Abstract
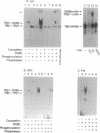
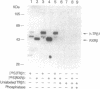
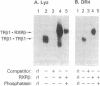
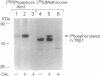
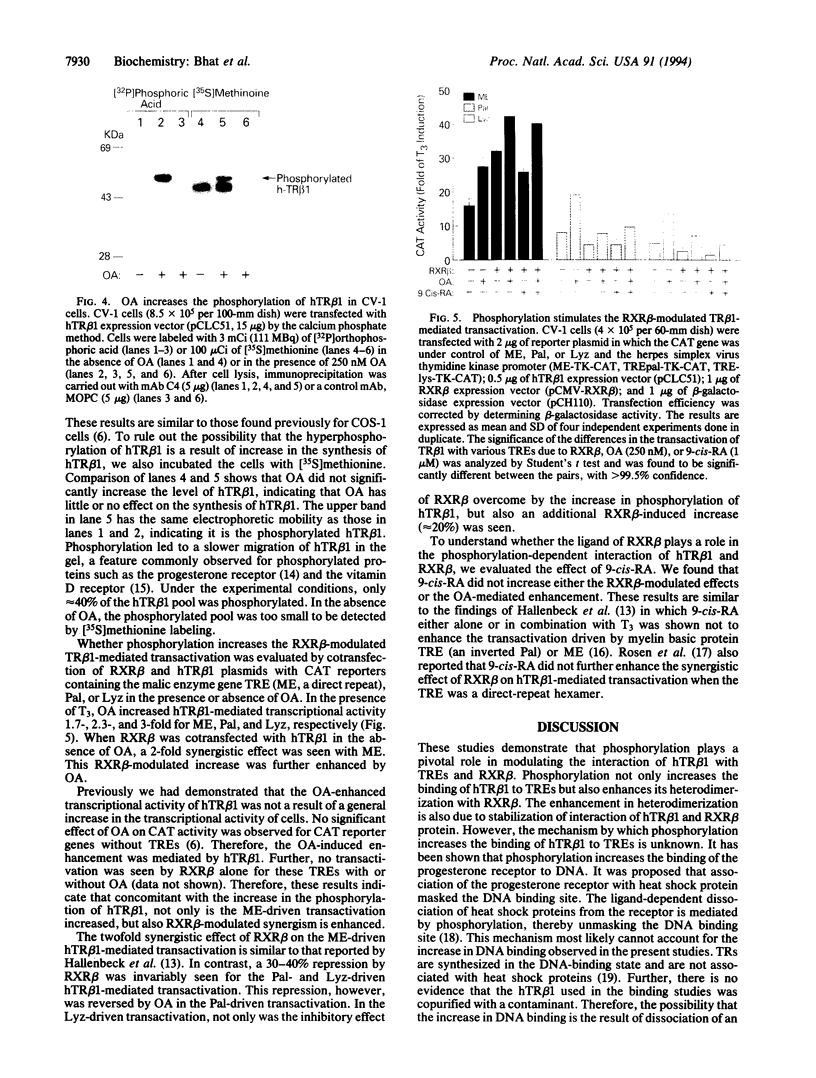
To understand the molecular basis of the phosphorylation-enhanced transcriptional activity of human thyroid hormone nuclear receptor subtype beta 1 (hTR beta 1), we studied the effect of phosphorylation on the interaction of hTR beta 1 with the retinoid X receptor beta (RXR beta), we studied the effect of phosphorylation on the interaction of hTR beta 1 with the retinoid X receptor beta (RXR beta). In vitro, the extent of hTR beta 1.RXR beta heterodimer bound to various thyroid hormone response elements (TREs) was compared before and after phosphorylation of hTR beta 1. Without phosphorylation, hTR beta 1.RXR beta heterodimer was barely detectable under the experimental conditions. After phosphorylation of hTR beta 1, heterodimer bound to (i) the chicken lysozyme gene TRE, (ii) a TRE consisting of direct repeats of half-site binding motifs separated by four gaps, and (iii) a palindromic TRE was enhanced by approximately 10-, 7-, and 6-fold, respectively. The effect of phosphorylation on hTR beta 1.RXR beta heterodimerization was reversible. Dephosphorylation of the phosphorylated hTR beta 1 by alkaline phosphatase led to loss of the ability of hTR beta 1 to form a heterodimer with RXR beta in either the absence or the presence of DNA. These results indicate that the heterodimerization is enhanced by phosphorylation. To evaluate the effect of phosphorylation on the interaction of hTR beta 1 with RXR beta in vivo, we cotransfected hTR beta 1, RXR beta and TRE-chloramphenicol acetyltransferase (CAT) expression plasmids into CV-1 cells. CAT activity was assessed in the presence or absence of okadaic acid. Okadaic acid is a potent inhibitor of phosphatases 1 and 2A and increases the in vivo phosphorylation of hTR beta 1 by approximately 10-fold. Using the CAT reporter gene under control of the TRE from the malic enzyme gene, we found that RXR beta increased the okadaic acid-enhanced hTR beta 1-mediated CAT activity by 2- to 3-fold in the presence of 3,3',5-triiodo-L-thyronine. However, 9-cis-retinoic acid did not enhance the effect of okadaic acid. Our results indicate that phosphorylation is essential for the interaction of hTR beta 1 with RXR beta. Thus, phosphorylation plays a pivotal role in the gene-regulating activity of hTR beta 1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashizawa K., Cheng S. Y. Regulation of thyroid hormone receptor-mediated transcription by a cytosol protein. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9277–9281. doi: 10.1073/pnas.89.19.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Ligand and DNA-dependent phosphorylation of human progesterone receptor in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2664–2668. doi: 10.1073/pnas.89.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogazzi F., Hudson L. D., Nikodem V. M. A novel heterodimerization partner for thyroid hormone receptor. Peroxisome proliferator-activated receptor. J Biol Chem. 1994 Apr 22;269(16):11683–11686. [PubMed] [Google Scholar]
- Dalman F. C., Koenig R. J., Perdew G. H., Massa E., Pratt W. B. In contrast to the glucocorticoid receptor, the thyroid hormone receptor is translated in the DNA binding state and is not associated with hsp90. J Biol Chem. 1990 Mar 5;265(7):3615–3618. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Schrader W. T., O'Malley B. W. Hormone-dependent regulation of chicken progesterone receptor deoxyribonucleic acid binding and phosphorylation. Endocrinology. 1989 Dec;125(6):3051–3058. doi: 10.1210/endo-125-6-3051. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. L., Marks M. S., Lippoldt R. E., Ozato K., Nikodem V. M. Heterodimerization of thyroid hormone (TH) receptor with H-2RIIBP (RXR beta) enhances DNA binding and TH-dependent transcriptional activation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5572–5576. doi: 10.1073/pnas.89.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Phyillaier M., Nikodem V. M. Divergent effects of 9-cis-retinoic acid receptor on positive and negative thyroid hormone receptor-dependent gene expression. J Biol Chem. 1993 Feb 25;268(6):3825–3828. [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Jurutka P. W., Hsieh J. C., MacDonald P. N., Terpening C. M., Haussler C. A., Haussler M. R., Whitfield G. K. Phosphorylation of serine 208 in the human vitamin D receptor. The predominant amino acid phosphorylated by casein kinase II, in vitro, and identification as a significant phosphorylation site in intact cells. J Biol Chem. 1993 Mar 25;268(9):6791–6799. [PubMed] [Google Scholar]
- Kurokawa R., Yu V. C., När A., Kyakumoto S., Han Z., Silverman S., Rosenfeld M. G., Glass C. K. Differential orientations of the DNA-binding domain and carboxy-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993 Jul;7(7B):1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Lin K. H., Ashizawa K., Cheng S. Y. Phosphorylation stimulates the transcriptional activity of the human beta 1 thyroid hormone nuclear receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7737–7741. doi: 10.1073/pnas.89.16.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. H., Willingham M. C., Liang C. M., Cheng S. Y. Intracellular distribution of the endogenous and transfected beta form of thyroid hormone nuclear receptor visualized by the use of domain-specific monoclonal antibodies. Endocrinology. 1991 May;128(5):2601–2609. doi: 10.1210/endo-128-5-2601. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Levi B. Z., Segars J. H., Driggers P. H., Hirschfeld S., Nagata T., Appella E., Ozato K. H-2RIIBP expressed from a baculovirus vector binds to multiple hormone response elements. Mol Endocrinol. 1992 Feb;6(2):219–230. doi: 10.1210/mend.6.2.1569965. [DOI] [PubMed] [Google Scholar]
- Meier C. A., Parkison C., Chen A., Ashizawa K., Meier-Heusler S. C., Muchmore P., Cheng S. Y., Weintraub B. D. Interaction of human beta 1 thyroid hormone receptor and its mutants with DNA and retinoid X receptor beta. T3 response element-dependent dominant negative potency. J Clin Invest. 1993 Oct;92(4):1986–1993. doi: 10.1172/JCI116793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Park J. B., Ashizawa K., Parkison C., Cheng S. Y. One-step immunoaffinity purification of human beta 1 thyroid hormone receptor with DNA and hormone binding activity. J Biochem Biophys Methods. 1993 Sep;27(2):95–103. doi: 10.1016/0165-022x(93)90053-q. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., O'Donnell A. L., Koenig R. J. Ligand-dependent synergy of thyroid hormone and retinoid X receptors. J Biol Chem. 1992 Nov 5;267(31):22010–22013. [PubMed] [Google Scholar]
- Sugawara A., Yen P. M., Apriletti J. W., Ribeiro R. C., Sacks D. B., Baxter J. D., Chin W. W. Phosphorylation selectively increases triiodothyronine receptor homodimer binding to DNA. J Biol Chem. 1994 Jan 7;269(1):433–437. [PubMed] [Google Scholar]
- Wilson T. E., Paulsen R. E., Padgett K. A., Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992 Apr 3;256(5053):107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]