Abstract
Background:
Dental and medical practitioners encounter wide spectrum of oral lesions in their day-to-day practice. Many of the lesions such as leukoplakia, oral submucous fibrosis (OSF), etc., are associated with tobacco and betel nut chewing. Oral leukoplakia, OSF, oral lichen planus and oral squamous cell carcinoma (OSCC) are the most commonly occurring oral diseases associated with characteristic clinical and histological features and are associated with chronic inflammation at some stage of the disease process.
Aims:
To study and compare the number, morphology and topographical distribution of mast cells in oral epithelial dysplasia (OED), OSF and OSCC and to correlate different types of mast cells with the inflammatory infiltrate and vascularity of the lesions.
Materials and Methods:
Total number of subjects was 120 and equally divided into four groups of 30 as controls, OED, OSF and OSCC cases. Two sections of from each tissue embedded in paraffin wax block were made which were stained with hematoxylin and eosin and toluidine blue stain. Mast cells were counted in five different zones.
Results:
In the present study, increased numbers of mast cells were seen in all lesions. The cases with mild, moderate and severe inflammation showed increased number of typical (TMCs), atypical (AMCs) and granular mast cells (GMCs), respectively.
Conclusion:
The result of the present study concludes that the mast cells play a key role in mediating the cross links between external angiogenic agent and local immunologic factors.
Keywords: Angiogenesis, inflammation, malignant lesions, mast cells, premalignant lesions
INTRODUCTION
Dental and medical practitioners encounter wide spectrum of oral mucosal lesions in their day-to-day practice. These mucosal lesions vary in nature from simple to life-threatening ones. Many of the lesions such as leukoplakia, oral submucous fibrosis (OSF), etc., are associated with the habit of using tobacco and betel nut.
Oral leukoplakia, OSF, oral lichen planus and oral squamous cell carcinoma (OSCC) are the most commonly occurring oral lesions associated with characteristic clinical and histological features and are associated with chronic inflammation at some stage of the disease process.[1] Mast cells are the cells commonly seen during inflammation.
Mast cells arise from a multipotent CD34+ precursor in the bone marrow and circulate in the peripheral blood. They are distributed throughout the body which acts locally and systemically by releasing variety of potent mediators through degranulation. Mast cells are also known as “unicellular endocrine glands” due to their ability to release the chemical mediators of inflammation.[2] Mast cells have also been reported to play a role in tumor progression and metastases by promoting angiogenesis.[3]
Since inflammation is common in oral epithelial dysplasia (OED), OSF and OSCC, a retro-prospective study was carried out to evaluate the morphological and topographical distribution of mast cells in oral leukoplakia, OSF and OSCC and to find their association with inflammation.
MATERIALS AND METHODS
The present study was a retroprospective study. Study sample included tissue specimens embedded in paraffin wax blocks of reported case diagnosed as OED, OSF and OSCC that were retrieved from the archives of the Department of Oral Pathology and Microbiology. There was complete availability of records with relevant details of these cases.
For prospective study all the subjects without systemic disorders were included. The subjects were informed about the study and written consent for the same was obtained. After the necessary instructions, the most representative site for biopsy was selected and incisional biopsy was performed under aseptic conditions and local anesthesia. Biopsy specimens were fixed and preserved in 10% neutral buffered formalin solution.
Total number of subjects was 120 and was divided into four groups:
Group 1: Control group, comprises of 30 healthy volunteers (normal oral mucosa)
Group 2: Comprise of 30 cases of OED
Group 3: Comprise of 30 cases of OSF
Group 4: Comprise of 30 cases of OSCC.
Two sections of 5 μm thickness from each tissue embedded in paraffin wax block were made. One section was stained with hematoxylin and eosin and another section was stained with toluidine blue stain. The sections were observed under x4, x10 and x40 magnification objectives fitted with 1 cm2 graticule.
The area selected for counting mast cells included the most cellular part of the tissue and mast cells were counted in five different zones in all sections starting from subepithelial connective tissue from one end, proceeding along the entire length of sections. The average of each type of mast cells was taken from five different zones.
The following criteria were established for the morphological study of mast cells:
Typical (TMC): When the cytoplasmic membrane, the nucleus and the granules of cytoplasm were clearly seen as shown in Figures 1 and 2
Atypical (AMC): When the nucleus and the granules were clearly seen but the cytoplasm was not defined [Figures 1 and 2]
Granular (GMC): The presence of three or more aggregations of granules resulting from degranulated AMCs without the presence of their nucleus [Figures 1 and 2].
Figure 1.
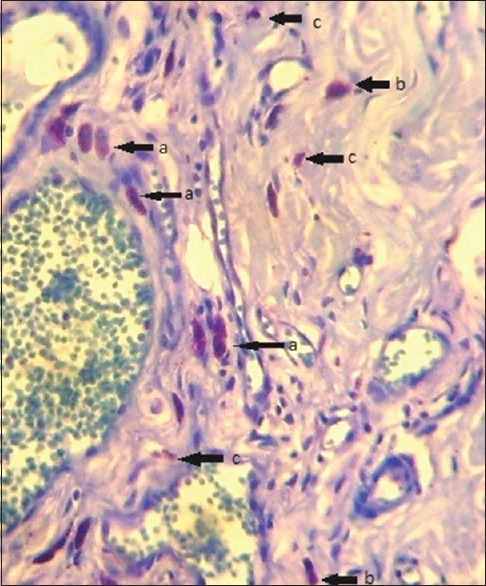
Photomicrograph of toluidine blue stain showing different types of mast cells: (a) Typical mast cells, (b) atypical mast cells and (c) granular mast cells (Toluidine blue stain, x100)
Figure 2.
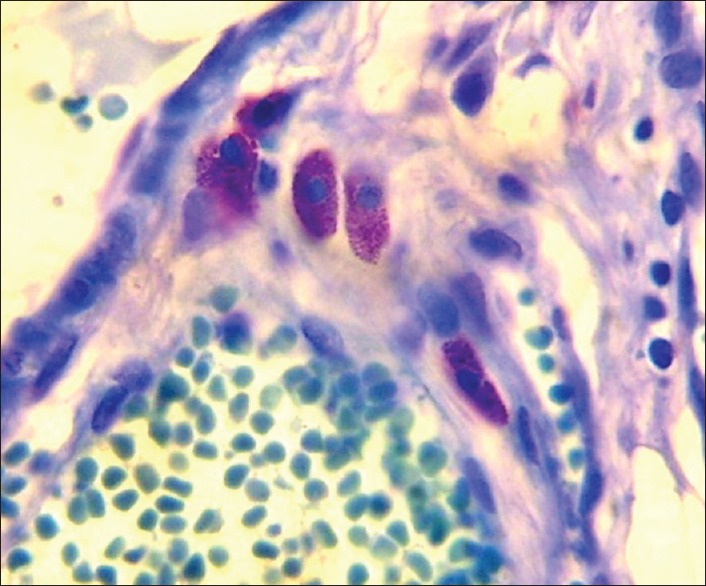
Photomicrograph of toluidine blue stain showing mast cells (Toluidine blue stain, x100)
Statistical analysis
Results were expressed as mean ± standard deviation and range values. One-way analysis of variance (ANOVA) was used for multiple group comparisons followed by Post-Hoc Tukey's for group wise comparisons.
A P value of 0.05 or less was considered for statistical significance.
RESULTS
One-way ANOVA test was applied to compare TMC, AMC and GMC between control, OED, OSF and OSCC which showed a significant difference (P < 0.02) [Table 1].
Table 1.
Comparison of individual type of mast cells in OED, OSF and OSCC and control group per person
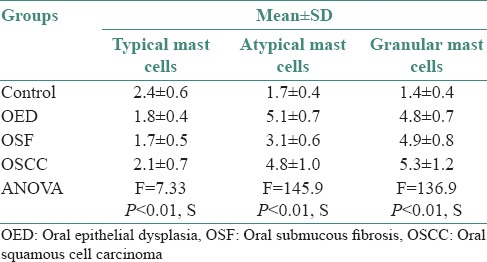
In the present study, increased numbers of mast cells were seen in all lesions. Malignant lesions showed highest number of mast cells as compared with the premalignant lesions. The cases with mild, moderate and severe inflammation showed increased number of TMCs, AMCs and GMCs respectively.
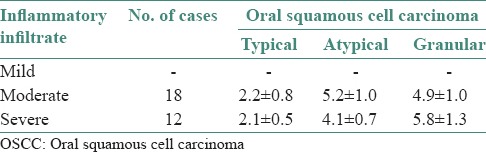
In OED, cases with mild inflammatory infiltrate showed increased number of TMCs (5.1 ± 0.4), moderate inflammatory cases showed increased number of AMCs (5.2 ± 0.6) and severe inflammatory cases showed increased number of GMCs (5.6 ± 0.7) as shown in Figures 3–5 and Table 2. In OSF, cases with mild inflammatory infiltrate showed increased number of TMCs (5.0 ± 0.8) and moderate inflammatory and severe inflammatory cases showed increased number of GMCs (4.7 ± 0.9) and (5.2 ± 0.0), respectively as shown in Figures 4–5 and Table 3. OSCC cases with moderate inflammatory response showed increased number of AMCs (5.2 ± 1.0) and cases of severe inflammatory response showed increased number of GMCs (5.8 ± 1.3) as shown in Figures 4–5 and Table 4.
Figure 3.
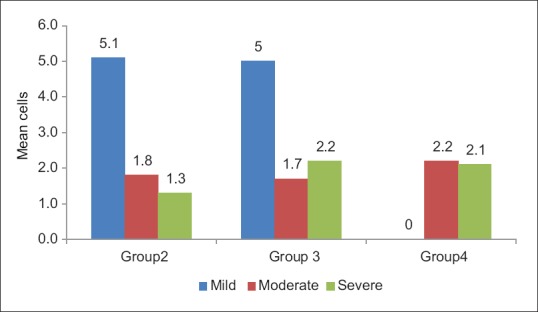
Graph showing comparison of typical mast cells with inflammatory infiltrate
Figure 5.
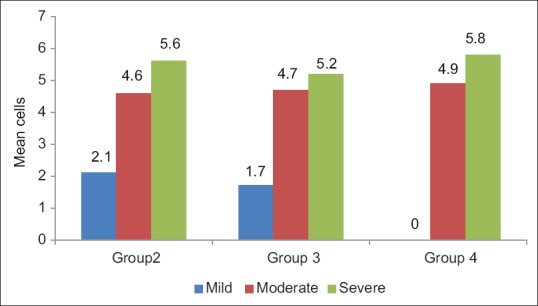
Graph showing comparison of granular mast cells with inflammatory infiltrate
Table 2.
Comparison of individual types of mast cells in OED with inflammatory infiltrate
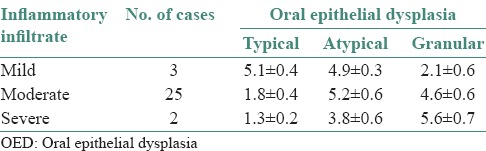
Figure 4.
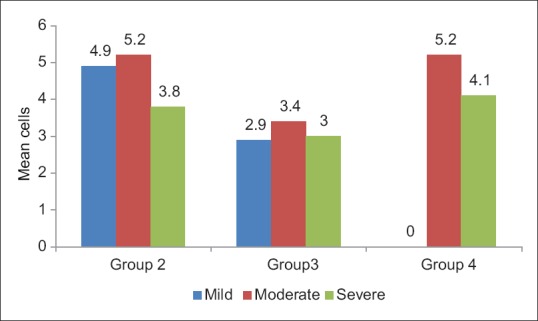
Graph showing comparison of atypical mast cells with inflammatory infiltrate
Table 3.
Comparison of individual types of mast cells in OSF with inflammatory infiltrate
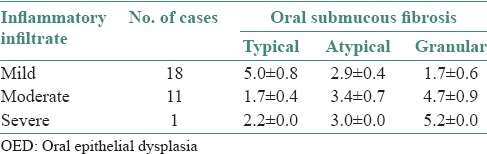
Table 4.
Comparison of individual types of mast cells in OSCC with inflammatory infiltrate
DISCUSSION
Mast cells are the local residents of the connective tissue. They are said to be proinflammatory and immune-amplifying in action and they produce mitogenic cytokines.[3]
Mast cells can release numerous proinflammatory, immunoregulatory and angiogenic molecules through different stimulation pathways. The activation of mast cells has been proved to have many biological consequences such as mitogenesis, extracellular matrix degradation, angiogenesis and augmentation of microvascular hyperpermeability and recruitment of inflammatory cells including macrophages. Increased angiogenesis has been associated with neoplastic progression, metastasis as seen in several studies and also is associated with increase in number of malignancies.[4]
The density of the mast cells in a tissue can be studied histochemically by using stains like toluidine blue and alcian blue; and immunohistochemically by using mast cell tryptase, heparin, chymase and carboxypeptidase A.[3]
Various studies have been conducted to assess the role of mast cells in precancerous and cancerous conditions. The present study was done for the quantitative and qualitative measurement of mast cells in OED and OSCC and an attempt was made to correlate their number and type with the density of inflammatory cells. This has enabled in the assessment of role of these cells in the etiopathogenesis of the above mentioned lesions. As mast cell degranulation predominantly occurs during inflammatory condition, these cells may play a role in the regulation of inflammatory responses.[5]
The mean number of mast cells in OED was found to be 1.8 for TMCs, 5.1 for AMCs and 4.8 for GMCs. Ankle et al., showed a mean increase in the number of mast cells/unit and concluded that the biologically and pharmacologically active agents in the mast cells might contribute to inflammatory reaction seen in leukoplakia. Interleukin-1 contribute to increased epithelial proliferation seen in leukoplakia and the release of proangiogenic and angiogenic factors, such as histamine and heparin, chymase, beta-fibroblast growth factors (βFGF) and vascular endothelial growth factors (VEGF) by mast cells may lead to increase in density of microvessels significantly between normal oral mucosa and oral leukoplakia without dysplasia, oral leukoplakia with mild, moderate or severe dysplasia and OSCC.[1]
The mean number of TMCs were 1.7, AMCs were 3.1 and GMCs type were 4.9 in OSF which is in accordance with the studies done by Bhatt and Dholakia who noted abundant mast cells in grade I and II of OSF. They attributed vesicle formation and symptoms of itching sensation to histamine release from mast cells. Prostaglandins and leukotrienes secreted by mast cells are potent secretagogues for serous and mucous cells which would attribute to increased salivation.[6]
The mean TMC, AMC and GMC count was 2.1, 4.8 and 5.3, respectively for OSCC. Mast cells are attracted at the lesion site and may turn on an angiogenic switch during tumorigenesis in OSCC. Moreover, the mast cells were shown to induce neovascularization through the carcinogenesis of the squamous cells.[7] The angiogenic factors which include VEGF, bFGF and the platelet-derived growth factors have been reported to stimulate mast cell migration. Various angiogenic factors which are secreted by the mast cells either directly promote angiogenesis by stimulating the migration and/or proliferation of the mast cells or indirectly through the degradation of the extracellular matrix.[3] Several factors secreted by mast cells, such as histamine, heparin, VEGF, βFGF and tryptase, have been shown to exert an activity on the migration and/or proliferation of endothelial cells.[4]
There is significant mast cell hyperplasia in all the oral lesions considered. The mediators of mast cells vary with variation in microenvironment in various diseases. Thus, mast cells play a key role in mediating the cross links between external angiogenic agent and local immunologic factors.
It has been suggested by Walsh et al., that cytokines released by tissue mast cells may be the triggering factor for induction of vascular adhesion molecules to allow entry of mast cells to the extravascular compartment. In the present study, increased number of mast cells were found corresponding to an increase in inflammatory cell response. Hence, concentration of inflammatory cells in tissue is the reflection of vascularity.[8]
Increased number of TMCs was observed in OED and OSMF with mild inflammatory infiltrate. Increased number of AMCs was observed in OED and OSCC with moderate inflammatory infiltrate. Increased number of GMCs was observed in OSMF with moderate and severe inflammatory infiltrate and in OED and OSCC with severe inflammatory infiltrate.
CONCLUSION
The decrease in the mast cell numbers might possibly reflect an important modification in the microenvironment during tumor initiation and progression. Currently, the exact functional relevance of the mast cells which surround various tumors is unclear. However, the accumulated evidence indicates that the mast cells may induce the tumor progression by providing a mitogenic stimulation or angiogenesis-the hallmark of the tumor growth and metastasis through the release of various mediators.
An increased number of TMCs in less inflamed areas and increased number of AMCs and GMCs in more inflamed areas contribute to the active participation of mast cells in various phases of inflammatory process manifested by their degranulation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ankle MR, Kale A, Nayak R. Mast cells are increased in leukoplakia, oral submucous fibrosis, oral lichen planus and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2007;11:18–22. [Google Scholar]
- 2.Sudhakar R, Ramesh V, Balamurali PD, Nirima O, Premalatha B, Karthikshree V. Incidence of mast cells in oral inflammatory lesions: A pilot study. J Oral Maxillofac Pathol. 2005;9:12–15. [Google Scholar]
- 3.Cheema VS, Ramesh V, Balamurali PD. Relevance of mast cells in oral squamous cell carcinoma. J Clin Diagn Res. 2012;6:1803–7. doi: 10.7860/JCDR/2012/4503.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholl P, Byers RM, Batsakis JG, Wolf P, Santini H. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue: Prognostic and therapeutic implications. Am J Sur. 1986;152:354–60. doi: 10.1016/0002-9610(86)90304-1. [DOI] [PubMed] [Google Scholar]
- 5.Michailidou EZ, Markopoulos AK, Antiniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008;2:126–32. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt AP, Dholakia HM. Mast cell density in oral submucous fibrosis. J Indian Dent Assoc. 1977;49:187–91. [Google Scholar]
- 7.Coussens LM, Raymond WW, Bergers G, Laig-webster M, Behrendtsen O, Werb Z. The inflammatory mast cells up-regulate the angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh LJ, Savage NW, Ishii T, Seymour GJ. Immunopathogenesis of oral lichen planus. J Oral Pathol Med. 1990;19:389–96. doi: 10.1111/j.1600-0714.1990.tb00866.x. [DOI] [PubMed] [Google Scholar]


