion regulation suggests that one type of regulatory strategy preferred by older adults appears to be attentional deployment, which involves directing one’s attention toward or away from particular aspects of emotional stimuli (Gross, 1998; Urry & Gross, 2010). Attentional deployment may be reflected in the positive gaze preferences displayed by older adults.
Given that age-related declines in cognitive resources are common, Urry and Gross (2010) suggested that older adults become more selective and optimize particular emotion-regulation strategies that can compensate for age-related losses in cognitive resources. Attentional deployment relies less on cognitive-control resources than other regulatory strategies, such as cognitive reappraisal, that happen later in the emotion regulation process and require direct engagement with negative stimuli (e.g., Sheppes & Meiran, 2008; Shiota & Levenson, 2009). For example, older adults were less successful than younger adults at using cognitive reappraisal strategies when they were instructed to direct their gaze toward unpleasant emotional images and to reduce negative emotions (Opitz, Rauch, Terry, & Urry, 2012). This age-related effect was mediated by reduced activation in the dorsomedial and left ventrolateral prefrontal regions, which have been implicated in cognitive control. Thus, activating positive gaze as a form of attentional deployment appears to be a relatively undemanding and simple regulatory tool that older adults utilize in their everyday lives. It is critical to further assess age-related positive gaze preferences under a variety of distracting attentional conditions, in order to determine which conditions are either conducive or prohibitive to positive gaze preferences among older adults.
The Present Study
In contrast to the previous studies examining the effect of divided attention (Allard & Isaacowitz, 2008; Knight et al., 2007), the present study explores the influence of environmental noise on the positivity effect in visual attention. The physical environment is full of potential distractions to our senses. Noise, such as viewing visually degraded stimuli (i.e., blurry screen presentations) while listening to background noise (i.e., watching television in a busy airport) can pose a challenge for information processing (e.g., Gao, Stine-Morrow, Noh, & Eskew, 2011). Previous studies have shown that irrelevant environmental distractions can interfere with the recollection of goal-relevant information (Wais & Gazzaley, 2011; Wais, Rubens, Boccanfuso, & Gazzaley, 2010) and event details (Perfect et al., 2012; Vredeveldt, Hitch, & Baddeley, 2011), and can hinder conceptual integration during reading (Gao, Levinthal, & Stine-Morrow, 2012; Gao, Stine-Morrow, Noh, & Eskew, 2011). External noise appears to induce domain-general interference, whether within or across sensory domains, which disrupts goal-related processing (Wais et al., 2010 Wais et al., 2011). Moreover, Gao and colleagues (2012) demonstrated that visual noise may have greater adverse effects on older adults, as evidenced by their reduced conceptual processing during reading and poorer rates of recall compared to younger adults (Gao, Levinthal, & Stine-Morrow, 2012; Speranza, Daneman, & Schneider, 2000). The greater challenge of processing information while experiencing noise and distractions has been accounted for by the effortfulness hypothesis (Dickinson & Rabbitt, 1991; Rabbitt, 1968, 1991; Wingfield, Tun, & McCoy, 2005), which holds that sensory challenges, created by environmental conditions or by aging, require additional information-processing resources, which are then more limited for other higher cognitive functions. Therefore, varying contextual demands by manipulating background noise appears to be an alternative, effective means to assess whether the positivity effect in visual attention requires full attentional control.
Following previous research, we also tested the role of attentional control in age-related positive gaze preferences in a naturally implicit regulatory context without providing explicit instruction for deliberate emotion regulation (Allard et al., 2008, 2010; Knight et al., 2007; Mather & Knight, 2005). Because it is commonplace for older adults to utilize regulatory strategies, and the positivity effect is a “preference” of older adults (Reed et al., 2014), using an implicit regulatory context is necessary to provide a naturalistic context to these effects in this population.
On the basis of results of previous eye-tracking research of age-related positive gaze preferences under unconstrained processing conditions, we predicted that older adults would demonstrate positive gaze preferences (looking more toward positive and less toward negative stimuli; e.g., Isaacowitz et al., 2006a, 2006b; Isaacowitz & Choi, 2011; Nikitin & Freund, 2011). However, taking into consideration the effects of attentional control on the positivity effect (Knight et al., 2007; Mather & Knight, 2005; Reed & Carstensen, 2012), we predicted that greater levels of visual noise or auditory distraction would interfere with or even reverse this positive gaze pattern in older adults. As noted in the methods section below, we added a cue for the high visual-noise condition in order to make the task more manageable, reasoning that we could only manipulate noise to the extent that the emotion of each stimulus was still perceivable (otherwise there would be no way to test age-related differences). Previous findings have shown that older adults rely on environmental cues to support their information processing in the face of internal noise (Spieler, Mayr, & LaGrone, 2006) and declining self-initiated processing (Craik, 1983; Craik & Jennings, 1992). Thus, we are aware that providing cues could diminish the potential impact of noise on the positivity effect in visual attention. However, control of gaze, as a form of attentional deployment, is thought to require less effort than other proactive emotion-regulation strategies (e.g., reappraisal, suppression; Allard et al., 2008, 2010; Gross, 1998; Urry & Gross, 2010), therefore we predicted that older adults would demonstrate positive gaze preferences irrespective of noise condition. Finally, we varied the age of the face stimuli, including younger and older faces, because recent evidence suggests that perceived age may have an effect on the processing of face stimuli (Ebner, He, Fichtenholtz, McCarthy, & Johnson, 2011a; Ebner, He, & Johnson, 2011b; He, Ebner, & Johnson, 2011). We predicted that participants would look more at facial stimuli that were of a similar age to themselves (Ebner et al., 2011; He et al., 2011). However, we did not form a hypothesis with regard to the effect of age of stimuli on the positivity effect in visual attention, given the lack of previous findings regarding this relationship.
Method
Participants
Participants in this study were 60 younger adults (17–23 years) and 34 older adults (57–81 years). The younger adults were recruited from an undergraduate participant pool at Brandeis University using flyers posted on campus. The older adults were recruited from the Brandeis University Emotion Laboratory participant pool and advertisements. Participants received either course credit or a monetary stipend as compensation. Fifty-six percent of the older sample held graduate-level degrees, 18% bachelor’s degrees, 21% two-year college degrees, and 6% high-school diplomas. Seventy-five percent of the sample identified as White, 15% Asian, 3% Hispanic, and 2% other. An additional five younger and 33 older adults participated in the study but were not included in data analyses because their eye movements were not trackable or because their eye-tracking data were not collected for at least 25% of the time that the slide was shown for each noise condition.1 Seven additional older adults were excluded because of a failure to meet a cut-off score of 26 on the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), because of poor hearing (e.g., wearing hearing aids), or because of an inability to complete the task due to fatigue. Table 1 provides the participants’ demographic information, sensory and cognitive functioning levels, and affective characteristics. Self-reported overall health and scores on the MMSE did not differ between the two age groups. The older adults had lower visual acuity (near vision, contrast sensitivity) and hearing acuity than younger adults. Compared to the younger adults, older adults had poorer executive function (as measured by the Trail Making Test, Part B) but better verbal ability. Consistent with previous findings on affective experience and aging (Carstensen et al., 2011; Charles, Reynolds, & Gatz, 2001; Mroczek & Kolarz, 1998), the older adults reported more positive and less negative affect than the younger adults. Sensory, cognitive, and affective measures that showed age differences were included as covariates in the eye-tracking analyses described below.
Table 1.
Demographic Information and Means and Standard Deviations for Sensory, Cognitive, and Affect Measures for Younger and Older Adults
| Variable | Younger (n = 60)
|
Older (n = 34)
|
||
|---|---|---|---|---|
| M | SD | M | SD | |
| % female | 62 | 79 | ||
| Age | 19.23 | 1.28 | 68.41 | 6.16 |
| Self-rating of health* | 4.22 | 0.74 | 3.79 | 0.98 |
| Sensory functioning | ||||
| Rosenbaum near vision** | 22.50 | 3.12 | 27.50 | 3.08 |
| Pelli-Robson contrast sensitivity** | 1.70 | 0.14 | 1.50 | 0.16 |
| Pure-tone average** a | 8.26 | 3.84 | 16.85 | 4.72 |
| Cognitive functioning | ||||
| MMSE | 29.40 | 0.91 | 29.39 | 1.00 |
| WAIS digit symbol substitutionb | 0.48 | 0.15 | 0.50 | 0.10 |
| TMT-B time in seconds**b | 65.50 | 25.04 | 96.73 | 44.19 |
| Vocabulary** | 13.50 | 2.14 | 16.24 | 2.54 |
| Affective functioning | ||||
| PANAS positive affect* | 30.12 | 6.74 | 34.19 | 6.59 |
| PANAS negative affect* | 15.05 | 5.10 | 12.06 | 2.95 |
Note. Self-reported current health, with scoring options ranging from 1 (poor) to 5 (excellent). Rosenbaum Pocket Vision Screener for near vision (Rosenbaum, 1984); Pelli-Robson Contrast Sensitivity Chart (Pelli, Robson, & Wilkins, 1988); MMSE = Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975) maximum score = 30; WAIS = Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981); TMT-B = Trail Making Test Part B (Reitan, 1992); Shipley Vocabulary Test (Zachary, 1986) maximum score = 21; PANAS = Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988).
The pure-tone average was calculated by averaging the values at 500, 1000 and 2000 Hz from the pure-tone audiometry (American National Standards Institute [ANSI], 1989).
Lower scores indicate better performance.
p < .05,
p < .01 for group differences.
Stimuli
Facial stimuli were taken from the FACES Database (for detailed information, see Ebner, Riediger, & Lindenberger, 2010). Five sets of 48 face pairs (total = 240 pairs), consisting of an emotional face and its neutral counterpart (24 fear–neutral and 24 happy–neutral pairs), were constructed for the eye-tracking presentation. Each set consisted of equal numbers of younger (18–31 years) and older (69–80 years) face pairs, half male and half female. The five sets of face pairs were randomly assigned to one of the five noise conditions (no noise, three visual levels [low, medium, high], and auditory noise). Face pairs within each noise condition were blocked for presentation and were further blocked for the types of noise (no, visual, and auditory) for presentation. The side of screen (left or right) on which the emotional or neutral face appeared was counterbalanced across participants. The presentation order of the noise blocks (no, visual, and auditory) was counterbalanced across participants. The presentation of levels of visual noise within the visual noise block was also counterbalanced across participants. Within each block, face pairs were pseudo-randomized such that no two faces alike in both age and valence appeared consecutively (e.g., younger–fear followed by younger–fear).
Static visual noise was generated using MATLAB software in conjunction with the Psychophysics Toolbox (Brainard, 1997; Kleiner, Brainard, & Pelli, 2007). The images were rendered in the CMYK color space (created using combinations of the colors “C” for cyan, “M” for magenta, “Y” for yellow, and “K” for black), and the visual noise was created by setting the “C,” “M,” and “Y” to identical luminance values at each pixel and varying the levels of “K.” Varying the proportion of color (“C,” “M,” and “Y”) to black “K” created a series of random pixels across the image, ranging from white to black (and grays in between). The blend function was also used to produce a smooth blending of the image and the static noise by combining the generated static noise with the underlying facial image. Without completely obscuring the underlying face, three levels of visual noise were created by varying the contrast of the noise pixels (0.3 = low noise, 0.5 = medium noise, 0.7 = high noise). While our aim was to create progressive task difficulty by varying the amount of noise, it was also important to ensure that the visual noise did not entirely impair participants’ ability to decode emotions from facial expressions, because that would have rendered our manipulation moot. Our pilot study (nyounger = 14, nolder = 15) indicated that the accuracy of identification of facial emotions was differentially reduced in the high-visual-noise context, resulting in lower than 90% accuracy. Therefore, for the high-visual-noise condition, a clear emotional–neutral face pair was presented very briefly (see Procedure for details). Then, that same emotional–neutral pair was presented with noise. This was done to cue the valence of the emotional–neutral face pairs.2
A recorded track of multiple voices speaking simultaneously (i.e., babble produced by 20 voices) was used for the auditory background noise. Further information on the speech babble can be found in Tun (1998). The track was presented binaurally through headphones at a normal conversation speech level of 65 dB (Olsen, 1998). Sample face pair stimuli are shown in Figure 1.
Figure 1.
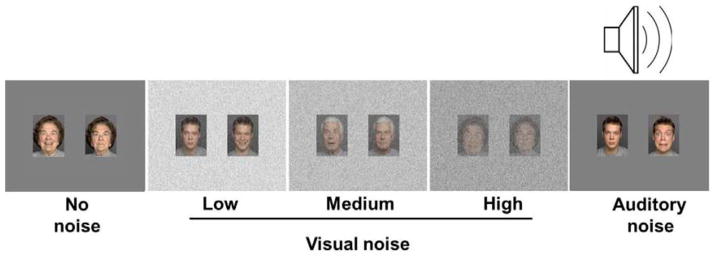
Sample pictures of younger and older faces used in the five noise conditions.
Equipment
An Applied Science Laboratories (Bedford, MA) EYE-TRAC 6 eye tracker was used to record eye movements at a rate of 60 Hz. The EYE-TRAC 6 tracks gaze by sending illumination from a remote unit to the pupil of the participant’s selected eye. A fixation was defined as an interval during which a participant’s gaze focused within 1° of the visual angle for at least 100 ms (Manor & Gordon, 2003) on predesignated areas of interest (AOIs). AOIs in this study were the areas of a square box containing each face; the area surrounding the boxes was designated as outside the AOI. The percentage of fixation time within an AOI relative to total fixation duration across all AOIs was calculated. Face stimuli were presented using GazeTracker software (EyeTellect, LLC, Charlottesville, VA) on a 17-inch monitor with a pixel resolution of 1280×1024.
Procedure
After obtaining informed consent, demographic, sensory, and affect measures were administered. Cognitive tasks were administered at the end of each experiment session. Participants were seated in front of the eye tracker and performed a 17-point eye calibration procedure to ensure accurate measurement of gaze across various areas on the screen. The eye-tracking task instructions were as follows:
“You will be viewing pairs of emotional–neutral faces that will appear simultaneously on the screen…across blocks of the pairs, the image pairs are embedded in either a visual mask or a background of auditory noise…for the visual mask blocks you will be viewing the image pairs presented with visual disruptions (like the static or snow on a fuzzy television picture)…the disruption will be greater for some pairs than others…for the images that have the most disruption, there will be a brief cue, whereby you will first briefly see the same pair normally before the disruption takes place…for the auditory noise block, you will be viewing images while hearing background noise (like you might experience when reading newspapers in a busy café or at the airport)…your task is to view the images as naturally as possible, as if you were watching a television show.”
Participants were also told that they would wear headphones while viewing images with a background of auditory noise. The eye-tracking session began with practice trials containing 24 face pairs, followed by five experimental blocks of face pairs. Each emotional–neutral face pair was displayed for five seconds and was followed by a 0.5 s crosshair slide to realign the gaze to the center of the screen. For the high-visual-noise condition, each face pair embedded in visual noise was presented after that same pair had been briefly shown without any visual noise for 0.5 s, in order to provide a cue of the emotional valence of the upcoming face pair. Each block was 5–6 minutes in duration. Upon completion of the eye-tracking session, participants were given a valence rating task to ensure they could identify the intended emotional valence of facial expressions despite the visual noise background. The task contained a subset of 72 faces selected from the eye-tracking stimulus sets (24 faces expressing fear, happiness, or neutral emotion, and 18 faces from the no disruption visual-noise blocks). In this task, single faces appeared one at a time for 5 s (in the case of high-visual-noise faces, a clear version of the same face was cued for 0.5 s). Each participant was then directed to a rating screen where they rated the valence of each face from 1 (negative) to 9 (positive) at his or her own pace, by selecting the corresponding number using the computer’s mouse. Following the valence task, participants completed cognitive tasks and were debriefed.
Results
Because the face stimuli were presented in pairs, a ratio score was used to assess the relative looking preference for one face over the other (Isaacowitz et al., 2006a, 2006b; Isaacowitz et al., 2008). For each emotional–neutral pair, the ratio score was computed by subtracting the fixation duration for the neutral face from that of its emotional counterpart and then dividing the difference by the total duration of fixation on both faces (i.e., [emotional − neutral]/[emotional + neutral]). Scores of zero indicate no preference for either face. A positive ratio score indicates a preference for the emotional face, while a negative ratio score indicates a preference for the neutral face.
Age Differences in Emotional Gaze Preferences with Noise Distraction
The ratio scores were entered into a 2 (Age Group: younger, older) × 5 (Noise: none, visual [low (L), medium (M), high (H)], auditory) × 2 (Emotion Type: fearful, happy) × 2 (Age of face: younger, older) mixed-model analysis of variance (ANOVA), with age group as a between-subjects factor and the rest as within-subjects factors.3 The descriptive statistics for younger and older participants’ gaze ratio scores in each noise condition are presented in Table 2. There was a significant main effect of Age Group, F(1, 92) = 6.07, p < .05, ηp2 = .06, with younger adults (M = .12, SE = .02) preferring emotional faces (overall) to neutral faces more than older adults (M = .04, SE = .03). There was also a main effect of Emotion Type, F(1, 92) = 25.26, p < .001, ηp2 = .22, as participants overall tended to look away from fearful faces (M = .02, SE = .02) and toward happy faces (M = .16, SE = .02). A number of significant two-way interactions emerged: Age Group × Emotion Type, F(1, 92) = 16.17, p < .001, ηp2 = .15; Age Group × Age of Face, F(1, 92) = 5.48, p < .05, ηp2 = .06; and Noise × Emotion Type, F(3.51, 322.70) = 4.23, p < .01, ηp2 = .04. No other significant main effects or interactions were found.
Table 2.
Mean (and Standard Deviation) of Fixation Ratio Scores by Age Group, Age of Face, Emotional Face Type (fearful, happy), and Noise Conditionf
| Noise | Younger group | Older group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Younger faces | Older faces | Total | Younger faces | Older faces | Total | |||||||
|
|
|
|
|
|
|
|||||||
| Fearful | Happy | Fearful | Happy | Fearful | Happy | Fearful | Happy | Fearful | Happy | Fearful | Happy | |
| No | ||||||||||||
| M | .06 | .13 | .14 | .14 | .10 | .13 | −.13 | .18 | −.10 | .19 | −.12 | .18 |
| SD | .26 | .25 | .26 | .21 | .24 | .21 | .33 | .30 | .32 | .28 | .31 | .27 |
| Visual L | ||||||||||||
| M | .11 | .17 | .15 | .15 | .13 | .16 | −.10 | .20 | −.07 | .13 | −.08 | .16 |
| SD | .33 | .26 | .30 | .25 | .30 | .22 | .34 | .37 | .34 | .35 | .30 | .33 |
| Visual M | ||||||||||||
| M | .13 | .11 | .15 | .13 | .14 | .12 | −.04 | .14 | −.07 | .16 | −.06 | .15 |
| SD | .29 | .21 | .23 | .28 | .22 | .21 | .32 | .36 | .33 | .33 | .28 | .33 |
| Visual H | ||||||||||||
| M | .06 | .15 | .09 | .16 | .09 | .15 | −.15 | .26 | −.11 | .27 | −.13 | .26 |
| SD | .30 | .29 | .29 | .27 | .27 | .24 | .33 | .35 | .32 | .31 | .30 | .29 |
| Auditory | ||||||||||||
| M | .07 | .10 | .12 | .16 | .09 | .13 | −.12 | .20 | −.16 | .14 | −.14 | .17 |
| SD | .27 | .26 | .24 | .24 | .13 | .21 | .34 | .32 | .36 | .29 | .32 | .28 |
Note. L = low; M = medium; H = high.
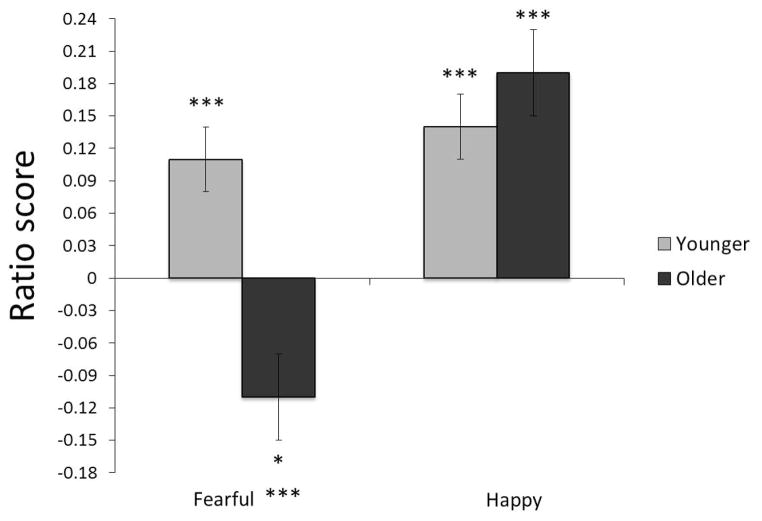
The Age Group × Emotion Type interaction was followed by one-sample t tests to determine which ratio scores were significantly different from zero, according to age group and emotion type (Isaacowitz et al., 2006a, 2006b, 2008). We calculated effect sizes for the one-sample t tests using Cohen’s d (M/SD; Cohen, 1988). Significant t scores indicated the presence of an attentional preference, with positive scores reflecting a preference for emotional faces and negative scores reflecting a preference for neutral faces (i.e., looking away from the emotional face). As shown in Figure 2, younger adults showed significant positive t scores for both fearful faces, t(59) = 3.98, p < .001, d = .52, and happy faces, t(59) = 6.13, p < .001, d = .78, indicating a looking preferences for both face types. Older adults, on the other hand, showed a significant negative t score for fearful faces, t(33) = −2.41, p < .05, d = .42, but a significant positive t score for happy faces, t(33) = 4.25, p < .001, d = .76. Age differences for each emotion type were tested using simple effects analyses. The older adults looked at fearful faces significantly less than their younger counterparts, F(1, 92) = 19.14, p < .001, ηp2= .17, d = .93; however, no age group difference emerged for happy faces, F(1, 92) = 1.03, p = .31, ηp2 = .01.4
Figure 2.
Fixation ratios by age group and emotional face type (fearful, happy). Bars indicate standard errors. Notations of significance (*p < .05, ***p < .001) next to each bar indicate that the ratio score for that condition is significantly different from zero. Notations of significance next to the label for an emotional face type indicate a significant difference between age groups for that face type.
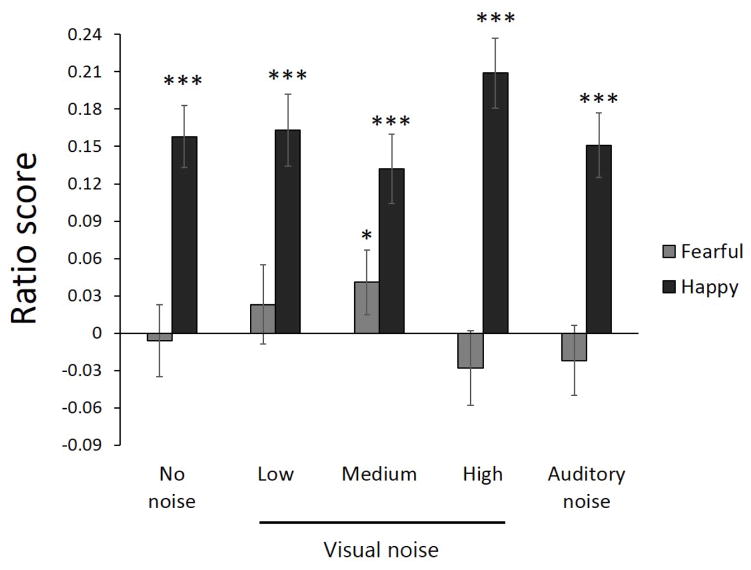
The Age Group × Age of Face interaction reflected that younger adults gazed longer at older emotional faces (M = .14, SE = .02) than emotional faces more similar to their own age (M = .11, SE = .02), F(1, 92) = 10.65, p < .01, ηp2 = .10. Older adults showed no significant difference in duration of gazing at older and younger faces, F < 1. The Noise × Emotion Type interaction was followed by simple main effects tests, the results of which indicated that when collapsed across age groups, participants showed stronger preferences for happy faces than fearful faces across all conditions (Fs > 6) (see Figure 3). One-sample t tests within noise conditions also confirmed significant attentional preferences for happy faces across all conditions, tno(93) = 6.24, p < .001, d = .63; tL(93) = 5.95, p < .001, d = .62; tM(93) = 4.77, p < .001, d = .49; tH(93) = 7.11, p < .001, d = .73; tauditory(93) = 5.93, p < .001, d = .61, but a significant preference toward fearful faces only in the medium visual-noise condition, t(93) = 2.54, p < .05, d = .26.
Figure 3.
Fixation ratios by noise condition and emotional face type (fearful, happy). Bars indicate standard errors. Notations of significance (*p < .05, ***p < .001) next to each bar indicate that the ratio score for that condition is significantly different from zero.
Despite the lack of a significant three-way Age Group × Noise × Emotion Type interaction, we conducted planned one-sample t tests to examine which ratio scores were different from zero according to age group, noise, and emotion type, all of which were collapsed across age of face. For younger adults, significant preferences toward both fearful faces, tno(59) = 3.39, p < .01, d = .44; tL(59) = 3.36, p < .01, d = .43; tM(59) = 4.85, p < .001, d = .63; tH(59) = 2.11, p < .05, d = .26; tauditory(59) = 3.27, p < .01, d = .42, and happy faces, tno(59) = 4.91, p < .001, d = .63; tL(59) = 5.62, p < .001, d = .73; tM(59) = 4.25, p < .001, d = .57; tH(59) = 5.00, p < .001, d = .65; tauditory(59) = 4.78, p < .001, d = .62, were found across all conditions. Older adults, on the other hand, displayed a significant preference toward avoiding fearful faces in the no-noise, high-visual-noise, and auditory-noise conditions, but they did not show any significant patterns for fearful faces in the low- and medium-visual-noise conditions, tno(33) = −2.16, p < .05, d = −.39; tL(33) = −1.64, p = .11, d = −.27; tM(33) = −1.15, p = .26, d = −.21; tH(33) = −2.49, p < .05, d = −.43; tauditory(33) = −2.50, p < .05, d = −.44. Older adults showed a significant preference toward happy faces in all conditions, tno(33) = 3.89, p < .001, d = .67; tL(33) = 2.89, p < .01, d = .48; tM(33) = 2.61, p < .05, d = .45; tH(33) = 5.22, p < .001, d = .90; tauditory(33) = 3.56, p < .01, d = .61. Thus, there was little evidence that noise influenced the gaze preferences of younger adults. In contrast, the gaze preferences of older adults (the propensity to look away from fearful faces) disappeared in the low- and medium-visual-noise conditions. Interestingly, the effect sizes indicate that older adults demonstrated their strongest preference toward happy faces in the high-visual-noise condition (see Table 2).
The Effects of Noise Block Order on Age Differences in Emotional Gaze Preferences
Another way to test the effects of noise on age differences in emotional gaze preferences is to examine whether the order in which the noise blocks were presented had an impact on gaze preferences. If noise and distractions reduce cognitive resources, and if displaying positive gaze preferences requires cognitive control, gaze preferences should be less evident when participants gaze on emotional faces with noise first, regardless of noise type. Therefore, the potential influence of noise block order during the experiment on gaze preference was tested by comparing fixation ratio scores when participants viewed emotional faces under the no-noise block either first, in the middle, or last.5
A 3 (Block Order: no-noise first, no-noise middle, no-noise last) × 2 (Age Group) × 5 (Noise) × 2 (Emotion Type) mixed-model ANOVA on fixation ratio scores was conducted collapsed across age of face. Block order and age group were included as between-subject factors and the rest were within-subject factors. The main effects of Age Group and Emotion Type were significant, F(1, 88) = 6.09, p < .05, ηp2 = .07 and F(1, 88) = 5.38, p < .001, ηp2 = .23, respectively. The Age Group × Emotion Type and the Noise × Emotion Type interactions remained significant, F(1, 88) = 16.79, p < .001, ηp2 = .16, and F(3.54, 311.16) = 4.28, p < .01, ηp2 = .05, respectively. The main effect of Block Order was not significant, F(2, 88) = 2.24, p = .11, ηp2 = .05, but a significant Block Order × Emotion Type interaction emerged, F(2, 88) = 4.30, p < .05, ηp2 = .09. Of particular importance, the Block Order × Age Group × Emotion Type interaction was significant, F(2, 88) = 4.14, p < .05, ηp2 = .09. All other effects were nonsignificant.
The Block Order × Age Group × Emotion Type interaction was decomposed using t tests to examine whether ratio scores were significantly different from zero, according to block order, age group, and emotion type. When the no-noise block came first, younger adults showed significant preferences for both fearful faces, t(17) = 2.28, p < .05, d = .53, and happy faces, t(17) = 2.99, p < .01, d = .68 (Mfearful = .10, SE = .05; Mhappy = .13, SE = .05). In contrast, older adults showed a significant preference away from fearful faces, t(11) = −2.42, p < .05, d = .70, and toward happy faces, t(11) = 3.48, p < .01, d = 1.00 (Mfearful = −.21, SE = .06; Mhappy = .32, SE = .06). This pattern, however, changed when participants viewed either the visual- or auditory-noise blocks first, with the no-noise block in the middle. In this case, younger adults did not show a preference toward fearful faces, t(21) = 1.48, p = .15, but did still show a preference toward happy faces, t(21) = 4.05, p < .01, d = .86. Older adults, on the other hand, did not show any preferences for either of these emotion types, ts < 2 (Mfearful = −.07, SE = .06; Mhappy = .07, SE = .06). When the no-noise block came last (i.e., after both the visual and auditory noise blocks), younger adults showed similar gaze patterns as in the no-noise-first block by exhibiting a significant preference toward both fearful faces, t(19) = 3.23, p < .01, d = .72, and happy faces, t(19) = 4.28, p < .001, d = .96 (Mfearful = .16, SE = .05; Mhappy = .21, SE = .04). Older adults, in contrast, did not show a significant preference away from fearful faces, t < 1 and showed only a marginally significant preference toward happy faces, t(9) = 2.26, p = .05, d = .71 (Mfearful = −.01, SE = .07; Mhappy = .16, SE = .06). Taken together, these results indicate that the order of noise blocks influenced gaze preferences to a greater extent for older adults. Further, the gaze preferences found in older adults disappeared or became less strong when they viewed emotional faces with a noise distraction first.
Valence Ratings
To assess whether participants could perceive the intended valence of the face pictures embedded in the varying levels of visual noise, we conducted in a 2 (Age Group) × 4 (Noise: no, visual [L, M, H]) × 2 (Emotion Type) × 2 (Age of Face) mixed-model ANOVA on valence rating scores. Age Group was included as a between-subjects factor and the rest were within-subjects factors. There was no main effect of Age Group, F(1, 89) = 2.09, p = .152, ηp2 = .02, but there were significant main effects of Noise, F(3, 267) = 14.60, p < .001, ηp2 = .14, Emotion Type, F(1, 146.23) = 859.89, p < .001, ηp2 = .91, and Age of Face, F(1, 89) = 40.64, p < .001, ηp2 = .31. These main effects were qualified by a number of significant two-way interactions: Noise × Emotion Type, F(4.98, 443.23) = 23.50, p < .001, ηp2 = .21; Noise × Age of Face, F(3, 267) = 23.66 p < .001, ηp2 = .2; and Emotion Type × Age of Face, F(1.90, 168.97) = 35.70, p < .001, ηp2 = .29. These two-way interactions were further qualified by significant three-way interactions for Age Group × Emotion Type × Age of Face, F(2, 178) = 3.12, p < .05, ηp2 = .03, and Noise × Emotion Type × Age of Face, F(5.00, 444.71) = 22.42, p < .001, ηp2=.20. No other effects were significant. Of particular relevance, the Noise × Emotion Type × Age of Face interaction was deconstructed using simple main effects across Age of Face and Emotion Type within Noise conditions. Happy younger faces were rated as more positive than neutral younger faces, and fearful younger faces were rated as more negative than neutral younger faces for each noise condition (No noise: Mhappy = 7.60, SE = .07; Mneutral = 4.74, SE = .06; Mfearful = 3.34, SE = .10; Low noise: Mhappy = 7.61, SE = .09; Mneutral = 4.66, SE = .06; Mfearful = 3.09, SE = .10; Medium noise: Mhappy = 7.35, SE = .08; Mneutral = 4.76, SE = .05; Mfearful = 3.04, SE = .09; High noise: Mhappy= 7.47, SE = .09; Mneutral = 4.58, SE = .06; Mfearful = 2.97, SE = .10), Fs(2, 88) > 512.34, ps < .001. Similarly, happy older faces were rated as more positive than neutral older faces, and fearful older faces were rated as more negative than neutral older faces for each noise condition (No noise: Mhappy = 7.71, SE = .08; Mneutral = 3.99, SE = .08; Mfearful = 2.78, SE = .10; Low noise: Mhappy = 7.54, SE = .09; Mneutral = 4.44, SE = .08; Mfearful = 2.81, SE = .10; Medium noise: Mhappy = 7.27, SE = .10; Mneutral = 4.35, SE = .07; Mfearful = 3.74, SE = .10; High noise: Mhappy = 7.35, SE = .09; Mneutral = 4.11, SE = .09; Mfearful = 2.88, SE = .11), Fs(2, 88) > 354.89, ps < .001. Thus, participants were able to accurately perceive the emotional valence of faces embedded in varying levels of visual-noise background and this did not vary by age group.6
Discussion
Older adults display positive preferences in their gaze patterns, focusing more on positive and less on negative emotional material, which appears to help with emotion regulation under certain circumstances (for a review, see Isaacowitz, 2012). This phenomenon, known as the age-related positivity effect, is theorized to be a top-down controlled process that taxes attentional resources (Mather & Carstensen, 2005). Therefore, some have argued that positive preferences, as reflected by visual attentiveness, are more likely to occur when the context allows for full control of visual attention (Knight et al., 2007; Mather & Carstensen, 2005). However, findings are mixed regarding the role of attentional control in age-related positive gaze preferences (Allard et al., 2008, 2010; Knight et al., 2007). The present study examined whether full attentional control is necessary to elicit positive gaze preferences in older adults. In order to test this, contextual demands were imposed on the participants during emotional processing, including visual and auditory noise. Happy–neutral and fearful–neutral face pairs were embedded in one of the following three backgrounds: a clear background (no noise), visual noise (low, medium, or high) or a background of auditory babble. Gaze preferences were assessed utilizing eye-tracking to record the percentage of time the eye fixates on a particular point or points in a visual field. By comparing the percentages of fixation on happy and fearful faces versus the percentages of fixation on neutral faces (i.e., a preference for the emotional face over the neutral face or vice versa), this study was able to assess how participants viewed the facial stimuli.
In line with previous eye-tracking research (Isaacowitz et al., 2006a, 2006b, 2008, 2011), an Age group × Emotion Type interaction was observed. Older adults demonstrated positive gaze preferences, looking more toward happy faces and away from fearful faces. Younger adults, on the other hand, showed gaze preferences toward both happy and fearful faces. Between-groups differences for each emotion indicated that older adults fixated for a significantly shorter period of time on fearful faces than younger adults, while no age group difference emerged for happy faces. Thus, these findings support the positivity effect in visual attention for older adults.
Noise Distraction and Age-Related Positive Gaze Preferences
In the present study, participants gazed more toward happy faces than fearful faces when they were experiencing a noise distraction. That is, overall both age groups showed a significant preference for happy faces in all conditions, while they showed a significant preference toward fearful faces only in the medium-visual-noise condition. Younger adults showed gaze preferences toward both fearful and happy faces irrespective of noise conditions, and thus their gaze preferences were less likely to be influenced by any form or level of noise. For older adults, robust gaze preferences (looking toward happy faces) were observed regardless of the type or level of noise, however, their tendency to look away from fearful faces disappeared under the low- and medium-visual-noise conditions. Thus, while older adults preferred to gaze at happy faces, regardless of type or intensity of noise distraction, their tendency to look away from fearful faces did not emerge with low or medium levels of visual distraction. One possible explanation for this finding is that the visual distractions imposed additional demands on emotional processing. Unlike the findings of Knight et al. (2007), we did not observe a reversal of the positive gaze preferences in the older group under any distracting conditions. Thus, the gaze patterns of the older adults in this study provide only partial support for the assertion that attentional control is necessary for the positivity effect to emerge (Knight et al., 2007; Mather & Carstensen, 2005).
It is possible that visual noise may have made fearful facial expressions appear less fearful. In this case, similar preferential patterns could have emerged for fearful as well as neutral facial stimuli. However, as shown in the valence ratings, participants were able to perceive the intended emotional valence of each facial expression across all noise conditions. Further, there were no age differences in the ability to detect the valences of facial expressions as a function of noise distraction. Therefore, it seems unlikely that noise distraction obscured valence-driven attentional effects. However, it is still possible that noise distractions may have reduced any difference in arousal between fearful and neutral facial stimuli. Nevertheless, older adults demonstrated preferences toward happy faces despite noise distractions.
Interestingly, in the high-visual-noise condition, older adults showed the strongest gaze preferences for happy faces, and also displayed the tendency to turn their gaze away from fearful faces. Thus, when clear facial cues were provided prior to visual distraction, older adults were able to demonstrate the typical positive gaze preferences even under highly distracting visual noise. This result is in line with previous research showing that older adults rely more on environmental cues for maintaining task presentations, thereby supporting information processing (Craik, 1983; Spieler et al., 2006; Wang et al., 2011). Of further interest, the preference for positive stimuli among older adults tended to be more pronounced in the high-visual-noise condition (.26) than in the no-noise condition (.18), though we were unable to detect statistical significance between these conditions. It is likely that older adults are more motivated to regulate their emotions when experiencing a high level of visual distraction. However, this study did not utilize mood measures; This phenomenon could not be directly assessed and remains an important possible question for further research.
The role of attentional control was also tested by looking at whether the order of the noise blocks had an impact on gaze patterns. The results revealed that older adults showed positive gaze preferences both toward positive and away from negative stimuli when they began with a block of stimuli that was free from noise. Conversely, positive gaze preferences did not emerge among older adults when the facial stimuli were presented with either a visual or an auditory distraction in the first block. When older adults were exposed to blocks of both forms of distraction prior to the block that was free of distraction, they showed no tendency to avoid fearful faces; however, they still showed a preference for looking toward happy faces. It is important to note, though, that the size of this effect was reduced when older adults were exposed to distractions first.
The noise-block order also influenced younger adults’ gaze patterns. The preference for fearful faces that had been previously noted among younger adults disappeared when they were exposed to a noise block before the block that was free of noise. These changes in the gaze preferences of younger adults are consistent with Knight et al.’s (2007) findings, which showed that, among younger adults, the proportion of fixation directed to negative faces in negative–neutral pairs was smaller in a divided-attention condition than the proportion of fixation directed to positive faces in positive–neutral pairs. Similarly, Pruzan and Isaacowitz (2006) compared visual attention to emotional stimuli among two groups of younger adults (graduating seniors and freshmen). Graduating seniors showed a tendency to avoid negative stimuli, a process similar to that displayed by older adults. Interestingly, the perceived social ending of graduation did not have an influence on the processing of positive stimuli. Thus, it appears that younger adults utilize a similar positive gaze preference for the processing of negative stimuli as that found in older adults.
The Role of Attentional Control in Positive Gaze
Previous studies by Mather and Knight (2005) and Knight et al. (2007) found that older adults in the divided-attention condition showed a reversal of the positivity effect in their memory or gaze. These findings support the argument that a substantial amount of attentional control is required to use gaze as a strategy for emotion regulation. Knight et al. (2007) argued that while emotional goals may be perpetually activated among older adults, the use of positive gaze as a tool of motivation likely requires the allocation of a substantial portion of attentional control resources for older adults. The present study provided some support for this argument because it showed that the tendency among older adults to look away from negative stimuli was no longer prevalent under conditions of low and medium visual noise. Moreover, while the order of noise blocks did influence gaze patterns for both age groups, its effect was exaggerated in the older group. When either of the noise distraction blocks came before the block without a distraction, the preferential gaze patterns of both looking away from negative stimuli and looking toward positive stimuli waned among older adults. These results indicate that the noise manipulation, which increased processing and created distraction, had a greater impact on older participants. However, we found no evidence of a reversal of the positivity effect in the older group, contrary to the findings of Knight et al. (2007).
If displaying positive gaze preferences places a strain on the resources required for attentional control, then older adults should have utilized the positive gaze pattern less during the final, distraction-free block because their resources would have already been taxed during the initial noise blocks. However, this was not the case. When the no-noise block was last, older adults still demonstrated gaze preferences for positive stimuli, though the effect was only marginally significant. It may be that continuous noise distraction increased the older adults’ motivation to regulate their emotions by viewing the positive rather than the negative pictures. It seems that the greater motivation to regulate emotion may have actually compensated for the additional attentional resources required. Tis idea is only speculative and further research is necessary to examine the link between gaze and mood under noise distraction.
The present study does not provide convincing evidence that positive gaze requires full control of visual attention. A preferential gaze toward positive stimuli seems more likely to be exhibited by older adults even when the noise distraction disrupts attentional control. However, unlike Allard and colleagues (2008, 2010), we found that the propensity to avoid negative stimuli among older adults was indeed less likely to occur under some types of noise distraction. Given that Allard et al. (2008) used a less demanding secondary task than Knight et al. (2007) and still found positive gaze preferences among older adults, it could be that the use of positive gaze preferences among older adults is dependent on the difficulty of the task or contextual demands. It is also interesting that, in the present study, noise distraction was more likely to disrupt selective attention to negative stimuli in both age groups.
The present findings appear to be in line with a recent proposal by Foster, Davis, and Kisley (2013) that there are different pathways by which older adults can achieve a positivity effect in emotion processing. In an event-related brain potential study, they showed that the late positive potential amplitude in response to negative images during an early phase of emotion processing peaked near 600 ms following stimulus onset among older adults with better cognitive abilities. On the other hand, older adults with worse cognitive abilities exhibited reduced neural reactivity to negative images, indicating that they had difficulties in processing negative information in an early phase of emotion processing. On the basis of these findings, Foster et al. (2013) argued that there may be different pathways for individuals with relatively poorer cognitive abilities compared to individuals with better cognitive abilities. Older adults with more limited cognitive abilities may utilize a positive gaze preference as a compensatory strategy (for example, Labouvie-Vief, Grühn, & Studer, 2010). In contrast, those with better cognitive abilities may utilize a positive gaze preference for regulatory reasons. In the latter case, older adults may focus more on positive and less on negative information in the later phases of emotion processing because emotion regulation strategies may take some time to yield the desired regulatory effect (Isaacowitz, Allard, Murphy, & Schlangel, 2009). Thus, our findings seem to extend beyond those of Foster et al. (2013), suggesting that the path used by older adults to achieve a positivity effect in emotion processing may also depend on context.
Visual Attention toward Emotional Faces of Difference Ages
Contrary to some previous eye-tracking studies, in which participants preferred facial stimuli that were of a similar age (Ebner et al., 2011; He et al., 2011), our younger adults spent more time looking at other-age (older) faces than younger faces. Older adults, on the other hand, showed no age-of-face preferences in their gaze patterns. Thus, older adults displayed the positivity effect in their gaze regardless of the age of the stimuli. It is possible that viewing faces embedded in visual noise may have reduced the perceived differences in age-related facial features of the stimuli, thus weakening gaze patterns associated with the age of the faces. Ebner et al. (2011) and He et al. (2011) asked participants to judge the emotional expressions of faces, which may have rendered own-age faces more relevant than other-age faces. Most previous studies on age differences in selective attention for emotional faces did not vary the age of the facial stimuli. Future studies need to include faces of different age groups to compare the positivity effect in visual attention toward own- and other-age facial stimuli.
Limitations and Future Directions
One limitation of the present study is that we used an implicit regulatory context. SST predicted that older adults chronically activate emotional goals and that these goals lead their gaze patterns toward goal-consistent information (i.e., more positive). Participants in the present study were instructed to look “naturally” at emotional stimuli. Therefore, it is unclear whether the positive gaze preferences found in older adults actually reflect an underlying motivation to regulate emotions and maintain a positive affective state. A future avenue of research would be to directly link age-related gaze patterns, mood, and attentional control to elucidate whether the use of positive gaze actually helps older adults enhance their mood.
Future research is also needed to ascertain whether positive gaze patterns are moderated by attentional control constraints. One study directly investigating this relationship in an implicit regulatory context (i.e., passive free viewing) showed that older adults with better executive function were able to resist mood decline throughout an eye-tracking session by displaying positive gaze preferences (Isaacowitz, Toner, & Neupert, 2009). On the other hand, Noh et al. (2011) created an explicit regulatory context by instructing participants to deliberately manage how they felt while viewing highly arousing, unpleasant images, and they found that alerting (i.e., using external warning cues to guide attention), but not executive function, predicted age differences in the link between gaze patterns and mood changes. That is, only older adults with well-developed alerting abilities were able to resist mood decline over time. They accomplished this by looking less at the most negative areas of images. Thus, examining gaze-mood links across various regulatory contexts, as well as considering different types of attentional ability, can provide a comprehensive picture of the role of attentional control in the positivity effect.
A limitation of the present study is that the noise distractions used may not have been intense enough to reduce attentional-control resources and prevent older adults from being able to implement their emotion-regulation strategies. Unlike previous studies, which examined positivity effects under a dual-task load, the present study used external noise which may more accurately reflect how older adults experience the demands of emotion regulation in everyday life. While our goal was to vary contextual demands, we needed our participants to be able to decode the emotions in the stimuli (even with highly distracting visual noise) in order to identify age-related gaze preferences for some emotions over others. For this reason, emotional cues had to be provided in the high-visual-noise condition, and older participants were able to demonstrate a positivity effect in their gaze despite such a significant distraction. Therefore, future research should examine the effects of varying intensities of noise distractions to provide a greater understanding of the conditions under which older adults display gaze preferences and how much attentional control is required for older adults to display positive gaze preferences. Only a small number of studies have examined the role of attentional control in the positivity effect in visual attention. The present study highlights the need for further examination of the interaction between attentional control and the positivity effect. This interaction could be illuminated by systematically varying the demands on attentional control of visual attention.
Time-sensitive measures would also be helpful to delineate the role of attentional control in the positivity effect on gaze. Depending on their attentional-control resources, the path used by older adults to achieve a positivity effect in emotion processing might differ (Foster et al., 2013; Isaacowitz et al., 2009). As we did not measure the time course of positive gaze preferences in the present study, we were unable to further investigate this potential way of further differentiating the path. This would be an important area for future study.
In sum, the present findings extend previous investigations on the age-related positivity effect in visual attention (Allard et al., 2008, 2009; Knight et al., 2007). This study provides evidence that eliciting positive gaze may require some attentional control, yet positive gaze preferences can still appear in a highly demanding context when external cues support emotional information processing. In light of the previous and current findings, it seems that while some types of attentional distractions or contexts may support age-related positivity effects, others may not. Conceptual models used to explain the mechanism and function of these age-related processes will need to incorporate a more complex understanding of the contexts within which age-related phenomena occur.
Acknowledgments
This study was supported by the National Institutes of Health, Grant R01 AG026323 and R01 AG026323, offered to Derek M. Isaacowitz. This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2013R1A1A2008016).
We are grateful to the Brandeis University Memory and Cognition Lab for allowing us to use their auditory-babble stimuli and audiometer, and to Raymond Stanley for his MATLAB programming for creating visual noise stimuli. We would also like to thank Brian Levinthal and Rhea Eskew for their advice and suggestions on earlier stages of programming the visual noise stimuli.
Footnotes
The relatively high percentage of exclusion of the older adults from the eye-tracking analyses (39% of the sample) is due to their wearing reflective eyewear or having other eye abnormalities, including small pupil diameter, and pupil obfuscation; eye tracking under visual noise distraction also contributed to the unsuccessful tracking rate for older adults. However, it is important to note that the exclusion rate for older adults in the present study is comparable to that of previous eye-tracking studies (e.g., Isaacowitz et al., 2006a, 2006b; Isaacowitz et al., 2008; Knight et al., 2007). Nonetheless, no significant differences emerged between trackable (n =34) and non-trackable (n = 26) older participants on self-reported health, cognitive, and affective measures, but non-trackable older participants had poorer near vision (for the Rosenbaum: Mtrackable = 27.50, SD = 3.08; Mnon-trackable = 30.58, SD = 5.35), t(58) = −2.80, p < .01, and poorer hearing than those of trackable older participants (for the pure-tone average: Mtrackable = 16.85, SD = 4.72; Mnon-trackable = 25.93, SD = 10.22), t(54) = −4.40, p < .001. Degrees of freedom for t tests do not match due to incomplete data for each measure.
The emotional–neutral face pair cues were presented for 0.5 s. We chose this duration based on the results of our pilot test, which showed that both age groups were able to identify the valence of the emotional–neutral faces with high visual noise (above 90% accuracy) when a cue was given for 0.5 s, and also on some previous research reporting that for very brief cues (shorter than 0.5 s), it was harder for older, compared to younger, adults to sustain their attention (Fernandez-Duque & Black, 2006; Jennings, Dagenbach, Engle, & Funke, 2007).
Whenever appropriate, Greenhouse-Geisser corrected degrees of freedom are used to adjust for violations of sphericity.
Among the potential covariates that showed age differences (see Table 1), only three sensory and vocabulary measures correlated significantly with gaze ratio scores, and this was true only for fearful face pairs. The Age Group × Emotion Type interaction remained significant when the scores on the sensory functioning measures, F(1, 84) = 4.35, p < .05, ηp2= .05, and vocabulary scores, F(1, 91) = 7.16, p < .01,ηp2 = .07, were included as covariates.
Because there were many combinations of orders by noise blocks (no, visual, auditory) and three levels of visual noise (low, medium, high), leaving few participants in each cell, we focused on whether the no-noise block came first, in the middle, or last (collapsing across the order of visual and auditory noise blocks).
Following up on the Age Group × Emotion Type × Age of Face interaction, simple main effects analyses were conducted separately for each age group across emotion type and age of face. Older adults rated happy faces as more positive than neutral faces, and rated fearful faces as more negative than neutral faces for both younger and older faces (Younger faces: Mhappy = 7.53, SE = .12; Mneutral = 4.71, SE = .08; Mfearful = 3.12, SE = .14; Older faces: Mhappy = 7.46, SE = .12; Mneutral = 4.32, SE = .10; Mfearful = 3.18, SE = .14), F(2, 88) = 289.70, p < .001,ηp2 = .87 and F(2, 88) = 279.95, p < .001, ηp2 = .86, respectively. Younger adults showed a similar pattern (Younger faces: Mhappy = 7.49, SE = .09; Mneutral = 4.66, SE = .06; Mfearful = 3.10, SE = .10; Older faces: Mhappy = 7.48, SE = .10; Mneutral = 4.13, SE = .08; Mfearful = 2.93, SE = .10), F(2, 88) = 488.59, p < .001, ηp2 = .92 and F(2, 88) = 529.62, p < .001, ηp2 = .92, respectively. Thus, both younger and older adults were able to perceive emotional valence of faces in a similar manner.
References
- Allard ES, Isaacowitz DM. Are preferences in emotional processing affected by distraction? Examining the age-related positivity effect in visual fixation within a dual-task paradigm. Aging, Neuropsychology, and Cognition. 2008;15:725–743. doi: 10.1080/13825580802348562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard ES, Wadlinger HA, Isaacowitz DM. Positive gaze preferences in older adults: Assessing the role of cognitive effort with pupil dilation. Aging, Neuropsychology, and Cognition. 2010;17:296–311. doi: 10.1080/13825580903265681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American National Standards Institute (ANSI) American national standards for audiometers (ANSI S3. 6-1989) New York: American National Standards Institute; 1989. [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz D, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the Intersection of Emotion and Cognition: Aging and the Positivity Effect. Current Directions in Psychological Science. 2005;14:117–121. doi: 10.1111/j.0963-7214.2005.00348.x. [DOI] [Google Scholar]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society, Series B. 1983;302:341–359. doi: 10.1098/rstb.1983.0059. [DOI] [Google Scholar]
- Dickinson CVM, Rabbitt PMA. Simulated visual impairment: Effects on text comprehension and reading speed. Clinical Vision Sciences. 1991;6:301–308. [Google Scholar]
- Ebner NC, He Y, Johnson MK. Age and emotion affect how we look at a face: Visual scan patterns differ for own-age versus other-age emotional faces. Cognition & Emotion. 2011;25:983–997. doi: 10.1080/02699931.2010.540817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, Lindenberger U. FACES—A database of facial expressions in young, middle-aged, and older women and men. Development and validation. Behavior Research Methods. 2010;42:351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster SM, Davis HP, Kisley MA. Brain responses to emotional images related to cognitive ability in older adults. Psychology and Aging. 2013;28:179–190. doi: 10.1037/a0030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer’s disease. Neuropsychology. 2006;20:133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- Gao X, Levinthal B, Stine-Morrow EAL. The effects of aging and visual noise on conceptual integration during reading. Quarterly Journal of Experimental Psychology. 2012;65:1833–1847. doi: 10.1080/17470218.2012.674146. [DOI] [PubMed] [Google Scholar]
- Gao X, Stine-Morrow EAL, Noh SR, Eskew R. Visual noise disrupts conceptual integration in reading. Psychonomic Bulletin and Review. 2011;18:83–88. doi: 10.3758/s13423-010-0014-4. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Grühn D, Smith J, Baltes PB. No aging bias favoring memory for positive material: Evidence from a heterogeneity-homogeneity list paradigm using emotionally toned words. Psychology and Aging. 2005;20:579–588. doi: 10.1037/0882-7974.20.4.579. [DOI] [PubMed] [Google Scholar]
- He Y, Ebner NC, Johnson MK. What predicts the own-age bias in face recognition memory? Social Cognition. 2011;29:97–119. doi: 10.1521/soco.2011.29.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM. Mood regulation in real time: Age differences in the role of looking. Current Directions in Psychological Science. 2012;21:237–242. doi: 10.1177/0963721412448651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Allard E, Murphy NA, Schlangel M. The time course of age related preferences toward positive and negative stimuli. Journal of Gerontology: Psychological Sciences. 2009;64B:188–192. doi: 10.1093/geronb/gbn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Blanchard-Fields Fredda. Linking process and outcome in the study of emotion and aging. Perspectives on Psychological Science. 2012;7:3–17. doi: 10.1177/1745691611424750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Choi Y. The malleability of age-related positive gaze preferences: Training to change gaze and mood. Emotion. 2011;11:90–100. doi: 10.1037/a0021551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Neupert SD. Use of gaze for real-time mood regulation: Effects of age and attentional functioning. Psychology and Aging. 2009;24:989–994. doi: 10.1037/a0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson HR. Looking while unhappy: Mood-congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19:848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006a;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006b;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Dagenbach D, Engle CM, Funke LJ. Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Aging, Neuropsychology, and Cognition. 2007;14:353–369. doi: 10.1080/13825580600788837. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D. What’s new in Psychtoolbox-3? Perception. 2007;36 doi: 10.1068/v070821. ECVP Abstract Supplement. [DOI] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Kryla-Lighthall N, Mather M. The role of cognitive control in older adults’ emotional well-being. In: Berngtson V, Gans D, Putney N, Silverstein M, editors. Handbook of Theories of Aging. 2. Springer Publishing; 2009. pp. 323–344. [Google Scholar]
- Labouvie-Vief G, Grühn D, Studer J. Dynamic integration of emotion and cognition: Equilibrium regulation in development and aging. In: Lerner RM, Lamb ME, Freund AM, editors. The handbook of life-span development: Vol. 2. Social and emotional development. Hoboken, NJ: Wiley; 2010. pp. 79–115. [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of Neuroscience Methods. 2003;128:85–93. doi: 10.1016/S0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Nikitin J, Freund AM. Age and motivation predict gaze behavior for facial expressions. Psychology and Aging. 2011;26:695–700. doi: 10.1037/a0023281. [DOI] [PubMed] [Google Scholar]
- Noh SR, Lohani M, Isaacowitz DM. Deliberate real-time mood regulation in adulthood: The importance of age, fixation, and attentional functioning. Cognition and Emotion. 2011;25:998–1013. doi: 10.1080/02699931.2010.541668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen WO. Average speech levels and spectra in various speaking/listening conditions. American Journal of Audiology. 1998;7:21–25. doi: 10.1044/1059-0889(1998/012). [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- Pruzan K, Isaacowitz DM. An attentional application of socioemotional selectivity theory in college students. Social Development. 2006;15:326–338. doi: 10.1046/j.1467-9507.2006.00344.x. [DOI] [Google Scholar]
- Rabbitt PMA. Channel capacity, intelligibility and immediate memory. The Quarterly Journal of Experimental Psychology. 1968;20:241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Oto-Laryngologica, Supplement. 1991;476:167–176. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- Reed AE, Carstensen LL. The theory behind the age-related positivity effect. Frontiers in Emotion Science. 2012;3:1–9. doi: 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Trail-making test: Manual for administration and scoring. South Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Rosenbaum JG. The biggest reward for my invention isn’t money. Medical Economics. 1984;61:152–163. [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8:870–874. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Shiota M, Levenson R. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza F, Daneman M, Schneider BA. How aging affects the reading of words in noisy backgrounds. Psychology and Aging. 2000;15:253–258. doi: 10.1037/0882-7974.15.2.253. [DOI] [PubMed] [Google Scholar]
- Tun PA. Fast noisy speech: Age differences in processing rapid speech with background noise. Psychology and Aging. 1998;13:424–434. doi: 10.1037/0882-7974.13.3.424. [DOI] [PubMed] [Google Scholar]
- Urry HL, Gross JJ. Emotion regulation in older age. Current Directions in Psychological Science. 2010;19:352–357. doi: 10.1177/0963721410388395. [DOI] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang X, Laubach M, Mazer JA, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Development. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- Wingfield A, Tun PA, McCoy SL. Hearing loss in older adults: What it is and how it interacts with cognitive performance. Current Directions in Psychological Science. 2005;14:144–148. doi: 10.1111/j.0963-7214.2005.00356.x. [DOI] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale–Revised manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]