Abstract
Background
Although implantable cardioverter-defibrillators (ICDs) reduce mortality in patients with out-of-hospital cardiac arrest, their effectiveness in survivors of in-hospital cardiac arrest—a population with different arrest etiologies and higher illness acuity than out-of-hospital cardiac arrest—is unknown. We therefore sought to conduct a comparative effectiveness study of ICD therapy in survivors of in-hospital cardiac arrest.
Methods
We linked data from a national inpatient cardiac arrest registry with Medicare files and identified 1200 adults from 267 hospitals between 2000 and 2008 who were discharged after surviving an in-hospital cardiac arrest due to ventricular fibrillation or pulseless ventricular tachycardia and who otherwise met traditional inclusion and exclusion criteria for secondary prevention ICD trials. The association between ICD treatment and long-term mortality was evaluated using an optimal match (≤ 4 controls for each ICD patient) propensity-score analysis.
Results
Of 1200 survivors, 343 (28.6%) received an ICD during the index hospitalization. Overall, 3-year mortality was 44.2%, with higher unadjusted mortality in the non-ICD vs. the ICD group (46.9% vs. 37.3%; log-rank P<0.001). After successfully matching 343 patients treated with ICDs with 823 untreated patients by propensity score, ICD treatment was associated with a 24% lower mortality rate (adjusted HR, 0.76; 95% CI, 0.60–0.97; P=0.025). This lower mortality was mediated by lower rates of out-of-hospital deaths among ICD-treated patients (22.1% vs. 30.8%; adjusted HR, 0.71 [0.52–0.96], P=0.019), whereas deaths occurring during a readmission were similar (15.2% vs. 16.1%; adjusted HR, 0.89 [95% CI, 0.60–1.32], P=0.56).
Conclusions
ICD therapy in survivors of in-hospital cardiac arrest due to a pulseless ventricular rhythm is used uncommonly but associated with lower long-term mortality. Given that fewer than 3 in 10 eligible survivors are treated with ICDs after surviving an in-hospital cardiac arrest, our findings highlight a potentially modifiable process of care which could improve long-term survival in this high-risk population.
Implantable cardioverter-defibrillators (ICDs) reduce mortality in survivors of out-of-hospital cardiac arrest.1, 2 Although cardiac arrest occurs almost as commonly within hospitals,3 no studies have examined the use or effectiveness of ICDs among survivors of in-hospital cardiac arrest. As a result, guidelines on ICD implantation for in-hospital survivors do not currently exist. The lack of studies and guidelines may be due, in part, to historically greater attention devoted to improving out-of-hospital resuscitation care, with best practices for in-hospital cardiac arrest only recently emerging.4, 5 It is also possible that ICDs are variably used in patients with in-hospital cardiac arrest due to perceived competing mortality risks, as hospitalized patients with cardiac arrest have higher illness acuity and comorbidity burden than those with out-of-hospital cardiac arrest. Given this gap in knowledge, there is a critical need to evaluate the benefit of ICDs in this population. If ICDs lower long-term mortality for survivors but remain underused, it would highlight a potentially modifiable target to improve the long-term survival of patients with in-hospital cardiac arrest. Moreover, in the absence of a randomized trial, a comparative effectiveness study of current practice can provide valuable insight into contemporary patterns of care, including ICD treatment differences among vulnerable populations (e.g., by race and sex).
To address these gaps in knowledge, we evaluated the effectiveness of ICD therapy and treatment patterns among survivors of in-hospital cardiac arrest in a national registry. Additionally, because of potential concerns about heterogeneity of treatment effect (e.g., by neurological and functional status at discharge), we examined whether the effect of ICD therapy on long-term mortality differed by patients’ demographics, primary discharge diagnosis, discharge neurological status, and hospital disposition.
Methods
Data Sources and Linkage
Get With the Guidelines®-Resuscitation (GWTG-Resuscitation), formerly the National Registry of Cardiopulmonary Resuscitation, is a large prospective quality-improvement registry of in-hospital cardiac arrests. Its design has been previously described in detail.6 In brief, trained quality-improvement hospital personnel enroll all patients with cardiac arrest (defined as the absence of a palpable central pulse, apnea, and unresponsiveness) and without do-not-resuscitate orders. Cases are identified by multiple methods, including centralized collection of cardiac arrest flow sheets, reviews of hospital paging system logs, and routine checks of code carts, pharmacy tracer drug records, and hospital billing charges for resuscitation medications.6 The registry uses standardized Utstein-style definitions for patient variables and outcomes to facilitate uniform reporting across hospitals.7, 8 Data accuracy is further ensured by rigorous certification of hospital staff and use of standardized software with data checks for completeness and accuracy.
In a prior report, we linked data from GWTG-Resuscitation to Medicare inpatient files.9 Briefly, patient-level data from GWTG-Resuscitation from January 1, 2000, through December 31, 2008, were linked to Medicare inpatient files using 6 identifiers: dates of hospital admission and discharge, patient age and sex, the hospital to which the patient was admitted (deidentified), and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes for cardiac arrest (427.5), ventricular fibrillation (427.41), or ventricular flutter (427.42) or procedure codes for cardiopulmonary resuscitation (99.60), defibrillation (99.62), or closed chest massage (99.63). For each linked patient, we obtained Medicare denominator and inpatient files from 2000 through 2010.
Study Population
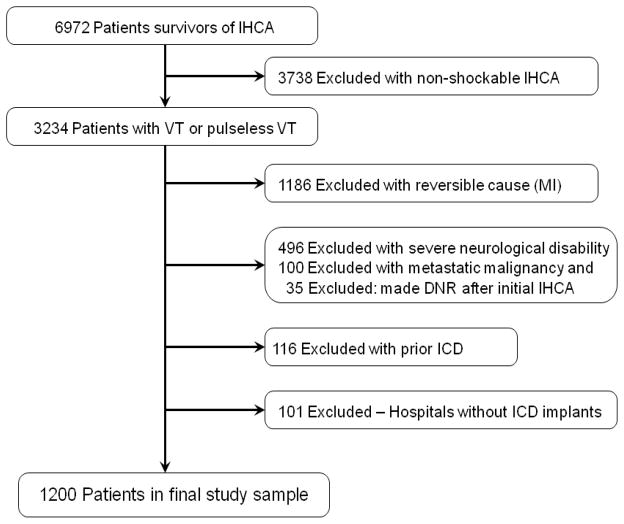
We derived the study cohort from hospitals that submitted data to GWTG-Resuscitation between January 1, 2000, and December 31, 2008 (Figure 1). We did not include patients after 2008 to allow for at least 2 years of follow-up in the Medicare data set. Based on the linkage described above, we were able to link to Medicare files 6972 GWTG-Resuscitation registry patients 65 years of age or older who survived to hospital discharge after an in-hospital cardiac arrest.9 As our study was focused on ICD implantation during the index hospitalization for cardiac arrest, we excluded 3,738 survivors with an initial cardiac arrest rhythm of asystole or pulseless electrical activity, as these patients would not benefit from ICD therapy.
Figure 1.
Definition of the Study Cohort
Consistent with prior secondary prevention trials for out-of-hospital cardiac arrest, we also excluded 1,186 patients with a myocardial infarction during the index hospitalization, as the cause of their cardiac arrest was treatable.10, 11 Since ICD therapy would not be offered to patients with poor functional status or limited life expectancy, we further excluded 496 patients with severe neurological disability (defined by a Cerebral Performance Category score of >2) at hospital discharge,12 100 patients with advanced cancer, and 35 patients who were made do-not-resuscitate after the initial acute resuscitation event and prior to hospital discharge. Additionally, we excluded 116 patients with a pre-existing ICD at the time of cardiac arrest. Finally, we excluded 101 patients who were admitted to hospitals with no documented ICD implants within Medicare inpatient files in the year prior to their arrest. The final study cohort comprised 1200 patients from 267 hospitals with the ability to implant ICDs.
Study Exposure and Outcomes
The primary outcome was long-term mortality, which was determined from Medicare denominator files, and the main independent variable was ICD implantation prior to hospital discharge, as determined by ICD-9-CM codes 37.94 or 00.51. As ICD therapy would be expected to decrease arrhythmic mortality (which typically occurs outside the hospital), we further categorized deaths as occurring out-of-hospital and during readmission. Readmission deaths were determined from a patient’s hospital disposition in Medicare inpatient files, and an out-of-hospital death was defined as one which did not occur during a readmission. In addition, we examined for the presence of sex and racial differences in ICD treatment and whether there was heterogeneity of ICD treatment effect on mortality by patients’ demographics (age, sex, and race), principal discharge diagnosis, neurological status at discharge, and hospital disposition.
Statistical Analysis
Baseline differences between patients in whom ICDs were and were not implanted were evaluated using X2 tests for categorical variables and Student’s t-tests for continuous variables. We constructed survival curves using Kaplan-Meier estimates to determine unadjusted rates of mortality.
Because of potential indication bias, we conducted a propensity score analysis to evaluate the association of ICD treatment with mortality.13–15 To accomplish this, we first constructed a multivariable logistic regression model to derive a patient’s propensity of ICD treatment during the index hospitalization for cardiac arrest. All patient covariates were included in the model to generate a non-parsimonious propensity score and included: age (categorized as 65–74, 75–84, and ≥85 years), sex, race (white, black, other), initial cardiac arrest rhythm (ventricular fibrillation or pulseless ventricular tachycardia), principal discharge diagnosis (cardiac vs. non-cardiac), discharge neurological status (discharge CPC score of 1 [little to no neurological disability] vs. 2 [moderate disability])12, and discharge destination (home, home with home health care, skilled nursing facility, rehabilitation site, and other). In addition, we included co-morbidities or medical conditions present within 24 hours prior to cardiac arrest (prior history of congestive heart failure, history of myocardial infarction, atrial arrhythmia, and diabetes mellitus; acute heart failure exacerbation during the index hospitalization with cardiac arrest; renal, hepatic, or respiratory insufficiency; baseline evidence of motor, cognitive, or functional deficits [CNS depression]; acute stroke; pneumonia; hypotension; sepsis; major trauma; and metabolic or electrolyte abnormality) and interventions in place at the time of cardiac arrest (mechanical ventilation, dialysis). The propensity score model showed good discrimination, with a c-statistic of 0.744.
We then conducted an optimal propensity score match between patients treated and not treated with an ICD, using an algorithm match with a caliper width no greater than 0.2 times the standard deviation of the logit of the propensity score.16 We confirmed that ICD and non-ICD patients were well-balanced in covariates after propensity score matching by ensuring that standardized differences between the ICD groups for each covariate were <10%.17 The association between ICD use and mortality was then assessed using a Cox regression model stratified on matched patient sets.18 As a sensitivity analysis, we examined how many patients received an ICD within 90 days after discharge and repeated the analyses above after reclassifying these patients with delayed ICD placement into the ICD group.
To evaluate the likelihood that our mortality findings could be confounded by unmeasured severity of illness despite our propensity matching, we repeated the above propensity score analyses separately for out-of-hospital deaths and deaths from readmission, as ICD treatment would be expected to only reduce out-of-hospital mortality. Furthermore, we compared 3-year cumulative readmission rates between the propensity-matched ICD and non-ICD groups. Since prior trials have found that ICD treatment would not be expected to influence readmission rates,19 finding similar rates of readmission between the two groups would suggest that our propensity score match adjusted well the burden of illness between the groups.
In addition, we examined whether there were differences in ICD treatment patterns by certain patient factors (e.g., race and sex) from the propensity score model. Finally, we evaluated whether the effectiveness of ICD therapy differed by the pre-specified subgroups of age, sex, race, principal discharge diagnosis category, neurological status at discharge, and hospital disposition by constructing multivariable Cox regression models, which adjusted for ICD status, hospital site, the variables used in the derivation of the aforementioned propensity score, and an interaction term between ICD therapy and each pre-specified factor, evaluated separately.
For each analysis, we evaluated the null hypothesis at a 2-sided significance level of 0.05 and calculated 95% confidence intervals (CIs) using robust standard errors. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina) and R version 2.10.0 (R Foundation for Statistical Computer, Vienna, Austria).20 The institutional review board of the Mid America Heart Institute approved the study and waived the requirement for informed consent, as the data was de-identified.
Results
Of 1200 survivors of an in-hospital cardiac arrest eligible for secondary prevention ICD therapy, 343 (28.6%) received an ICD. Table 1 compares baseline characteristics between those who did and did not receive an ICD. Patients receiving an ICD were, on average, 1.2 years younger than those who did not and were more likely to be men. Patients with a prior history of heart failure or myocardial infarction, heart failure during the index hospitalization, a history of an atrial arrhythmia, or a principal cardiac discharge diagnosis were also more likely to undergo ICD implantation at discharge. In contrast, patients with respiratory insufficiency, metabolic abnormalities, or pneumonia within 24 hours prior to cardiac arrest and those on a mechanical ventilator at the time of cardiac arrest were less likely to undergo ICD implantation. Finally, patients with minimal to no neurological disability at discharge or those who were discharged home were more likely to receive an ICD.
Table 1.
Baseline Characteristics According to ICD Status.
| ICD (N = 343) | No ICD (N = 857) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD, years | 74.5 ± 6.0 | 75.7 ± 7.1 | 0.005 |
| Male sex | 216 (63.0) | 453 (52.9) | 0.001 |
| Race | |||
| White | 283 (89.3) | 715 (89.0) | 0.59 |
| Black | 25 (7.9) | 72 (9.0) | |
| Other | 9 (2.8) | 16 (2.0) | |
| Missing | 26 | 54 | |
| Pre-existing conditions | |||
| Diabetes mellitus | 119 (34.7) | 254 (29.6) | 0.09 |
| History of heart failure | 132 (38.5) | 246 (28.7) | <0.001 |
| Heart failure this admission | 110 (32.1) | 173 (20.2) | < 0.001 |
| History of myocardial infarction | 116 (33.8) | 237 (27.7) | 0.03 |
| Atrial arrhythmia | 221 (64.4) | 466 (54.4) | 0.001 |
| Respiratory insufficiency | 59 (17.2) | 240 (28.0) | < 0.001 |
| Renal insufficiency | 68 (19.8) | 191 (22.3) | 0.35 |
| Hypotension | 37 (10.8) | 135 (15.8) | 0.03 |
| Metabolic or electrolyte abnormality | 14 (4.1) | 97 (11.3) | < 0.001 |
| Pneumonia | 15 (4.4) | 70 (8.2) | 0.02 |
| Baseline depression in CNS function | 16 (4.7) | 70 (8.2) | 0.02 |
| Septicemia | 10 (2.9) | 43 (5.0) | 0.11 |
| Acute stroke | 8 (2.3) | 19 (2.2) | 0.90 |
| Hepatic insufficiency | 2 (0.6) | 14 (1.16) | 0.26 |
| Major trauma | 1 (0.3) | 10 (1.2) | 0.19 |
| Interventions in place | |||
| Mechanical ventilation | 35 (10.2) | 185 (21.6) | < 0.001 |
| Dialysis | 2 (0.6) | 12 (1.4) | 0.37 |
| Event characteristics | |||
| Hospital-wide response activation | 292 (85.1) | 628 (73.3) | <0.001 |
| Use of automated external defibrillator | 48 (14.0) | 109 (12.7) | 0.55 |
| Initial cardiac arrest rhythm | 0.07 | ||
| Ventricular fibrillation | 201 (58.6) | 550 (64.2) | |
| Pulseless ventricular tachycardia | 142 (41.4) | 307 (35.8) | |
| Discharge status | |||
| Principal discharge diagnosis | <0.001 | ||
| Cardiac | 284 (82.8) | 573 (66.9) | |
| Non-cardiac | 59 (17.2) | 284 (33.1) | |
| Neurological disability | 0.003 | ||
| Minimal to none | 238 (69.4) | 517 (60.3) | |
| Moderate | 105 (30.6) | 340 (39.7) | |
| Disposition | <0.001 | ||
| Home self-care | 155 (45.2) | 281 (32.8) | |
| Home with home health care | 78 (22.7) | 155 (18.1) | |
| Skilled nursing facility | 64 (18.7) | 203 (23.7) | |
| Other inpatient facility (e.g., rehabilitation) | 42 (12.2) | 207 (24.2) | |
| Other | 4 (1.2) | 11 (1.3) | |
Abbreviations: CNS, central nervous system
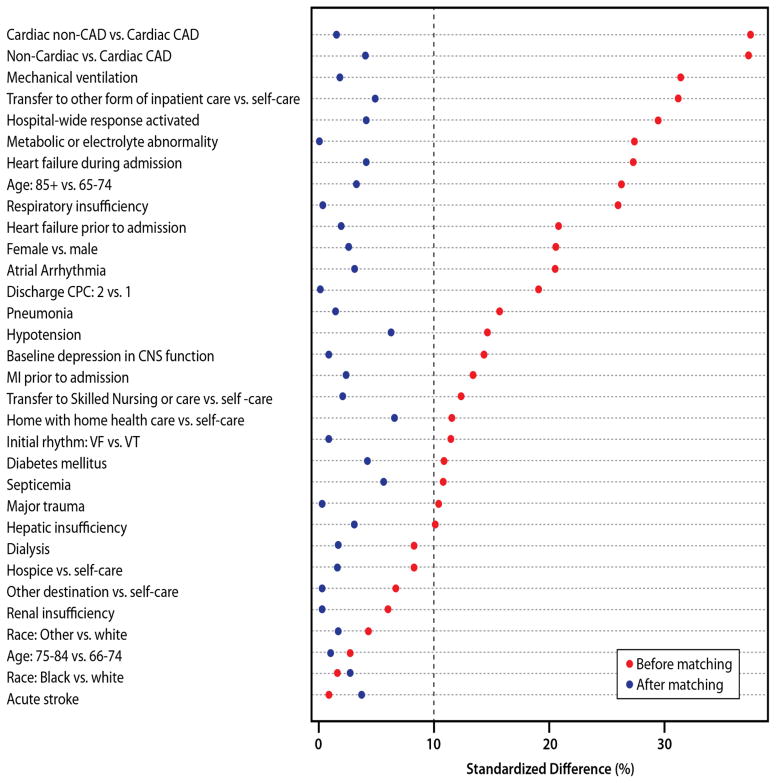
Overall, the mortality rate at 3 years was 44.2%, with a significantly higher unadjusted mortality rate in the non-ICD group than the ICD group (46.9% vs. 37.3%; log-rank P<0.001). Prior to matching by propensity score, the distribution of patient case-mix was uneven (standardized differences >10%) for many covariates between the ICD and non-ICD patient groups, but these differences were eliminated after propensity matching, with all standardized differences ≤10% (Figure 2). A successful match was accomplished for 1166 (97.2%) patients, with 34 non-ICD patients who were not matched. After matching by propensity score, we found that ICD treatment was associated with a 24% lower mortality rate at 3 years (adjusted Hazard Ratio [HR], 0.76; 95% CI, 0.60–0.97; P=0.025). Based on the 46.1% 3-year mortality rate in the non-ICD group, this would translate to an 11% lower absolute mortality rate (number needed to treat of 9) in patients treated with an ICD.
Figure 2. Covariate Balance Between ICD Groups Before and After Propensity Score Matching.
Significant differences (standardized difference [SD] >10%) in characteristics existed between ICD and non-ICD patients prior to propensity score matching. After the match, all characteristics were balanced (SD <10%) between the ICD and non-ICD groups. Covariates are ranked by magnitude of standardized differences prior to propensity score matching.
We queried Medicare inpatient files after hospital discharge and determined that only 5 (0.6%) and 14 (1.6%) non-ICD patients were subsequently implanted with an ICD at 30 and 90 days follow-up, respectively. In sensitivity analyses, repeating our propensity score analyses after reclassifying these 14 patients into the ICD group did not materially change our findings (HR, 0.74 [0.58–0.94]; P=0.01). When we categorized deaths as occurring outside the hospital or during a readmission, we found that patients treated with an ICD had a lower 3-year rate of out-of-hospital mortality (ICD group, 22.1%; non-ICD group, 30.8%; HR, 0.71 [0.52–0.96], P=0.019) but no differences in deaths during readmission (15.2% vs. 16.1%; HR, 0.89 [95% CI, 0.60–1.32], P=0.56) (Figure 3). Moreover, we found no difference in the 3-year cumulative readmission incidence rates between the 2 groups: ICD group, 3.39 readmissions; non-ICD group: 3.09 readmissions; P=0.21 (Figure 4). Both of these latter findings suggest that the propensity methods adequately adjusted for competing comorbidities.
Figure 3. Cumulative Mortality Rates.
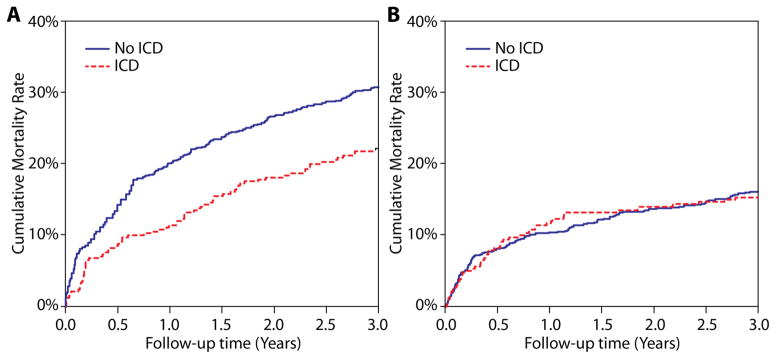
When comparing the propensity-matched patient groups, rates of out-of-hospital deaths were lower in patients treated with ICDs (panel A), whereas deaths during readmission were similar (panel B).
Figure 4. Cumulative Readmission Incidence Rates.
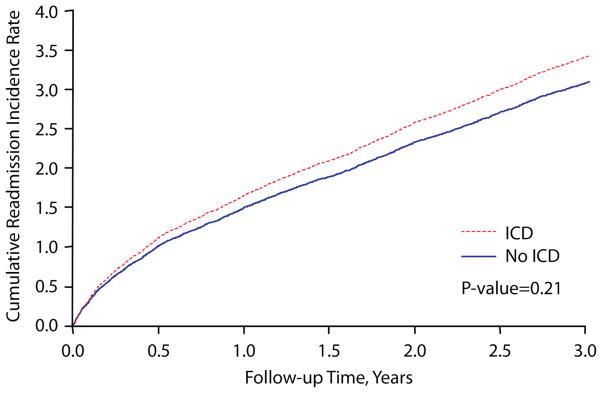
There were no significant differences in 3-year all-cause readmission rates between the propensity-matched ICD and non-ICD groups.
Key predictors of ICD treatment are summarized in Table 2 (see Supplementary Appendix eTable 1 for full model results). Notably, women and the very elderly were less likely to be treated with an ICD at hospital discharge, whereas there were no differences by race. Patients with a discharge disposition to a skilled nursing or inpatient (e.g., rehabilitation) facility were less likely to be treated with an ICD, but there were no differences in ICD treatment by discharge neurological status. Despite these treatment patterns, there was no evidence of heterogeneity of treatment effect, as interaction terms between ICD therapy and key patient factors were not significant (Table 3). For instance, ICD therapy was effective both in patients with minimal to no neurological disability at discharge (HR, 0.81 [0.61–1.06]) and those with moderate neurological disability (HR, 0.68 [0.48–0.95]; interaction P-value of 0.42). Similarly, there were no differences in the effect of ICD therapy by age group, sex, race, principal discharge diagnosis category, and hospital disposition, although the hazard ratio for patients ≥85 years of age was greater than 1.
Table 2.
Selected Predictors of ICD Treatment Among IHCA Survivors.*
| Hazard Ratio (95% CI) | P value | |
|---|---|---|
| Age group, years | ||
| 65 to 74 | Reference | Reference |
| 75 to 84 | 0.96 (0.71–1.29) | 0.77 |
| ≥ 85 | 0.29 (0.16–0.52) | <0.001 |
| Female sex | 0.67 (0.50–0.89) | 0.007 |
| Race | ||
| White | Reference | Reference |
| Black | 1.07 (0.66–1.73) | 0.78 |
| Other | 1.30 (0.50–3.38) | 0.59 |
| Initial rhythm of ventricular fibrillation (vs. VT) | 0.78 (0.59–1.05) | 0.10 |
| Non-cardiac primary discharge diagnosis | 0.76 (0.50–1.16) | 0.21 |
| Moderate neurological disability at discharge | 0.82 (0.60–1.13) | 0.22 |
| Discharge disposition | ||
| Home self-care | Reference | Reference |
| Home with home health care | 0.94 (0.65–1.36) | 0.74 |
| Skilled nursing facility | 0.70 (0.47–1.05) | 0.08 |
| Other inpatient facility (e.g., rehabilitation) | 0.39 (0.25–0.60) | <0.001 |
| Other | 1.75 (0.30–10.33) | 0.54 |
Abbreviations: VT, ventricular tachycardia
Full model results are summarized in Supplementary Appendix, eTable 1
Table 3. Interaction Analyses in Pre-Specified Subgroups.
* The lower mortality associated with ICD therapy was consistent across pre-specified patient factors, including demographics, primary discharge diagnosis category, discharge neurological status, and hospital disposition.
| Hazard Ratio (95% CI) | P for Interaction | |
|---|---|---|
| Overall cohort | 0.76 (0.60–0.97) | |
|
| ||
| Age groups, years | 0.39 | |
| 65 to 74 | 0.66 (0.48–0.91) | |
| 75 to 84 | 0.80 (0.59–1.08) | |
| ≥ 85 | 1.08 (0.54–2.14) | |
| Sex | 0.41 | |
| Men | 0.70 (0.53–0.92) | |
| Women | 0.83 (0.60–1.15) | |
| Principal discharge diagnosis | 0.41 | |
| Cardiac | 0.69 (0.43–1.10) | |
| Non-cardiac | 0.86 (0.64–1.14) | |
| Discharge neurological status | 0.42 | |
| Minimal to no disability | 0.81 (0.61–1.06) | |
| Moderate disability | 0.68 (0.48–0.95) | |
| Hospital disposition | 0.87 | |
| Home with home health care | 0.79 (0.54–1.15) | |
| Skilled nursing facility | 0.64 (0.41–0.99) | |
| Other inpatient facility (e.g., rehabilitation) | 0.79 (0.53–1.18) | |
An interaction P value of >0.05 suggests no difference in the effect of ICD therapy for the strata in that subgroup.
Discussion
Among patients 65 years or older who survived an in-hospital cardiac arrest due to ventricular fibrillation or pulseless ventricular tachycardia, we found that fewer than 3 in 10 eligible patients were treated with an ICD for secondary prevention prior to hospital discharge. Importantly, ICD treatment was associated with a 24% lower mortality risk over 3 years of follow-up. Older patients and women, as well as those requiring further skilled nursing or inpatient treatment, were less likely to be treated, but there were no observed differences in the association between ICD treatment and survival by demographics, neurological disability at discharge, or hospital disposition. Collectively, our findings provide initial empirical evidence for the clinical effectiveness of ICD therapy among survivors of in-hospital cardiac arrest, and, given the low rate of adoption, highlight a potentially important opportunity to further improve the care and outcomes of this population.
Little is known about the benefit of ICDs for in-hospital cardiac arrest. Although clinical trials have demonstrated that ICDs reduce mortality in survivors of out-of-hospital cardiac arrest,1, 2 patients with in-hospital cardiac arrest typically have a greater burden of comorbidities. As a result, in-hospital cardiac arrest may represent a clinically different population of patients with higher competing mortality risks that may limit the effectiveness of secondary prevention ICD treatment. To our knowledge, our study is the first to examine the effectiveness of ICD therapy in survivors of an in-hospital cardiac arrest, in part because research on this condition has been limited by the lack of a clinical registry with longitudinal data on survival. We were able to overcome this challenge by leveraging the rich detailed data (including patients’ severity of illness, discharge neurological impairment, and functional status [as measured by hospital disposition]) collected within GWTG-Resuscitation and by linking this registry to Medicare inpatient files to provide a robust assessment of long-term outcomes. We found that secondary prevention ICDs reduced long-term mortality by 24%—an effect size similar to the 28% relative risk reduction from secondary prevention ICD trials for out-of-hospital cardiac arrest.2 Importantly, the benefit was consistent in men and women (a population in which the benefit of ICDs has been questioned in other contexts21, 22), as well as in those with moderate neurological or functional disability (e.g., disposition to acute nursing or rehabilitation facilities).
Several aspects of our study merit further comment. In our analyses, we used propensity scores to ensure balance in patient covariates. However, our findings still could have been influenced by unmeasured factors. Since ICD treatment would not be expected to reduce morbidity unrelated to a life-threatening ventricular arrhythmia, we evaluated rates of readmission to examine whether or not our propensity methods were able to achieve good balance of patients’ overall health for those who did and did not receive an ICD. Reassuringly, ICD treatment was not associated with lower readmission rates, which is consistent with prior primary prevention trials of patients at risk of out-of-hospital cardiac arrest.19 Moreover, we found that the mortality differences in the ICD group were due to lower rates of deaths outside the hospital rather than during a readmission, which is aligned with the mechanism of expected benefit with ICD treatment – reduction of out-of-hospital arrhythmic deaths. In addition, we restricted our analyses to only those patients who could have been treated by excluding untreated patients who were admitted at hospitals without an ICD implant in the year prior to their cardiac arrest. Finally, we found that if patients were not treated with an ICD prior to discharge, the likelihood of subsequent ICD implant after discharge was exceedingly low.
We recently reported that only 43% of Medicare-aged in-hospital cardiac arrest survivors were still alive at 3 years.9 While the adoption rate of ICD treatment in our study was low, it also suggests a potential opportunity to further increase the long-term survival of in-hospital cardiac arrest survivors whose arrest was due to a ventricular arrhythmia—as these rhythms comprise 46% of all cardiac arrest survivors from the hospitalized setting.9 The 11% absolute mortality reduction associated with ICD treatment in this study suggests that 9 survivors of in-hospital cardiac arrest may need to be treated for 3 years to save one life. As our comparative effectiveness study was observational in nature, there may still be a need for a randomized trial to definitively establish the clinical benefit of ICDs in patients with in-hospital cardiac arrest and evaluate which patient groups are most likely to benefit.
Our study has some potential limitations. First, GWTG-Resuscitation is a quality-improvement registry that collects cardiac arrest data from a diverse group of hospitals; therefore, long-term outcomes in nonparticipating hospitals may differ. Nonetheless, the effectiveness of ICD treatment would not be expected to be affected since we examined post-discharge outcomes. Second, we restricted the analysis to fee-for-service, elderly Medicare beneficiaries. While outcomes in patients younger than 65 years may differ, prior concerns on the benefit of ICD treatment have primarily focused on the elderly (the focus of our study), given competing non-cardiac mortality risks. Third, despite use of propensity scores, unmeasured confounders may still bias our findings. Nonetheless, we indirectly assessed for this possibility by examining rates of rehospitalization and deaths during readmission as proxies for competing risks. Fourth, although we found that rates of ICD implanatation in non-ICD patients were low (1.6%) at 3 months after discharge, we were not able to evaluate outpatient implants. Nonetheless, in our experience, only one-quarter of ICD implants are coded as outpatient procedures (Personal Communication, Dr. Lesley Curtis, Duke University); therefore, the rate of outpatient ICD implants after discharge is likely to be <1%. Moreover, if ICDs are indeed associated with lower mortality, any ‘crossover’ leading to misclassification of ICD status would be expected to bias our findings toward the null. Fifth, although we found no evidence for heterogeneity of effect, some subgroups were likely underpowered. Therefore, our interaction analyses should be interpreted with caution. Finally, we did not have information on cause of death.
In conclusion, we found that ICD treatment in survivors of in-hospital cardiac arrest was associated with substantially lower long-term mortality. Given that only about 3 in 10 eligible survivors are treated with ICDs after an in-hospital cardiac arrest, our findings highlight a potentially modifiable process of care which could improve long-term survival in this high-risk population.
Acknowledgments
Funding/Support:
Dr. Chan is supported by a Career Development Grant Award (K23HL102224) and an R01 Award (1R01HL123980) from the National Heart Lung and Blood Institute.
Dr. Krumholz was supported, in part, by a grant (1U01HL105270-02) from the National Heart, Lung, and Blood Institute to the Center for Cardiovascular Outcomes Research at Yale University.
GWTG-Resuscitation is sponsored by the American Heart Association, which had no role in the study design, data analysis or manuscript preparation and revision.
Footnotes
Disclosures: None of the authors have any relevant conflicts of interest or disclosures.
Authorship: Dr. Chan had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and design: Chan
Acquisition of Data: Chan, Curtis
Statistical Analysis: Li, Hammill
Analysis and interpretation of data: Chan, Krumholz, Spertus, Curtis, Li, Hammill, Nallamothu
Drafting of the manuscript: Chan, Nallamothu
Critical revision of the manuscript for important intellectual content: Chan, Krumholz, Spertus, Curtis, Li, Hammill, Nallamothu
Study Supervision: Chan
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, Greene HL, Boczor S, Domanski M, Follmann D, Gent M, Roberts RS. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 3.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, Groeneveld PW. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358:9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 5.Morrison LJ, Neumar RW, Zimmerman JL, Link MS, Newby LK, McMullan PW, Jr, Hoek TV, Halverson CC, Doering L, Peberdy MA, Edelson DP. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127:1538–1563. doi: 10.1161/CIR.0b013e31828b2770. [DOI] [PubMed] [Google Scholar]
- 6.Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane-Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D’Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 8.Cummins RO, Chamberlain D, Hazinski MF, Nadkarni V, Kloeck W, Kramer E, Becker L, Robertson C, Koster R, Zaritsky A, Bossaert L, Ornato JP, Callanan V, Allen M, Steen P, Connolly B, Sanders A, Idris A, Cobbe S. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital ‘Utstein style’. American Heart Association. Circulation. 1997;95:2213–2239. doi: 10.1161/01.cir.95.8.2213. [DOI] [PubMed] [Google Scholar]
- 9.Chan PS, Nallamothu BK, Krumholz HM, Spertus JA, Li Y, Hammill BG, Curtis LH. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368:1019–1026. doi: 10.1056/NEJMoa1200657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallstrom AP, Greene HL, Wyse DG, Zipes D, Epstein AE, Domanski MJ, Schron EB. Antiarrhythmics Versus Implantable Defibrillators (AVID)--rationale, design, and methods. Am J Cardiol. 1995;75:470–475. [PubMed] [Google Scholar]
- 11.Wyse DG, Friedman PL, Brodsky MA, Beckman KJ, Carlson MD, Curtis AB, Hallstrom AP, Raitt MH, Wilkoff BL, Greene HL. Life-threatening ventricular arrhythmias due to transient or correctable causes: high risk for death in follow-up. J Am Coll Cardiol. 2001;38:1718–1724. doi: 10.1016/s0735-1097(01)01597-2. [DOI] [PubMed] [Google Scholar]
- 12.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate propensity scores. The American Statistician. 1985;39:33–38. [Google Scholar]
- 17.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. Journal of Clinical Epidemiology. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 18.Walker GA. Common Statistical Methods for Clinical Research with SAS® Examples. 2. Cary, N.C: 2002. [Google Scholar]
- 19.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 20.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2008. [Accessed August 2, 2014]. http://www.R-project.org. [Google Scholar]
- 21.Dhruva SS, Redberg RF. Evaluating sex differences in medical device clinical trials: time for action. JAMA. 2012;307:1145–1146. doi: 10.1001/jama.2012.254. [DOI] [PubMed] [Google Scholar]
- 22.Ghanbari H, Dalloul G, Hasan R, Daccarett M, Saba S, David S, Machado C. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:1500–1506. doi: 10.1001/archinternmed.2009.255. [DOI] [PubMed] [Google Scholar]