Abstract
We determined whether pretreatment with (1) the μ-/δ-opioid receptor (μ-/δ-OR) antagonist, naloxone, (2) the δ1,2-OR antagonist, naltrindole, or (3) the peroxynitrite scavenger, D-penicillamine, affects the development of tolerance to the ventilatory depressant effects of morphine in rats. The injection of morphine in vehicle-pretreated rats decreased minute ventilation predominantly via decreases in tidal volume. Pretreatment with naloxone blunted the responses to morphine whereas pretreatment with naltrindole or D-penicillamine did not. A second injection of morphine, given one day later, elicited markedly smaller responses in vehicle rats whereas it elicited pronounced ventilatory depression in rats that were pretreated with naloxone, naltrindole or D-penicillamine (prior to morphine) the day before. Moreover, the ventilatory responses elicited by subsequent exposure to a hypoxic-hypercapnic challenge were markedly depressed in naloxone- or D-penicillamine-pretreated rats compared to vehicle-pretreated rats. These findings suggest that activation of μ- and δ-ORs causes tolerance to the ventilatory depressant effects of morphine at least partly via the generation of peroxynitrite.
Keywords: morphine, ventilatory depression, tolerance, opiate receptors, peroxynitrite, rats
1. Introduction
The analgesic and ventilatory depressant effects of morphine involve activation of μ-opioid receptors (μ-ORs) and δ-ORs (Kilpatrick and Smith, 2005; Trescot et al., 2008; Dahan et al., 2010). In addition, the activation of central or peripheral μ-ORs blunts the hypoxic ventilatory response (Zhang et al., 2009), and opioids inhibit carotid body chemoafferent activity and depress the responses of these afferents to hypoxic and hypercapnic challenges (McQueen and Ribeiro, 1980; Kirby and McQueen, 1986; Mayer et al., 1989). The analgesic (Bailey and Conner, 2005; Salvemini and Neumann, 2009) and ventilatory-depressant (Bowen et al., 1979; Hepburn et al., 1997; Freye and Latasch, 2003) actions of morphine are subject to tolerance upon chronic administration. The development of tolerance to the analgesic actions of opioids involves down-regulation and/or desensitization of μ-ORs (Connor et al., 2004; Bailey and Connor, 2005; Raehal and Bohn, 2005; Ueda and Ueda, 2009) by numerous effectors (Raith and Hochhaus, 2004; Bailey et al., 2009; Salvemini and Neumann, 2009) and alterations in gene expression (Ammon-Treiber and Höllt, 2005). The mechanisms by which tolerance develops to the ventilatory depressant effects of morphine may be similar to those responsible for tolerance to morphine analgesia. However, there is evidence that whereas δ-OR antagonists diminish tolerance to the analgesic actions of μ-OR agonists (Hepburn et al., 1997; Ananthan, 2006), they do not prevent tolerance to the ventilatory depression (Hepburn et al., 1997). As such, δ-OR antagonists represent a potential therapy that allows for the maintenance of opioid analgesia while allowing tolerance to the negative ventilatory effects (Hepburn et al., 1997).
The generation of the potent oxidant/nitrating agent, peroxynitrite, is a key factor in the development of tolerance to opioid-induced analgesia (Salvemini, 2009; Salvemini and Neumann, 2009). It is unknown whether peroxynitrite is involved in tolerance to opioid-induced ventilatory depression and whether peroxynitrite is generated via stimulation of μ- and/or δ-ORs. Moreover, the question arises as to whether tolerance to the ventilatory actions of opioids also translates into lesser suppression of the responses to hypoxic and/or hypercapnic challenges and whether this involves the generation of peroxynitrite. As such, we determined whether (1) the μ-/δ-OR antagonist, naloxone (DeHaven-Hudkins and Dolle, 2004; Ananthan, 2006), (2) the δ1,2-OR antagonist, naltrindole (Portoghese, 1993; Ananthan, 2006), or (3) the peroxynitrite scavenger, D-penicillamine (D-PEN) (Singh et al., 2007), modulate the development of tolerance to the ventilatory depressant effects of morphine in conscious rats. We also determined whether tolerance to morphine is associated with diminished suppression of the ventilatory responses to hypoxic-hypercapnic (H-H) challenge, and whether the initial activation of μ- and/or δ-ORs, and the generation of peroxynitrite, is involved in the altered responses to the H-H challenge. The present studies provide evidence that similar to the development of tolerance to the analgesic actions of opioids (Salvemini, 2009; Salvemini and Neumann, 2009), peroxynitrite may be a key player in the development of tolerance to the ventilatory actions of morphine.
2. Methods
2.1. Rats and surgeries
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the Animal Care and Use Committee of the University of Virginia. Adult male Sprague-Dawley rats (Harlan, Madison, WI, USA) were implanted with jugular vein catheters under 2% isoflurane anesthesia. The rats were allowed a minimum of 4 days to recover from surgery before use. All catheters were flushed with sterile isotonic saline at least 4h before commencement of experiments. All of the studies were performed in a quiet laboratory with relative humidity of 51 ± 2% and room temperature of 21.2 ± 0.2 °C.
2.2. Ventilatory parameters
Ventilatory parameters were continuously recorded in conscious rats using a whole-body 12-chamber plethysmography system (PLY 3223; BUXCO Inc., Wilmington, NC, USA), as described previously (Kanbar et al., 2010). The parameters were frequency of breathing (fR), tidal volume (VT), and minute ventilation (V̇ = fR X VT). Specialized software provided by BUXCO Inc., constantly corrected digitized values for changes in chamber temperature and humidity and a rejection algorithm excluded motion-induced artifacts.
2.3. Protocols for ventilation studies
On each experimental day, 12 rats (6 vehicle-treated and 6 drug-treated rats) were placed in the chambers and allowed 45-60 min to acclimatize before commencing the protocols (see below). Data was continuously recorded (i.e., breath by breath) throughout the acclimatization and experimental periods. There were a total six rats in each experimental group.
2.3.1 Naloxone study
One group of rats received an injection of vehicle (saline, i.v.). Another group received naloxone (1.5 mg/kg, i.v.). After 15 min, all rats received morphine (10 mg/kg, i.v.). On day 2, the rats were placed in the chambers and after acclimatization, they received morphine (10 mg/kg, i.v.). Beginning 45 min after the injection of morphine, all rats were exposed to a H-H challenge for 30 min via the re-breathing method (Hayashi et al., 1982). Air-flow to the chambers was stopped allowing the rats to re-breathe their own air (inbuilt soft-ware adjusted flow-derived values for increases in chamber temperature and humidity). A major benefit of this model is that the rats breathe chamber air which becomes progressively more hypoxic and hypercapnic, thereby mimicking clinical scenarios (Lévy et al., 2009; Dempsey et al., 2010). Moreover, hypercapnia is a potent arousal stimulus when delivered rapidly, and as such, a gradual increase in environmental CO2 limits the degree of arousal (Fewell and Konduri, 1988). A group of naïve rats also underwent a H-H challenge for 30 min.
2.3.1 Naltrindole study
Day 1: One group of rats received a bolus injection of vehicle (saline, i.v.) whereas the second group received an injection of naltrindole (1.5 mg/kg, i.v.). After 15 min, all rats received a bolus injection of morphine (10 mg/kg, i.v.). Day 2: All rats received an injection of vehicle and 15 min later an injection of morphine (10 mg/kg, i.v.).
2.3.1 D-penicillamine study
Day 1: One group of rats received an injection of vehicle (saline, i.v.). Another group received DPEN (1 mmol/kg, i.v.). After 15 min, all rats received an injection of morphine (10 mg/kg, i.v.). Day 2: Rats were a placed in the chambers and after acclimatization, they received morphine (10 mg/kg, i.v.). Beginning 45 min after the injection of morphine, all rats were exposed to a H-H challenge for 30 min. The use of D-PEN was based on our studies showing that similar to L-PEN (Graves et al., 2006), D-PEN is an effective peroxynitrite scavenger when given systemically (Lewis et al., unpublished observations) and that D-PEN does not have the toxicities associated with systemically administered L-PEN (Walshe, 2011).
2.4. Drugs
Injectable (liquid) form of (+)-morphine sulfate (10 mg/ml) was obtained from Baxter Healthcare Corporation (Deerfield, IL, USA). Naloxone hydrochloride dihydrate, naltrindole hydrochloride and D-PEN were obtained from Sigma-Aldrich (St. Louis, MO, USA). The dosages for naloxone and naltrindole are expressed in terms of the salt.
2.5. Statistics
The recorded data (collected into 1 min bins) and derived Response Areas (cumulative percent changes from pre-values) were taken for statistical analyses. The data are presented as mean ± SEM and were analyzed by one-way or two-way analysis of variance followed by Student's modified t test with Bonferroni corrections for multiple comparisons between means (Wallenstein et al., 1980). A value of P < 0.05 was taken to denote statistical significance.
Results
3.1. Effects of test drugs on resting ventilatory parameters – Day 1
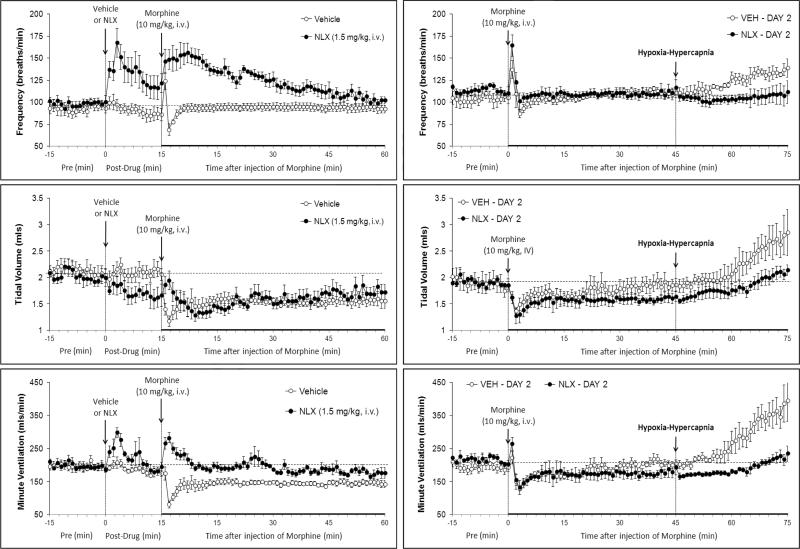
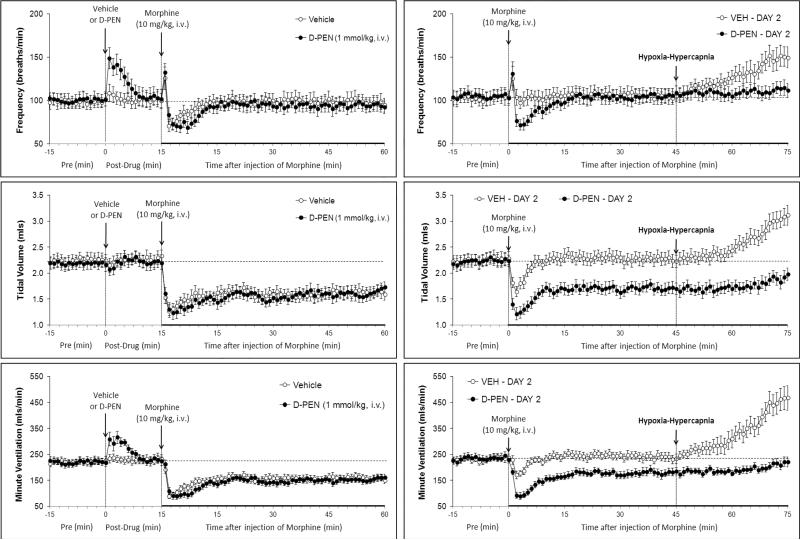
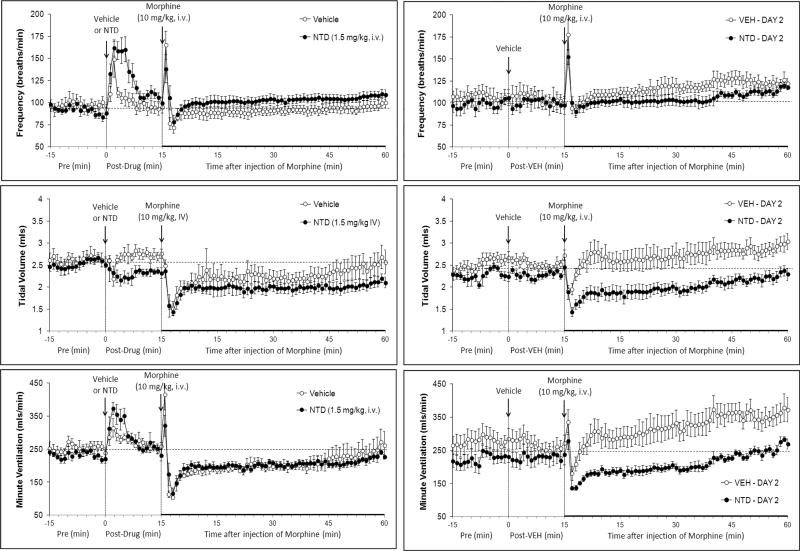
Resting ventilatory parameters were similar between all groups of rats on Day 1 and the values recorded on Day 2 were similar to those on Day 1 with one exception (Table 1). Specifically, in the naloxone study, resting fR recorded on Day 2 was higher than on Day 1 in the rats that received vehicle and in those that received naloxone. The injection of vehicle elicited transient changes in ventilatory parameters that had fully subsided by the time morphine was injected (Figs 1-3, left-hand columns; Table 2). Naloxone elicited a substantial and sustained increase in fR that was accompanied by a sustained decrease in VT (Fig. 1, Table 2). As such, naloxone elicited a relatively transient increase in V̇ (Fig. 1, Table 2). Naltrindole elicited a substantial increase in fR that was accompanied by a decrease in VT (Fig. 2, Table 2). These responses had largely subsided by the time morphine was injected. As such, naltrindole elicited an increase in V̇ of about 5 min in duration (Fig. 2, Table 2). The injection of D-PEN elicited an increase in fR of 7-8 min in duration (Fig. 3, Table 2). There were minimal changes in VT. As such, the increases in V̇ paralleled the increases in fR (Fig. 3, Table 2).
Table 1.
Resting ventilatory parameters and body weights in the two groups of rats
| Vehicle |
Drug |
||||
|---|---|---|---|---|---|
| Study | Parameter | DAY 1 | DAY 2 | DAY 1 | DAY 2 |
| Naloxone | Body Weights, grams | 294 ± 3 | 290 ± 2 | 291 ± 3 | 288 ± 2 |
| Frequency, breaths/min | 93 ± 5 | 104 ± 6* | 99 ± 4 | 114 ± 3* | |
| Tidal Volume, mls | 2.13 ± 0.12 | 1.91 ± 0.15 | 2.03 ± 0.10 | 1.92 ± 0.13 | |
| Minute Ventilation, mls/min | 197 ± 8 | 195 ± 13 | 198 ± 8 | 216 ± 9 | |
| Naltrindole | Body Weights, grams | 287 ± 3 | 283 ± 3 | 288 ± 4 | 286 ± 4 |
| Frequency, breaths/min | 97 ± 5 | 105 ± 8 | 92 ± 3 | 100 ± 7 | |
| Tidal Volume, mls | 2.63 ± 0.14 | 2.54 ± 0.08 | 2.53 ± 0.18 | 2.28 ± 0.07 | |
| Minute Ventilation, mls/min | 253 ± 13 | 273 ± 26 | 243 ± 8 | 228 ± 20 | |
| D-penicillamine | Body Weights, grams | 290 ± 3 | 287 ± 3 | 293 ± 3 | 294 ± 3 |
| Frequency, breaths/min | 99 ± 7 | 101 ± 6 | 107 ± 7 | 105 ± 8 | |
| Tidal Volume, mls | 2.23 ± 0.12 | 2.20 ± 0.11 | 2.26 ± 0.12 | 2.24 ± 0.11 | |
| Minute Ventilation, mls/min | 223 ± 13 | 221 ± 14 | 233 ± 13 | 236 ± 14 | |
The data are presented as mean ± SEM. There were six rats in each group.
P < 0.05, Day 2 versus Day 1.
Fig. 1.
Left-hand panels: Changes in frequency of breathing, tidal volume and minute ventilation elicited by injection of vehicle or naloxone (NLX, 1.5 mg/kg, i.v.) and subsequent injection of morphine (10 mg/kg, i.v.) in conscious rats. Right-hand panels. Changes in frequency of breathing, tidal volume and minute ventilation elicited by injection of morphine (10 mg/kg i.v.) and subsequent exposure to hypoxic-hypercapnic challenge on Day 2 in rats that received vehicle plus morphine or NLX plus morphine, 1 day earlier. Data are mean ± SEM. There were 6 rats in each group.
Fig. 3.
Left-hand panels: Changes in frequency of breathing, tidal volume and minute ventilation elicited by injection of vehicle or D-penicillamine (D-PEN, 1 mmol/kg, i.v.) and subsequent injection of morphine (10 mg/kg, i.v.) in conscious rats. Right-hand panels. Changes in frequency of breathing, tidal volume and minute ventilation elicited by injection of morphine (10 mg/kg i.v.) and subsequent exposure to hypoxic-hypercapnic challenge on Day 2 in rats that received vehicle plus morphine or D-PEN plus morphine, 1 day earlier. Data are mean ± SEM. There were 6 rats in each group.
Table 2.
Effects of bolus injection of vehicle or test drugs on resting ventilatory parameters
| Peak Response, % |
Cumulative Response, % |
||||
|---|---|---|---|---|---|
| Study | Parameter | Vehicle | Drug | Vehicle | Drug |
| Naloxone | Frequency, breaths/min | +4 ± 4 | +71 ± 19*,† | −2 ± 2 | +36 ± 6*,† |
| Tidal Volume, mls | −9 ± 9 | +1 ± 4 | −2 ± 1 | −15 ± 3*,† | |
| Minute Ventilation, mls/min | +6 ± 8 | +52 ± 11*,† | −4 ± 2 | +15 ± 4*,† | |
| Naltrindole | Frequency, breaths/min | +52 ± 8* | +77 ± 13* | +7 ± 3 | +39 ± 4*,† |
| Tidal Volume, mls | −16 ± 3* | −15 ± 3* | +3 ± 1 | −8 ± 2*,† | |
| Minute Ventilation, mls/min | +24 ± 3* | +54 ± 7*,† | +9 ± 2* | +20 ± 2*,† | |
| D-penicillamine | Frequency, breaths/min | +10 ± 6 | +48 ± 6 | +3 ± 4 | +17 ± 4*,† |
| Tidal Volume, mls | +1 ± 3 | +6 ± 4 | +1 ± 3 | +1 ± 4 | |
| Minute Ventilation, mls/min | +6 ± 4 | +43 ± 7*,† | +3 ± 2 | +18 ± 5*,† | |
The data are presented as mean ± SEM. There were six rats in each group.
P < 0.05, significant response.
P < 0.05, drug-treated versus vehicle-treated rats.
Fig. 2.
Left-hand panels: Changes in frequency of breathing, tidal volume and minute ventilation elicited by injection of vehicle or naltrindole (NTD, 1.5 mg/kg, i.v.) and subsequent injection of morphine (10 mg/kg, i.v.) in conscious rats. Right-hand panels. Changes in frequency of breathing, tidal volume and minute ventilation elicited by injection of vehicle and then morphine (10 mg/kg i.v.) on Day 2 in rats that received vehicle plus morphine or NTD plus morphine, 1 day earlier. Data are mean ± SEM. There were 6 rats in each group.
3.2. Effects of test drugs on the ventilatory responses to morphine – Day 1
In the naloxone study, morphine elicited transient fluctuations in fR in vehicle-treated (vehicle) rats that were accompanied by sustained decreases in VT and therefore V̇ (Fig. 1, left-hand panels; Fig. 4). Morphine elicited prompt and sustained increases in fR in naloxone-treated (naloxone) rats that were accompanied by sustained decreases in VT that except for the first few minutes were similar to those in vehicle rats (Fig. 1, left-hand panel; Fig. 4). As such, morphine elicited a transient increase in V̇ in naloxone rats (Fig. 1, left-hand panel; Fig. 4). Neither naltrindole (Fig. 2, left-hand panels; Fig. 5) nor D-PEN (Fig. 3, left-hand panels; Fig. 6) affected the morphine-induced transient changes in fR or the sustained decreases in VT and V̇
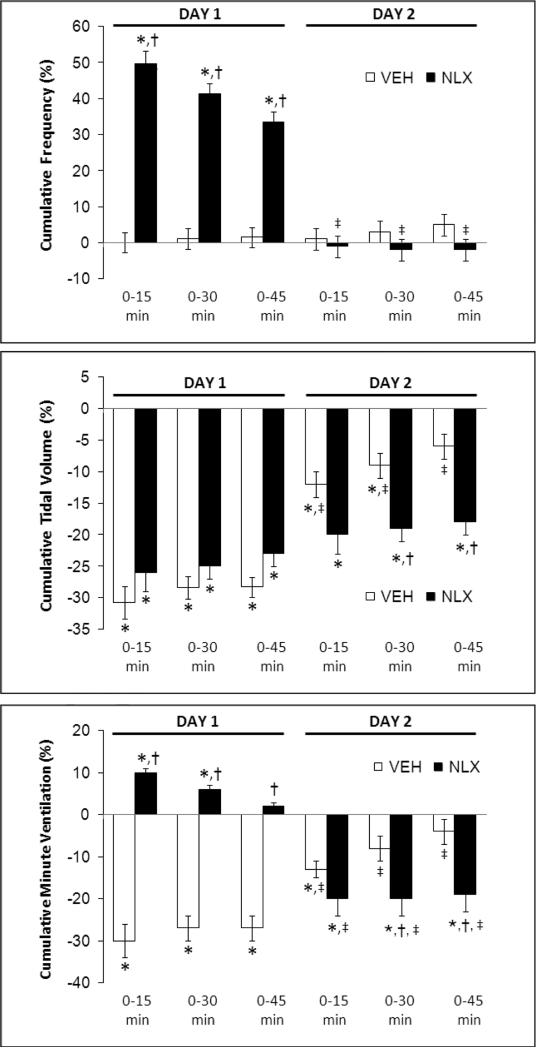
Fig. 4.
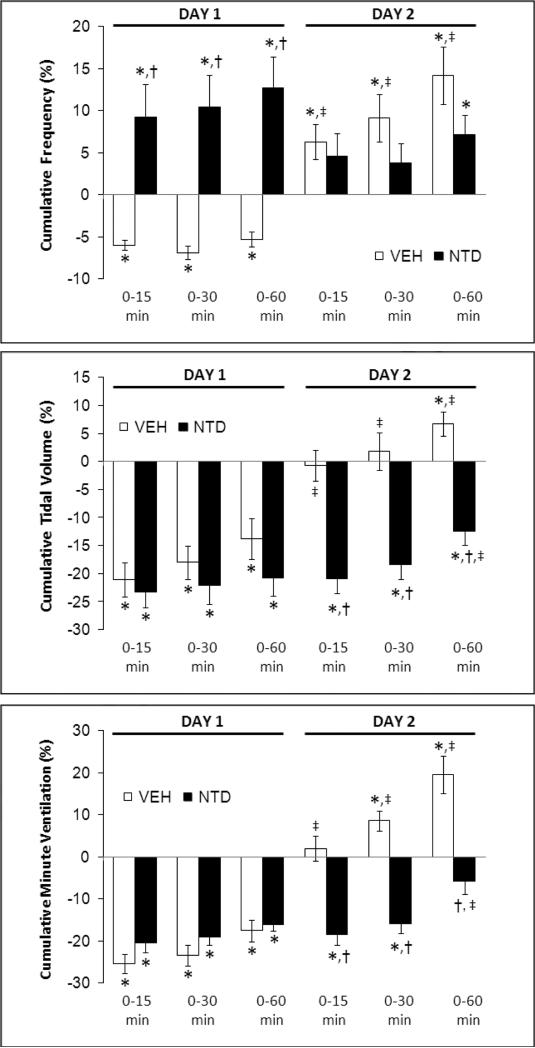
Cumulative percent changes in frequency of breathing (top panel), tidal volume (middle panel) and minute ventilation (bottom panel) elicited by morphine (10 mg/kg, i.v.) in conscious rats pretreated with vehicle (VEH) or naloxone (NLX, 1.5 mg/kg, i.v.) on Day 1, and again on Day 2 in these rats. Data are mean ± SEM. There were six rats in each group. *P < 0.05, significant cumulative response. †P < 0.05, NLX-treated versus vehicle-treated rats. ‡P < 0.05, Day 2 versus Day 1.
Fig. 5.
Cumulative percent changes in frequency of breathing (top panel), tidal volume (middle panel) and minute ventilation (bottom panel) elicited by morphine (10 mg/kg, i.v.) in conscious rats pretreated with vehicle (VEH) or naltrindole (NTD, 1.5 mg/kg, i.v.) on Day 1, and again on Day 2 in these rats. Data are mean ± SEM. There were six rats in each group. *P < 0.05, significant cumulative response. †P < 0.05, NTD-treated versus vehicle-treated rats. ‡P < 0.05, Day 2 versus Day 1.
Fig. 6.
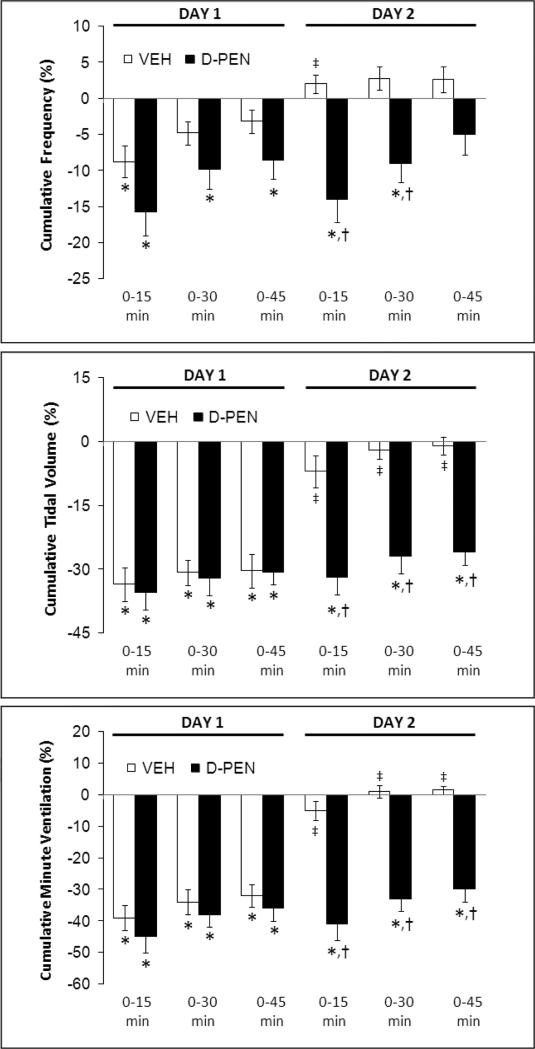
Cumulative percent changes in frequency of breathing (top panel), tidal volume (middle panel) and minute ventilation (bottom panel) elicited by morphine (10 mg/kg, i.v.) in conscious rats pretreated with vehicle (VEH) or D-penicillamine (D-PEN, 1 mmol/kg, i.v.) on Day 1, and again on Day 2 in these rats. Data are mean ± SEM. There were six rats in each group. *P < 0.05, significant cumulative response. †P < 0.05, NTD-treated versus vehicle-treated rats. ‡P < 0.05, Day 2 versus Day 1.
3.3. Effects of morphine on Day 2
Effects of naloxone
The injection of morphine in vehicle rats on Day 2 (i.e., those that received vehicle plus morphine on Day 1) elicited similar initial changes in fR, VT and V̇ as on Day 1 (Fig. 1, right-hand panels; Fig. 4). However, the duration of the decreases in VT and V̇ on Day 2 were substantially shorter than on Day 1 (note that comparisons are valid until 45 min) (Figs. 1 and 4). The depressant affects of morphine on VT, and to a lesser degree V̇, were of longer duration in the rats that received naloxone plus morphine on Day 1 (Fig. 1, right-hand panels; Fig. 4). Effects of naltrindole: The injection of morphine in vehicle rats on Day 2 elicited smaller reductions in VT and V̇ than on Day 1, and indeed steady increases in fR and VT resulted in a substantial increase in V̇ (Fig. 2, right-hand panels; Fig. 5). The injection of morphine in the naltrindole rats on Day 2 elicited changes in fR, VT and V̇ that were similar to those on Day 1. Effects of D-PEN: the injection of morphine in vehicle rats on Day 2 elicited smaller reductions in fR, VT and V̇ than on Day 1 (Fig. 3, right-hand panels; Fig. 6). The injection of morphine in the D-PEN rats on Day 2 elicited changes in fR, VT and V̇ that were virtually identical to those on Day 1.
3.4. Ventilatory responses elicited by exposure to H-H challenge
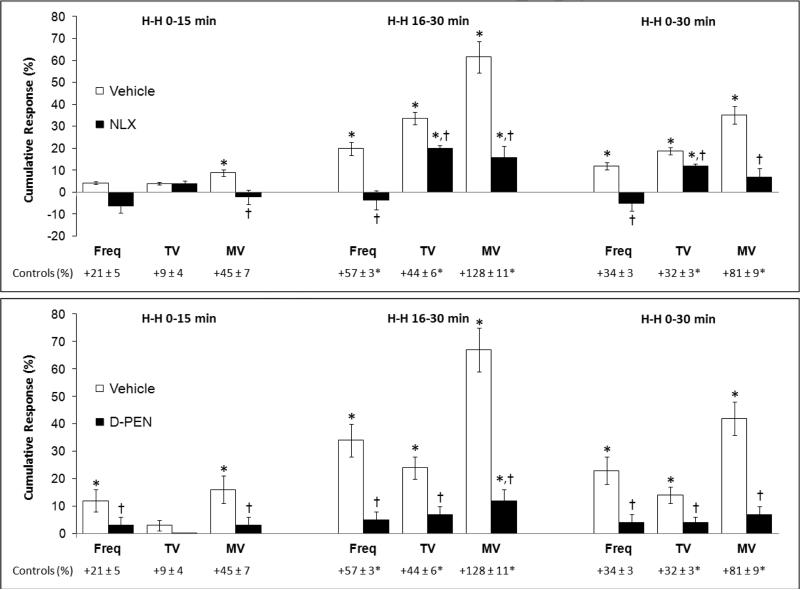
In the naloxone study, H-H challenge elicited increases in fR, VT and V̇ (Fig. 1, right-hand panel) in the vehicle rats but markedly smaller responses in the naloxone rats. In the D-penicillamine study, H-H challenge elicited increases in fR, VT, V̇ (Fig. 3, right-hand panel) in vehicle rats but minimal responses in D-PEN rats. The cumulative responses during H-H challenge were substantially smaller in the naloxone or D-PEN rats than in the respective vehicle rats (Fig. 7). Note, the ventilatory responses in vehicle-treated rats that received morphine were substantially smaller than in a naïve group of rats (see legend of Fig. 7).
Fig. 7.
Cumulative percent changes in frequency of breathing (Freq), tidal volume (TV), and minute ventilation (MV) during hypoxic-hypercapnic challenge following injection of morphine (10 mg/kg, i.v.) in conscious rats on Day 2. These rats were pretreated with (a) vehicle plus morphine, or naloxone (NLX, 1.5 mg/kg, i.v.) plus morphine, on Day 1 (upper panel), or (b) vehicle plus morphine, or D-penicillamine (D-PEN, 1 mmol/kg, i.v.) plus morphine, on Day 1 (lower panel). Data are mean ± S.E.M. There were six rats in each group. *P < 0.05, significant cumulative response. †P < 0.05, naloxone-treated or D-PEN-treated rats versus vehicle-treated rats. The “control” values under each panel represent the responses elicited by the hypoxichypercapnic challenge in a naïve group of rats (n=6, 288 ± 2 g). The asterisks on the control values denote significance (P < 0.05) from the responses in vehicle + morphine-treated rats.
4. Discussion
The key findings of this study were that development of tolerance to the ventilatory depressant effects of morphine was attenuated by naloxone, naltrindole, and D-PEN. This is consistent with the concept that the co-activation of μ- and δ-ORs triggers the production of peroxynitrite, which directly down-regulates μ- and δ-ORs and/or their intracellular signaling mechanisms (Salvemini, 2009; Salvemini and Neumann, 2009).
4.1. Effects of test agents on ventilatory parameters
Naloxone readily enters the brain (DeHaven-Hudkins and Dolle, 2004; Ananthan, 2006; Dahan et al., 2010) and is an antagonist of μ-, δ- and κ-ORs. Naloxone has twice the affinity for μ-ORs than for δ-ORs and 15 times greater affinity for μ-ORs than κ-ORs (Lewanowitsch and Irvine, 2003; Janecka et al., 2004). Naloxone increased V̇ via increases in fR, consistent with evidence that it exerts ventilatory excitant effects in conscious (Isom and Elshowihy, 1982a,b) and in anesthetized (Mauser and Chapman, 1987) rats. The δ1,2-OR antagonist, naltrindole (Portoghese, 1993; Ananthan, 2006) also increased V̇ via increases in fR, consistent with evidence that activation of δ-ORs depresses ventilation (Shook et al., 1990; Chen et al., 1991; Haji et al., 2000) and genioglossus muscle activity (Hajiha et al., 2009). The responses elicited by naloxone and naltrindole suggest that endogenous opioid peptides (e.g., enkaphalins, endomorphins) with activity at μ- and/or δ-ORs (Shook et al., 1990; Keresztes et al., 2010) play a tonic role in the control of breathing (Dahan et al., 2010). To our knowledge, the effects of D-PEN on ventilatory parameters have not been reported. D-PEN elicited increases in V̇ via increases in fR rather than VT, which may involve its redox properties (Graves et al., 2006). We presume that the effects of naloxone and naltrindole on ventilatory parameters are due to their actions in the brain although actions in peripheral structures such as the carotid bodies cannot be discounted.
4.2. Effects of morphine on Day 1
Morphine elicited a transient increase in fR at 1 min in vehicle rats that was associated with a decrease in VT and therefore a minimal change in V̇. Lower systemic doses or central injections of morphine stimulate ventilation via activation of μ-ORs (Szeto et al., 1991; Cheng et al., 1993). Since these excitatory responses were rapidly followed by ventilatory depression, transient increase in fR may have been due to initially lower concentrations of morphine activating μ-ORs in the brain, and as the tissue levels of morphine increased, the ventilatory depressant effects of morphine and/or its major metabolite, morphine-3-glucurodide (Hasegawa et al., 2010) began to dominate. The transient decreases in fR elicited by morphine were accompanied by sustained decreases in VT and therefore V̇, consistent with findings from other laboratories (van den Hoogen and Colpaert, 1986; Czapla et al., 2000). The ventilatory responses elicited by morphine were markedly affected by pretreatment with naloxone but not naltrindole or D-PEN. Specifically, morphine elicited robust and long-lasting increases in fR in the naloxone rats whereas, except for the first few minutes, the morphine-induced decreases in VT were minimally affected. The lack of effects of naloxone (1.5 mg/kg) on the morphine (10 mg/kg)-induced decrease in VT may simply be due to insufficient blockade of μ-ORs since the same dose of the selective μ1-OR receptor antagonist, naloxonazine, markedly attenuated morphine-induced suppression of VT (Lewis et al., unpublished findings). Since the ventilatory excitant effects of morphine in naloxone rats did not occur in naltrindole rats, it is possible that they involve activation of κ-ORs (Kilpatrick and Smith, 2005; Yamada et al., 2006).
4.3. Effects of morphine on Day 2 in naloxone or naltrindole rats
The ventilatory depressant responses elicited by morphine on Day 2 in vehicle rats were much less than on Day 1 confirming that the actions of morphine are subject to tolerance (McGilliard and Takemori, 1978; Bowen et al., 1979; Roerig et al., 1987; Hepburn et al., 1997; Freye and Latasch, 2003). Roerig et al. (1987) determined that mice implanted subcutaneously with morphine pellets developed tolerance to the central effects of this opioid by demonstrating that the ventilatory depressant effects elicited by subsequent bolus injections of morphine given subcutaneously or intracerebroventricularly elicited markedly smaller responses than in naïve mice. Moreover, McGilliard and Takemori (1978) demonstrated that the ability of naloxone to affect the analgesic and ventilatory depressant effects of subcutaneously administered morphine in mice changed with the duration of subcutaneous morphine treatment providing evidence that narcotic-induced respiratory depression and analgesia may be mediated by different receptor interactions.
Since tolerance was less in naloxone-pretreated rats it is likely that μ-OR activation is involved in the down-regulation of the mechanisms mediating the ventilatory depressant effects of morphine. Although naloxone did not markedly attenuate the morphine-induced falls in VT, it prevented the development of tolerance. Our findings with naltrindole, suggest that the ability of naloxone to block δ-ORs (DeHaven-Hudkins and Dolle, 2004; Ananthan, 2006) may have been the key to preventing tolerance. Despite the inability of naltrindole or D-PEN to blunt the responses to morphine, both markedly attenuated the development of tolerance. This suggests that activation of δ1,2-ORs generates peroxynitrite, causing the down-regulation of the G protein-coupled μ-/δ1,2-ORs that mediate the ventilatory depressant effects of morphine. Our findings support evidence that peroxynitrite is a key factor in the development of tolerance to opioid-induced analgesia (Salvemini and Neumann, 2009) and that peroxynitrite elicits the down-regulation of a variety of G protein-coupled receptors (Benkusky et al., 1998, 1999), ion-channels (Graves et al., 2005a) and S-nitrosothiol recognition sites (Graves et al., 2005b; Lewis et al., 2005). Our data contrast with evidence that tolerance to the ventilatory depressant effects of morphine occurs in naltrindole-treated rats or that tolerance to respiratory depression reflects actions independent of μ-/δ-ORs populations (Hepburn et al., 1997). There may be several reasons for the discrepancy in these findings including the different effects of morphine. For example, in our study, morphine had transient effects on fR (although it decreased VT) whereas in the study of Hepburn et al (1997), morphine induced a sustained depression of fR (no other parameters were recorded). To our knowledge, the use of δ1,2-OR antagonists to prevent tolerance to the analgesic actions of opioids while allowing for tolerance to develop to the respiratory depression has not progressed as a viable clinical approach (Ananthan, 2006).
4.4. Effects of morphine on Day 2 in D-penicillamine rats
L-PEN (Althaus et al., 1994, 1995) and D-PEN (Singh et al., 2007) scavenge peroxynitrite in vitro. The reaction of peroxynitrite with PEN yields a single S-nitro-PEN adduct (Althaus et al., 1994). Similar to L-PEN (Graves et al,. 2006), D-PEN is an effective peroxynitrite scavenger when given systemically (Lewis, unpublished observations). Oral administration of D-PEN results in detectable amounts of this thiol in blood and plasma (Abounassif and Jefferies, 1983). It is feasible that D-PEN prevents tolerance to the effects of morphine on peripheral structures such as the carotid bodies and neuromuscular elements in the chest wall and diaphragm as well as brain structures devoid of a blood-brain barrier (Duvernoy and Risold, 2007), which would be readily accessible to naloxone, naltrindole and D-PEN. These circumventricular organs express neuronal μ-, δ-and κ-ORs (Atweh and Kuhar, 1977; Snyder and Pasternak, 2003), and direct injections of OR agonists into these structures elicit physiological responses (Bhandari et al., 1992; Fregoneze and Antunes-Rodrigues, 1992). Although the effects of direct injections of opioids into these brain structures on ventilatory parameters are not known, these structures are involved in ventilatory control (Gatti et al., 1985; Ferguson et al., 1989). In addition, D-PEN may enter the cerebrospinal fluid and brain tissue, since intravenous D-PEN improves neurological recovery in mice after traumatic brain injury, a condition characterized by the generation and deleterious effects of peroxynitrite (Hall et al., 1999). Accordingly, D-PEN may prevent tolerance to the effects of morphine on brain neurons involved in ventilatory control (Kilpatrick and Smith, 2005; Trescot et al., 2008; Dahan et al., 2010).
4.5. Ventilatory responses to H-H challenge – day 2
The ventilatory responses elicited by H-H challenge in vehicle rats that received morphine were diminished compared to naïve rats. This is consistent with evidence that morphine suppresses ventilatory responses to hypoxia and hypercapnia by actions in the brain and carotid bodies (McQueen and Ribeiro, 1980; Kirby and McQueen, 1986; Mayer et al., 1989; Zhang et al., 2009). Despite development of tolerance to the ventilatory depressant effects of morphine per se (and that parameters were at pre-morphine levels when the H-H challenge was delivered), morphine still was able to suppress the responses to H-H challenge. These insidious effects of morphine are consistent with evidence that the morphine metabolite/opiate-receptor agonist, morphine-6-glucurodide, blunts ventilatory response to hypercapnia challenge although it minimally affects resting ventilation (Peat et al., 1991). The ventilatory responses elicited by H-H challenge were suppressed in naloxone or D-PEN rats, suggesting that blockade of μ-ORs and (to a lesser degree) δ-ORs by naloxone prevents peroxynitrite-induced down-regulation and/or desensitization of the receptors that depress the responses to H-H challenge.
4.6. Conclusions
Our studies show that the activation of μ- and δ-ORs plays a major role in the development of tolerance to the ventilatory depressant actions of morphine. Similar to analgesic tolerance (Salvemini, 2009; Salvemini and Neumann, 2009), our studies with D-PEN suggest that peroxynitrite may be a major player in tolerance development to morphine-induced ventilatory depression. The ability of D-PEN to chelate metals (Aposhian, 1961; Levine, 1975), thereby diminishing peroxynitrite-induced oxidation and nitration reactions (Ischiropoulos et al., 1992), may also be involved. The mechanisms by which activation of μ- and δ-ORs leads to the generation of peroxynitrite, which requires the interaction of nitric oxide and superoxide anion, have received substantial attention (Salvemini, 2009; Salvemini and Neumann, 2009). It remains to be determined whether the generation of peroxynitrite in the brain and/or peripheral structures participates in the development of tolerance to the ventilatory depressant effects of morphine.
Highlights.
The μ-/δ-opioid receptor antagonist naloxone decreased tolerance to morphine.
The δ1,2-opioid receptor antagonist naltrindole decreased tolerance to morphine.
The peroxynitrite scavenger D-penicillamine decreased tolerance to morphine.
Co-activation of μ-/δ1,2-opioid receptors impairs the effects of morphine via generation of peroxynitrite.
Acknowledgements
This study was supported by grants from Galleon Pharmaceuticals (to S.J.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abounassif MA, Jefferies TM. The determination of D-penicillamine and its disulphide in plasma by reversed-phase ion-pair high-performance liquid chromatography. J Pharm Biomed Anal. 1983;1:65–72. doi: 10.1016/0731-7085(83)80009-0. [DOI] [PubMed] [Google Scholar]
- Althaus JS, Oien TT, Fici GJ, Scherch HM, Sethy VH, VonVoigtlander PF. Structure activity relationships of peroxynitrite scavengers an approach to nitric oxide neurotoxicity. Res Commun Chem Pathol Pharmacol. 1994;83:243–254. [PubMed] [Google Scholar]
- Althaus JS, Andrus PK, Hall ED, Vonvoigtlander PF. Improvements in the salicylate trapping method for measurement of hydroxyl radical levels in the brain. In: Ohnishi ST, Ohnishi T, editors. Central Nervous System Trauma Research Techniques. CRC Press; Boca Raton, FL: 1995. p. 437444. [Google Scholar]
- Ammon-Treiber S, Höllt V. Morphine-induced changes of gene expression in the brain. Addict Biol. 2005;10:81–89. doi: 10.1080/13556210412331308994. [DOI] [PubMed] [Google Scholar]
- Ananthan S. Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics. AAPS J. 2006;8:E118–E125. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aposhian HV. Biochemical and pharmacological properties of the metal-binding agent penicillamine. Fed Proc Fed Am Soc Exp Biol. 1961;20(Suppl 10):185188. [PubMed] [Google Scholar]
- Atweh SF, Kuhar MJ. Autoradiographic localization of opiate receptors in rat brain. III. The telencephalon. Brain Res. 1977;134:393–405. doi: 10.1016/0006-8993(77)90817-4. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, Henderson G. Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Brit J Pharmacol. 2009;158:157–164. doi: 10.1111/j.1476-5381.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkusky NA, Lewis SJ, Kooy NW. Attenuation of vascular relaxation after development of tachyphylaxis to peroxynitrite in vivo. Am J Physiol. 1998;275:H501–H508. doi: 10.1152/ajpheart.1998.275.2.H501. [DOI] [PubMed] [Google Scholar]
- Benkusky NA, Lewis SJ, Kooy NW. Peroxynitrite-mediated attenuation of alpha- and beta-adrenoceptor agonist-induced vascular responses in vivo. Eur J Pharmacol. 1999;364:151–158. doi: 10.1016/s0014-2999(98)00791-2. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Bingham S, Andrews PL. The neuropharmacology of loperamide-induced emesis in the ferret: the role of the area postrema, vagus, opiate and 5-HT3 receptors. Neuropharmacology. 1992;31:735–742. doi: 10.1016/0028-3908(92)90034-m. [DOI] [PubMed] [Google Scholar]
- Bowen SR, Carpenter FG, Sowell JG. Ventilatory depression in naive and tolerant rats in relation to plasma morphine concentration. Brit J Pharmacol. 1979;65:457–463. doi: 10.1111/j.1476-5381.1979.tb07851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci. 1991;48:2165–2171. doi: 10.1016/0024-3205(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Wu D, Soong Y, McCabe S, Decena JA, Szeto HH. Role of μ1- and δ-opioid receptors in modulation of fetal EEG and respiratory activity. Am J Physiol Regul Integr Comp Physiol. 1993;265:R433–R438. doi: 10.1152/ajpregu.1993.265.2.R433. [DOI] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. μ-opioid receptor desensitization: is morphine different? Brit J Pharmacol. 2004;143:685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapla MA, Gozal D, Alea OA, Beckerman RC, Zadina JE. Differential cardiorespiratory effects of endomorphin 1, endomorphin 2, DAMGO, and morphine. Am J Respir Crit Care Med. 2000;162:994–999. doi: 10.1164/ajrccm.162.3.9911102. [DOI] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Dolle RE. Peripherally restricted opioid agonists as novel analgesic agents. Curr Pharm Des. 2004;10:743–757. doi: 10.2174/1381612043453036. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell C. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM, Risold PY. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev. 2007;56:119–147. doi: 10.1016/j.brainresrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Beckmann LM, Fisher JT. Effects of subfornical organ stimulation on respiration in the anesthetized rat. Can J Physiol Pharmacol. 1989;67:1097–1101. doi: 10.1139/y89-173. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Konduri GG. Repeated exposure to rapidly developing hypoxemia influences the interaction between oxygen and carbon dioxide in initiating arousal from sleep in lambs. Pediatr Res. 1988;24:28–33. doi: 10.1203/00006450-198807000-00008. [DOI] [PubMed] [Google Scholar]
- Fregoneze JB, Antunes-Rodrigues J. Role of opioid peptides and subfornical organ in the renal function of intact and hypophysectomized rats. Physiol Behav. 1992;51:287–292. doi: 10.1016/0031-9384(92)90142-o. [DOI] [PubMed] [Google Scholar]
- Freye E, Latasch L. Development of opioid tolerance - molecular mechanisms and clinical consequences. Anasthesiol Intensivmed Notfallmed Schmerzther. 2003;38:14–26. doi: 10.1055/s-2003-36558. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Dias Souza J, Taveira Da Silva AM, Quest JA, Gillis RA. Chemical stimulation of the area postrema induces cardiorespiratory changes in the cat. Brain Res. 1985;346:115–123. doi: 10.1016/0006-8993(85)91100-x. [DOI] [PubMed] [Google Scholar]
- Graves JE, Lewis SJ, Kooy NW. Loss of K+ATP-channel-mediated vasodilation after induction of tachyphylaxis to peroxynitrite. J Cardiovasc Pharmacol. 2005a;46:646–652. doi: 10.1097/01.fjc.0000181716.79580.dd. [DOI] [PubMed] [Google Scholar]
- Graves JE, Bates JN, Kooy NW, Lewis SJ. Vasodilator actions of the endothelium-derived relaxing factor L-S-nitrosocysteine in anaesthetized rats are markedly diminished by peroxynitrite. Clin Exp Pharmacol Physiol. 2005b;32:1137–1141. doi: 10.1111/j.1440-1681.2005.04310.x. [DOI] [PubMed] [Google Scholar]
- Graves JE, Kooy NW, Lewis SJ. L-β,β-dimethylcysteine attenuates the hemodynamic responses elicited by systemic injections of peroxynitrite in anesthetized rats. Brit J pharmacological. 2006;148:7–15. doi: 10.1038/sj.bjp.0706692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther. 2000;86:277–304. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol. 2009;587:2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Kupina NC, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann N Y Acad Sci. 1999;890:462–468. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Kishimoto S, Shibatani N, Nomura H, Ishii Y, Onishi M, Inotsume N, Takeuchi Y, Fukushima S. The pharmacokinetics of morphine and its glucuronide conjugate in a rat model of streptozotocin-induced diabetes and the expression of MRP2, MRP3 and UGT2B1 in the liver. J Pharm Pharmacol. 2010;62:310–314. doi: 10.1211/jpp.62.03.0004. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Yoshida A, Fukuda Y, Honda Y. CO2-ventilatory response of the anesthetized rat by rebreathing technique. Pflugers Arch. 1982;393:77–82. doi: 10.1007/BF00582395. [DOI] [PubMed] [Google Scholar]
- Hepburn MJ, Little PJ, Gingras J, Kuhn CM. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J Pharmacol Exp Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- Janecka A, Fichna J, Janecki T. Opioid receptors and their ligands. Curr Top Med Chem. 2004;4:1–17. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Zhu L, Chen J, Tsai JHM, Martin JC, Smith SD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Isom GE, Elshowihy RM. Naloxone-induced enhancement of carbon dioxide stimulated respiration. Life Sci. 1982a;31:113–118. doi: 10.1016/0024-3205(82)90422-2. [DOI] [PubMed] [Google Scholar]
- Isom GE, Elshowihy RM. Interaction of acute and chronic stress with respiration: modification by naloxone. Pharmacol Biochem Behav. 1982b;16:599–603. doi: 10.1016/0091-3057(82)90422-1. [DOI] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A, Borics A, Tóth G. Recent advances in endomorphin engineering. ChemMedChem. 2010;5:1176–1196. doi: 10.1002/cmdc.201000077. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Smith TW. Morphine-6-glucuronide: actions and mechanisms. Med Res Rev. 2005;25:521–544. doi: 10.1002/med.20035. [DOI] [PubMed] [Google Scholar]
- Kirby GC, McQueen DS. Characterization of opioid receptors in the cat carotid body involved in chemosensory depression in vivo. Brit J Pharmacol. 1986;88:889–898. doi: 10.1111/j.1476-5381.1986.tb16263.x. 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy P, Bonsignore MR, Eckel J. Sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–260. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- Levine WG. Heavy metal antagonists. In: Goodman LS, Gilman A, editors. The Pharmacological Basis of Therapeutics. MacMillan Publishing Company; New York: 1975. pp. 912–923. [Google Scholar]
- Lewanowitsch T, Irvine RJ. Naloxone and its quaternary derivative, naloxone methiodide, have differing affinities for mu, delta, and kappa opioid receptors in mouse brain homogenates. Brain Res. 2003;964:302–305. doi: 10.1016/s0006-8993(02)04117-3. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Graves JE, Bates JN, Kooy NW. Peroxynitrite elicits dysfunction of stereoselective S-nitrosocysteine recognition sites. J Cardiovasc Pharmacol. 2005;46:637–645. doi: 10.1097/01.fjc.0000181717.87204.2f. [DOI] [PubMed] [Google Scholar]
- Mayer N, Zimpfer M, Raberger G, Beck A. Fentanyl inhibits the canine carotid chemoreceptor reflex. Anesth Analg. 1989;69:756–762. [PubMed] [Google Scholar]
- Mauser PJ, Chapman RW. Role of endogenous opioids on ventilation and chemical control of breathing in pentobarbitone-anesthetized rats. Pharmacology. 1987;35:317–326. doi: 10.1159/000138356. [DOI] [PubMed] [Google Scholar]
- McGilliard KL, Takemori AE. Alterations in the antagonism by naloxone of morphine-induced respiratory depression and analgesia after morphine pretreatment. J Pharmacol Exp Ther. 1978;207:884–891. [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Inhibitory actions of methionine-enkephalin and morphine on the cat carotid chemoreceptors. Brit J Pharmacol. 1980;71:297–305. doi: 10.1111/j.1476-5381.1980.tb10939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat SJ, Hanna MH, Woodham M, Knibb AA, Ponte J. Morphine-6-glucuronide: effects on ventilation in normal volunteers. Pain. 1991;45:101–104. doi: 10.1016/0304-3959(91)90170-3. [DOI] [PubMed] [Google Scholar]
- Portoghese PS. The design of delta-selective opioid receptor antagonists. Farmaco. 1993;48:243–251. [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–E591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raith K, Hochhaus G. Drugs used in the treatment of opioid tolerance and physical dependence: a review. Int J Clin Pharmacol Ther. 2004;42:191–203. doi: 10.5414/cpp42191. [DOI] [PubMed] [Google Scholar]
- Roerig SC, Fujimoto JM, Lange DG. Development of tolerance to respiratory depression in morphine- and etorphine-pellet-implanted mice. Brain Res. 1987;400:278–284. doi: 10.1016/0006-8993(87)90627-5. [DOI] [PubMed] [Google Scholar]
- Salvemini D. Peroxynitrite and opiate antinociceptive tolerance: a painful reality. Arch Biochem Biophys. 2009;484:238–244. doi: 10.1016/j.abb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Neumann WL. Peroxynitrite: a strategic linchpin of opioid analgesic tolerance. Trends Pharmacol Sci. 2009;30:194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Shook JE, Watkins WD, Camporesi EM. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am. Rev. Respir. Dis. 1990;142:895–909. doi: 10.1164/ajrccm/142.4.895. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J Neurosci Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Pasternak GW. Historical review: Opioid receptors. Trends Pharmacol Sci. 2003;24:198–205. doi: 10.1016/S0165-6147(03)00066-X. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Cheng PY, Dwyer G, Decena JA, Wu DL, Cheng Y. Morphine-induced stimulation of fetal breathing: role of mu 1-receptors and central muscarinic pathways. Am J Physiol Regul Integr Comp Physiol. 1991;261:R344–R350. doi: 10.1152/ajpregu.1991.261.2.R344. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11(2 Suppl):S133–S153. [PubMed] [Google Scholar]
- Ueda H, Ueda M. Mechanisms underlying morphine analgesic tolerance and dependence. Front Biosci. 2009;14:5260–5272. doi: 10.2741/3596. [DOI] [PubMed] [Google Scholar]
- van den Hoogen RH, Colpaert FC. Respiratory effects of morphine in awake unrestrained rats. J Pharmacol Exp Ther. 1986;237:252–259. [PubMed] [Google Scholar]
- Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Walshe JM. Penicillamine neurotoxicity: an hypothesis. ISRN Neurol. 2011;2011:464572. doi: 10.5402/2011/464572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Shimoyama N, Sora I, Uhl GR, Fukuda Y, Moriya H, Shimoyama M. Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors. Brain Res. 2006;1083:61–69. doi: 10.1016/j.brainres.2006.01.095. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Opioid μ-receptors in medullary raphe region affect the hypoxic ventilation in anesthetized rats. Respir Physiol Neurobiol. 2009;168:281–288. doi: 10.1016/j.resp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]