Abstract
Rationale: Limited data exist about the international burden of severe sepsis in critically ill children.
Objectives: To characterize the global prevalence, therapies, and outcomes of severe sepsis in pediatric intensive care units to better inform interventional trials.
Methods: A point prevalence study was conducted on 5 days throughout 2013–2014 at 128 sites in 26 countries. Patients younger than 18 years of age with severe sepsis as defined by consensus criteria were included. Outcomes were severe sepsis point prevalence, therapies used, new or progressive multiorgan dysfunction, ventilator- and vasoactive-free days at Day 28, functional status, and mortality.
Measurements and Main Results: Of 6,925 patients screened, 569 had severe sepsis (prevalence, 8.2%; 95% confidence interval, 7.6–8.9%). The patients’ median age was 3.0 (interquartile range [IQR], 0.7–11.0) years. The most frequent sites of infection were respiratory (40%) and bloodstream (19%). Common therapies included mechanical ventilation (74% of patients), vasoactive infusions (55%), and corticosteroids (45%). Hospital mortality was 25% and did not differ by age or between developed and resource-limited countries. Median ventilator-free days were 16 (IQR, 0–25), and vasoactive-free days were 23 (IQR, 12–28). Sixty-seven percent of patients had multiorgan dysfunction at sepsis recognition, with 30% subsequently developing new or progressive multiorgan dysfunction. Among survivors, 17% developed at least moderate disability. Sample sizes needed to detect a 5–10% absolute risk reduction in outcomes within interventional trials are estimated between 165 and 1,437 patients per group.
Conclusions: Pediatric severe sepsis remains a burdensome public health problem, with prevalence, morbidity, and mortality rates similar to those reported in critically ill adult populations. International clinical trials targeting children with severe sepsis are warranted.
Keywords: multiple organ failure, sepsis, pediatrics
At a Glance Commentary
Scientific Knowledge on the Subject
In existing large, multicenter epidemiological studies of pediatric severe sepsis, researchers have relied on retrospective case identification using administrative codes and have reported minimal data about treatment and nonmortal outcomes. To date, no studies have prospectively applied consensus criteria to identify critically ill children with severe sepsis within a large, international, multicenter network of pediatric intensive care units or included comprehensive data about treatment strategies and outcomes.
What This Study Adds to the Field
The present study provides the largest international characterization of pediatric severe sepsis epidemiology. We demonstrate that severe sepsis remains a highly prevalent public health problem for critically ill children worldwide that is associated with substantial morbidity and mortality. The prevalence of 8.2% was comparable to that reported in adult studies, and the hospital mortality rate of 25% was higher than previously estimated in retrospective studies that used administrative databases. These data support the view that well-designed trials targeting both morbidity and mortality outcomes in pediatric severe sepsis should be feasible with international, multicenter cooperation.
Severe sepsis is a life-threatening condition commonly treated in pediatric intensive care units (PICUs) worldwide (1–5). It is estimated that over one-third of children who die in tertiary care PICUs within the United States have severe sepsis (6). Recent reports of a rising prevalence of pediatric sepsis (1, 6, 7) may reflect an expanding vulnerable population with chronic comorbidities (8, 9), increasing rates of multidrug-resistant organisms and opportunistic infections (10, 11), and a surge in sepsis surveillance (12, 13). However, shifting patterns of diagnostic coding may confound current epidemiological estimates that are based largely on retrospectively identified cases in administrative databases (5, 14), and prior studies using prospective case identification have been limited to few sites within single countries, thus precluding broad generalizations (2, 5, 15). To date, no pediatric studies have examined the epidemiology and global burden of severe sepsis across multiple countries using a prospective methodology.
Consensus guidelines emphasize basic principles of goal-directed resuscitation, prompt antimicrobial administration, and supportive care of organ dysfunction in pediatric sepsis (16, 17). However, few large clinical trials have addressed the management of critically ill children with severe sepsis (18–23). Consequently, debate remains about the optimal approach to both basic and adjuvant therapies. For example, vasoactive strategies, immune stimulation, and plasma exchange would all benefit from further evaluation in rigorous pediatric trials (21, 24, 25). The low frequency of pediatric sepsis at any one institution necessitates broad—ideally international—collaboration across many sites to achieve adequate enrollment and generalizability (26). Global epidemiologic data are essential to characterize existing treatment variability, identify meaningful outcome targets, and calculate realistic sample sizes for such trials (27).
The objective of the Sepsis Prevalence, Outcomes, and Therapies (SPROUT) study was to determine the point prevalence of pediatric severe sepsis and characterize the microbiology, current therapeutic interventions, and patient-centered outcomes within a broad international network of PICUs. Modeled after similar epidemiological studies in adults (28, 29), SPROUT prospectively screened and collected data on a large number of critically ill children who met consensus criteria for severe sepsis (16). The study authors sought to provide generalizable data about the global epidemiology of pediatric severe sepsis to inform the design of future multicenter interventional trials. Some of the results of this study have been reported previously in the form of an abstract (30).
Methods
Overview
The SPROUT study was a prospective, cross-sectional study of the point prevalence, therapies, and outcomes for pediatric patients with severe sepsis admitted to a PICU, conducted on 5 days over the course of 1 year: June 5, 2013; September 17, 2013; November 6, 2013; January 22, 2014; and March 19, 2014. Sites were recruited by open invitation and participation was voluntary, with no funding provided to sites. Ethics approval was obtained at all sites, with waiver of informed consent granted at all but three sites, at which written consent was required for data collection.
Inclusion and Exclusion Criteria
All patients younger than 18 years of age treated in a participating PICU at 9:00 a.m. local time on each study day were screened for severe sepsis using the 2005 International Pediatric Sepsis Consensus Conference criteria: (1) two or more systemic inflammatory response syndrome criteria, (2) confirmed or suspected invasive infection, and (3) cardiovascular dysfunction, acute respiratory distress syndrome, or two or more organ dysfunctions (16). To determine the point prevalence of active severe sepsis, only those patients who met consensus criteria for severe sepsis within the 24-hour period from 9:00 a.m. the day before the study day through 9:00 a.m. on the study day were included. Patients who had previously met criteria for severe sepsis at a prior point during their PICU hospitalization but no longer had manifestations of active severe sepsis within this 24-hour time window were not included in this point prevalence analysis, even if they were still being treated for an infection (e.g., with antibiotics). Exclusion criteria were corrected gestational age less than 42 weeks, age 18 years or older, or surgery involving cardiopulmonary bypass in the preceding 5 days.
Data Collection
Data were collected using the web-based Research Electronic Data Capture (31) on PICU characteristics, patient demographics, comorbidities, site of infection, and microbiological isolates for all patients who met the criteria for severe sepsis. Laboratory results, antimicrobial administration, vasoactive infusions, mechanical ventilation, and adjuvant therapies within a 48-hour period (from 9:00 a.m. the day before to 9:00 a.m. the day after the study day) were also captured. Definitions for the primary site of infection were adapted from published criteria (32). The day of severe sepsis recognition was identified by retrospectively reviewing the medical charts to determine the first calendar day on which a patient met consensus criteria for severe sepsis (16). The presence of new or progressive multiorgan dysfunction syndrome (MODS) was measured for 7 days following severe sepsis recognition according to previously published criteria (20, 33). For severity of illness, the Pediatric Index of Mortality (PIM)-3 score (34) was calculated at PICU admission, and the Pediatric Logistic Organ Dysfunction score (35) was collected on the study day. To facilitate consistent and accurate data collection, we used separate pilot and training phases, embedded quality checks within the electronic case report form, and secondarily validated missing or outlying data values (see Table E1 in the online supplement).
Patients with severe sepsis were followed for 90 days or until death or hospital discharge. Outcomes included vasoactive- and ventilator-free days from the day of severe sepsis recognition through Day 28, new or progressive MODS, change in functional status from admission to hospital discharge (using the Pediatric Overall Performance Category [POPC] 1–6 ordinal scale [36]), and all-cause mortality at PICU and hospital discharge. For vasoactive- and ventilator-free days, one point was assigned for each day following sepsis recognition up to 28 days that patients were both alive and free from use of vasoactive medications or invasive mechanical ventilation, respectively. A calendar day counted toward the total number of vasoactive days if the patient received an infusion of dopamine or dobutamine >5 μg/kg/min or any infusion of epinephrine, norepinephrine, vasopressin/terlipressin, phenylephrine, milrinone, or continuous vasodilator (e.g., nitroglycerin) for any part of that day. A calendar day counted toward the total number of ventilator days if mechanical ventilation was delivered via an endotracheal or tracheostomy tube during any part of that day. For patients who died within 28 days of sepsis recognition, vasoactive- and ventilator-free days equaled the number of days free from use of vasoactive infusions or invasive mechanical ventilation between sepsis recognition and death (26). Patients surviving to hospital discharge were classified as having “at least mild disability” for any increase in POPC and “at least moderate disability” if discharge POPC score was ≥3 and increased ≥1 from baseline (37). A composite outcome of death or moderate to severe disability at hospital discharge was also determined.
Statistical Analyses
Data from the five study dates were merged and analyzed using STATA software (Version 12.1; StataCorp, College Station, TX). Categorical data, expressed as frequency (%), were analyzed using Fisher’s exact test. Continuous data, expressed as median (interquartile range [IQR]), were analyzed using the Kruskal-Wallis test. Point prevalence was calculated as the number of patients with severe sepsis divided by the total number screened and is presented with the 95% confidence interval (CI). Data across ordered groups were compared using a nonparametric test of trend of ranks. Multivariable logistic regression was used to determine the association of corticosteroid and albumin use with mortality, controlling for relevant covariates. We identified relevant covariates a priori based on biological plausibility, feasibility of data collection, and prior studies (38, 39). To assess for potential confounders, we separately added each covariate to our base bivariate model, in which PICU mortality was the outcome and corticosteroid or albumin was the primary independent variable. Covariates that changed the base model OR by ≥10% were considered to be true confounders and were included in the final multivariate model after assessing for collinearity (40). In addition, we determined that age, sex, and PIM-3 severity of illness score would be included as covariates in the final multivariable models, even if these variables did not reach the threshold for confounding. Sample sizes were calculated to report a range of clinically meaningful differences for future interventional trials. Statistical significance was defined as a P value < 0.05.
Results
SPROUT recruited 128 PICUs in 26 countries: 59 in North America, 39 in Europe, 10 in South America, 10 in Asia, 7 in Australia/New Zealand, and 3 in Africa (see Figure E1). Ninety-eight percent were staffed by pediatric intensivists, 87% were affiliated with academic institutions, and 46% were part of free-standing children’s hospitals. Sites had a median (IQR) of 16 (10–23) PICU beds and 718 (500–1,300) annual admissions.
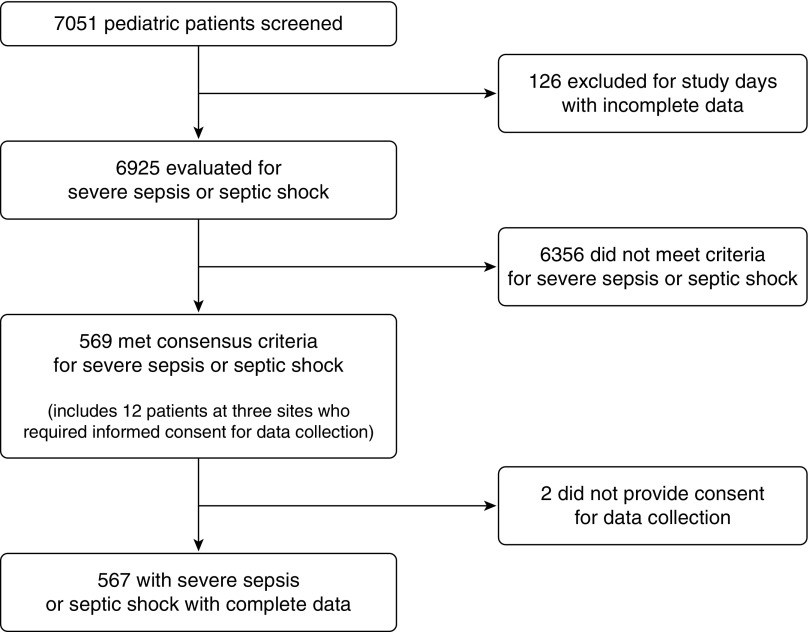
Overall, 6,925 children were screened and 569 met consensus criteria for severe sepsis (Figure 1), yielding a point prevalence of 8.2% (95% CI, 7.6–8.9%). Point prevalence varied across regions (P < 0.001) (Figure E2): North America 7.7% (95% CI, 6.9–8.5%), Europe 6.2% (5.0–7.6%), Australia/New Zealand 6.8% (4.4–9.8%), Asia 15.3% (11.7–19.5%), South America 16.3% (12.1–21.3%), and Africa 23.1% (13.5–35.2%). Detailed data were collected on 567 of the 569 patients with severe sepsis (2 patients did not provide consent for data collection out of the 12 with severe sepsis from three sites that required consent for data collection). Patient characteristics are presented in the aggregate in Table 1 and by age in Table E2. The median age was 3 years (IQR, 0.7–11.0), and 77% had comorbid conditions, with the most common being respiratory illness (30.3%). Medical admissions accounted for 81.1% of patients, with approximately even distribution of admissions from the hospital’s emergency department (ED), general hospital ward, or transfer from another hospital. Patients transferred from a ward had a higher rate of comorbidities than ED patients (86% vs. 70%, P = 0.001). The majority of patients exhibited respiratory (82.7%) or cardiovascular dysfunction (70.2%) at screening.
Figure 1.
Schematic depicting screening and patient enrollment. For sites with incomplete screening or patient data, all data from that site on the study day were excluded from the analysis (total of 126 patients from four sites). A waiver of consent was approved at all sites for screening and at all but three sites for data collection.
Table 1.
Characteristics of Patients with Severe Sepsis
| Characteristic | Value |
|---|---|
| Age, yr | 3.0 (0.7–11.0) |
| Male sex, n (%) | 302 (53.3) |
| Race/ethnicity, n (%) | |
| White | 245 (43.2) |
| Hispanic | 97 (17.1) |
| Black | 79 (13.9) |
| Asian | 75 (13.2) |
| Other | 39 (6.9) |
| Unknown | 32 (5.6) |
| Comorbid conditions, n (%) | |
| Respiratory | 172 (30.3) |
| Gastrointestinal | 141 (24.9) |
| Cardiovascular | 136 (24.0) |
| Genetic | 115 (20.3) |
| Hematologic/immunologic | 114 (20.1) |
| Neuromuscular | 97 (17.1) |
| Neoplastic | 80 (14.1) |
| Prematurity | 76 (13.4) |
| Metabolic | 62 (10.9) |
| Renal | 55 (9.7) |
| Solid organ/stem cell transplant | 54 (9.5) |
| Number of comorbid conditions, n (%) | |
| None | 128 (22.6) |
| 1 | 141 (24.9) |
| ≥2 | 298 (52.6) |
| Admission POPC, n (%) | |
| Good performance | 290 (51.2) |
| Mild disability | 85 (15.0) |
| Moderate disability | 90 (15.9) |
| Severe disability or coma | 102 (18.0) |
| Lactate, maximum, mmol/L* | 1.8 (1.1–3.5) |
| ScvO2, minimum, %† | 66 (55–75) |
| PaO2/Fio2, minimum, mm Hg‡ | 158 (96–251) |
| PIM-3 score§ | 4.1 (1.7–8.7) |
| PELOD score║ | 11 (2–12) |
| Type of PICU admission, n (%) | |
| Medical | 460 (81.1) |
| Surgical, scheduled | 53 (9.4) |
| Surgical, unscheduled | 34 (6.0) |
| Trauma | 20 (3.5) |
| Source of admission, n (%) | |
| Emergency department¶ | 167 (29.5) |
| Hospital floor | 158 (27.9) |
| Operating room | 50 (8.8) |
| Other hospital** | 166 (29.3) |
| Other | 26 (4.6) |
| Organ dysfunction present at screening,†† n (%) | |
| Respiratory | 469 (82.7) |
| Cardiovascular | 398 (70.2) |
| Hematologic | 175 (30.9) |
| Hepatic | 143 (25.2) |
| Neurologic | 119 (21.0) |
| Renal | 93 (16.4) |
Definition of abbreviations: PELOD = Pediatric Logistic Organ Dysfunction; PICU = pediatric intensive care unit; PIM-3 = Pediatric Index of Mortality 3; POPC = Pediatric Overall Performance Category; ScvO2 = central venous oxygen saturation.
Data are presented as median (interquartile range), unless noted otherwise.
Lactate was measured in 391 of 567 patients.
ScvO2 was measured in 200 of 567 patients.
PaO2/Fio2 ratio was measured in 352 of 567 patients; it was not collected in patients with unpalliated cyanotic heart disease.
PIM-3 was measured at time of PICU admission.
PELOD score was calculated from data within a 48-hour time window around the study day (9:00 a.m. on the day before to 9:00 a.m. the day after the study day).
Emergency department at the same hospital as the PICU.
“Other hospital” includes emergency department at another hospital.
Based on organ dysfunction criteria defined by the 2005 International Pediatric Sepsis Consensus Conference (16).
The most common primary sites of infection (Table 2) were respiratory (40%) and bloodstream (19%). An infectious organism was isolated in 65% of patients, and blood cultures were positive in 26%, including patients with secondary bacteremia (Table 2). There was a similar proportion of Gram-positive (26.5%; 95% CI, 22.9–30.3%) and Gram-negative (27.9%; 95% CI, 24.2–31.8%) infections, with Staphylococcus aureus being the most commonly isolated bacteria. Fungi, mainly Candida species, were isolated in 13.4%.
Table 2.
Site of Infection and Microbiologic Etiology of Severe Sepsis
| Characteristic | n (%) |
|---|---|
| Primary site of infection | |
| Respiratory | 228 (40.2) |
| Primary bloodstream | 108 (19.1) |
| Abdominal | 47 (8.3) |
| Central nervous system | 25 (4.4) |
| Genitourinary | 21 (3.7) |
| Skin | 20 (3.5) |
| Other | 29 (5.1) |
| Unknown | 89 (15.7) |
| Microbiology* | |
| Total patients with positive isolate† | 371 (65.4) |
| Gram-negative bacteria | 158 (27.9) |
| Pseudomonas species | 45 (7.9) |
| Klebsiella species | 36 (6.4) |
| Escherichia coli | 32 (5.6) |
| Enterobacter species | 17 (3.0) |
| Acinetobacter species | 14 (2.5) |
| Other | 55 (9.7) |
| Gram-positive bacteria | 150 (26.5) |
| Staphylococcus aureus | 65 (11.5) |
| Methicillin-resistant Staphylococcus aureus | 20 (3.5) |
| Enterococcus species | 25 (4.4) |
| Staphylococcus epidermis | 21 (3.7) |
| Streptococcus pneumonia | 10 (1.8) |
| Other | 45 (7.9) |
| Anaerobic bacteria | 1 (0.2) |
| Other bacteria | 3 (0.5) |
| Fungi | 76 (13.4) |
| Candida species | 67 (11.8) |
| Aspergillus species | 3 (0.5) |
| Other | 8 (1.4) |
| Parasites | 3 (0.5) |
| Viruses | 119 (21.0) |
| Rhinovirus | 32 (5.6) |
| Respiratory syncytial virus | 22 (3.9) |
| Adenovirus | 20 (3.5) |
| Cytomegalovirus | 13 (2.3) |
| Influenza | 12 (2.1) |
| Human metapneumovirus | 12 (2.1) |
| Epstein-Barr virus | 8 (1.4) |
| Other virus | 27 (4.8) |
Categories do not add up to 100% as some infections were polymicrobial.
Sources of positive isolates include blood, urine, cerebrospinal fluid, respiratory system (nasopharynx, tracheal, and bronchoalveolar lavage), stool, wound, and other normally sterile body fluids (pleural, pericardial, and peritoneal).
Ninety-eight percent of patients were treated with antibiotics, 19% with antivirals, and 33% with antifungals. Other sepsis-related therapies used are shown in Table 3. For the 421 patients (74%) who received invasive mechanical ventilation, median duration was 13 (IQR, 6–28) days. Vasoactive medications were used in 55%, with significant variation across age groups (P = 0.01) and the highest use in infants aged 29 days to 1 year (see Table E3). Vasoactive medications were used for a median of 7 (IQR, 3–17) days. Epinephrine and norepinephrine were the most commonly used medications, at 43% and 42% of patients treated with vasoactive medications, respectively, and dopamine was used in 32%. Epinephrine use decreased with age (Ptrend = 0.03), and there was a tendency to use norepinephrine in older patients (Ptrend = 0.095). Thirty percent were treated with milrinone, and use of other vasodilators was rare (3%). Although the majority had central venous (84%) or arterial catheters (62%), 6% and 24% of those treated with vasoactive medications did not have a central venous or arterial catheter, respectively. Nearly half (45%) of the patients were treated with corticosteroids, with no differences across age groups (P = 0.45). Other adjuvant therapies, including insulin, granulocyte/granulocyte–monocyte colony–stimulating factor (G/GM-CSF), immunoglobulin, plasma exchange, and extracorporeal membrane oxygenation (ECMO) were used in ≤10% of patients, with most of these therapies used more frequently in nonsurvivors (see Table E4).
Table 3.
Therapies Used within the 48-Hour Data Collection Window
| Therapy | Total |
|---|---|
| Vasoactive infusions* | 314 (55) |
| Dopamine† | 102 (32) |
| Dobutamine† | 20 (6) |
| Epinephrine† | 134 (43) |
| Norepinephrine† | 133 (42) |
| Vasopressin† | 31 (10) |
| Phenylephrine† | 2 (<1) |
| Milrinone† | 95 (30) |
| Vasodilator†‡ | 9 (3) |
| Invasive mechanical ventilation | 421 (74) |
| Corticosteroids | 242 (45) |
| Albumin | 135 (24) |
| Synthetic colloid | 22 (4) |
| Blood products§ | 232 (41) |
| Nutrition, enteral | 331 (58) |
| Nutrition, parenteral | 221 (39) |
| Gastric stress ulcer prophylaxis | 445 (78) |
| Insulin║ | 57 (10) |
| G/GM-CSF | 23 (4) |
| IVIG | 38 (7) |
| RRT¶ | 81 (14) |
| Plasma exchange | 5 (1) |
| ECMO | 30 (5) |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; G/GM-CSF = granulocyte/granulocyte–monocyte colony–stimulating factor; IVIG = intravenous immunoglobulin; RRT = renal replacement therapy.
Data are presented as n (%).
Includes dopamine >5 μg/kg/min, dobutamine >5 μg/kg/min, or any dose of epinephrine, norepinephrine, vasopressin, phenylephrine, milrinone, levosimendan, or a vasodilator.
Denominator is the number of patients receiving any vasoactive infusion (n = 314).
Vasodilators include nitroprusside, nitroglycerin, and nicardipine.
Includes packed red blood cells, platelets, fresh frozen plasma, cryoprecipitate, granulocytes, and whole blood.
Includes intravenous insulin by continuous infusion only.
Includes hemodialysis, all continuous renal replacement modalities, and peritoneal dialysis.
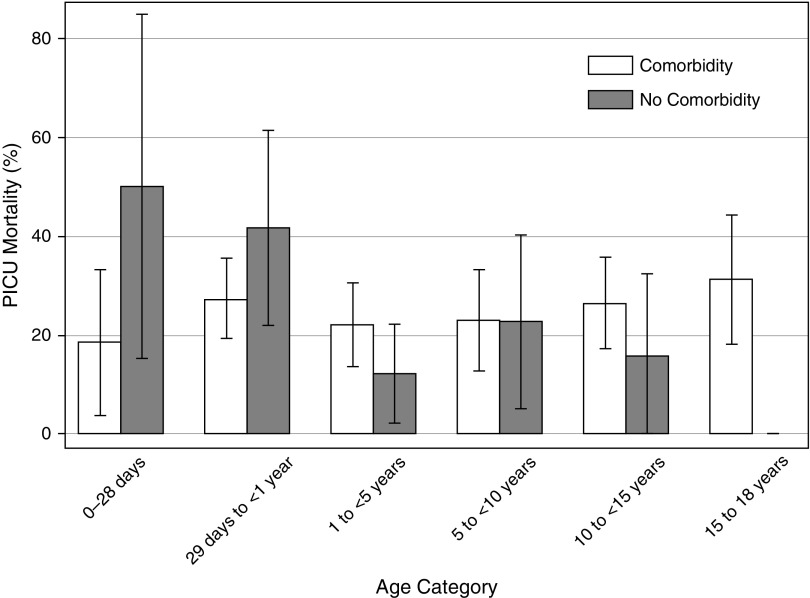
PICU and hospital mortality rates were 24% and 25%, respectively, and did not vary by age (Table 4). The presence of any comorbidity did not affect PICU mortality (P = 0.35) (Figure 2), but mortality was highest in patients with solid organ/stem cell transplant (48.2%), malignancy (41.3%), renal disease (38.2%), and hematologic/immunologic conditions (37.7%). Only one child died (0.7% of PICU deaths) within the first day of referral to the PICU. Cumulative deaths over the first 2 weeks of PICU admission were 7 (5.0%) within 2 days, 23 (16.6%) within 7 days, and 56 (40.3%) within 14 days. Corticosteroids were associated with PICU mortality after controlling for age, sex, PIM-3, geographic region, and comorbidities (neuromuscular, hematologic/immunologic, malignancy, and solid organ/stem cell transplant) linked to increased corticosteroid use (adjusted OR, 1.58; 95% CI, 1.01–2.49). Albumin use was also associated with PICU mortality after controlling for age, sex, PIM-3, geographic region, and number of comorbid conditions (adjusted OR, 2.50; 95% CI, 1.54–4.05). Fifteen of the 30 patients treated with ECMO died.
Table 4.
Outcomes for Total Cohort and by Age Category
| Age Categories |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total | 0–28 d | 29 d to <1 yr | 1 to <5 yr | 5 to <10 yr | 10 to <15 yr | 15 to <18 yr | P Value* | |
| Vasoactive-free days, median (IQR) | 23 (12–28) | 20 (5–26) | 21 (1–27) | 25 (19–28) | 26 (20–28) | 25 (16–28) | 22 (12–28) | <0.001 |
| Ventilator-free days, median (IQR) | 16 (0–25) | 14 (0–23) | 4 (0–21) | 19 (2–25) | 22 (7–28) | 16 (0–28) | 16 (1–28) | <0.001 |
| New or progressive MODS† | 171 (30) | 10 (29) | 48 (34) | 37 (27) | 25 (29) | 32 (30) | 19 (31) | 0.89 |
| PICU mortality | 139 (24) | 9 (26) | 43 (31) | 26 (19) | 20 (23) | 26 (25) | 15 (24) | 0.42 |
| Hospital mortality | 145 (25) | 9 (26) | 43 (31) | 28 (21) | 20 (23) | 28 (26) | 17 (27) | 0.54 |
| At least mild disability‡ | 116 (28) | 8 (31) | 29 (30) | 31 (29) | 18 (27) | 16 (21) | 14 (31) | 0.73 |
| At least moderate disability§ | 73 (17) | 5 (19) | 17 (17) | 17 (16) | 13 (19) | 9 (12) | 12 (27) | 0.40 |
| Death or disability║ | 218 (38) | 14 (40) | 60 (43) | 45 (33) | 33 (38) | 37 (35) | 29 (47) | 0.41 |
Definition of abbreviations: IQR = interquartile range; MODS = multiorgan dysfunction syndrome; PICU = pediatric intensive care unit.
Data are presented as n (%), unless otherwise noted.
Kruskal-Wallis test or Fisher’s exact test across age categories.
New or progressive MODS was considered starting the day after sepsis recognition.
Any increase in Pediatric Overall Performance Category (POPC) from baseline to hospital discharge in the 422 hospital survivors.
Discharge POPC ≥ 3 and an increase of ≥1 from baseline in the 422 hospital survivors.
Death or at least moderate disability at hospital discharge.
Figure 2.
Pediatric intensive care unit (PICU) mortality by age and presence of at least one comorbid condition. Bars represent PICU mortality, with error bars denoting the 95% confidence interval. PICU mortality did not differ significantly across age categories (P = 0.42) or by presence of comorbid conditions (P = 0.35).
PICU mortality varied across geographic regions: 21% in North America, 29% in Europe, 32% in Australia/New Zealand, 40% in Asia, 11% in South America, and 40% in Africa (P = 0.004). To assess whether mortality estimates were skewed by inclusion of resource-limited countries, we performed a sensitivity analysis excluding Asia, Africa, and South America. In that analysis, PICU and hospital mortality remained largely unchanged at 23% and 24%, respectively. Additionally, mortality was not significantly different for patients treated in North America, Europe, and Australia/New Zealand compared with Asia, Africa, and South America (PICU mortality: 23% vs. 29%, P = 0.23; hospital mortality: 24% vs. 31%; P = 0.19).
Vasoactive-free and ventilator-free days exhibited a prominent bimodal distribution, with nonsurvivors clustered at zero and survivors clustered at >20 days, yielding wide IQRs (Table 4). MODS was present in 67% on the day of severe sepsis recognition, mostly due to concurrent cardiovascular and respiratory dysfunction. Despite this high rate of initial MODS, 30% developed new or progressive multiorgan dysfunction within the ensuing 7 days. Twenty-eight percent of survivors had developed at least mild disability and 17% at least moderate disability at hospital discharge, without variation by age. Thirty-eight percent met the composite outcome of death or moderate to severe disability at hospital discharge.
Estimated sample sizes needed to detect 5% and 10% absolute risk reductions for dichotomous outcome measures in pediatric severe sepsis clinical trials are provided in Table E5. Assuming a 50% consent rate, between 165 and 1,437 patients per group would need to be enrolled across 9–79 PICUs over the course of 3 years to achieve 80% power and a standard type I error rate of 5% in clinical trials. Specifically, 165 patients per group would be necessary to detect a 10% absolute risk reduction in proportion to at least new moderate functional disability at hospital discharge, whereas 1,437 patients per group would be needed to detect a 5% absolute risk reduction in death or moderate disability. For PICU mortality—the most commonly used outcome in clinical trials—1,059 patients per group enrolled from 58 PICUs over the course of 3 years would be required for 80% power to detect a 5% absolute risk reduction.
Discussion
This large international point prevalence study of nearly 7,000 children across 128 sites demonstrates that pediatric severe sepsis remains highly prevalent, accounting for >8% of all critically ill children. Although the absolute number of pediatric severe sepsis cases has been reported as 10-fold less than adults (4, 41), the 8.2% prevalence in PICU patients is remarkably similar to the proportion of critically ill adults with severe sepsis (42). These results suggest that a typical 16-bed PICU is likely to be treating at least one critically ill child for severe sepsis at any given time. Moreover, hospital mortality, often considered to be too low to practically study in pediatric severe sepsis, was 25% and exceeded prior epidemiological estimates that relied on retrospective administrative data (1, 4, 43). As in other studies, comorbid conditions were common (1, 5, 6, 18, 44–46), and children with immunosuppressive conditions and preexisting renal disease exhibited the highest mortality. However, we could not determine whether death was attributable to sepsis or to an underlying comorbid condition. Morbidity was also common, with one-third developing progressive organ dysfunction and nearly one in five survivors exhibiting new functional disability.
Over one-third of patients were transferred to the PICU from hospital locations other than the ED. This finding suggests that sepsis improvement efforts, which have overwhelmingly focused on the ED setting, may need to be expanded in scope (12, 13, 45). For example, a prior study demonstrated that antimicrobial administration took >1 hour longer for pediatric patients receiving initial sepsis therapy on a hospital ward than in the ED, with delayed antimicrobial administration independently associated with mortality (46). Our data support the need to better attend to patients with severe sepsis that develops during hospitalization, as this population represents a large proportion of critically ill children with severe sepsis who are more likely to have comorbidities and may face barriers to sepsis recognition and management distinct from those of ED patients.
Adjuvant therapies, other than corticosteroids and albumin, were rarely used in this study. The extent to which this finding reflects a perceived lack of benefit or the absence of sufficient supporting data is not known. However, both corticosteroids and albumin were independently associated with mortality (38, 39), and the use of insulin, renal replacement therapies, and G/GM-CSF was higher in patients who died. Although we cannot exclude the possibility that use of these therapies themselves contributed to increased mortality, our data do support at least the willingness to use adjunctive therapies in patients with more severe illness. Thus, even at a time when enthusiasm for novel pharmacologic agents in sepsis has been tempered, there remains an imperative for adequately powered clinical trials to define these agents’ efficacy and optimize the timing of existing therapies in pediatric severe sepsis.
Several important insights can be gleaned from the outcomes in this study. The hospital mortality rate of 25% confirms that severe sepsis remains a critical public health problem, even in children. This finding contrasts with reports of several large epidemiological studies of pediatric sepsis with mortality rates closer to 4–10% (1, 4, 43). However, these studies used International Classification of Diseases, Ninth Revision (ICD-9), codes, which have been shown to capture a lower severity of illness and thereby underestimate mortality (5, 7). In a recent database study using expanded ICD-9 coding, researchers reported a PICU mortality of 26.4% (6), and authors of prospective studies have reported short-term mortality rates up to 24% (2, 5, 19, 46, 47). The mortality in the present study also reflects the high rate of multiorgan dysfunction. In previous studies, investigators have reported 19–57% mortality for pediatric patients with sepsis-associated MODS (2, 3, 48). Thus, the mortality reported in our present study is indicative of the critically ill subset of children with sepsis-associated MODS who should be targeted for enrollment into interventional trials. Finally, our finding that almost one-fifth of survivors developed at least moderate functional disability underscores the need to include morbidity endpoints in pediatric sepsis trials.
To conduct interventional trials in pediatric severe sepsis, we estimate that it would take 3 years and ≥58 PICUs to enroll 2,118 children with the aim of a pragmatic 5% reduction in all-cause PICU mortality. Although such a large study of pediatric severe sepsis may seem ambitious, we believe that well-designed trials of this magnitude could—and should—be undertaken using a wide network of international PICUs. Consideration of using new or progressive multiorgan dysfunction, functional disability, or a composite endpoint of death plus disability would reduce sample size requirements while capturing meaningful outcomes. Although relevant exclusion criteria would need to be accounted for in planning future trials, patients at high risk of death, who may be most likely to benefit, should not be excluded from clinical trials (27).
The SPROUT study has several strengths. The point prevalence design facilitated efficient prospective data collection over a short time within a diverse network of international PICUs. Second, because the criteria used to define severe sepsis in this study were the precise criteria recommended for enrollment into clinical trials (16), SPROUT provides unique baseline data representative of the target population. Finally, modifying the traditional point prevalence design to include a 90-day follow-up period facilitated the study of multiple patient-centered outcomes (49).
The SPROUT study provides several important insights to inform the conduct of future clinical trials in pediatric sepsis. First, the overall burden of severe sepsis in children is similar to that in the adult critically ill population, thus justifying the need to undertake large-scale international trials with children with severe sepsis. Second, given variable use of primary and adjunctive therapies for severe sepsis, future trials that seek to optimize currently available therapies will be as important as investigations of novel agents. Third, quality improvement and research initiatives need to include more entry locations beyond the ED, in recognition of the fact that up to two-thirds of children treated in the PICU for severe sepsis originate from other inpatient locations.
An important limitation of the present study is the cross-sectional nature of data collection, which, though it improved site participation by minimizing burdensome data collection, likely underestimated the overall use of different sepsis therapies over the entire course of illness. We may have also missed some cases of new or progressive multiorgan dysfunction by measuring organ dysfunction for only 7 instead of 28 days following sepsis recognition. However, any underestimate is likely to be small because more than 95% of organ dysfunction in pediatric sepsis has been shown to develop within 7 days (33), and this approach greatly improved efficiency of data collection. Additionally, although we compiled one of the largest cohorts of prospectively identified pediatric severe sepsis cases to date, the sample was not sufficient to compare outcomes according to etiology of infection or use of therapies other than corticosteroids. Statistical power was also insufficient to detect a possible increase in mortality in resource-limited countries, owing to the skewed enrollment of 83.2% of patients at sites in developed regions. Moreover, despite global participation, the majority of PICUs were located in developed countries and affiliated with academic institutions; thus, the generalizability of these data to resource-limited and community-based settings may be limited. However, the network of sites participating in this study remains representative of the type of PICUs most likely to take part in future clinical trials. Finally, funding constraints limited our ability to capture longer-term outcomes, such as mortality and functional status at 1 year.
In conclusion, this large international study demonstrates that pediatric severe sepsis remains a highly prevalent public health problem in critically ill children and is associated with substantial morbidity and mortality. The data provided by the SPROUT study provide a unique picture of the global epidemiology of pediatric severe sepsis that can be used to directly inform the design of future multicenter interventional trials.
Acknowledgments
Acknowledgment
The authors thank the World Federation of Pediatric Intensive and Critical Care Societies for help in site recruitment. The authors also thank the Australian and New Zealand Intensive Care Society for review of the study protocol and for their support of site recruitment within Australia and New Zealand. We are grateful to all local investigators at participating SPROUT sites for their contributions to this study.
SPROUT Study Investigators
North America
Canada: P. Fontela (Montreal Children’s Hospital–McGill); M. Tucci and M. Dumitrascu (Sainte-Justine Hospital); and P. Skippen and G. Krahn (BC Children’s Hospital). United States: M. Bigham, T. Polanski, S. Latifi, D. Giebner, and H. Anthony (Akron Children’s Hospital); J. Hume, A. Galster, and L. Linnerud (Amplatz Children’s Hospital); R. Sanders, G. Hefley (Arkansas Children’s Hospital); K. Madden (Boston Children’s Hospital); A. Thompson and S. Shein (Children’s Hospital of Pittsburgh); S. Gertz (Children’s Hospital–Hackensack); Y. Han, T. Williams, and A. Hughes-Schalk (Children’s Mercy Hospital); H. Chandler (Children’s Healthcare of Atlanta); A. Orioles, E. Zielinski, and A. Doucette (Children’s Hospital in Minnesota); A. Orioles, E. Zielinski, and A. Doucette (Children’s Hospital St. Paul); C. Zebuhr and T. Wilson (Children’s Hospital Colorado); C. Dimitriades, J. Ascani, S. Layburn, and S. Valley (Children’s Hospital New Orleans); B. Markowitz, J. Terry, and R. Morzov (Children’s Hospital of Los Angeles); A. McInnes (Children’s Hospital of Monmouth); J. McArthur, K. Woods, and K. Murkowski (Children’s Hospital of Wisconsin); M. Spaeder and M. Sharron (Children’s National Medical Center); D. Wheeler, E. Beckman, E. Frank, and K. Howard (Cincinnati Children’s Medical Center); C. Carroll (Connecticut Children’s Medical Center); S. Nett and D. Jarvis (Dartmouth Hitchcock Medical Center); V. Patel (Dayton Children’s Hospital); R. Higgerson and L. Christie (Dell Children’s Medical Center); K. Typpo and J. Deschenes (Diamond Children’s Hospital); A. Kirby (Doernbecher Children’s Hospital); T. Uhl, K. Rehder, I. Cheifetz, and S. Wrenn (Duke Children’s Hospital); K. Kypuros (El Paso Children’s Hospital); K. Ackerman (Golisano Children’s Hospital); F. Maffei and G. Bloomquist (Geisinger/Janet Weis Children’s Hospital); E. Bezares (Hospital Cardiovascular de Puerto Rico y el Caribe); N. Rizkalla (Johns Hopkins Hospital); D. Kimura, S. Shah, and C. Tigges (Le Bonheur Children’s Hospital); F. Su and C. Barlow (Lucile Packard Children’s Hospital); K. Michelson, K. Wolfe, D. Goodman, L. Campbell, and L. Sorce (Lurie Children’s Hospital of Chicago); K. Bysani and T. Monjure (Medical City Children’s–Dallas); M. Evans (Medical University of South Carolina); B. Totapally, M. Chegondi, and C. Rodriguez (Miami Children’s Hospital); J. Frazier and L. Steele (Nationwide Children’s Hospital); S. Viteri and A. Costarino (Nemours/Alfred I. duPont Children’s Hospital); N. Thomas and D. Spear (Penn State Milton S. Hershey Medical Center); E. Hirshberg and J. Lilley (Primary Children’s Medical Center); C. Rowan and C. Rider (Riley Hospital for Children); J. Kane (Rush Children’s Hospital); G. Puig and A. Puig-Ramos (San Jorge Children’s Hospital); J. Zimmerman and C. Greeley (Seattle Children’s Hospital); J. Lin and R. Jacobs (St. Louis Children’s Hospital); M. Parker and K. Culver (Stony Brook University); L. Loftis, N. Jaimon, and M. Goldsworthy (Texas Children’s Hospital); J. Fitzgerald, S. Weiss, V. Nadkarni, J. Bush, and M. Diliberto (The Children’s Hospital of Philadelphia); C. Alen and M. Gessouroun (Oklahoma University Medical Center); A. Sapru, T. Lang, and M. Alkhouli (University of California, San Francisco); S. Kamath, D. Friel, and J. Daufeldt (University of Iowa); R. Garcia and M. Villar (University Pediatric Hospital); D. Hsing, C. Carlo, and S. Pon (Weill Cornell Medical Center); J. Scimeme and A. Shaheen (Wolfson Children’s Hospital); A. Hassinger and H. Qiao (Women and Children’s Hospital of Buffalo); and J. Giuliano and J. Tala (Yale Children’s Hospital).
South America
Argentina: D. Vinciguerra and A. Fernandez (Hospital Durand). Colombia: R. Carrero (Clínica Infantil Colsubsidio); P. Hoyos (Hospital de San Jose); J. Jaramillo and A. Posada (Hospital General de Medellín); L. Izquiierdo (Hospital Military Central); and B. Olave and J. Donado (Pablo Tobón Uribe). Chile: R. Dalmazzo and S. Rendich (Clínica Las Condes); L. Palma and M. Lapadula (Clínica Santa María); C. Acuña (Hospital Luis Calvo Mackenna); and P. Cruces (Hospital Padre Hurtado).
Europe
Belgium: S. Clément De Cléty, M. Dujardin, C. Berghe, and S. Renard (St. Luc University Hospital). Czech Republic: J. Zurek (Masaryk University). Germany: H. Steinherr (Klinikum Augsburg). Greece: K. Mougkou (Aghia Sophia Children’s Hospital); and E. Critselis and K. Mougkou (P. & A. Kyriakou Children’s Hospital). Italy: M. Di Nardo, S. Picardo, and F. Tortora (Bambino Gesu Area Rossa); E. Rossetti (Bambino Gesu Children’s Hospital); T. Fragasso, P. Cogo, and R. Netto (Bambino Gesu Pediatrico). Lithuania: A. Dagys, V. Gurskis, and R. Kevalas (Lithuanian University of Health Sciences). Netherlands: C. Neeleman, J. Lemson, and C. Luijten (Radboud University Medical Centre). Poland: K. Wojciech and I. Pagowska-Klimek (Polish Mother’s Memorial Hospital); and M. Szczepanska and J. Karpe (Szyszko Śląskiego University). Portugal: P. Nunes and H. Almeida (Hospital Professor Doutor Fernando Fonseca); and J. Rios and M. Vieira (Centrol Hospitalar Lisboa Norte). Spain: P. Revilla (Children’s Hospital Miguel Servet); J. Villaescusa, J. Lopez-Herce, and A. Bustinza (Hospital General Universitario Gregorio Marañón); A. Cuesta and S. Hofheinz (Hospital 12 de Octubre); A. Rodriguez-Nunez (Hospital Clínico Universitario); S. Sanagustin and E. Gonzalez (Hospital de la Sant Creu Sant Pau); M. Riaza and R. Piaya (Hospital Universitario Madrid); P. Soler (Hospital Carlos Haya Materno Infantil); E. Esteban (Hospital Sant Joan de Déu); J. Laraudogoitia and C. Monge (Hospital Universitario Donostia); V. Herrera and J. Granados (Hospital Universitario Salamanca); and C. Gonzalez (Hospital Virgen de la Arrixaca). Turkey: T. Koroglu and E. Ozcelik (Dokuz Eylul University). United Kingdom: P. Baines (Alder Hey Children’s Hospital); and A. Plunkett (Birmingham Children’s Hospital); P. Davis and S. George (Bristol Royal Hospital for Children); S. Tibby and J. Harris (Evelina Children’s Hospital); R. Agbeko and R. Lampitt (Great North Children’s Hospital–Newcastle); J. Brierly, M. Peters, A. Jones, T. Dominguez, and T. Thiruchelvam (Great Ormond Street Hospital); A. Deep, L. Ridley, and W. Bowen (King’s College Hospital); R. Levin and I. Macleod (Royal Hospital for Sick Children); M. Gray and N. Hemat (St George’s Hospital); J. Alexander and S. Ali (University Hospital of North Staffordshire NHS Trust); J. Pappachan and J. McCorkell (University Hospital Southampton NHS Foundation Trust); and P. Fortune, M. MacDonald, and P. Hudnott (Royal Manchester Children’s Hospital).
Asia
China: Q. Suyun (Beijing Children’s Hospital). India: S. Singhi and K. Nallasamy (Advanced Pediatrics); and R. Lodha (All India Institute of Medical Sciences). Japan: N. Shime and Y. Tabata (Kyoto Prefectural University of Medicine); O. Saito and T. Ikeyama (Tokyo Metropolitan Children’s Hospital); and T. Kawasaki (Shizuoka Children’s Hospital). Malaysia: L. Lum, A. Abidin, and S. Kee (University Malaya Medical Center); S. Tang and R. Jalil (Kebangsaan Malaysia Medical Center). Singapore: Y. Guan and L. Yao (KK Women’s and Children’s Hospital); and K. Lin and J. Ong (National University Hospital).
Africa
South Africa: A. Salloo, L. Doedens, and L. Mathivha (Chris Hani Baragwanath Hospital); G. Reubenson and S. Moaisi (Rahima Moosa Mother and Child Hospital); and A. Pentz and R. Green (Steve Biko Academic Hospital).
Australia
A. Schibler and A. Fernandez (Mater Children’s Hospital); S. Erickson (Princess Margaret Hospital); J. Millar and C. Delzoppo (Royal Children’s Hospital Melbourne); G. Williams and M. Morritt (Sydney Children’s Hospital); J. McEniery, D. Long, T. Dorofaeff, and M. Coulthard (Royal Children’s Hospital Brisbane); and N. Watts and M. Morritt (Children’s Hospital at Westmead).
New Zealand
J. Beca, C. Sherring, and T. Bushell (Starship Children’s Hospital).
Footnotes
Supported by the Endowed Chair, Department of Anesthesia and Critical Care, University of Pennsylvania Perelman School of Medicine, and the Center for Pediatric Clinical Effectiveness at The Children’s Hospital of Philadelphia. S.L.W. is also supported by National Institutes of Health grant K12 HD047349-10. Financial support for data collection in all U.K. centers was provided by the National Institute for Health Research (NIHR) Clinical Research Network and in Southampton by the Southampton NIHR Wellcome Trust Clinical Research Facility. None of the funders participated in the design and conduct of study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Author Contributions: S.L.W. and J.C.F.: had full access to all of the data in the study and take complete responsibility for the integrity of the data and the accuracy of the data analysis; S.L.W., J.C.F., V.M.N., J.A.R., and N.J.T.: conducted and are responsible for the data analysis; S.L.W., J.C.F., V.M.N., and N.J.T.: study concept and design; S.L.W., J.C.F., J.P., D.W., J.C.J.-B., A.S., S.C.S., S.E., J.A.R., J.L.B., V.M.N., and N.J.T.: acquisition, analysis, or interpretation of data; S.L.W., J.C.F., V.M.N., and N.J.T.: drafting of the manuscript; S.L.W., J.C.F., J.P., D.W., J.C.J.-B., A.S., S.C.S., S.E., J.A.R., J.L.B., V.M.N., and N.J.T.: critical revision of the manuscript for important intellectual content; S.L.W., J.C.F., J.A.R., V.M.N., and N.J.T.: statistical analysis; S.L.W., J.C.F., and V.M.N.: obtained funding; J.L.B.: administrative, technical, or material support; S.L.W., J.C.F., V.M.N., and N.J.T.: study supervision.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201412-2323OC on March 4, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med. 2013;14:686–693. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 2.Jaramillo-Bustamante JC, Marín-Agudelo A, Fernández-Laverde M, Bareño-Silva J. Epidemiology of sepsis in pediatric intensive care units: first Colombian multicenter study. Pediatr Crit Care Med. 2012;13:501–508. doi: 10.1097/PCC.0b013e31823c980f. [DOI] [PubMed] [Google Scholar]
- 3.Kutko MC, Calarco MP, Flaherty MB, Helmrich RF, Ushay HM, Pon S, Greenwald BM. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4:333–337. doi: 10.1097/01.PCC.0000074266.10576.9B. [DOI] [PubMed] [Google Scholar]
- 4.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SL, Parker B, Bullock ME, Swartz S, Price C, Wainwright MS, Goodman DM. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13:e219–e226. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 6.Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med. 2014;15:828–838. doi: 10.1097/PCC.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 7.Balamuth F, Weiss SL, Neuman MI, Scott H, Brady PW, Paul R, Farris RW, McClead R, Hayes K, Gaieski D, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med. 2014;15:798–805. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magee JC, Krishnan SM, Benfield MR, Hsu DT, Shneider BL. Pediatric transplantation in the United States, 1997–2006. Am J Transplant. 2008;8:935–945. doi: 10.1111/j.1600-6143.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- 9.Wen SW, Smith G, Yang Q, Walker M. Epidemiology of preterm birth and neonatal outcome. Semin Fetal Neonatal Med. 2004;9:429–435. doi: 10.1016/j.siny.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Gudiol C, Bodro M, Simonetti A, Tubau F, González-Barca E, Cisnal M, Domingo-Domenech E, Jiménez L, Carratalà J. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect. 2013;19:474–479. doi: 10.1111/j.1469-0691.2012.03879.x. [DOI] [PubMed] [Google Scholar]
- 11.Kunz AN, Brook I. Emerging resistant Gram-negative aerobic bacilli in hospital-acquired infections. Chemotherapy. 2010;56:492–500. doi: 10.1159/000321018. [DOI] [PubMed] [Google Scholar]
- 12.Cruz AT, Perry AM, Williams EA, Graf JM, Wuestner ER, Patel B. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127:e758–e766. doi: 10.1542/peds.2010-2895. [DOI] [PubMed] [Google Scholar]
- 13.Larsen GY, Mecham N, Greenberg R. An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127:e1585–e1592. doi: 10.1542/peds.2010-3513. [DOI] [PubMed] [Google Scholar]
- 14.Rhee C, Murphy MV, Li L, Platt R, Klompas M Centers for Disease Control and Prevention Epicenteres Program. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis. 2015;60:88–95. doi: 10.1093/cid/ciu750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfler A, Silvani P, Musicco M, Antonelli M, Salvo I Italian Pediatric Sepsis Study (SISPe) group. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care Units: a prospective national survey. Intensive Care Med. 2008;34:1690–1697. doi: 10.1007/s00134-008-1148-y. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein B, Giroir B, Randolph A International Consensus Conference on Pediatric Sepsis. International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 17.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira CF, de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, Fernandes JC, Vaz FA, Carcillo JA, Rivers EP, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–1075. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 19.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, et al. REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 20.Karam O, Tucci M, Ducruet T, Hume HA, Lacroix J, Gauvin F Canadian Critical Care Trials Group; PALISI Network. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med. 2011;12:512–518. doi: 10.1097/PCC.0b013e3181fe344b. [DOI] [PubMed] [Google Scholar]
- 21.Choong K, Bohn D, Fraser DD, Gaboury I, Hutchison JS, Joffe AR, Litalien C, Menon K, McNamara P, Ward RE Canadian Critical Care Trials Group. Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. Am J Respir Crit Care Med. 2009;180:632–639. doi: 10.1164/rccm.200902-0221OC. [DOI] [PubMed] [Google Scholar]
- 22.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 23.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, Yeh T, Kim SS, Cafaro DP, Scannon PJ, et al. rBPI21 Meningococcal Sepsis Study Group. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet. 2000;356:961–967. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 24.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, Carcillo JA. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen TC, Han YY, Kiss JE, Hall MW, Hassett AC, Jaffe R, Orr RA, Janosky J, Carcillo JA. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36:2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curley MA, Zimmerman JJ. Alternative outcome measures for pediatric clinical sepsis trials. Pediatr Crit Care Med. 2005;6(3 Suppl):S150–S156. doi: 10.1097/01.PCC.0000161582.63265.B6. [DOI] [PubMed] [Google Scholar]
- 27.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C? Crit Care Med. 2014;42:1714–1721. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald JC, Weiss SL, Nadkarni V, Bush JL, Thomas N SPROUT and PALISI Investigators. Global epidemiology and outcomes of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies (SPROUT) study [abstract] Crit Care Med. 2013;41(12 Suppl):A137. [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025–1031. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, Slater A ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network. Paediatric Index of Mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med. 2013;14:673–681. doi: 10.1097/PCC.0b013e31829760cf. [DOI] [PubMed] [Google Scholar]
- 35.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study Lancet 2003362192–197.. [Published erratum appears in Lancet 2006;367:902.] [DOI] [PubMed] [Google Scholar]
- 36.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 37.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013;14:835–842. doi: 10.1097/PCC.0b013e3182a551c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkinson SJ, Cvijanovich NZ, Thomas NJ, Allen GL, Anas N, Bigham MT, Hall M, Freishtat RJ, Sen A, Meyer K, et al. Corticosteroids and pediatric septic shock outcomes: a risk stratified analysis. PLoS One. 2014;9:e112702. doi: 10.1371/journal.pone.0112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markovitz BP, Goodman DM, Watson RS, Bertoch D, Zimmerman J. A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: what is the role of steroids? Pediatr Crit Care Med. 2005;6:270–274. doi: 10.1097/01.PCC.0000160596.31238.72. [DOI] [PubMed] [Google Scholar]
- 40.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 41.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 43.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119:487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 44.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123:849–857. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 45.Paul R, Neuman MI, Monuteaux MC, Melendez E. Adherence to pals sepsis guidelines and hospital length of stay. Pediatrics. 2012;130:e273–e280. doi: 10.1542/peds.2012-0094. [DOI] [PubMed] [Google Scholar]
- 46.Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, Grundmeier R, Nadkarni VM, Thomas NJ. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42:2409–2417. doi: 10.1097/CCM.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong HR, Salisbury S, Xiao Q, Cvijanovich NZ, Hall M, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16:R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proulx F, Joyal JS, Mariscalco MM, Leteurtre S, Leclerc F, Lacroix J. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 49.Weiss SL, Fitzgerald JC, Faustino EV, Festa MS, Fink EL, Jouvet P, Bush JL, Kissoon N, Marshall J, Nadkarni VM, et al. Pediatric Acute Lung Injury and Sepsis Investigators Network and Australia and New Zealand Intensive Care Society Investigators. Understanding the global epidemiology of pediatric critical illness: the power, pitfalls, and practicalities of point prevalence studies. Pediatr Crit Care Med. 2014;15:660–666. doi: 10.1097/PCC.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]