Abstract
OBJECTIVE:
This study aimed to determine whether inhaled prostaglandins are associated with improvement in pulmonary physiology or mortality in patients with ARDS and assess adverse effects.
METHODS:
The following data sources were used: PubMed, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, reference lists, conference proceedings, and ClinicalTrials.gov. Studies selected included randomized controlled trials and nonrandomized studies. For data extraction, two reviewers independently screened titles and abstracts for eligibility. With regard to data synthesis, 25 studies (two RCTs) published over 21 years (1993-2014) were included. The PROSPERO registration number was CRD42014013180.
RESULTS:
One randomized controlled trial showed no difference in the change in mean Pao2 to Fio2 ratio when comparing inhaled alprostadil to placebo: 141.2 (95% CI, 120.8-161.5) to 161.5 (95% CI, 134.6-188.3) vs 163.4 (95% CI, 140.8-186.0) to 186.8 (95% CI, 162.9-210.7), P = .21. Meta-analysis of the remaining studies demonstrated that inhaled prostaglandins were associated with improvement in Pao2 to Fio2 ratio (16 studies; 39.0% higher; 95% CI, 26.7%-51.3%), and Pao2 (eight studies; 21.4% higher; 95% CI, 12.2%-30.6%), and a decrease in pulmonary artery pressure (−4.8 mm Hg; 95% CI, −6.8 mm Hg to −2.8 mm Hg). Risk of bias and heterogeneity were high. Meta-regression found no association with publication year (P = .862), baseline oxygenation (P = .106), and ARDS etiology (P = .816) with the treatment effect. Hypotension occurred in 17.4% of patients in observational studies.
CONCLUSIONS:
In ARDS, inhaled prostaglandins improve oxygenation and decrease pulmonary artery pressures and may be associated with harm. Data are limited both in terms of methodologic quality and demonstration of clinical benefit. The use of inhaled prostaglandins in ARDS needs further study.
In terms of mortality and survivor morbidity, ARDS exacts a significant toll on patients and the health-care system.1 Shunt physiology drives hypoxemia; pulmonary hypertension is common and may have adverse prognostic significance.2‐5 The use of inhaled pulmonary vasodilators, which could improve oxygenation by preferentially improving perfusion to well-ventilated lung regions and reduce pulmonary pressures, therefore, has physiologic rationale. Inhaled nitric oxide (iNO) continues to be used for a significant minority of patients with ARDS.6,7 While shown to improve oxygenation, meta-analyses of randomized trials demonstrate no mortality benefit with iNO, and an association with harm.8,9 It is unknown whether other inhaled pulmonary vasodilators are associated with similar physiologic or clinical outcomes.
The inhaled prostaglandins epoprostenol (prostaglandin I2 [PGI2]; Flolan) and alprostadil (prostaglandin E1 [PGE1]) promote pulmonary vasodilation via a cyclic adenosine monophosphate-mediated decrease in intracellular calcium.10 They also have antiinflammatory and antiplatelet aggregation properties, providing further potential mechanistic benefit in ARDS.10‐15 One observational study demonstrated the use of inhaled epoprostenol in 22% of patients with severe ARDS treated with extracorporeal support.16 A systematic review that included only one randomized controlled trial (RCT) of 14 pediatric patients concluded that enough evidence did not exist to support or refute the use of inhaled epoprostenol in ARDS.17 However, other clinical studies have been completed since this review was published. As such, it is unknown whether the use of inhaled prostaglandins in ARDS provides any benefit.
Therefore, the objectives of this study were to perform a systematic review of the literature, including RCTs and observational studies, to determine whether the inhaled prostaglandins epoprostenol and alprostadil are associated with an improvement in pulmonary physiology (eg, oxygenation, pulmonary artery pressures) or mortality in postneonatal children and adults with ARDS. An assessment of the adverse effects associated with this therapy was also an aim of interest. Based on the existing data regarding iNO, the primary hypothesis was that the use of inhaled prostaglandins would be associated with an improvement in oxygenation and pulmonary artery pressures, but would not confer any mortality benefit.
Materials and Methods
This systematic review was designed, conducted, and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (e-Appendix 1 (792KB, pdf) ) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) (e-Appendix 2 (792KB, pdf) ) guidelines.18,19 It was registered with PROSPERO (registration number CRD42014013180). Ethical approval from the Human Research Protection Office at the principal investigator’s institution was not required.
Search and Identification of Studies
A written protocol (e-Appendix 3 (792KB, pdf) ) that was finalized prior to beginning the search was followed. The timeline was from 1976 (discovery of PGI2) through 2014, and searched PubMed, EMBASE, Cumulative Index of Nursing and Allied Health Literature (CINAHL), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cochrane Database of Systematic Reviews. Searches were completed in May 2014. A trained medical librarian (S. F.) experienced in systematic reviews assisted in designing the search strategy and in conducting the electronic search. Two authors (B. M. F. and N. M. M.) also manually screened reference lists of articles selected for inclusion to identify additional studies. To identify potential unpublished data, B. M. F. also (1) searched abstracts from the Society of Critical Care Medicine, European Society of Intensive Care Medicine, American Thoracic Society, CHEST, International Symposium on Intensive Care and Emergency Medicine, and Pharmacotherapy from 1999 to 2014 and (2) searched online for clinical trials registration (ClinicalTrials.gov). B. M. F. also contacted principal investigators of published and unpublished studies as needed.
Inclusion Criteria
RCTs were included, as well as nonrandomized studies (prospective interventional studies, prospective and retrospective cohort analyses, case series). The inclusion of nonrandomized studies was decided a priori for the following reasons: (1) high likelihood the question of interest could not be investigated strictly with RCTs secondary to lack of existing randomized trials; (2) to provide an explicit evaluation of strengths and weaknesses of the current literature; (3) to assess evidence of effects (benefit and harm); and (4) to provide evidence for the undertaking of randomized trials.20 The intervention was inhaled epoprostenol or inhaled alprostadil; the comparison was placebo or no intervention/usual care, as well as iNO, provided that all crossover studies reported data transparently. Studies of hypoxemic patients that did not explicitly state the population was ARDS were excluded. Studies that did not report preintervention and postintervention data, such as the effect on oxygenation, were excluded. Papers that were reviews, correspondences, editorials, and nonhuman studies were also excluded. The reference list of all review articles was screened to identify additional studies for inclusion.
Study Selection and Data Abstraction
Two reviewers (B. M. F. and N. M. M.) independently screened titles and abstracts of identified studies for eligibility. After this relevance screen, full text articles were assessed for eligibility, and the two reviewers compared their exclusion logs to determine whether there was disagreement. All studies deemed potentially relevant after the screen were obtained and the full manuscripts were reviewed (B. M. F., N. M. M., and L. S.). In cases of disagreement, a consensus was reached among the three reviewers.
Assessment of Study Quality
The quality of clinical trials selected for inclusion was assessed by using the Cochrane Collaboration Tool for assessing the risk of bias in clinical trials.21 High quality was defined as a grade of “A” in at least three of the four methodology domains. For studies of observational design, quality was assessed with the Newcastle-Ottawa Scale, assigning a maximum of nine points.22,23 Five or fewer points indicated a high risk of bias.
Assessment of Publication Bias
A graphic display (funnel plot) of the size of the treatment effect against the precision of the trial was used to evaluate for potential publication bias.24
Data Analysis
During the conduct of the systematic review, a scoping review of the literature revealed a lack of controls from which to compare mortality or adverse events.25 Therefore, the decision was made to assess physiologic end points as the primary outcomes, including oxygenation parameters (Pao2 to Fio2 ratio and Pao2), and mean pulmonary artery pressure (mPAP). Secondary outcomes included mortality and adverse effects.
Meta-analysis:
Review Manager (RevMan, Version 5.1; The Nordic Cochrane Centre, The Cochrane Collaboration) was used to conduct the meta-analysis. A generic inverse variance, random effects model was used. Continuous data are reported as mean difference (measure of absolute change). Overall effect estimates were generated using a Z test and presented as mean differences (measures of absolute change). A P value of ≤ .05 was considered statistically significant. The decision to combine the data on epoprostenol and alprostadil was made a priori. The decision to not combine evidence from randomized trials and nonrandomized studies was also made a priori, as per expert recommendation.20 Stratified subgroup analyses were performed, as were sensitivity analyses, which excluded the study with the largest mean difference in Pao2 to Fio2 ratio and the largest number of patients.26,27
Heterogeneity between studies was assessed using the I2 statistic, with suggested thresholds for low (25%-49%), moderate (50%-74%), and high (≥ 75%) values.28,29 During the systematic review, it was evident that the secondary outcomes (mortality and adverse effects) could not be assessed quantitatively. A post hoc decision was, therefore, made to report overall mortality and reported adverse effects in a descriptive, qualitative fashion. A post hoc decision to use a χ2 test to compare differences in the rate of hypotension between the observational cohort studies (longer exposure to inhaled prostaglandins) and the prospective studies (very brief exposure to inhaled prostaglandins) was also made.
Meta-Regression:
The I2 statistic indicated significant heterogeneity among the entire collection of data. Subgroup analysis and meta-regression were performed to explain some of the heterogeneous effect sizes between studies. Possible sources of heterogeneity tested included baseline oxygenation, pulmonary vasodilator dosing, source of ARDS (pulmonary vs nonpulmonary), and study year. A linear meta-regression model weighted to reflect the variance of the individual studies was used to model the data. OpenMeta [Analyst] (Center for Evidence-Based Medicine, Brown School of Public Health) was used for regression with continuous covariates.30
Results
Search and Selection
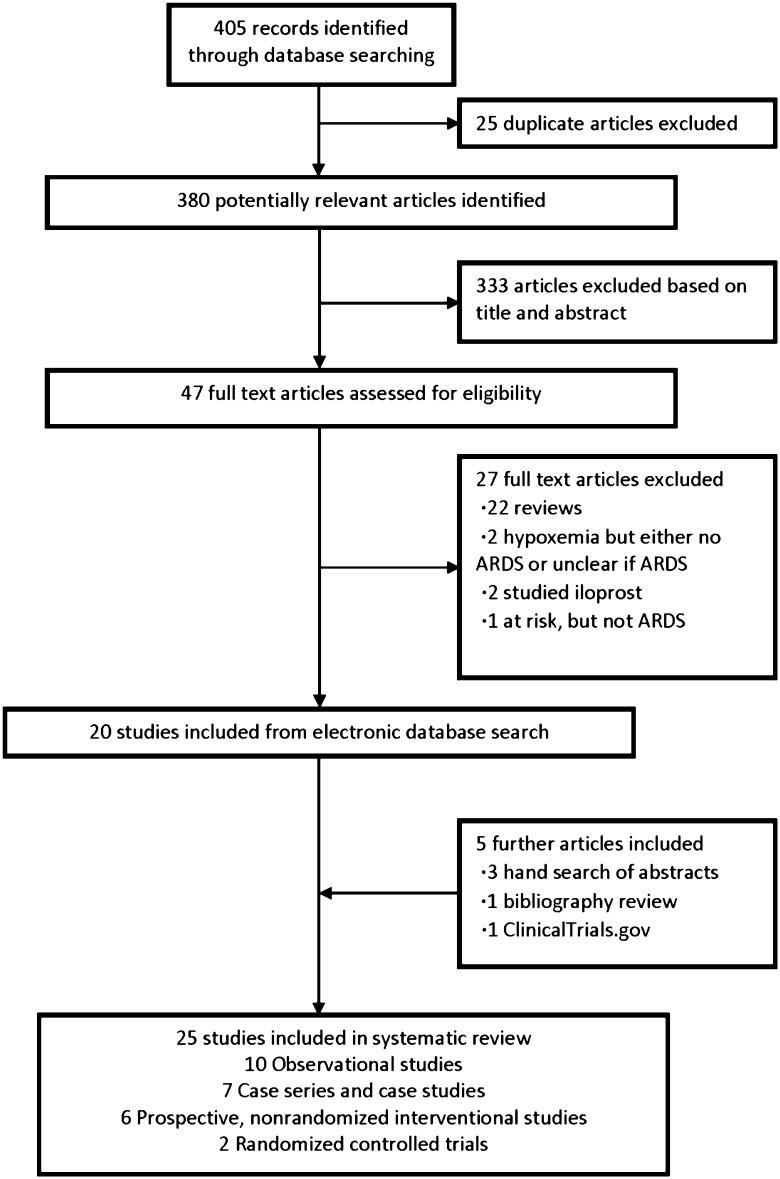
The comprehensive search yielded a total of 380 potentially relevant publications. Details regarding the search, study selection, and reason for exclusion are shown in Figure 1.
Figure 1 –
Search, inclusion, and exclusion flow diagram.
Inclusion
After the relevance search, a complete manuscript review was performed on the remaining 47 articles. Twenty-five studies were included in the final analysis.
Study Characteristics and Outcomes Reporting
The characteristics of the included studies are shown in Tables 1 and 2.26,27,31‐53 Two studies were RCTs, six were prospective, nonrandomized interventional studies, 10 were observational studies, and seven were case series. The total number of patients across studies was 606 (n = 497 epoprostenol, n = 109 alprostadil, median 11 patients per study).
TABLE 1 ] .
Study Characteristics of Randomized Controlled Trials
| Study/Year | N | Therapy | Duration of Therapy | Timing of Therapya | High-Quality RCT?b | Primary Outcomec | Secondary Outcomesc | Comments |
| Dahlem et al34/2004 | 14 | PGI2 | ≈ 125 min | ICU day 3 | Yes | OI | Hemodynamics, ventilator settings | Crossover with normal saline placebo |
| Siddiqui et al43/2013 | 67 | PGE1 | 30 min | Within 24 h | Yes | Diastolic dysfunction, LVEDP | Pao2:Fio2 | Double-blind, with normal saline placebo control |
Continuous data are presented as mean (SD) unless otherwise noted. LVEDP = left ventricular end-diastolic pressure; PGE1 = alprostadil; PGI2 = epoprostenol; RCT = randomized controlled trial.
Timing of therapy reported variably across studies and is referenced either to onset of ARDS, respiratory failure, or ICU day.
As assessed by the Cochrane Collaboration Tool for assessing risk of bias in clinical trials. Four domains were assessed: random sequence generation, concealment of allocation, blinding, and selective outcome reporting. High quality was defined as a grade of “A” in at least three-fourths of the methodology domains. To explain, for trials where blinding is not feasible at the point of intervention, a grade of “A” would be assigned if the investigator collecting the primary outcome was blinded to the treatment allocation.
Some outcomes were not explicitly stated or defined in the manuscript as primary or secondary outcomes, but reported as such in the table.
TABLE 2 ] .
Study Characteristics of Nonrandomized Studies
| Study/Year | N | Therapy | Duration of Therapy | Timing of Therapya | Risk of Bias (NOS Score)b | Primary Outcomec | Secondary Outcomesc | Comments |
| Prospective, nonrandomized interventional studies | ||||||||
| Walmrath et al52/1996 | 16 | PGI2 | < 70 min | 1-4 d | High (5) | Pao2:Fio2, Pao2, shunt |
mPAP PVR |
Crossover study with iNO |
| Van Heerden et al48/1996 | 5 | PGI2 | 30 min | Not reported | High (4) | Pao2, mPAP |
None stated | Crossover study with iNO |
| Zwissler et al53/1996 | 8 | PGI2 | 45 min | 10.3 d | High (5) | Pao2, mPAP, PVR, shunt |
Establish dose-response curve and optimal safe dose | … |
| Putensen et al39/1998 | 10 | PGE1 | 100 min | 16 (1) | High (5) | Pao2 | mPAP PVR RVEF |
Crossover with iNO and IV PGE1 |
| van Heerden et al50/2000 | 9 | PGI2 | 150 min | 5.8 d | High (5) | Pao2:Fio2, A-a gradient |
6-keto PGF1α, platelet aggregation |
… |
| Domenighetti et al35/2001 | 15 | PGI2 | 75 min | 32 (2) h | High (5) | Pao2:Fio2, Pao2 |
mPAP, PVR |
Examined difference between pulmonary and nonpulmonary ARDS |
| Observational cohort studies | ||||||||
| Meyer et al26/1998 | 15 | PGE1 | 103 (17) h, range 1-7 d | 42 (8) h | High (5) | Pao2, Pao2:Fio2 |
None stated | … |
| Siobal et al45/2003 | 11 | PGI2 | Mean 41 h, range 9-116 h | 3.9 (3.4) d | High (5) | Pao2:Fio2, Spo2 |
None stated | … |
| Rovira et al42/2004 | 5 | PGI2 | 1-3 d | 1-2 d | High (4) | Pao2:Fio2 | None stated | Abstract only |
| Camamo et al33/2005 | 27 | PGI2 (n = 10), PGE1 (n = 17) |
5.9 (7.6) d, 4.6 (3.1) d |
Not reported | High (4) | Pao2:Fio2, Pao2 |
Differences between the two drugs on clinical outcomes | … |
| Raheem40/2009 | 15 | PGI2 | 23 (1-46) h | Not reported | High (4) | Pao2:Fio2 | None stated | Abstract only |
| Ross et al41/2012 | 12 | PGI2 | 4.2 (2.5) d, range < 1-9 d | Not reported | High (4) | Pao2:Fio2, Spo2, A-a gradient |
Not stated | Abstract only Compared with iNO |
| Dunkley et al36/2013 | 16 | PGI2 | 4.8 (6.0) d | Not reported | High (3) | Pao2:Fio2 at 4 h, medication errors |
Dose response, therapy duration, adverse events, mortality |
… |
| Pacheo et al27/2014 | 216 | PGI2 | Survivors 118.5 (85.1) h; nonsurvivors 99.1 (108.7) h |
Survivors 55.2 (76.8) h; nonsurvivors 69.6 (93.8) h |
Low (7) | Hospital mortality, 90-d mortality |
Not stated | … |
| Torbic et al46/2013 | 32 | PGI2 | 3.2 (2.6) d | Not reported | Low (6) | Pao2:Fio2 at 1 h | ICU LOS, HLOS, Duration of therapy, MV duration, adverse events, cost | Compared PGI2 to iNO |
| Singh et al44/2014 | 98 | PGI2 | Not reported | Not reported | High (5) | Pao2:Fio2 | Not stated | Abstract only |
| Case series and case studies | ||||||||
| Walmrath et al51/1993 | 3 | PGI2 | ≈ 90 min | 2-3 d | N/A | Pao2:Fio2, mPAP |
Shunt | … |
| Bein et al32/1994 | 1 | PGI2 | 30 min | Not reported | N/A | Pao2, mPAP |
Not stated | … |
| Pappert et al38/1995 | 3 | PGI2 | Not reported | Day 15-55 | N/A | Pao2:Fio2 | Not stated | Studied children |
| Van Heerden et al49/1996 | 2 | PGI2 | ≈ 48 h | Not reported | N/A | Pao2:Fio2 | Not stated | … |
| van Heerden et al47/1997 | 1 | PGI2 | ≈ 5 d | Not reported | N/A | Pao2 | 6-keto PGF1α, platelet aggregation |
… |
| Allan et al31/2010 | 1 | PGI2 | ≈ 31 h | ≈ 1.5 d | N/A | Pao2:Fio2, Pao2 |
… | … |
| McMillen et al37/2011 | 4 | PGI2 | 72.5 (58.8-99.8) h | Day 1-8 | N/A | Pao2:Fio2 | … | … |
Continuous data are presented as mean (SD) unless otherwise noted. A-a = alveolar-arterial oxygenation; HLOS = hospital length of stay; iNO = inhaled nitric oxide; LOS = length of stay; mPAP = mean pulmonary artery pressure; MV = mechanical ventilation; N/A = not applicable; NOS = Newcastle-Ottawa Scale; OI = oxygenation index [(Fio2 × mean airway pressure)/Pao2]; PGF1α = prostaglandin F1α; PVR = pulmonary vascular resistance; RVEF = right ventricular ejection fraction; Spo2 = peripheral oxygen saturation. See Table 1 legend for expansion of other abbreviations.
Timing of therapy reported variably across studies and is referenced either to onset of ARDS, respiratory failure, or ICU day.
As assessed by the Newcastle-Ottawa Quality Assessment Scale.
Some outcomes were not explicitly stated or defined in the manuscript as primary or secondary outcomes, but reported as such in the table.
The RCTs were rated as high quality by the Cochrane Collaboration Tool for assessing the risk of bias in clinical trials. On the nine-point Newcastle-Ottawa Scale, the median risk of bias score was 5, indicating a high risk of bias. The main risk of bias was selection bias (eg, lack of a nonexposed cohort) and information bias (eg, lack of description in outcome assessment).
The primary outcome was physiologic in 24 of 25 studies (ie, oxygenation, pulmonary artery pressures) and clinical (ie, lengths of stay, mortality) in four of 25 studies. There was a wide range of delivered doses (e-Table 1 (792KB, pdf) ).
Effect of Inhaled Prostaglandins on Physiologic Outcomes
The results of the two RCTs are reported separately and qualitatively.20,34,43 One crossover randomized trial, using nebulized normal saline placebo, assessed the effect of epoprostenol on oxygenation index [(Fio2 × mean airway pressure)/Pao2] in 14 children. Preintervention oxygenation index was 10.0 (95% CI, 7.8-14.5), which decreased to 7.4 (95% CI, 6.5-9.7) after epoprostenol therapy was titrated to 30 ng/kg/min (P = .001). The effect on oxygenation was not reported. The other randomized trial assessed the effect of alprostadil vs placebo on 67 adults. Alprostadil was associated with an increase in mean Pao2 to Fio2 ratio from 141.2 (95% CI, 120.8-161.5) to 161.5 (95% CI, 134.6-188.3); this was not significant when compared with the increase in mean Pao2 to Fio2 ratio that occurred in the placebo (163.4 [95% CI, 140.8-186.0] to 186.8 [95% CI, 162.9-210.7]) (P = .21).
Meta-analysis:
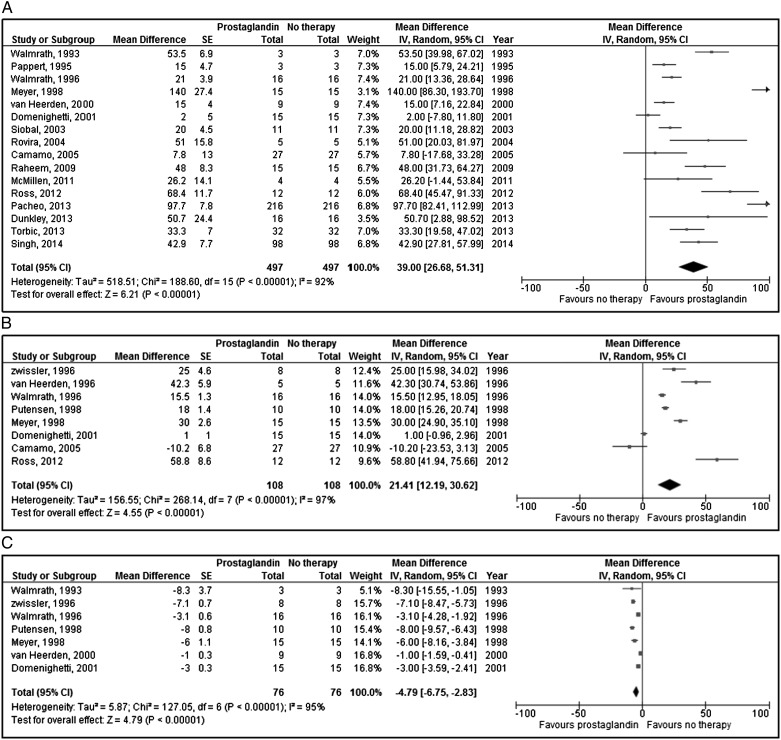
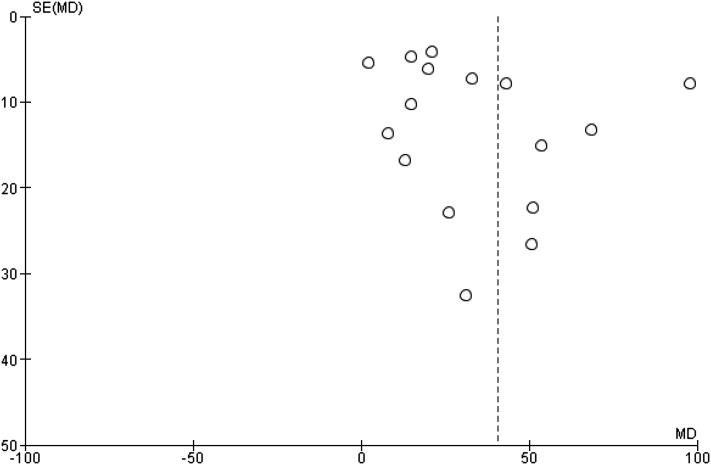
Aggregate meta-analysis of the remaining datasets (excluding the two RCTs) is presented in Table 3 and Figure 2. This analysis demonstrated that inhaled prostaglandins were associated with improved Pao2 to Fio2 ratio (16 studies, 497 patients, 994 measurements; 39.0% higher; 95% CI, 26.7%-51.3%), and Pao2 (eight studies, 108 patients, 216 measurements; 21.4% higher; 95% CI, 12.2%-30.6%), and decrease in mPAP (seven studies, 76 patients, 152 measurements; −4.8 mm Hg; 95% CI, −6.8 mm Hg to −2.8 mm Hg). Funnel plot analysis (Fig 3) revealed possible reporting bias with asymmetric skew to the left.54 There was significant statistical heterogeneity for each outcome.
TABLE 3 ] .
Stratified Summary Values for Meta-analyses
| Stratification | No. of Studies (Patients), Meta-analysis | Mean Difference [95% CI] | P Value | I2 % |
| All datasets | ||||
| Pao2:Fio2 | 16 (497) | 39.00 [26.68, 51.31] | < .0001 | 92 |
| Pao2 | 8 (108) | 21.41 [12.19, 30.62] | < .0001 | 97 |
| mPAP | 7 (76) | −4.79 [-6.75, −2.83] | < .0001 | 95 |
| Epoprostenol | ||||
| Pao2:Fio2 | 15 (465) | 35.68 [23.67, 47.69] | < .0001 | 92 |
| Pao2 | 6 (66) | 20.72 [9.15, 32.29] | .0004 | 97 |
| mPAP | 5 (51) | −3.75 [−5.71, −1.78] | .0002 | 95 |
| Alprostadil | ||||
| Pao2:Fio2 | 2 (32) | 77.45 [−42.67, 197.57] | .21 | 92 |
| Pao2 | 3 (42) | 16.79 [4.27, 29.32] | .009 | 92 |
| mPAP | 2 (25) | −7.14 [−9.08, −5.20] | < .0001 | 54 |
| Prospective, interventional studies | ||||
| Pao2:Fio2 | 3 (40) | 13.07 [2.78, 23.35] | .01 | 78 |
| Pao2 | 5 (54) | 19.17 [9.26, 29.07] | .0002 | 98 |
| mPAP | 5 (58) | −4.35 [−6.52, −2.19] | < .0001 | 97 |
| Cohort studies | ||||
| Pao2:Fio2 | 13 (457) | 46.91 [31.33, 62.49] | < .0001 | 91 |
| Pao2 | 3 (54) | 25.89 [−5.23, 57.01] | .10 | 96 |
| mPAP | 2 (18) | −6.19 [−8.25, −4.12] | < .0001 | 0 |
| Publication y, 1993-2000 | ||||
| Pao2:Fio2 | 6 (50) | 32.30 [17.12, 47.47] | < .0001 | 89 |
| Pao2 | 5 (54) | 24.59 [17.98, 31.19] | < .0001 | 91 |
| mPAP | 4 (43) | −4.75 [−8.17, −1.34] | .006 | 97 |
| Publication y, 2001-2014 | ||||
| Pao2:Fio2 | 11 (451) | 40.24 [22.01, 58.46] | < .0001 | 93 |
| Pao2 | 3 (54) | 15.66 [−14.38, 45.71] | .31 | 96 |
| mPAP | N/A | … | … | … |
| High risk of bias | ||||
| Pao2:Fio2 | 11 (239) | 33.73 [21.64,45.83] | < .0001 | 87 |
| Pao2 | 8 (108) | 21.41 [12.19, 30.62] | < .0001 | 97 |
| mPAP | 6 (73) | −4.60 [−6.61, −2.59] | < .0001 | 96 |
| Low risk of bias | 65.41 [2.30, 128.52] | .04 | 97 | |
| Pao2:Fio2 | 2 (248) | … | … | … |
| Pao2 | N/A | … | … | … |
| mPAP | N/A | … | … | … |
| Exclusion of case series | ||||
| Pao2:Fio2 | 13 (487) | 41.16 [26.60, 55.73] | < .0001 | 93 |
| Pao2 | 8 (108) | 21.41 [12.19, 30.62] | < .0001 | 97 |
| mPAP | 6 (73) | −4.60 [−6.61, −2.59] | < .0001 | 96 |
See Table 2 legend for expansion of abbreviations.
Figure 2 –
A-C, Effect of inhaled prostaglandins on Pao2 to Fio2 ratio (A), Pao2 (B), and mean pulmonary artery pressure (C). These parameters were assessed in a before-after fashion with respect to prostaglandin therapy. Therefore, the term “Total” refers to the number of measurements taken, which is exactly double the number of total patients in the each study. df = degrees of freedom.
Figure 3 –
Funnel plot for outcome of Pao2 to Fio2 ratio in studies of inhaled prostaglandins for ARDS. MD = mean difference.
To examine sources of heterogeneity in the aggregate meta-analysis and sources of variation in individual study results, additional stratified meta-analyses were performed. For these subgroups, analyses were restricted to (1) type of inhaled pulmonary vasodilator (epoprostenol or alprostadil), (2) publication year, (3) study type, (4) risk of bias, and (5) exclusion of case series. Meta-analysis of the data from the prospective, nonrandomized interventional studies was conducted separately from the observational studies and case series, in accordance with guideline recommendations of meta-analyses of nonrandomized studies.20 This was done to decrease heterogeneity across study types, as the interventional studies were more homogeneous with respect to size (n = 5-16 patients) and duration of intervention (very brief exposure to inhaled prostacyclins). The subgroup analyses are presented in Table 3. A similar effect on physiology was seen in the subgroup analyses. After exclusion of the study with the largest mean difference in Pao2:Fio2 ratio, analysis demonstrated that inhaled prostaglandins were associated with improved Pao2 to Fio2 ratio (15 studies, 482 patients, 964 measurements; 35.7% higher, 95% CI, 23.7%-47.7%).26 A similar result was obtained when excluding the study with the largest number of patients (15 studies, 281 patients, 562 measurements; 33.0% higher, 95% CI, 23.2%-42.9%).27
Meta-regression:
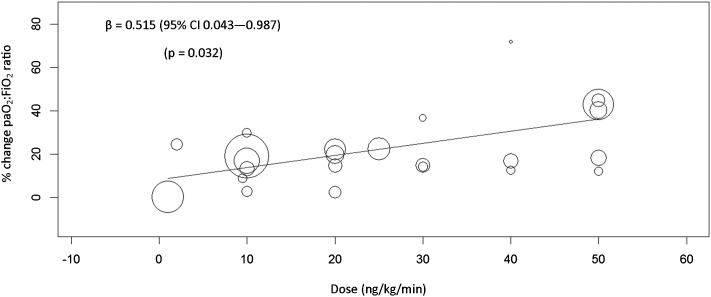
Linear meta-regression was used to assess the impact of continuous covariates on treatment effect. Year of publication (P = .862), baseline Pao2 to Fio2 ratio (P = .106), and proportion of nonpulmonary ARDS (P = .816) were not associated with the treatment effect. A dose-response relationship was tested among studies that reported data separately for cohorts with a defined dose, and higher doses of inhaled prostaglandins increased Pao2 to Fio2 ratio linearly (Fig 4).
Figure 4 –
Meta-regression analysis demonstrating a dose-response relationship between oxygenation and increasing dose of inhaled pulmonary vasodilator.
Adverse effects
Adverse events were variably reported overall. Twenty studies mentioned adverse events, or a lack of adverse effects (eg, “no effect on blood pressure”), somewhere in the manuscript (e-Table 2 (792KB, pdf) ). Eleven studies reported no effect on systemic hemodynamics, while five studies reported hypotension, ranging from an incidence of 12.5% to 33.3%. There was a statistically significant difference in the rate of hypotension between the prospective studies vs the observational studies, 0.69% (1 of 144) vs 27 of 155 (17.4%) (P < .001). Three studies reported thrombocytopenia, anemia, or transfusion requirement.
Mortality
Mortality was reported in 17 of 25 studies (e-Table 3 (792KB, pdf) ). Due to lack of controls, an investigation into an association of inhaled prostaglandin with mortality could not be ascertained. The overall reported mortality in patients with ARDS receiving inhaled prostaglandins was 295 of 522 (56.5%).
Discussion
In patients with ARDS, the traditional inhaled pulmonary vasodilator of choice has been iNO, with little data on inhaled prostaglandins. This systematic review and meta-analysis was, therefore, undertaken to assess outcomes associated with inhaled prostaglandins. The first finding is that inhaled prostaglandins appear to be used with some frequency in ARDS. This is demonstrated by the 25 publications included in the analysis, as well as the discovery of several other studies not meeting the inclusion criteria.55‐62 The data would also suggest that use is increasing in frequency, as approximately 75% of the patients were from studies published in the last 3 years. This is an interesting phenomenon when put into context of other findings in this analysis: (1) a lack of clinical outcome data demonstrating benefit, (2) overall low quality for the majority of data, and (3) significant heterogeneity in the data that does exist.
Only one study, to our knowledge, reported a clinical outcome as a primary analysis of interest. The two RCTs that exist had very brief exposure to study drug and did not study patient-centered outcomes. Furthermore, one RCT included only children, a potentially unique population with respect to ARDS incidence, outcome, and response to therapy.63,64 The majority of observational studies were low quality. This suggests a lack of transparency and significant potential for bias in the published literature. Heterogeneity was demonstrated not only statistically, but also in a clinical overview of the reported data with respect to dosing, duration of exposure, and timing of therapy.
Aggregate meta-analysis and stratified subgroup analyses show improved oxygenation in ARDS. Similar results have been demonstrated with iNO, yet there is a lack of correlation between changes in oxygenation and outcome benefit in ARDS.8,9,65,66 Furthermore, the majority of studies measured oxygenation changes in a before-after fashion, suggesting that the oxygenation benefit should be interpreted with caution. Without a placebo, it is impossible to assess whether oxygenation benefit was secondary to the use of inhaled prostaglandins. Consistency across data suggests this, but in a dose-finding study of iNO, 24% of the placebo group had an increase in Pao2 of ≥ 20%.67 Similar placebo effects were seen in one RCT included in this review.43 Furthermore, some of the cohort studies specifically excluded patients whose oxygenation did not respond to therapy, and although averaged measures of oxygenation were found to improve for the group overall, multiple studies report that a significant percentage of patients were nonresponders.34‐36,39,44 So, it is possible that inhaled prostaglandins confer no oxygenation benefit, and these results reflect improved oxygenation secondary to a change in Fio2 or other concomitant therapies that were not reported (eg, prone positioning, positive end-expiratory pressure setting).
Descriptive analysis of cohort studies suggests that patients dosed with inhaled prostaglandins experience adverse events that are serious and fairly common. Specifically, hypotension was reported in 17.4% of patients in the cohort studies. This is in contrast to the prospective interventional studies, which reported adverse events with less frequency. This may be secondary to the difference in drug exposure between the two study types, as the treatment duration in the cohort studies was significantly longer. There is biologic plausibility, as a prostaglandin metabolite, of 6-keto PGF1α, has been measured in the systemic circulation and demonstrates that the effect of inhaled prostaglandins is not isolated to the lung.50 The lack of a control group in these studies also makes it difficult to conclude that the reported adverse events were related to inhaled prostaglandin therapy. Little data were provided on other ARDS treatments, such as adherence to lung-protective ventilation, and selective reporting of adverse events was common. However, the reported rate of hypotension in the cohort studies suggests that inhaled prostaglandins may be associated with possible harm and raises concern about prolonged exposure in the routine setting of ARDS treatment.
iNO does not reduce mortality in patients with ARDS.9 Inasmuch as inhaled prostaglandins may have a similar effect on hypoxemia and pulmonary hypertension as iNO, if the only effect of inhaled prostaglandins is on this physiology, then it is unlikely that they will improve long-term clinical outcome either. However, there is also biologic plausibility that a potential effect of inhaled prostaglandins could be derived from their antiplatelet and antiinflammatory properties.10‐15 This may be more impactful as far as meaningful clinical outcome is concerned, but needs to be studied further. Reported ARDS mortality rate was 56.5% in patients treated with inhaled prostaglandins. While no inference on causation can be drawn, with this mortality rate exceeding that in reported ARDS literature, it is unclear that any benefit is derived.
There are important limitations in this systematic review. Due to a lack of RCTs, unpublished and nonrandomized studies were included in the analysis.68,69 This decision has several implications. By including nonrandomized trials, biases in the primary data are likely to be greater.20 An attempt to control for this was done by systematically grading each study for bias and reporting these results transparently. Nonrandomized trials often lead to increased heterogeneity, which was demonstrated in a clinical overview of the data reported, as well as statistically. Stratified subgroup meta-analyses were conducted in an attempt to control for this, and these gave similar results as the aggregate data. Meta-regression analysis was also performed. Confounding is also an issue with nonrandomized studies. It is possible that clinicians dosed patients with ARDS with inhaled prostaglandins based on a higher likelihood of clinical response or survival (ie, confounding by indication). A mortality rate of 56.5% speaks against this. On the other hand, it is also possible that clinicians chose to dose patients with the most severe ARDS with inhaled prostaglandins, and the high mortality rate is a reflection of ARDS severity and a lower chance of survival. It is also possible that the search did not uncover all of the published literature in this domain, as nonrandomized studies are indexed poorly and have a lack of study registries. The search was exhaustive, rigorous, and reproducible, giving confidence that the largest amount of data on this topic to date was uncovered. Finally, while ARDS was an explicit inclusion criterion for this systematic review, not every individual study stated how ARDS was defined. An assumption would be that consensus definitional criteria for ARDS were used, but without an explicit statement to this fact in each publication, we are unsure.70,71 It is recognized that these limitations make drawing conclusions on the use of inhaled prostaglandins for ARDS difficult. It, therefore, must be emphasized that due to the paucity of quality data, this analysis cannot discern whether there is truly any benefit or harm. However, this analysis provides an explicit evaluation of the strengths and weaknesses of the current literature to date, and by demonstrating a signal in the data for both benefit (ie, physiologic effects) and harm (ie, rate of hypotension), evidence for the need for randomized trials in this area has been provided.
Conclusions
The data regarding the use of inhaled prostaglandins for ARDS are limited both in terms of methodologic quality and demonstration of clinical benefit. Meta-analysis demonstrates that inhaled prostaglandins improve oxygenation and decrease pulmonary artery pressures and may be associated with adverse events. The use of inhaled prostaglandins in ARDS is in need of further study.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: B. M. F. takes responsibility for the content of the manuscript, including the data and analysis. B. M. F. also conceived the study and contributed to its design, obtained research funding, supervised the conduct of the study, screened studies for inclusion in the analysis, and managed, analyzed, and interpreted data; N. M. M. and L. S. contributed to the design of the study, screened studies for inclusion in the analysis, and analyzed and interpreted data; S. F. contributed to the design of the search strategy, conducted the electronic search, and analyzed and interpreted data; M. H. K. contributed to the design of the study, as well as data analysis and interpretation; C. R. C. contributed to the design of the study, analyzed and interpreted data, and provided methodologic oversight; and all authors contributed to the drafting of the manuscript, revision of the manuscript, and approval of the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding sources played no role in the design, conduct, analysis, or interpretation of these data and played no role in the drafting, revision, or submission of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any of the other supporting bodies.
Other contributions: This work was performed at the following institutions: Washington University School of Medicine in St. Louis and The University of Iowa College of Medicine. We would like to thank the authors of several of the manuscripts that were included and excluded in this review. Their time and generosity in responding to our inquiries is very much appreciated.
Additional information: The e-Appendixes and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- iNO
inhaled nitric oxide
- mPAP
mean pulmonary artery pressure
- PGE1
prostaglandin E1
- PGI2
prostaglandin I2
- RCT
randomized controlled trial
Footnotes
FUNDING/SUPPORT: Dr Fuller was supported by the Emergency Medicine Grant-in-Aid from the Department of Emergency Medicine, Washington University School of Medicine in St. Louis and the KL2 Career Development Award. Dr Mohr was supported by the Emergency Medicine Foundation Research Fellowship. Dr Kollef was supported by the Barnes-Jewish Hospital Foundation. This research was supported by the Washington University Emergency Care Research Core, which receives funding from the Barnes-Jewish Hospital Foundation, as well as the Washington University Institute of Clinical and Translational Sciences [Grants UL1 TR000448 and KL2 TR000450] from the National Institutes of Health National Center for Advancing Translational Sciences.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute. lung injury. N Engl J Med. 2005;353(16):1685-1693. [DOI] [PubMed] [Google Scholar]
- 2.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296(9):476-480. [DOI] [PubMed] [Google Scholar]
- 3.Jardin F, Gueret P, Dubourg O, Farcot JC, Margairaz A, Bourdarias JP. Two-dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med. 1985;13(11):952-956. [DOI] [PubMed] [Google Scholar]
- 4.Squara P, Dhainaut J-F, Artigas A, Carlet J. Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intensive Care Med. 1998;24(10):1018-1028. [DOI] [PubMed] [Google Scholar]
- 5.Sloane PJ, Gee MH, Gottlieb JE, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992;146(2):419-426. [DOI] [PubMed] [Google Scholar]
- 6.Rachmale S, Li G, Ahmed A, et al. Use of rescue therapies for ARDS patients: population-based study in Olmstead County, Minnesota. Crit Care Med. 2010;38(suppl 12):377. [Google Scholar]
- 7.Rachmale S, Ding S, Wilson G, Li G, Franco PM. Rescue therapies in patients with refractory hypoxemia. Am J Respir Crit Care Med. 2011;183:A1643. [Google Scholar]
- 8.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adhikari NK, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014;42(2):404-412. [DOI] [PubMed] [Google Scholar]
- 10.Puri N, Dellinger RP. Inhaled nitric oxide and inhaled prostacyclin in acute respiratory distress syndrome: what is the evidence? Crit Care Clin. 2011;27(3):561-587. [DOI] [PubMed] [Google Scholar]
- 11.Riva CM, Morganroth ML, Ljungman AG, et al. Iloprost inhibits neutrophil-induced lung injury and neutrophil adherence to endothelial monolayers. Am J Respir Cell Mol Biol. 1990;3(4):301-309. [DOI] [PubMed] [Google Scholar]
- 12.Rose F, Hattar K, Gakisch S, et al. Increased neutrophil mediator release in patients with pulmonary hypertension—suppression by inhaled iloprost. Thromb Haemost. 2003;90(6):1141-1149. [DOI] [PubMed] [Google Scholar]
- 13.Burghuber OC, Silberbauer K, Haber P, Sinzinger H, Elliott M, Leithner C. Pulmonary and antiaggregatory effects of prostacyclin after inhalation and intravenous infusion. Respiration. 1984;45(4):450-454. [DOI] [PubMed] [Google Scholar]
- 14.Fantone JC, Marasco WA, Elgas LJ, Ward PA. Stimulus specificity of prostaglandin inhibition of rabbit polymorphonuclear leukocyte lysosomal enzyme release and superoxide anion production. Am J Pathol. 1984;115(1):9-16. [PMC free article] [PubMed] [Google Scholar]
- 15.Fantone JC, Kinnes DA. Prostaglandin E1 and prostaglandin I2 modulation of superoxide production by human neutrophils. Biochem Biophys Res Commun. 1983;113(2):506-512. [DOI] [PubMed] [Google Scholar]
- 16.Davies A, Jones D, Bailey M, et al. ; Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888-1895. [DOI] [PubMed] [Google Scholar]
- 17.Afshari A, Brok J, Moller AM, Wetterslev J. Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev. 2010;(8):CD007733. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [DOI] [PubMed] [Google Scholar]
- 20.Reeves B, Deeks JJ, Higgins JPT, Wells GA, on behalf of the Cochrane Non-Randomised Studies Methods Group. Including non-randomised studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. London, England: The Cochrane Collaboration; 2008. [Google Scholar]
- 21.Higgins J, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. London, England: The Cochrane Collaboration; 2008. [Google Scholar]
- 22.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2008. Ottawa Hospital Research Institute website http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 17, 2014.
- 24.Egger M, Smith GD, Altman DG, eds. Systematic Reviews in Health Care: Meta-analysis in Context. 2nd ed London, England: BMJ Publishing Group; 2001. [Google Scholar]
- 25.Armstrong R, Hall BJ, Doyle J, Waters E. Cochrane Update. ‘Scoping the scope’of a cochrane review. J Public Health (Oxf). 2011;33(1):147-150. [DOI] [PubMed] [Google Scholar]
- 26.Meyer J, Theilmeier G, Van Aken H, et al. Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg. 1998;86(4):753-758. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco J, Arnold H, Skrupky L, Watts P, Micek ST, Kollef MH. Predictors of outcome in 216 subjects with ARDS treated with inhaled epoprostenol. Respir Care. 2014;59(8):1178-1185. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1-15. [Google Scholar]
- 31.Allan PF, Codispoti CA, Womble SG, et al. Inhaled prostacyclin in combination with high-frequency percussive ventilation. J Burn Care Res. 2010;31(2):347-352. [DOI] [PubMed] [Google Scholar]
- 32.Bein T, Pfeifer M, Riegger GA, Taeger K. Continuous intraarterial measurement of oxygenation during aerosolized prostacyclin administration in severe respiratory failure. N Engl J Med. 1994;331(5):335-336. [DOI] [PubMed] [Google Scholar]
- 33.Camamo JM, McCoy RH, Erstad BL. Retrospective evaluation of inhaled prostaglandins in patients with acute respiratory distress syndrome. Pharmacotherapy. 2005;25(2):184-190. [DOI] [PubMed] [Google Scholar]
- 34.Dahlem P, van Aalderen WM, de Neef M, Dijkgraaf MG, Bos AP. Randomized controlled trial of aerosolized prostacyclin therapy in children with acute lung injury. Crit Care Med. 2004;32(4):1055-1060. [DOI] [PubMed] [Google Scholar]
- 35.Domenighetti G, Stricker H, Waldispuehl B. Nebulized prostacyclin (PGI2) in acute respiratory distress syndrome: impact of primary (pulmonary injury) and secondary (extrapulmonary injury) disease on gas exchange response. Crit Care Med. 2001;29(1):57-62. [DOI] [PubMed] [Google Scholar]
- 36.Dunkley KA, Louzon PR, Lee J, Vu S. Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: a pilot study. Ann Pharmacother. 2013;47(6):790-796. [DOI] [PubMed] [Google Scholar]
- 37.McMillen JC, Burke CF, Dhingra A, Dudney TM, Faircloth BE. Use of inhaled epoprostenol in patients with H1N1 influenza-associated acute respiratory distress syndrome: a case series. Ann Pharmacother. 2011;45(5):e26. [DOI] [PubMed] [Google Scholar]
- 38.Pappert D, Busch T, Gerlach H, Lewandowski K, Radermacher P, Rossaint R. Aerosolized prostacyclin versus inhaled nitric oxide in children with severe acute respiratory distress syndrome. Anesthesiology. 1995;82(6):1507-1511. [DOI] [PubMed] [Google Scholar]
- 39.Putensen C, Hörmann C, Kleinsasser A, Putensen-Himmer G. Cardiopulmonary effects of aerosolized prostaglandin E1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157(6 pt 1):1743-1747. [DOI] [PubMed] [Google Scholar]
- 40.Raheem S, Dzierba A. Aerosolized epoprostenol as adjunct therapy for acute respiratory distress syndrome. Crit Care Med. 2009;37(12):A195. [Google Scholar]
- 41.Ross B, Miller M, Oliveira P. A retrospective comparison of an inhaled epoprostenol dosing protocol versus conventional therapy with inhaled nitric oxide on outcomes in patients with severe acute respiratory distress syndrome. Crit Care Med. 2012;40(12 suppl 1):418. [Google Scholar]
- 42.Rovira I, Fabregas N, Martinez J, Alcon A, Adalia R, Zavala E. Use of selective pulmonary vasodilator therapy in a surgical ICU: analysis of 10 years. Intensive Care Med. 2004;30(suppl 1):S206. [Google Scholar]
- 43.Siddiqui S, Salahuddin N, Zubair S, et al. Use of inhaled PGE1 to improve diastolic dysfunction, LVEDP, pulmonary hypertension and hypoxia in ARDS—a randomised clinical trial. Open J Anesthesiol. 2013;3:109. [Google Scholar]
- 44.Singh A, Girdhar A, Treger K, et al. Impact of inhaled epoprostenol on oxygenation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:A3087. [Google Scholar]
- 45.Siobal MS, Kallet RH, Pittet J-F, et al. Description and evaluation of a delivery system for aerosolized prostacyclin. Respir Care. 2003;48(8):742-753. [PubMed] [Google Scholar]
- 46.Torbic H, Szumita PM, Anger KE, Nuccio P, LaGambina S, Weinhouse G. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care. 2013;28(5):844-848. [DOI] [PubMed] [Google Scholar]
- 47.van Heerden PV. Systemic levels of 6-keto-prostaglandin F1 alpha following administration of inhaled aerosolized prostacyclin. Anaesth Intensive Care. 1997;25(6):701-703. [DOI] [PubMed] [Google Scholar]
- 48.Van Heerden PV, Blythe D, Webb SA. Inhaled aerosolized prostacyclin and nitric oxide as selective pulmonary vasodilators in ARDS—a pilot study. Anaesth Intensive Care. 1996;24(5):564-568. [DOI] [PubMed] [Google Scholar]
- 49.Van Heerden PV, Webb SA, Hee G, Corkeron M, Thompson WR. Inhaled aerosolized prostacyclin as a selective pulmonary vasodilator for the treatment of severe hypoxaemia. Anaesth Intensive Care. 1996;24(1):87-90. [DOI] [PubMed] [Google Scholar]
- 50.van Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL. Dose-response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest. 2000;117(3):819-827. [DOI] [PubMed] [Google Scholar]
- 51.Walmrath D, Schneider T, Pilch J, Grimminger F, Seeger W. Aerosolised prostacyclin in adult respiratory distress syndrome. Lancet. 1993;342(8877):961-962. [DOI] [PubMed] [Google Scholar]
- 52.Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153(3):991-996. [DOI] [PubMed] [Google Scholar]
- 53.Zwissler B, Kemming G, Habler O, et al. Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154(6 pt 1):1671-1677. [DOI] [PubMed] [Google Scholar]
- 54.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 55.Peter M, Jaffe B, Gilbert C, Baram M. Effect of inhaled epoprostenol on oxygenation, outcome, and diuresis in critically ill patients.[abstract]. Chest. 2011;140(4_MeetingAbstracts):287A. [Google Scholar]
- 56.Tonelli A, Sasidhar M, Heresi-Davila G, Mullin R, Conci D. Oxygenation change in patients with severe hypoxia, shunt and rescue inhaled epoprostenol. Am J Respir Crit Care Med. 2013;187:A4475. [Google Scholar]
- 57.McMillen J, Krumenacker L, Faircloth B, Rowe A. Effect of low dose corticosteroids on response to inhaled epoprostenol in patients with septic shock and ARDs. Crit Care Med. 2012;40(12):102. [Google Scholar]
- 58.Scoville BA, Tan P, Johnson DW, Aaronson P. Comparison of short-term pulmonary improvement associated with inhaled nitric oxide and inhaled epoprostenol for acute respiratory distress syndrome. Pharmacotherapy. 2011;31(10):324e. [Google Scholar]
- 59.Mullin R, Lam S, Conci D, Heresi G, Sasidhar M. Inhaled aerosolized prostacyclin as an efficacious and economic alternative to inhaled nitric oxide. Chest. 2012;142(4_MeetingAbstracts):401A.22459772 [Google Scholar]
- 60.Tabrizi MB, Schinco MA, Tepas JJ, III, Hwang J, Spiwak E, Kerwin AJ. Inhaled epoprostenol improves oxygenation in severe hypoxemia. J Trauma Acute Care Surg. 2012;73(2):503-506. [DOI] [PubMed] [Google Scholar]
- 61.Manickavel S, Mathew J, Africano J, Khan M, Aboeed A. Comparison Of Inhaled Nitric Oxide With Epoprostenol In Hypoxic Respiratory Failure. Am J Respir Crit Care Med. 2014;189:A4473. [Google Scholar]
- 62.De Wet CJ, Affleck DG, Jacobsohn E, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004;127(4):1058-1067. [DOI] [PubMed] [Google Scholar]
- 63.Dahlem P, van Aalderen WM, Bos AP. Pediatric acute lung injury. Paediatr Respir Rev. 2007;8(4):348-362. [DOI] [PubMed] [Google Scholar]
- 64.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124(1):87-95. [DOI] [PubMed] [Google Scholar]
- 65.Taylor RW, Zimmerman JL, Dellinger RP, et al. ; Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603-1609. [DOI] [PubMed] [Google Scholar]
- 66.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308. [DOI] [PubMed] [Google Scholar]
- 67.Dellinger RP, Zimmerman JL, Taylor RW, et al. ; Inhaled Nitric Oxide in ARDS Study Group. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Crit Care Med. 1998;26(1):15-23. [DOI] [PubMed] [Google Scholar]
- 68.Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA. 1993;269(21):2749-2753. [PubMed] [Google Scholar]
- 69.Hopewell S, McDonald S, Clarke M, Egger M. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;2(2):MR000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818-824. [DOI] [PubMed] [Google Scholar]
- 71.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526-2533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement