Cannibalism is the term used to describe “Cell-Eat-Cell” phenomenon. In Spanish, the word “Canibal” refers to cannibalism among the caribs, whereas in Greek the term used is ‘anthropophagy’ where ‘anthropos’ means ‘man’ and ‘phagein’ is ‘to consume’. Thus, cannibalism is the act or practice of a human eating another human in the actual sense.[1] The cellular cannibalism do occur, generally defined as the ability of a cell to engulf or phagocytose another cell of its own type or any other. It was first described by Leydenin 1904, who coined those cells as “bird's-eye cells”.[2] Cannibalistic cells (CCs) were initially noticed in cytological smears where the cell that had ingested another cell consisted of a vacuole containing the ingested cell and this vacuole pushed the nucleus to the periphery of the cell. This unusual property of tumor cells gets an advantage over survival at low nutrient adverse conditions.[1]
Cellular cannibalism fundamentally differs from phagocytosis, entosis, empripolesis and autophagy; though it may mimic these phenomenon.[3] Self-cannibalism (macroautophagy) is a well-regulated process of cell repair as well as of molecule and organelle recycling that allows the cells to survive. It also represents a cell death pathway characterized by specific features that differentiate autophagy from other cell death processes. The cells that are able to exert intense autophagic activity were also able to engulf and digest entire cell siblings. This phenomenon represents a sort of ‘xeno-cannibalism’. The two phenomenon: Self and xeno-cannibalism, could be related; the latter being an exacerbation of the first and providing further survival option to the cells.[4]
Cannibalism as described by Brouwer et al., is a process of successive steps, where the initial process starts with the attachment of CCs to a free cell pursued by gradual engulfment of the cell cytoplasm of the free cell, with alteration of the nucleus of the CC to semilunar shape, but the nucleus of the free cell remains unaltered. Eventually the engulfed or free cell gets completely interiorized within the CC and may finally die or can divide inside the vacuoles or sometimes manage to escape altogether, reemerging as cell that is indistinguishable from their un-engulfed counterparts.[5] The schematic representation of mechanism of cannibalism is presented in Figure 1, the histopathological images of the mechanism is highlighted in Figure 2 and the hand drawn illustration of the same is depicted in Figure 3.
Figure 1.
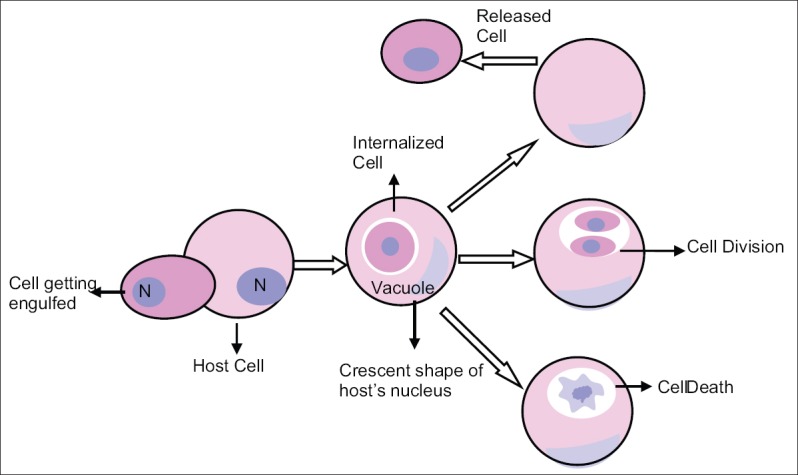
Mechanism of cannibalism represented schematically
Figure 2.
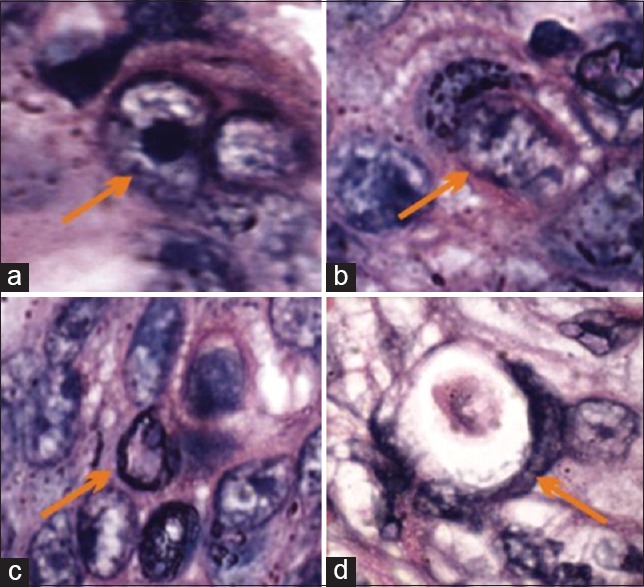
Photomicrograph showing stages of cannibalism: (a) Initial stage, (b) crescent shape nucleus of host cell and partial engulfment, (c) complete engulfment and (d) dead engulfed cell (H&E stain, ×1,000). H&E = Hematoxylin and eosin
Figure 3.

Hand drawn illustration of mechanism of cannibalism. (a) Cannibalistic cell in close proximity to tumor cell, (b) crescent shape nucleus of cannibalistic cell engulfing the tumor cell, (c) tumor cell totally engulfed by cannibalistic cell and (d) degeneration of tumor cell within the cannibalistic cell
Another mechanism of cannibalism has been proposed that states that engulfment may be initiated after the cells detach from the extracellular matrix due to lack of nutrition. This is a common occurrence among tumor cells in vivo. Cells proliferate from a single epithelial sheet to form a three-dimensional mass.
Various dynamics hold cells together at cell–cell junctions. Any imbalance in adhesion forces between two cells may result in engulfment, with one cell pulling the other cell more strongly where the cell that has been pulled strongly is engulfed.[6]
CCs can be easily identified on routine hematoxylin and eosin (H and E) stained sections and no special advanced diagnostic techniques are required. The CC has no selectivity. It can affect the dead or living cells and involve either homogenous (cells of same type) or heterogeneous (cells of different type) cells. It targets cells that are more passive. Most heterotypic live cell engulfment involves leukocytes ingested into a variety of host cells (epithelial cells, fibroblasts, neural cells and others). Homotypic live cell engulfment is most often reported between tumor cells in breast, lung and gastric carcinoma and other cancers.[7]
In our previous research, we noticed that the presence of CC in oral squamous cell carcinoma (OSCC) revealed its aggressive nature and increase in number of CC was significantly associated with lymph node metastasis.[8] It has been emphasized that cellular cannibalism is correlated with the aggressiveness, degree of anaplasia, invasiveness and metastatic potential of the malignancy.[9] Though cannibalism is generally used to differentiate between benign and malignant tumors, recently it has been demonstrated in benign tumors. Sarode and Sarode reported cannibalism in central and peripheral giant cell granuloma (CGCG and PGCG). Their aggressive and recurrent cases showed significantly more mean cannibalistic giant cells (GCs). They hypothesized that the assessment of frequency of CC in CGCG and PGCG will help in predicting the biological behavior of the tumor on simple histological ground. They demonstrated that the cannibalistic GCs as well as mononuclear stromal cells expressed histiocytic markers. The internalized cells did not express bcl-2, suggesting that the internalization induces apoptotic cell death.[10]
It has been reported that cannibalism in malignant tumor is caused due to a shift in the metabolic pathway that encourages selection of certain cell phenotypes that are able to survive in the caustic environment. These selected malignant cells are highly virulent and cannibalize other malignant cells to survive and progress in adverse condition within microenvironment such as hypoxia, low nutrient supply and acidity.[11] This pathogenic process is not applicable to benign tumors such as PGCG and CGCG. The GCs of these pathologies are derived from monocyte-macrophage lineage and resemble osteoclasts.[12] Hence, GCs in PGCG and CGCG possess inherent property of engulfment which is responsible for cannibalism of stromal tumor cells.
The CC is also responsible for resistance of tumors to specific immune reactions. However, new data also suggests that metastatic tumor cells may use this peculiar function to feed in conditions of low nutrient supply. This makes malignant cancer cells similar to microorganisms, rather than to normal cells undergoing malignant transformation.[1] Tumor microenvironment also plays an important role in the formation of CCs. These cells are particularly resistant to low pH and are formed in carcinogenesis in order to sustain or progress in unfavorable conditions such as low nutrient supply, hypoxia or starvation or as a tumor immune escape mechanism. A molecular framework of factors which contribute to the formation of CCs include the presence of an acidic environment that allows continuous activation of specific lytic enzymes such as cathepsin B, caveolin formation and the actin linker molecule ezrin. Each of these molecular factors involved in tumor cannibalism may be new possible targets in future antitumor therapies.[1,13]
Sarode et al., noted bizarre morphological appearances of cannibalism in OSCC, where they found one malignant cell was engulfing the other and this complex was further engulfed by another cell. In another area they noted one cell engulfing two cells at a time. As these features were distinctly different from those described in conventional cannibalism, they termed this phenomenon as “complex cannibalism”.[13]
To conclude, cannibalism by tumor cells can contribute either to their survival or proliferation. It is a phenomenon that resembles autophagic digestion of cellular organelles under starvation, where tumor cells can phagocytose other tumor cells. Moreover, when tumor cells engulf surrounding inflammatory cells; they further contribute to immune escape mechanisms. Cellular cannibalism has easily identifiable morphological features under light microscopy without the use of any advanced and expensive molecular techniques. Hence, aggressiveness of the neoplasm can be assessed on a routine basis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Fais S. Cannibalism: Away to feed on metastatic tumors. Cancer Lett. 2007;258:155–64. doi: 10.1016/j.canlet.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Bauchwitz MA. The bird’s eye cell: Cannibalism or abnormal division of tumor cells. Acta Cytol. 1981;25:92. [Google Scholar]
- 3.Sharma N, Dey P. Cell cannibalism and cancer. Diagn Cytopathol. 2011;39:229–33. doi: 10.1002/dc.21402. [DOI] [PubMed] [Google Scholar]
- 4.Malorni W, Matarrese P, Tinari A, Farrace MG, Piacentini M. Xeno-cannibalism A survival “escamotage”. Autophagy. 2007;3:75–7. doi: 10.4161/auto.3439. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer M, de Ley L, Feltcamp CA, Elema J, Jongsma AP. Serum dependent cannibalism and auto destruction in cultures of human small cell carcinoma of lung. Cancer Res. 1984;44:2947–51. [PubMed] [Google Scholar]
- 6.Krajcovic M, Overholtzer M. Mechanisms of ploidy increase in human cancers: A new role for cell cannibalism. Cancer Res. 2012;72:1596–601. doi: 10.1158/0008-5472.CAN-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- 8.Jose D, Mane DR, Datar U, Muttagi S, Hallikerimath S, Kale AD. Evaluation of cannibalistic cells: A novel entity in prediction of aggressive nature of oral squamous cell carcinoma. Acta Odontol Scand. 2014;72:418–23. doi: 10.3109/00016357.2013.798872. [DOI] [PubMed] [Google Scholar]
- 9.Sarode SC, Sarode GS. Neutrophil-tumor cell cannibalism in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43:454–8. doi: 10.1111/jop.12157. [DOI] [PubMed] [Google Scholar]
- 10.Sarode SC, Sarode GS. Cellular cannibalism in central and peripheral giant cell granuloma of the oral cavity can predict biological behavior of the lesion. J Oral Pathol Med. 2014;43:459–63. doi: 10.1111/jop.12119. [DOI] [PubMed] [Google Scholar]
- 11.Alfarouk KO, Muddathir AK, Shayoub ME. Tumor acidity as evolutionary spite. Cancers (Basel) 2011;3:408–14. doi: 10.3390/cancers3010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: A review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:603–15. doi: 10.1016/j.tripleo.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Sarode GS, Karmarkar S, Sarode SC. Complex cannibalism: An unusual finding in oral squamous cell carcinoma. Oral Oncol. 2012;48:e4–6. doi: 10.1016/j.oraloncology.2011.08.013. [DOI] [PubMed] [Google Scholar]


