Abstract
Background
The purpose of the present study was to evaluate the sensitivity, specificity, and accuracy of thermography in identifying patients with temporomandibular dysfunction (TMD).
Material/Methods
The study sample consisted of 50 patients (27 women and 23 men) ages 19.2 to 24.5 years (mean age 22.43±1.04) with subjective symptoms of TMD (Ai II–III) and 50 patients (25 women and 25 men) ages 19.3 to 25.1 years (mean age 22.21±1.18) with no subjective symptoms of TMD (Ai I).
The anamnestic interviews were conducted according to the three-point anamnestic index of temporomandibular dysfunction (Ai). The thermography was performed using a ThermaCAM TMSC500 (FLIR Systems AB, Sweden) independent thermal vision system. Thermography was closely combined with a 10-min chewing test.
Results
The results of our study indicated that the absolute difference in temperature between the right and left side (ΔT) has the highest diagnostic value. The diagnostic effectiveness of this parameter increased after the chewing test. The cut-off points for values of temperature differences between the right and left side and identifying 95.5% of subjects with no functional disorders according to the temporomandibular dysfunction index Di (specificity 95.5%) were 0.26°C (AUC=0.7422, sensitivity 44.3%, accuracy 52.4%) before the chewing test and 0.52°C (AUC=0.7920, sensitivity 46.4%, accuracy 56.3%) after it.
Conclusions
The evaluation of thermography demonstrated its diagnostic usefulness in identifying patients with TMD with limited effectiveness. The chewing test helped in increasing the diagnostic efficiency of thermography in identifying patients with TMD.
Keywords: Temperature, Temporomandibular Joint Disorders, Thermography
Background
Changes in body temperature have long been regarded as an important diagnostic factor. Recently, rapid development in infrared radiation technology and its conversion to a visible image have given rise to a new technique called thermography [1]. As a consequence, this method has made it possible to produce images reflecting the physiological processes of living organisms by observing the temperature distribution on the outer surface of an examined system in a dynamic way and without the need for any contact [1,2]. The use of the phrase “observation of temperature distribution on the outer surface” not only limits the area of investigation to the properties of this surface, but it also has deeper implications, especially if the observed system is a living organism [3]. Thermal heterogeneity (e.g., on the surface of facial skin) largely depends on the blood flow and the type of tissue located directly beneath it. Thus, the surface of the skin above the muscle tissue, which is characterized by high metabolic activity, emits more thermal radiation than the skin covering the bone or connective tissue. Therefore, thermography visualizes the thermal properties of tissue similar to how radiology illustrates anatomy [1–4]. The advanced analysis used in dynamic thermography thus makes it possible to detect vasomotor changes within layers of the skin, which may help explain vegetative pain syndrome [2,4,5].
The undeniable advantages of thermography, such as its non-invasive evaluation approach, the absence of ionizing radiation, and the relatively low costs involved, are sufficient to recommend its inclusion among the additional tests widely employed to diagnose temporomandibular dysfunction (TMD) [6]. The validity of using thermography for dental diagnostic purposes, especially in the diagnosis of temporomandibular dysfunctions of the masticatory motor system, is still under review [1,7–11]. Some previous studies confirmed the diagnostic efficiency of thermography in identifying subjects with TMD [12–14]. Infrared thermography is a tool that can be applied to individuals with myogenous TMD due to the changes in the microcirculatory dynamics (i.e., there is a decrease in the skin temperature due to compression of blood vessels conditioned by muscle hyperactivity) [12,15,16], whereas skin temperature over the TMJ is increased in individuals with joint pain [17]. Moreover, individuals with TMD exhibit greater asymmetry in skin temperature than do those without this condition [2,13,18].
This method has been also used for multiple purposes within medicine and in the field of dentistry [19,20]. Several studies have employed infrared thermography as an assessment tool for individuals with neck pain, breast cancer, and diabetic neuropathy [21–23]. Moreover, many studies have been performed on the use of thermography in the diagnosis of nerve injuries after surgery or trauma, in diagnosis of inflammation after the surgical removal of mandibular third molars, and in clinical research [19].
The purpose of the present study was to evaluate the sensitivity, specificity, and accuracy of thermography as a means of identifying patients with TMD. We hypothesized that there is no diagnostic efficiency of thermography in identifying patients with TMD.
Material and Methods
The research was approved by the Ethics Committee of the Pomeranian Medical University in Szczecin, Poland (number BN-001/45/07). All the patients were informed about the aim and research design and they gave their informed consent to all of the procedures performed.
The study sample comprised 50 patients (27 women and 23 men) ages 19.2 to 24.5 years (mean 22.43±1.04) with subjective symptoms of TMD (Ai II-III) and 50 patients (25 women and 25 men) ages 19.3 to 25.1 years (mean 22.21±SD 1.18) with no subjective symptoms of TMD (Ai I). Participants were selected from patients referred to the Orthodontic Department of the Pomeranian Medical University in Szczecin, Poland. The exclusion criteria for the study population were: depressive disorders, pain in other parts of the body, inflammations, taking painkillers and anti-depressants, periodontal diseases, and completed treatment of masticatory motor system dysfunctions. Patients who had already finished their orthodontic treatment and those who were undergoing treatment at the time of the study were also excluded.
The anamnestic interviews included the patients’ general medical history as well as detailed information about their masticatory motor system. They were conducted according to a 3-point anamnestic index of temporomandibular dysfunction – Ai (Table 1) [24,25].
Table 1.
Anamnestic index of temporomandibular dysfunction (Ai).
| Ai | Symptoms |
|---|---|
| I | No subjective symptoms of temporomandibular dysfunction – no symptoms reported by patient |
| II | Mild symptoms of temporomandibular dysfunction – temporomandibular joint noise, feeling of “jaw fatigue” (fatigue of masticatory muscles), feeling of “jaw rigidity” (increased tone of masticatory muscles) |
| III | Severe symptoms of temporomandibular dysfunction – restricted mouth opening, painful lower jaw movements, temporomandibular joint pain, masticatory muscle pain, temporomandibular joint luxation, lockjaw |
Masticatory motor system function was assessed on the basis of a clinical examination and thermography. The clinical examination, involving a visual and an auscultatory assessment as well as palpation methods, made it possible to qualitatively and quantitatively evaluate the functioning of the masticatory system. Data obtained from the clinical study was analyzed using the clinical temporomandibular dysfunction index (Di) (Table 2). The results of the clinical temporomandibular dysfunction index (Di), based on the total number of points obtained during the tests, were interpreted according to the model shown in Table 3 [24,25].
Table 2.
Clinical index of temporomandibular dysfunction (Di).
| Di | Symptoms |
|---|---|
| Mandibular movements | |
| 0 | Normal range |
| 1 | Small reduction in amplitude |
| 5 | Large reduction in amplitude |
| Temporomandibular joint function | |
| 0 | Smooth, noiseless abduction and adduction of mandible, trajectory asymmetry <2 mm |
| 1 | Noise in one or both joints during abduction and adduction of mandible, trajectory asymmetry >2 mm |
| 5 | Abduction of mandible impossible and/or luxation |
| Masticatory muscle pain | |
| 0 | No tenderness |
| 1 | Tenderness of 1–3 sites |
| 5 | Tenderness of 4 and more sites |
| Temporomandibular joint pain | |
| 0 | No tenderness |
| 1 | Unilateral or bilateral tenderness |
| 5 | Unilateral or bilateral tenderness of the dorsal surface of joint |
| Pain during movement of mandible | |
| 0 | No pain |
| 1 | Pain during one out of all possible movement directions |
| 5 | Pain during more than one out of all possible movement directions |
Table 3.
Interpretation of the clinical index of temporomandibular dysfunction (Di).
| Range | Severity of dysfunction | Description |
|---|---|---|
| 0 | Di 0 | No dysfunction |
| 1–4 | Di I | Mild dysfunction |
| 5–9 | Di II | Moderate dysfunction |
| 10–25 | Di III | Severe dysfunction |
Thermography was performed in tandem with a chewing test, during which the subject was asked to chew 8 g of Hubba Bubba gum (Wrigley, France) for 10 min. The chewing test helped to increase the diagnostic efficiency of thermography in identifying patients with TMD.
The thermographic examination was performed in accordance with the recommendations of the European Society of Thermology, by the same operator [14,26–28]. The thermographic procedures were conducted in a closed room at a controlled temperature of 22–24°C and with relative humidity of 50–70%. Use of heat-emitting equipment was kept to a minimum.
The participants in the study were told not to eat for at least 2 h prior to the test. Before the procedures, the patients were asked, if necessary, to pin up their hair to expose the area of the ear and temple. The facial skin was cleaned using a moistened tissue and cooled by a cold air-flow dryer for 15 s. A 20-min resting period was allowed for facial temperature equilibration. During the adaptation process, the patients did not undertake any physical activity, chew, or touch their facial skin.
Before the study, the participants underwent a dermatological examination to identify any possible skin inflammation (dermatitis) of various etiologies, such as inflammation of the hair follicles (folliculitis), allergic reactions, contact eczema, or enlarged veins.
The thermography was performed with a ThermaCAM TMSC500 (FLIR Systems AB, Sweden) independent thermal vision system equipped with a matrix composed of uncooled FPA (focal plane array) microvoltometer detectors. We used a built-in, 7.5-μm high-pass filter, which made it possible to measure infrared radiation in wavelengths ranging between 7.5 and 13 μm. The ThermaCAM TMSC500 thermovision unit had a thermal resolution of 0.07°C and an accuracy of 2%.
Both facial and neck thermograms were taken using right and left lateral projections in constant conditions with the patient sitting on the examination chair, with a distance of 1 m and a constant angle of 90° between the camera and the patient. To prevent reflections, a thick black material was placed directly behind the patient.
The recordings were performed prior to the chewing test (T0) and after the effort test (T10). To obtain the highest image quality, automatic calibration tools were used during the examinations, making it possible to optimize the level and range of the temperatures shown as well as the color palette and the highest contrast in all areas of the image. The emissability value of the skin considered for this study was 0.98.
Thermographic images were recorded using ThermaCAM Researcher 2001 and ThermaCAM Reporter 2000 Professional (FLIR Systems AB, Sweden) Software.
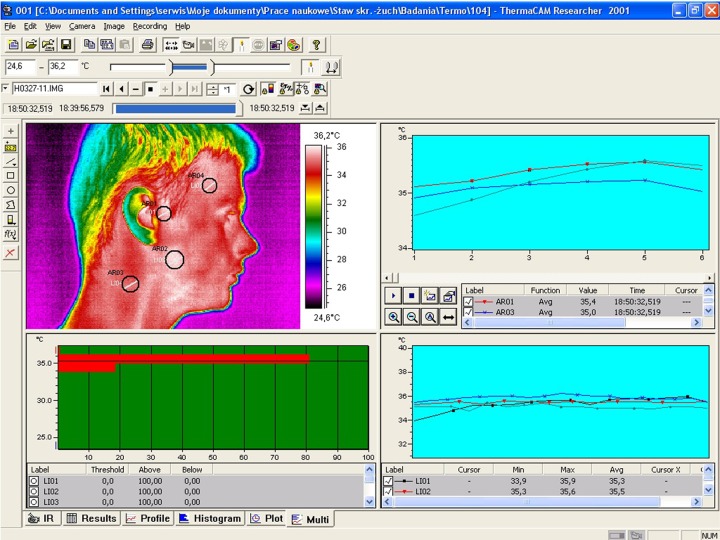
A metric analysis of the thermograms was carried out in selected regions of the face and neck marked by tools in a circle-shaped area with a 1-cm diameter (Figure 1):
Figure 1.
Thermogram analysis window of ThermaCAM Researcher software showing areas of measurement.
– in the projection of the lateral part of the temporomandibular joint,
– in the projection of the anterior part of temporal muscle,
– in the projection of the belly of the superficial part of the masseter muscle,
– in the projection of the sternocleidomastoid muscle.
Due to the possibility of shifts in individual thermograms of the examined subjects, each image was analyzed individually and corrections were made if necessary.
A quantitative assessment of the thermograms was performed using a “rain” scale. The analysis of the selected areas of the face and neck included absolute measurements specifying the maximum temperature values (Tmax). According to the literature [4,26], the difference between right and left zone temperatures (ΔT) was selected as the variable to identify patients with TMD. In subjects with bilateral symptoms, this variable also shows its diagnostic usefulness because the severity of symptoms on both sides is never identical.
The Kruskal-Wallis test, the median, and the Mann-Whitney U test were used to verify hypotheses regarding the presence or absence of differences between the mean values of the independent variables. A chi-square test was performed to identify relationships between discrete or qualitative variables. The accuracy of the classifier (a single variable or the whole model) together with a description of its sensitivity and specificity were calculated using receiver operating characteristic (ROC) curve analysis. An analysis of the ROC curve for variables such as maximum temperature values (Tmax) and absolute difference in temperature between the right and left side zones (ΔT) was based on mean measurements in 8 areas on the right and left side of the face and neck. This method made it possible to determine the optimal cut-off points for the specific misclassification costs and the a priori probabilities for the occurrence of the studied phenomenon. A level of P=0.05 was considered to be statistically significant.
Results
The results of the clinical examination of the functioning of the masticatory motor system according to the Di algorithm among patients with symptoms of temporomandibular dysfunction are presented in Table 4. This data analysis showed the severity of TMD in the study group. Mild temporomandibular dysfunction (Di I) was the most frequent form of dysfunction in women (24%) and men (22%). Moderate dysfunction (Di II) had by a similar frequency and occurred in 20% of women and 16% of men. However, severe dysfunction was observed far more rarely in the examined group (P<0.0426) in both women (10%) and men (8%).
Table 4.
Temporomandibular dysfunction index Di in the study group with symptoms of TMD.
| Gender | Di group | |||||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| n | (%) | n | (%) | n | (%) | |
| Females | 12 | 24 | 10 | 20 | 5 | 10 |
| Males | 11 | 22 | 8 | 16 | 4 | 8 |
| Total | 23 | 46 | 18 | 36 | 9 | 18 |
A mathematical analysis of the ROC curve was used to assess the diagnostic efficiency of thermography in identifying patients with TMD regardless of the severity of TMD. The analysis of the ROC curve showed that the highest diagnostic efficiency in thermography was achieved for estimators of distribution of variables such as the absolute difference in temperature between the right and left side zones (ΔT). The diagnostic efficiency in the case of thermography for this variable both before the test (area under the ROC curve [AUC]=0.7422, standard error of mean – SEM=0.0464, P<0.0378, Table 5), and after the test (AUC=0.7920, SEM=0.0466, P<0.0231) was significantly higher than in the case of the maximum temperature (Tmax); before the test AUC=0.5134, SEM=0.0731 and after the test AUC=0.6461, SEM=0.0522, (Table 5).
Table 5.
Data for some cut-off points in thermography as discriminators for patients without symptoms of temporomandibular dysfunction (Di 0) or with symptoms (Di I, II or III).
| Time/variable | Parameter | Sensitivity=Specificity | Specificity=95% | AUC (SEM) | |
|---|---|---|---|---|---|
| Before the chewing test | Maximal temperature* [Tmax] | Cut-off point | 35.11°C | 36.01°C | 0.5134# (0.0731)# |
| Sensitivity | 51.1% | 6.3% | |||
| Specificity | 51.8% | 95.1% | |||
| Accuracy | 50.8% | 20.3% | |||
| Absolute difference in temperature between right and left side* [ΔT] | Cut-off point | 0.21°C | 0.26°C | 0.7422# (0.0464)#,## | |
| Sensitivity | 68.4% | 44.3% | |||
| Specificity | 68.1% | 95.1% | |||
| Accuracy | 67.9% | 52.4% | |||
| After the chewing test | Maximal temperature* [Tmax] | Cut-off point | 35.82°C | 36.31°C | 0.6461#,## (0.0522)## |
| Sensitivity | 55.2% | 11.3% | |||
| Specificity | 55.9% | 95.4% | |||
| Accuracy | 54.9% | 32.7% | |||
| Absolute difference in temperature between right and left side* [ΔT] | Cut-off point | 0.31°C | 0.52°C | 0.7920## (0.0466)## | |
| Sensitivity | 70.3% | 46.4% | |||
| Specificity | 69.9% | 95.5% | |||
| Accuracy | 68.8% | 56.3% | |||
Based on measurements in 8 areas on the right and left side of face and neck.
P<0.0352;
P<0.0416.
AUC – area under ROC curve; SEM – standard error of mean.
The chewing test was a major factor that increased the efficiency of thermography in identifying patients with TMD. The results of examinations performed after the chewing test for the maximum temperature variable (Tmax, AUC=0.6461, P<0.0352, Table 5) and the absolute difference in temperature between the right and left side (ΔT, AUC=0.7920, P<0.0416, Table 5) showed a significant increase in diagnostic efficiency in patients with TMD compared with examinations made before the chewing test (Tmax, AUC=0.5134 and ΔT, AUC=0.7422, Table 5).
The thermographic examination showed 95.5% specificity in identifying patients with no symptoms of dysfunctions according to Di when the absolute difference in temperature between the right and left side (ΔT) zones was lower than 0.26°C (accuracy 52.4%) before the chewing test and 0.52°C (accuracy 56.3%) after the test (Table 5).
Discussion
This study was performed to evaluate the sensitivity, specificity, and accuracy of thermography as a means of identifying patients with TMD. The results of our study confirmed its limited diagnostic efficiency in identifying subjects with TMD – 95.5% specificity in identifying patients with no symptoms of dysfunctions according to Di when the absolute difference in temperature between the right and left side (ΔT) zones was lower than 0.26°C with sensitivity 44.3% and accuracy 52.4% before chewing test and 0.52°C with sensitivity 46.4% and accuracy 56.3% after test. The chewing test was a major factor that increased the efficiency of thermography in identifying patients with TMD. Further research is needed on the use of thermography in the diagnosis of TMD. Gratt et al. [12] showed 88% specificity in identifying healthy subjects, with 80% sensitivity in determining true-positive results, with an accuracy range within the range of 84%. Canavan and Gratt [14] reported 89% accuracy, 85% sensitivity, and 92% specificity at a cut-off point of 0.25°C using thermography of the TMJ region in the differentiation of asymptomatic individuals and those with mild-to-moderate TMD. McBeth and Gratt [13] reported 87% sensitivity at the cut-off point of ΔT 0.4°C and 86% specificity at the cut-off point of ΔT 0.1°C in a study involving control subjects, subjects in orthodontic treatment, and subjects with TMD. In contrast to these studies, a recent study reports low sensitivity (38.5–76.9%) and specificity (22.8–71.2%) with the use of infrared thermography on the masticatory muscles [29].
Thermography has many advantages – it is easy to use and non-invasive. However, there is a lack of standardized protocol for the temperature measurement of the masticatory muscles using infrared thermography. Gratt et al. [12] and Gratt and Sickles [18] evaluated the orofacial region by establishing 5 measurement areas: small TMJ, large TMJ, mandible, midface, and entire half-face. In another study by Dibai Filho et al. [29], the measurement of skin surface temperature was done in the muscle central point.
The literature extensively describes the use of thermography for the evaluation of individuals with TMD [4,16,27]. The symmetry of temperature distribution in the face and neck has been analyzed as a local prognostic factor in many studies. Based on 4000 measurements of thermograms of 100 healthy adults, Weinstein [26] described the cut-off point for identifying pathologies associated with pain in the head as the absolute value of the temperature differences between corresponding homonymous structures in excess of 0.5°C. This value was determined using spot measurements.
In a study conducted on a group of 30 subjects without functional dysfunctions, Gratt and Sickles [18] drew attention to the fact that absolute temperature differences in both temporomandibular joints did not exceed 0.2°C. In further studies carried out on a group of 102 healthy volunteers (48 women and 54 men), Gratt et al. [27] indicated that a high level of temperature compliance within univariate structures is a very important and common feature in all the analyzed thermal images. Lateral and frontal thermograms were performed using an Agema 870 thermal imaging camera, and the only condition that disqualified a patient from the study was the occurrence of facial pain. The results showed that the variation in temperature within the 25 measured areas in the right and left zones did not exceed 0.4°C. The authors proposed lowering the acceptable temperature symmetry of corresponding facial and neck structures from 0.5°C to 0.4°C. The consistent extension of the study group up to 50 patients with intra-joint disorders such as disc displacement or degenerative changes of the articular joint made it possible to draw conclusions and create a diagnostic algorithm [12]. The study showed 88% specificity in identifying healthy subjects, with 80% sensitivity in determining true-positive results with an accuracy range within the range of 84%. In addition, the discriminatory efficiency of thermography made it possible to correctly identify 3 groups of patients: those with osteoarthritis (30%), those with dislocation of the articular discs (47%), and healthy subjects (90%). The average temperature difference in small areas over temporomandibular joints provided greater discriminatory ability. The average absolute temperature differences between the right and left side of the area measured was 0.1°C for the healthy subjects and 0.4°C for the subjects with pathology. A second factor differentiating these 2 groups was the average temperature in the middle of the face. In the study group it was significantly lower (34.2°C) than in healthy subjects (34.9°C). The third differentiator was the point value of the temperature difference on the right and left sides, which was 0.15°C in the study group and 0.0°C in the healthy group. Based on the studies carried out, the authors determined the cut-off values for the individual areas, which are extremely useful in the clinical identification of patients and healthy subjects. Among the healthy subjects, the limit value for the average temperature differences within the temporomandibular joints was tagged at 0.2°C and the average temperature in half of the face was greater than or equal to 35.0°C in the healthy group. The point value of the average temperature of joints on the right and left sides had discriminatory value for patients with articular disc dislocation at temperatures of less than 35.9°C.
An assessment made by Canavan and Gratt [14] of the diagnostic efficiency of thermography in patients with mild-to-moderate functional disorders of the masticatory motor system also showed high values. The average absolute value of temperature differences at 1 cm2 above the lateral side of the temporomandibular joint among patients with dysfunctions was significantly higher (0.42°C±0.17°C) compared with the control group (0.13°C±0.13°C). The mathematical analysis showed 85% sensitivity in identifying sick patients and 92% specificity in identifying healthy subjects at a cut-off point of 0.25°C and an accuracy level of 89% (39 cases out of 44). In addition, absolute differences in temperatures in the joints had discriminatory value for patients experiencing moderate-to-medium pain. There were no significant differences in ΔT in subjects experiencing mild pain compared to the control group.
Another thermographic study conducted by McBeth and Gratt [13] on a group of 69 subjects confirmed that thermography was highly useful in identifying objective signs of functional disorders of the masticatory motor system. The diagnostic efficiency of thermography in identifying patients with acoustic symptoms of pain such as clicking was 87% at the cut-off point (ΔT 0.4°C), while its specificity in identifying those without functional disorders was 86% at the cut-off point (ΔT 0.1°C). The average absolute difference in temperature in the corresponding structures of both sides among those with functional disorders was 0.43°C (±0.19°C), whereas in patients with no such dysfunctions it was 0.13°C (±0.14°C). According to the authors of this study, the evidence presented was sufficient to recommend the use of the thermography in identifying the symptoms of functional disorders of the masticatory motor system.
A 6-year summary of thermographic research conducted by Gratt et al. [30] resulted in the systematization of the diagnostic algorithm applied to patients experiencing pain within the facial part of the skull. Based on 164 thermograms of patients with various diagnostic problems and 164 thermograms of healthy subjects, 4 important types of thermograms were listed: “Normal” thermograms with a temperature difference in contralateral areas from 0.0°C to 0.25°C ± characterized subjects with neuralgia or psychogenic facial pain syndrome; “Cold” thermograms, in which the temperature difference in the analyzed areas of the right and left side was lower than 0.35°C were characteristic of neuralgia with no vegetative symptoms; “Warm” thermograms in which the temperature difference in the univariate measuring areas exceeded 0.35°C were characteristic of neuralgia with vegetative symptoms, arthropathy of temporomandibular joints, and inflammation of the maxillary sinus; and the “Ambiguous” thermogram with a temperature difference of ±0.26 to 0.35°C. An analysis of the usefulness of a new classification system of thermograms showed 92% discriminatory efficiency, clearly confirming the usefulness of thermography in differential diagnosis of pain within the facial part of the skull.
Limitations
The present study has the following limitations: 1) relatively small sample size, non-allocation of individuals based on the menstrual cycle and period of the day (these factors can influence skin temperature [31]); and 2) the acclimation time of the volunteers was 20 min at 22–24°C, whereas other authors suggest a shorter time of 8–16 min [32]. The subtle evaluation of diagnostic efficiency of thermography in identifying subjects with TMD needs further research with larger sample sizes.
Conclusions
Despite the limitations of this study, the evaluation of thermography demonstrated its diagnostic usefulness in identifying patients with TMD with limited effectiveness.
The chewing test helped to increase the diagnostic efficiency of thermography in identifying patients with TMD.
Footnotes
Source of support: Self-financing
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Anbar M, Gratt BM, Hong D. Thermology and facial telethermography. Part I: History and technical review. Dentomaxillofac Radiol. 1998;27:61–67. doi: 10.1038/sj/dmfr/4600314. [DOI] [PubMed] [Google Scholar]
- 2.Gratt BM, Anbar M. Thermology and facial telethermography: Part II. Current and future clinical applications in dentistry. Dentomaxillofac Radiol. 1998;27:68–74. doi: 10.1038/sj/dmfr/4600324. [DOI] [PubMed] [Google Scholar]
- 3.Jędrusik-Pawłowska M, Niedzielska I, Bogucki R, Kajewski B. Effectiveness of hyperbaric oxygen therapy in mandibular osteoradionecrosis shown by thermography monitoring. Med Sci Monit. 2010;16(2):MT1–8. [PubMed] [Google Scholar]
- 4.Haddad DS, Brioschi ML, Arita ES. Thermographic and clinical correlation of myofascial trigger points in the masticatory muscles. Dentomaxillofac Radiol. 2012;41:621–29. doi: 10.1259/dmfr/98504520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holey LA, Dixon J, Selfe J. An exploratory thermographic investigation of the effects of connective tissue massage on autonomic function. J Manipulative Physiol Ther. 2011;34:457–62. doi: 10.1016/j.jmpt.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Wieckiewicz M, Zietek M, Nowakowska D, Wieckiewicz W. Comparison of Selected Kinematic Facebows Applied to Mandibular Tracing. Biomed Res Int. 2014;2014:818694. doi: 10.1155/2014/818694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biagioni PA, Longmore RB, McGimpsey JG, Lamey PJ. Infrared thermography. Its role in dental research with particular reference to craniomandibular disorders. Dentomaxillofac Radiol. 1996;25:119–24. doi: 10.1259/dmfr.25.3.9084259. [DOI] [PubMed] [Google Scholar]
- 8.Komoriyama M, Nomoto R, Tanaka R, et al. Application of thermography in dentistry-visualization of temperature distribution on oral tissues. Dent Mater J. 2003;22:436–43. doi: 10.4012/dmj.22.436. [DOI] [PubMed] [Google Scholar]
- 9.Biagioni PA, McGimpsey JG, Lamey PJ. Electronic infrared thermography as a dental research technique. Br Dent J. 1996;180:226–30. doi: 10.1038/sj.bdj.4809033. [DOI] [PubMed] [Google Scholar]
- 10.Kalili TK, Gratt BM. Electronic thermography for the assessment of acute temporomandibular joint pain. Compend Contin Educ Dent. 1996;17:979–93. [PubMed] [Google Scholar]
- 11.Fikackova H, Ekberg E. Can infrared thermography be a diagnostic tool for arthralgia of the temporomandibular joint? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:643–50. doi: 10.1016/j.tripleo.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 12.Gratt BM, Sickles EA, Ross JB, et al. Thermographic assessment of craniomandibular disorders: diagnostic interpretation versus temperature measurement analysis. J Orofac Pain. 1994;8:278–88. [PubMed] [Google Scholar]
- 13.McBeth SB, Gratt BM. Thermographic assessment of temporomandibular disorders symptomology during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1996;109:481–88. doi: 10.1016/s0889-5406(96)70132-4. [DOI] [PubMed] [Google Scholar]
- 14.Canavan D, Gratt BM. Electronic thermography for the assessment of mild and moderate temporomandibular joint dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:778–86. doi: 10.1016/s1079-2104(05)80316-6. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues-Bigaton D, Dibai-Filho AV, Packer AC, et al. Accuracy of two forms of infrared image analysis of the masticatory muscles in the diagnosis of myogenous temporomandibular disorder. J Bodyw Mov Ther. 2014;18:49–55. doi: 10.1016/j.jbmt.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Barão VA, Gallo AK, Zuim PR, et al. Effect of occlusal splint treatment on the temperature of different muscles in patients with TMD. J Prosthodont Res. 2011;55:19–23. doi: 10.1016/j.jpor.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues-Bigaton D, Dibai-Filho AV, Costa AC, et al. Accuracy and reliability of infrared thermography in the diagnosis or arthralgia in women with temporomandibular disorder. J Manipulative Physiol Ther. 2013;36:253–58. doi: 10.1016/j.jmpt.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Gratt BM, Sickles EA. Thermographic characterization of the asymptomatic temporomandibular joint. J Orofac Pain. 1993;7:7–14. [PubMed] [Google Scholar]
- 19.Christensen J, Matzen LH, Vaeth M, et al. Thermography as a quantitative imaging method for assessing postoperative inflammation. Dentomaxillofac Radiol. 2012;41:494–99. doi: 10.1259/dmfr/98447974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misiołek M, Namysłowski G, Czecior E. Thermography in the investigation of head and neck tumors. Med Sci Monit. 1999;5(6):1187–90. [Google Scholar]
- 21.Dibai Filho AV, Packer AC. Assessment of the upper trapezius muscle temperature in women with and without neck pain. J Manipulative Physiol Ther. 2012;35:413–17. doi: 10.1016/j.jmpt.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Wishart GC, Campisi M, Boswell M, et al. The accuracy of digital infrared imaging for breast cancer detection in women undergoing breast biopsy. Eur J Surg Oncol. 2010;36:535–40. doi: 10.1016/j.ejso.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bagavathiappan S, Philip J, Jayakumar T, et al. Correlation between plantar foot temperature and diabetic neuropathy: a case study by using an infrared thermal imaging technique. J Diabetes Sci Technol. 2010;4:1386–92. doi: 10.1177/193229681000400613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Näpänkangas R, Raunio A, Sipilä K, Raustia A. Effect of mandibular advancement device therapy on the signs and symptoms of temporomandibular disorders. J Oral Maxillofac Res. 2013;3(4):e5. doi: 10.5037/jomr.2012.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leite RA, Rodrigues JF, Sakima MT, Sakima T. Relationship between temporomandibular disorders and orthodontic treatment: a literature review. Dental Press J Orthod. 2013;18:150–57. doi: 10.1590/s2176-94512013000100027. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein SA, Weinstein G, Weinstein EL, Gelb M. Facial thermography, basis, protocol, and clinical value. Cranio. 1991;9:201–11. doi: 10.1080/08869634.1991.11678368. [DOI] [PubMed] [Google Scholar]
- 27.Gratt BM, Sickles EA. Electronic facial thermography: An analysis of asymptomatic adult subjects. J Orofacial Pain. 1995;9:255–65. [PubMed] [Google Scholar]
- 28.Ring E, Ammer K, Jung A, et al. Standardization of infrared imaging. Eng Med Biol Soc. 2004;2:1183–85. doi: 10.1109/IEMBS.2004.1403378. [DOI] [PubMed] [Google Scholar]
- 29.Dibai Filho AV, Packer AC, Costa AC, Rodrigues-Bigaton D. Accuracy of infrared thermography of the masticatory muscles for the diagnosis of myogenous temporomandibular disorder. J Manipulative Physiol Ther. 2013;36:245–52. doi: 10.1016/j.jmpt.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Gratt BM, Graff-Radford SB, Shetty V, et al. A 6-year clinical assessment of electronic facial thermography. Dentomaxillofac Radiol. 1996;25:247–55. doi: 10.1259/dmfr.25.5.9161178. [DOI] [PubMed] [Google Scholar]
- 31.Kurz A. Physiology of thermoregulation. Best Pract Res Clin Asaesthesiol. 2008;22:627–44. doi: 10.1016/j.bpa.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Roy RA, Boucher JP, Comtois AS. Digitized infrared segmental thermometry: time requirements for stable recording. J Manipulative Physiol Ther. 2006;29:468.e1–468.e10. doi: 10.1016/j.jmpt.2006.06.007. [DOI] [PubMed] [Google Scholar]