Abstract
Background
The mite Psoroptes cuniculi is a common worldwide ectoparasite and the most frequently found in rabbit farms. It causes significant economic losses on commercial rabbit breeding associated with poor leather quality, reduced conception rates, weight loss, poor growth and death. Several strategies have been proposed for the treatment of mange caused by this mite, ranging from the use of acaricides, entomopathogenic fungi, essential oils and vaccines. However, therapy and control of both human scabies and animal mange are still based mainly on the use of drugs and chemicals such as ivermectin, which involves disadvantages including genotoxic and cytotoxic effects, resistance and environmental damage. Bacillus thuringiensis is a bacterium, innocuous for human being, domestic animals and plants that produces highly biodegradable proteins, and has been used worldwide for biological control. The aim of this work was to find an alternative treatment based on biological control for scabies caused by Psoroptes cuniculi, using protein extracts from strains of Bacillus thuringiensis.
Methods
P. cuniculi mites were obtained from naturally infected New Zealand rabbits, and different doses of protein from B. thuringiensis were added to the mites. We measured mortality and obtained the median lethal concentration and median lethal times. For histological analysis, the mites were fixed in 10 % formalin, processed according to the paraffin embedded tissue technique. Sections were stained with hematoxylin-eosin to observe the general histological structure.
Results
We report here for the first time evidence about the in vitro acaricidal effect caused by the strain GP532 of B. thuringiensis on the mite Psoroptes cuniculi, with an LC50 of 1.3 mg/ml and a LT50 of 68 h. Histological alterations caused by B. thuringiensis on this mite, included the presence of dilated intercellular spaces in the basal membrane, membrane detachment of the peritrophic matrix and morphological alterations in columnar cells of the intestine.
Conclusions
Since this mite is an obligate ectoparasite that affects rabbits, goats, horses, cows and sheep, B. thuringiensis protein extracts are proposed as a potential treatment for biological control of mange in farm animals.
Keywords: Acaricidal, Bacillus thuringiensis, Rabbit, Psoroptes cuniculi, Mange
Background
The mite Psoroptes cuniculi (P. cuniculi) is a common worldwide ectoparasite and the most frequently found in rabbit farms. This mite causes otitis, the most common disease in domestic rabbits, and the main reason that the owners seek veterinarian. Thus, this parasite affects the general animal health and hygiene [1]. P. cuniculi infestation can cause considerable weight loss, less favorable feed conversion rates, vestibular dysfunction, and meningitis, frequently complicated by secondary bacterial infections [2]. It causes significant economic losses associated with poor leather quality, reduced conception rates, weight loss, poor growth and death [3].
Several strategies have been proposed for the treatment of mange caused by P. cuniculi, ranging from the use of acaricides, entomopathogenic fungi and essential oils, to vaccines [4, 5]. However, therapy and control of both human scabies and animal mange are still based mainly on the use of drugs and chemicals such as ivermectin [6]. The use of drugs, such as ivermectin, to control this parasite has disadvantages, since it has both, genotoxic and cytotoxic effects. In addition, their widespread use induces resistance, accompanied by environmental pollution [7–10]. These facts associated with the interest in the consumption of products ensuring good sanitary quality, hygiene, and proper handling have lead research efforts to discover new effective compounds.
Bacillus thuringiensis (B. thuringiensis) is a Gram-positive bacterium, innocuous for human being, domestic animals and plants. It produces highly biodegradable proteins, and has been used worldwide for biological control against several agriculture pests and some mosquitoes vectors of human diseases [11, 12], and more recently it has been proposed to be used against some parasites that affect animal and human health [13]. Over 90 % of the market for bio-insecticides include products based on this bacterium [14], and worldwide there are about 60,000 isolates of B. thuringiensis in public and private collections, that may have specific insecticidal activity [15–17]. The aim of this work was to study the acaricidal potential of protein extracts of strains of B. thuringiensis, finding that the strain GP532 had an LC50 of 1.3 mg/ml and acts at short times (LT50 = 68 h) in in vitro studies. Furthermore, we report that the acaricidal effect of B. thuringiensis protein extracts is by producing histological alterations on the gut of the mite.
Methods
Ethics statement
All animal procedures followed the animal care and experimentation practices recommended as per Mexican regulations (NOM-062-ZOO-1999). These regulations are in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH and The Weatherall Report), ensuring compliance with international regulations and guidelines. The present manuscript does not contain clinical studies or patient data.
Isolation of the B. thuringiensis strains
Corpses of P. cuniculi were taken from a sample of a scab from New Zealand infected rabbit and placed individually in a sterile microcentrifuge tube. They were washed twice with sodium hypochlorite 1 % and with sterile water. Then they were placed individually in a sterile microcentrifuge tube, 500 μl of liquid Luria-Bertani (LB) medium was added to each tube and the corpses were macerated with sterile tip. The tubes were incubated at 30 ° C during 72 h. After this, 100 μl of the culture were diluted with 800 μl of sterile water and subjected to a heat shock of 60° by one hour, to eliminate fungi or no spore-forming bacteria. A loop was taken, seeded in a petri dish with solid LB medium and incubated by 24 h, a single colony was taken and seeded on HCT media. The strains which produced crystals were selected and kept in LB and glycerol 60 % [11, 18]. The strains were deposited at the B. thuringiensis collection of the laboratory of Vegetal Parasitology of the Biological Research Center (LVP-BRC) at the University of the State of Morelos, Mexico.
Adult mites
P. cuniculi mites were obtained from five naturally infected New Zealand rabbits tranquilized with intramuscular administration of ketamine/xylazine (40 mg/kg and 10 mg/kg). Scabs containing mites were obtained by scraping from the external ear, placed in the bottom of 50 mL tubes, and kept at 28 ± 2 °C during 15 min, to stimulate migration of the mites off scabs [19]. Mites were identified using a stereomicroscope according to the morphological features of their species [20], and only adult mites migrating at least to the mark corresponding to 25 mL of height in the 50 ml tube were included in the bioassays.
Selection of strains and production of spore-crystal proteins
Strains of B. thuringiensis were obtained from the strain collection of the LVP-BRC. We tested 12 strains isolated from corpses of P. cuniculi, and the GP526 strain isolated from Meloidogyne sp, all of them grown in HCT medium at 28 ± 2 °C until complete sporulation (72 h). Total proteins (spores and crystals) were recovered with a bacteriological loop and suspended in sterile water containing protease inhibitor PMSF 100 μM. Total protein concentration was quantified by the Bradford technique [21].
In vitro toxicity bioassays
The immersion technique previously described was used [22], briefly, thirty adult mites were placed in microtubes and 1 ml of protein from B. thuringiensis was added by triplicate for 60 s. Mites were placed in agar petri dishes containing water and filter paper, and sealed with Parafilm® to avoid dehydration. Mortality was determinated and quantified microscopically, taking as dead mites those lacking of reaction to stimulation with a brush, with persistent immobility for 5 min [23, 24]. Bioassays were repeated 4 times on different days.
Median lethal concentration (LC50) and median lethal time (LT50)
We conducted a dose response curve performing 10 replicates for each dose, and LC50 was the concentrations inducing ≥50 % mortality. The LC50 was calculated using Probit analysis via the Polo Plus 2003 statistical program [25]. Mortality was quantified every 24 h, a linear regression analysis was performed, and data was expressed as net mortality [18].
Histological analysis
The mites were fixed in 10 % formalin, processed according to the paraffin embedded tissue technique, and histological sections of 6 μm were obtained using an MR2235 Leica microtome. Sections were stained with hematoxylin-eosin, ten microscope fields were evaluated in each section and photo-documented with an image analyzer [26].
Protein profile
Five μg of concentrated spore-crystal in disruption buffer were boiled for 10 min and centrifuged at 14,000 rpm for 5 min [27]. The supernatant was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) 10 % and the gel was stained with Coomassie blue.
Results
Selection of strains, production of spore-crystal proteins and in vitro bioassays
To analyze the acaricidal effect of total protein extracts obtained from 13 strains of B. thuringiensis, we soaked P. cuniculi mites in a solution with 1 mg/mL of total protein for 60 s (Fig. 1). The dose of 1 mg/mL induced mortality of several of the strains tested and it was tested for different times after exposure to B. thuringiensis. After 24, 48 and 72 h post-treatment, we found the maximum toxicity with strains GP532 and GP392 at 24 h (11.48 ± 4.6 and 11.11) and 48 h (18.88 ± 7.92 and 17.037 ± 4.41) (Table 1). After 72 h, we observed the highest toxicity with strains GP532, GP522, GP392, GP871 and GP873, inducing 34.62 % ± 5.95, 29.44 % ± 3.07, 29 % ± 3.39, 28.8 % ± 3.42 and 27.9 % ± 3.98 respectively, whereas in the control group we observed 16.4 % ± 2.72 of mortality.
Fig. 1.

Toxicity induced 72 h post incubation of P. cuniculi mites for 60 s with total proteins derived from 13 different strains of B. thuringiensis. Mean ± SD of the percentage of mortality is shown. *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, Kruskal-Wallis test
Table 1.
Time-response toxicity induced by strains of B. thuringiensis on mites incubated for 60 s
| Time post-treatment | |||
|---|---|---|---|
| Strain | 24 h | 48 h | 72 h |
| Control | 6.3 ± 1.73 | 9.92 ± 2.55 | 16.46 ± 2.72 |
| GP532 | 11.41 ± 4.6*** | 18.88 ± 7.92*** | 34.62 ± 5.96*** |
| GP522 | 8.51 ± 1.7** | 12.59 ± 2.44 | 29.44 ± 3.07*** |
| GP392 | 11.11 ± 4.85*** | 17.03 ± 4.41*** | 29.07 ± 3.39*** |
| GP871 | 7.96 ± 1.67 | 13.7 ± 3.59 | 28.88 ± 3.42 |
| GP873 | 8.51 ± 1.7** | 15.18 ± 3.27** | 27.96 ± 3.98*** |
| GP681 | 9.07 ± 3.58* | 12.03 ± 4.59 | 23.33 ± 3.23 |
| GP551 | 5.55 ± 1.61 | 11.11 ± 2.28 | 23.33 ± 2.55*** |
| GP526 | 9.07 ± 1.53*** | 12.03 ± 1.67 | 18.7 ± 1.67 |
| GP683 | 6.85 ± 2.13** | 14.07 ± 4.36*** | 22.22 ± 7.23*** |
| GP884 | 6.48 ± 2.67 | 10.03 ± 2.8 | 17.03 ± 3.77 |
| GP880 | 5.92 ± 3.14 | 8.7 ± 3.26 | 16.29 ± 3.59 |
| GP684 | 5.37 ± 3.45** | 10.37 ± 3.4 | 15.55 ± 3.96 |
| GP877 | 3.51 ± 2.13 | 8.88 ± 4.57*** | 15.37 ± 4.86* |
Mean ± SD of the percentage of mortality. *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, Kruskal-Wallis test
The strain that induced the highest toxicity was GP532. Then, we tested GP532 strain increasing the immersion time from 60 s to five minutes. With this increase in the immersion time, we observed an increase in toxicity from 34.64 % to 52.03 % after 72 h post-treatment, while in the control group we did not observe significant effect on mortality (from 16.46 % to 16.94 %). This increase up to 52 % represents more than 300 % (16.94 taken as 100 %), showing significant efficacy of the strain GP532 in the induction of toxicity of mites. Alternatively, we tested the efficacy of the commercial acaricide ivermectin, which produced 98 % of mortality after 48 h post treatment (Table 2).
Table 2.
Time-response toxicity induced by GP532 on mites incubated for 5 min
| Time post-treatment | |||
|---|---|---|---|
| Treatment | 24 h | 48 h | 72 h |
| Control | 6.20 ± 2.13 | 10.648 ± 3.54 | 16.94 ± 3.32 |
| GP532 | 10.74 ± 3.28** | 20.370 ± 3.79*** | 52.03 ± 6.04*** |
| Ivermectin | 78.33 ± 11.27*** | 98.88 ± 1.98*** | 99.81 ± 0.78*** |
Mean ± SD of the percentage of mortality. **P ≤ 0.01 and ***P ≤ 0.001, Kruskal-Wallis test
Median lethal concentration (LC50) and median lethal time (LT50)
Subsequently, we evaluated different concentrations of strain GP532 for 5 min of immersion and analyzed data with a Probit analysis of survival to determine the LC50. We found that LC50 is achieved with a concentration of 1.3 mg/mL (Fig. 2a).
Fig. 2.
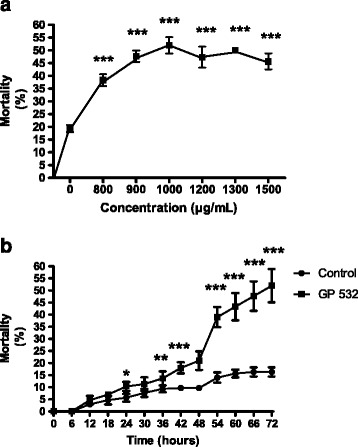
Dose (a) and time-dependent (b) cytotoxicity induced by immersion of mites in proteins of the strain GP532 for 5 min (mean ± SD, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001, Mann Whitney test)
To determine the toxicity of the strain GP532 at different times, TL50 was evaluated every six hours post immersion and analyzed by a linear regression analysis, finding a TL50 of 68 h with a correlation of 0.94 after immersion of mites during 5 min (Fig. 2b).
Histological analysis
Additionally, we analyzed the histoarchitecture of the mites (Fig. 3), observing the presence of dilated intercellular spaces in the peritrophic matrix of the intestine after 1 min of treatment with 1 mg/ml of GP532. Furthermore, we observed a greater amount of vacuole shaped fat stores in the ventricular zone. It was also observed that most of the treated mites had no intestinal content (Fig. 3c) while mites in the control group had no changes in the intestine and they had intestinal content (Fig. 3a). The ventricular tissue in control mites shown dispersed fat stores (Fig. 3b), while in treated mites we observed presence of dilated intercellular spaces in the basal membrane, detachment in the membrane of the peritrophic matrix, and complete emptying of the intestinal contents (Fig. 3c, arrows), and in the ventricle we observed the presence of fat pads (Fig. 3d, arrows). In the tissue treated with ivermectin, we observed absence of vacuolization and of fatty deposits in both the intestine and in the ventricle (Fig. 3 e, f).
Fig. 3.

Photomicrograph of longitudinal sections in control (a, b), treated mites with the strain GP532 of B. thuringiensis (c, d) or with ivermectin (e, f). LU: lumen of the intestine, MP: peritrophic matrix, EC: ectoperitrophic space, MB: basement membrane, CI: intestinal content, Va: vacuole, VEM: medium ventricle, Ov: ovary. Arrows indicate alteration on the intestinal basement membrane or vacuole shaped fat deposits in ventricle, H&E- X40
Moreover, we observed morphological alterations in columnar cells of the bowel in treated mites (Fig. 4b), whereas in the tissue of control mites we did not observe changes in these cells (Fig. 4a). In ivermectin-treated mites, we did not observe the changes induced by B. thuringiensis in the peritrophic matrix of intestine, in the ventricular zone, or in columnar cells (Fig. 4c).
Fig. 4.
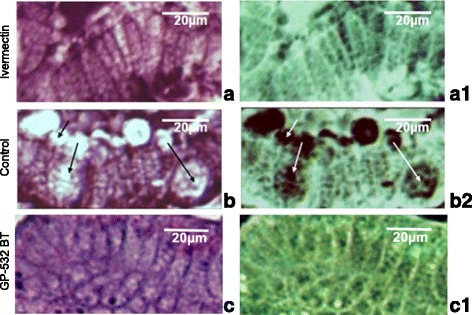
Representative image of the structure of the columnar cells in the mite P. cuniculi without treatment (a) or after treatment with the strain GP532 of B. thuringiensis (b) or ivermectin c, and their respective images through the relief filter (a1, b1, c1). Arrows indicate presence of dilated intercellular spaces of the columnar epithelium regarding the peritrophic matrix. Bar = 20 μm
In the central nervous system of the mite, the synganglion was observed histologically similar to the control group (Fig. 5a) in both ivermectin treated mites (Fig. 5b) and B. thuringiensis (Fig. 5c).
Fig. 5.

Photomicrograph of longitudinal sections in nervous system of control mites (a) or treated with the strain GP532 of B. thuringiensis (b) or ivermectin (c). Syn: Synganglion
Electrophoretic profile of GP532 strain of B. thuringiensis
Subsequently, we analyzed the protein profile of strain GP532, noting that the major bands in the protein profile of strain GP532 have molecular weights ranging from 25 to 135 kDa (Fig. 6).
Fig. 6.

Electrophoretic profile of GP532 strain of B. thuringiensis
Discussion
As it is well known, the insecticidal activity of B. thuringiensis is species specific. Here we isolated thirteen strains of B. thuringiensis, most of them from corpses of P. cuniculi, expecting to find a strain with acaricidal activity. We evaluated their potential acaricidal effect in vitro finding several strains causing mortality of the mite. One of the strain reached 52.03 % of mortality after 72 h post treatment, while the no treated mites had 16 % of mortality. This mortality was achieved with 1.3 mg mL-1, a dose relatively low comparative with doses reported for thick affecting livestock, where they reported that 1 mg/ml of B. thuringiensis kurstaki induced 100 % of mortality against engorged females of A. persicus after five days. In addition, 2.5 mg mL-1 of B. thuringiensis israelensis caused 100 % of mortality, and 5 mg mL-1 of B. thuringiensis thuringiensis caused 93.3 % of mortality on H. dromedarii [28–30]. These doses are low comparative with those used to diminish the 50 % of growth of synanthropic mites, where they range between 25 and 38 mg g-1 [31], 30 times higher than the CL50 reported in our study. Compared with the studies mentioned above, we also had a shorter time for effectiveness of B. thuringiensis against P. cuniculi. The estimated LT50 in our study was 2.8 days, similar to data reported in an in vitro study conducted with the entomopathogenic fungus M. anisopliae on Psoroptes spp, where the LT50 was 2.7 days at a concentration of 1x107 conidia mL-1, after 10 min of immersion [4]. Regarding the mortality reached, we obtained a dependable LC50, but we were not able to reach a 100 % of mortality. However, it is possible to test this strain in future studies, by applying several doses or longer times of treatment in vivo, trying the purified protein with major activity or using combined strains of B. thuringiensis as possible strategies. In addition, as it was proposed by Erban et al. [31], the combined application of B. thuringiensis with other compounds could increase the efficacy of mite control. Therefore, GP532 strain could be combined with diatomaceous earth [32], or using a mixture of B. thuringiensis with other acaricidal compounds. For example, it has been proven that a combination of chitinase and soybean trypsin protease inhibitor effectively suppresses population growth in Acarus siro [33], and many B. thuringiensis strains have chitinolytic activities [34] that could enhance the efficacy in mite control. Another possible combination is with other natural products as naphthoquinones, which could result in a decrease of the induction of long-term resistance, with short-term efficacy, and at a low cost [35].
Although in our study ivermectin showed a high effectiveness, several drawbacks of this drug have been reported, which calls for the search for alternative treatments. Ivermectin induced drug resistance has been documented in in vitro assays with S. scabei at a concentration as low as 100 μg g-1; as well, resistance has been documented for some intestinal helminths in animals, in arthropods and in humans [36–38]. Moreover, in cattle, the dose of subcutaneous ivermectin is 200 μg kg-1, while for rabbits the dose is 400 μg kg-1 to achieve serum concentrations that allow a pharmacological effect against the parasite, and with this dose, it has been proven that the drug remains at least after 348 h, representing a disadvantage in animals raised for human consumption [39]. The main concern using ivermectin is neurotoxicity, which in most mammalian species may manifest as central nervous system depression, and consequent ataxia, as it might be expected from promoters of inhibitory GABAergic synapses [40]. In addition, subcutaneous administration of ivermectin is painful for animals, and although we have the option of topical application, in rabbits it is unsuitable because of the natural behavior of licking each other in the ears as part of their hygiene, that may affect the pharmacokinetics of drugs, and prolonged treatment could cause intoxication [41, 42]. Additionally, in male rabbits it was demonstrated that repeated administration of ivermectin subcutaneously causes a decrease in the weight of the sexual organs, which can affect the animal production [43]. In future studies, the combined use of B. thuringiensis and ivermectin could also be analyzed, seeking a synergistic or additive effect with the possibility of lowering the dose of ivermectin and consequently its adverse effects.
Histologically, in mites treated with B. thuringiensis we observed significant differences with regard to the normal tissue of untreated mites [44]. In the tissue of mites treated with B. thuringiensis, we noticed an increase of fat deposits adipocyte-like on the contour of the ventricle. We found vacuolization in several cells, possibly due to an accumulation of water and fat (hydropic degeneration) caused by an inability of the cells to maintain ionic and fluid homeostasis due to glycogen degradation, that could explain the structural changes in the tissue, like vacuolization and adipocytes to trap toxins and other substances reported in ticks [45]. The morphological alterations found in the intestine were not caused by all the strains with acaricidal effect (not shown), but whether these effects of agglomeration of fat deposits were observed in the ventricular zone. The damage on columnar cells and intestine were only observed on mites treated with GP532 strain. Intestine alterations on columnar cells were similar to those of an histopathological study in intestinal tissue conducted with Spodoptera frugiperda treated with aizawai-Xentari® B. thuringiensis, and in other insects of the order of Lepidoptera, Manduca sexta and Anticarsia gemmatalis [46, 47] which suggests that the mechanism of action of B. thuringiensis on the mite could be similar to that described in Lepidoptera.
The electrophoretic profile of GP532 strain shows a major band of about 135 kDa. It has been reported that one of the proteins produced by B. thuringiensis serovariety kurstaki HD73 strain, is Cry1A that has an approximate molecular weight of 133.3 kDa [48]. Also, the action of B. thuringiensis on the mite of apicultural importance, Varroa destructor, was reported, and the active strain against this parasite has a 100 % identity with B. thuringiensis serovariety kurstaki HD73 strain [18].
For mites, the mechanism of action of B. thuringiensis is unknown. The presence of enzymes such as trypsin, alkaline phosphatase and some aminopeptidases on digestive physiology of the genus Psoroptes [49], suggest that alterations in intestinal columnar cells observed in P. cuniculi, may be due to activation of B. thuringiensis protoxins like Cry1A, which in brush border membrane vesicles of Lepidoptera induces alterations pore-like. Cry1A acts through a specific receptor, the “Receptor A”, an aminopeptidase-N with a molecular weight of 170 kDa that has been purified from brush border membranes of intestinal epithelial cells of H. virescens [50]. In other Lepidoptera, the molecular weight of receptors reported are 120, 140 and 170 kDa [51]. In the present study, we did not analyze the toxic action of specific proteins isolated from B. thuringiensis on the mite P. cuniculi, however, future studies will focus on these studies, as well as sequencing of the toxic protein against the mite P. cuniculi to know the mechanism of action of B. thuringiensis on mites.
In the in vitro tests, during the death of the mites induced by B. thuringiensis we observed contractions prior to death. This phenomenon has been reported in other in vitro assays with ivermectin. In mammals, according to the modeling of the mechanism of action, ivermectin binds and it is transported by a membrane P-glycoprotein, and activates glutamate-gated chloride channels interfering with nervous system and muscle function, enhancing inhibitory neurotransmission [36, 52]. However, in our study, we did not observe alterations in the synganglion, which regulates nerve impulses in the mite; therefore, it is possible to attribute the contractions to a degradation of muscle glycogen, coupled with the instability caused by B. thuringiensis homeostasis in the digestive system of the mite.
Conclusions
Several B. thuringiensis strains isolated from P. cuniculi tested in this work, had in vitro acaricidal effect on this mite. The GP532 strain had the highest effect under our experimental conditions, with a LC50 of 1.3 mg ml-1, LT50 of 68 h, and producing histological alterations on the gut of the mite.
Acknowledgements
Authors thank Maribel Nieto Miranda and Miriam Cecilia Guido Jiménez for technical assistance. EDG received a fellowship from Consejo Nacional de Ciencia y Tecnología (305290). The work was partially supported with grants PROMEP 103.5/11/3825 and Individual research project UAEM 2013 PII-36, both awarded to FIFP.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EDG carried out experiments, analysis and interpretation of data, and drafted the manuscript; GPC participated in the conception and the design of the study, acquisition of funding, and interpretation of data; CHC contributed to conception and design of the study, interpretation of data and in drafting the manuscript; MPM participated in the analysis of histological studies and interpretation of data; VMHV and JMM revised the manuscript critically making substantive intellectual contributions; FIFP participated in the conception and the design of the study, acquisition of funding, general supervision of the research group, analysis and interpretation of data. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributor Information
Emmanuel Dunstand-Guzmán, Email: dunstand_ipalogy@outlook.com.
Guadalupe Peña-Chora, Email: penacg@uaem.mx.
Claudia Hallal-Calleros, Email: challalc@gmail.com.
Mario Pérez-Martínez, Email: drmvzmariomtz@gmail.com.
Víctor Manuel Hernández-Velazquez, Email: vmanuelh@uaem.mx.
Jorge Morales-Montor, Email: jmontor66@biomedicas.unam.mx.
Fernando Iván Flores-Pérez, Email: ivan.flores@uaem.mx.
References
- 1.White SD, Bourdeau PJ, Meredith A. Dermatologic problems of rabbits. Sem Av Ex Pet Med. 2007;11:141–50. doi: 10.1053/saep.2002.123982. [DOI] [Google Scholar]
- 2.Ulutas B, Voyvoda H, Bayramli G, Karagenc T. Efficacy of topical administration of eprinomectin for treatment of ear mite infestation in six rabbits. Vet Dermatol. 2005;16:334–7. doi: 10.1111/j.1365-3164.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- 3.Shang X, Wang D, Miao X, Wang X, Li J, Yang Z, et al. The oxidative status and inflammatory level of the peripheral blood of rabbits infested with Psoroptes cuniculi. Parasit Vectors. 2014;7:124. doi: 10.1186/1756-3305-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George DR, Finn RD, Graham KM, Sparagano OA. Present and future potential of plant-derived products to control arthropods of veterinary and medical significance. Parasit Vectors. 2014;7:28. doi: 10.1186/1756-3305-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrucci S, Flamini G, Cioni PL, Morelli I, Macchioni F, Macchioni G. In vitro and in vivo efficacy of extracts of Artemisia verlotorum against Psoroptes cuniculi. Vet Rec. 2001;148:814–5. doi: 10.1136/vr.148.26.814. [DOI] [PubMed] [Google Scholar]
- 6.Borges FA, Almeida GD, Heckler RP, Lemes RT, Onizuka MK, Borges DG. Anthelmintic resistance impact on tropical beef cattle productivity: effect on weight gain of weaned calves. Trop Anim Health Prod. 2013;45(3):723–7. doi: 10.1007/s11250-012-0280-4. [DOI] [PubMed] [Google Scholar]
- 7.Coles TB, Dryden MW. Insecticide/acaricide resistance in fleas and ticks infesting dogs and cats. Parasit Vectors. 2014;7:8. doi: 10.1186/1756-3305-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AM, Stephen FB, Cawley GD, Bellworthy SJ, Groves BA. Resistance of the sheep scab mite Psoroptes ovis to propetamphos. Vet Rec. 1996;139:451. [PubMed] [Google Scholar]
- 9.O’Brien DJ. Treatment of psoroptic mange with reference to epidemiology and history. Vet Parasitol. 1999;83:177–85. doi: 10.1016/S0304-4017(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 10.Ündeger U, Başaran N. Effects of pesticides on human peripheral lymphocytes in vitro: induction of DNA damage. Arch Toxicol. 2005;79:169–76. doi: 10.1007/s00204-004-0616-6. [DOI] [PubMed] [Google Scholar]
- 11.Peña G, Miranda-Rios J, De la Riva AG, Pardo-Lopez L, Soberon M, Bravo A. Bacillus thuringiensis S-layer protein involved in toxicity against Epilachna varivestis (Coleoptera: Coccinellidae) Ap Env Microbiol. 2006;72:353–60. doi: 10.1128/AEM.72.1.353-360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dambach P, Louis VR, Kaiser A, Ouedraogo S, Sié A, Sauerborn R, et al. Efficacy of Bacillus thuringiensis var. israelensis against malaria mosquitoes in northwestern Burkina Faso. Parasit Vectors. 2014;7:371. doi: 10.1186/1756-3305-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peña G, Aguilar-Jiménez FA, Hallal-Calleros C, Morales-Montor J, Hernández-Velázquez VM, Flores-Pérez FI. In vitro ovicidal and cestocidal effects of toxins from Bacillus thuringiensis on the canine and human parasite Dipylidium caninum. BioMed Res Int. 2013;2013:1–7. doi: 10.1155/2013/174619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glare TR, O’Callaghan M. Bacillus thuringiensis. Biology, ecology and safety. Chichester, UK: Wiley J and sons; 2000. [Google Scholar]
- 15.Lecadet MM. Bacillus thuringiensis toxins: The proteinaceous crystal. In: Montie TC, Kadis S, Ajl SJ, editors. Microbial toxins. 1970. pp. 437–71. [Google Scholar]
- 16.Boucias DG, Pendland JC. Principles of insect pathology. Norwel, USA: Klumer Academic Plublishers; 1998. [Google Scholar]
- 17.Crickmore N, Zeigler DR, Feitelson E, Schnepf E, Van Rie J, Lereclus D, et al. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:807–13. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alquisira-Ramírez EV, Paredes-Gonzalez JR, Hernández-Velázquez VM, Ramírez-Trujillo JA, Peña-Chora G. In vitro susceptibility of Varroa destructor and Apis mellifera to native strains of Bacillus thuringiensis. Apidol. 2014;45(6):707–18. doi: 10.1007/s13592-014-0288-z. [DOI] [Google Scholar]
- 19.Walton SF, Currie BJ. Problems in diagnosing Scabies, a global disease in human and animal Populations. Clin Microbiol Rev. 2007;20:268–79. doi: 10.1128/CMR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates PG. Inter- and intra-specific variation within the genus Psoroptes (Acari: Psoroptidae) Vet Parasitol. 1999;83:201–17. doi: 10.1016/S0304-4017(99)00058-8. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Ruvalcaba M, Peña-Chora G, Romo-Martínez A, Hernández-Velázquez V, De la Parra AB, La Rosa DP. Evaluation of Bacillus thuringiensis pathogenicity for a strain of the tick, Rhipicephalus microplus, resistant to chemical pesticides. J Insect Sci. 2010;10:186. doi: 10.1673/031.010.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macchioni F, Perrucci S, Cecchi F, Cioni PL, Morelli I, Pampiglione S. Acaricidal activity of aqueous extracts of camomile flowers, Matricaria chamomilla, against the mite Psoroptes cuniculi. Med Vet Entomol. 2004;18:205–7. doi: 10.1111/j.0269-283X.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 24.Lekimme M, Mignon B, Leclipteux T, Tombeux S, Marechal F, Losson B. In vitro tests for evaluation of the hatchability of the eggs of Psoroptes mites following exposure to acaricidal compounds. Med Vet Entomol. 2006;20:102–5. doi: 10.1111/j.1365-2915.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 25.Robertson JL, Preisler HK, Russell RM: Polo Plus Probit and Logit analysis. LeOra software 2002-2003 P. 36.
- 26.Rosas-Velasco C, Perez-Martinez M, Castillo-Juarez H, Flores-Perez FI. Histological changes induced by medrox-yprogesterone acetate on the uterus of ovariectomized rabbits. Vet Mex. 2007;38:207–14. [Google Scholar]
- 27.Ibarra JE, del Rincón MC, Ordúz S, Noriega D, Benintende G, Monnerat R, et al. Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Appl Environ Microbiol. 2003;69(9):5269–74. doi: 10.1128/AEM.69.9.5269-5274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassanain MA, El Garhy FM, Abdel-Ghaffar AF, El-Sharaby A, Abdel Megeed NK. Biological control studies of soft and hard ticks in Egypt. I. The effect of Bacillus thuringiensi varieties on soft and hard ticks (Ixodidade) Parasitol Res. 1997;83:209–13. doi: 10.1007/s004360050235. [DOI] [PubMed] [Google Scholar]
- 29.Samish M, Rehacek J. Pathogens and predators of ticks and their potential in biological control. Ann Rev Entomol. 1999;44:15–182. doi: 10.1146/annurev.ento.44.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Zhioua E, Heyer K, Browning M, Ginsberg HS, LeBrun RA. Pathogenicity of Bacillus thuringiensis variety kurstaki to Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 1999;36:900. doi: 10.1093/jmedent/36.6.900. [DOI] [PubMed] [Google Scholar]
- 31.Erban T, Nesvorna M, Erbanova M, Hubert J. Bacillus thuringiensis var. tenebrionis control of synanthropic mites (Acari: Acaridida) under laboratory conditions. Exp App Acarol. 2009;49(4):339–46. doi: 10.1007/s10493-009-9265-z. [DOI] [PubMed] [Google Scholar]
- 32.Palyvos NE, Athanassiou CG, Kavallieratos NG. Acaricidal effect of a diatomaceous earth formulation against Tyrophagus putrescentiae (Astigmata: Acaridae) and its predator Cheyletus malaccensis (Prostigmata: Cheyletidae) in four grain commodities. J Econ Entomol. 2006;99:229–36. doi: 10.1093/jee/99.1.229. [DOI] [PubMed] [Google Scholar]
- 33.Sobotnik J, Kudlikova-Krizkova I, Vancova M, Munzbergova Z, Hubert J. Chitin in the peritrophic membrane of Acarus siro (Acari: Acaridae) as a target for novel acaricides. J Econ Entomol. 2008;101:1028–33. doi: 10.1093/jee/101.3.1028. [DOI] [PubMed] [Google Scholar]
- 34.Casique-Arroyo G, Bideshi D, Salcedo-Hernandez R, Barboza-Corona JE. Development of a recombinant strain of Bacillus thuringiensis subsp. kurstaki HD-73 that produces the endochitinase. ChiA74 A Van Leeuw. 2007;92:1–9. doi: 10.1007/s10482-006-9127-1. [DOI] [PubMed] [Google Scholar]
- 35.Lee CH, Lee HS. Acaricidal activity and function of mite indicator using plumbagin and its derivatives isolated from Diospyros kaki Thunb. roots (Ebenaceae) J Microbiol Biotechnol. 2008;18(2):314–21. [PubMed] [Google Scholar]
- 36.Xu M, Molento M, Blackhall W, Ribeiro P, Beech R, Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol Bioch Parasitol. 1998;91:327–35. doi: 10.1016/S0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 37.Walton SF, Myerscough MR, Currie BJ. Studies in vitro on the relative efficacy of current acaricides for Sarcoptes scabiei var hominis. Trans R Soc Trop Med Hyg. 2000;94:92. doi: 10.1016/S0035-9203(00)90454-1. [DOI] [PubMed] [Google Scholar]
- 38.Currie BJ, Harumal P, Mckinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis. 2004;39:8–12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- 39.McKellar QA, Midgley DM, Galbraith EA, Scott EW, Bradley A. Clinical and pharmacological properties of ivermectin in rabbits and guinea pigs. Vet Rec. 1992;130:71–3. doi: 10.1136/vr.130.4.71. [DOI] [PubMed] [Google Scholar]
- 40.Edwards G. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J. 2003;24(Suppl 1):S8. doi: 10.1186/1475-2883-2-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada Y, Haraguchi N, Uchida K, Meng Y. Jaw movements and EMG activities of limb-licking behavior during grooming in rabbits. Phys Behav. 1993;53:301–7. doi: 10.1016/0031-9384(93)90208-W. [DOI] [PubMed] [Google Scholar]
- 42.Laffont CM, Alvinerie M, Bousquet-Mélou A, Toutain PL. Licking behaviour and environmental contamination arising from pour-on ivermectin for cattle. Int J Parasitol. 2001;31:1687–92. doi: 10.1016/S0020-7519(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 43.El-Nahas E-A. Effect of ivermectin on male fertility and its interaction with P-glycoprotein inhibitor (verapamil) in rats. Environ Toxicol Pharmacol. 2008;26(2):206–11. doi: 10.1016/j.etap.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Rossi G, Donadio E, Perrucci S. Immunocytochemistry of Psoroptes cuniculi stained by sera from naive and infested rabbits: preliminary results. Parasitol Res. 2007;100:1281–5. doi: 10.1007/s00436-006-0400-z. [DOI] [PubMed] [Google Scholar]
- 45.Obenchain FD, Oliver JH. A qualitative analysis of the form, function and interrelationships of fat body and associated tissues in adult ticks (Acari-ixodoidea) J Exp Zoo. 1973;186:217–35. doi: 10.1002/jez.1401860302. [DOI] [PubMed] [Google Scholar]
- 46.Knaak N, Fiuza LM. Histopathology of Anticarsia gemmatalis hübner (Lepidoptera; Loctuidae) treated with nucleopolyhedrovirus and Bacillus thuringiensis serovar kurstaki. Braz J Microbiol. 2005;36:196–200. doi: 10.1590/S1517-83822005000200017. [DOI] [Google Scholar]
- 47.Levy SM, Falleiros AM, Gregório EA, Arrebola NR, Toledo LA. The larval midgut of Anticarsia gemmatalis (Hübner) (Lepidoptera: Noctuidae): light and electron microscopy studies of the epithelial cells. Braz J Biol. 2004;4:633–8. doi: 10.1590/s1519-69842004000400010. [DOI] [PubMed] [Google Scholar]
- 48.Adang MJ, Staver MJ, Rocheleau TA, Leighton J, Barker JRF, T’hompson DV. Characterized full-length and truncated plasmid clones of the crystal protein of Bacillus thuringiensis subsp. kurstaki HD-73 and their toxicity to Manduca sexta. Gene. 1985;36(3):289–300. doi: 10.1016/0378-1119(85)90184-2. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton KA, Nisbet AJ, Lehane MJ, Taylor MA, Billingsley PF. A physiological and biochemical model for digestion in the ectoparasitic mite, Psoroptes ovis (Acari: Psoroptidae) Int J Parasitol. 2003;33(8):773–85. doi: 10.1016/S0020-7519(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 50.Luo K, Sangadala S, Masson L, Mazza A, Brousseau R, Adang MJ. The Heliothis virescens 170 kDa aminopeptidase functions as “Receptor A” by mediating specific Bacillus thuringiensis Cry1A δ-endotoxin binding and pore formation. Insect Biochem Mol Biol. 1997;27(8):735–43. doi: 10.1016/S0965-1748(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 51.Gill S, Cowles EA, Francis V. Identification, isolation, and cloning of a Bacillus thuringiensis CryIAc toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J Biol Chem. 1995;270:27277–82. doi: 10.1074/jbc.270.45.27277. [DOI] [PubMed] [Google Scholar]
- 52.Burkhart CN. Ivermectin: an assessment of its pharmacology, microbiology and safety. Vet Hum Toxicol. 2000;42:30–5. [PubMed] [Google Scholar]


