Abstract
Remote ischemic preconditioning is often performed by limb ischemic preconditioning (LIPC), which has been demonstrated to be beneficial to various cells, including endothelial cells. The mechanisms underlying the protection have not been well clarified. The present study was designed to observe the effects of sera derived from rats after LIPC on human umbilical vein endothelial cells (HUVECs) injured by hydrogen peroxide (H2O2) -induced oxidative stress and explore the involvement of redox state in the protection. Incubation with 1 mM H2O2 for 2 h induced a significant reduction in HUVECs’ viability with increased production of malondialdehyde (MDA) and reactive oxygen species (ROS). Preincubation with early preconditioning serum (EPS) or delayed preconditioning serum (DPS) derived from rats subjected to LIPC alleviated these changes. Both EPS and DPS increased the nuclear translocation of transcription factor nuclear factor E2-related factor 2 (Nrf2) and the expression of antioxidases. The protective effects of EPS and DPS were blocked neither by MEK/ERK inhibitors U0126 nor by PI3K/Akt inhibitors LY294002. In conclusion, the present study provides the evidence that LIPC protects the HUVECs from H2O2-induced injury by, at least partially, enhancement of Nrf2 translocation and upregulation of antioxidases via signaling pathways independent of MEK/ERK and PI3K/Akt.
Introduction
Remote ischemic preconditioning is effective in mitigating injuries induced by both ischemia-reperfusion (I/R) and other hazardous factors in remote tissues or organs such as heart, liver, kidney, brain and intestine [1–6]. It is in most cases performed by effective, feasible, cost-effective and adverse-effect-free limb ischemic preconditioning (LIPC). The substances and the mechanisms underlying the protection have not been well clarified, although a variety of mechanisms [7–9].
Perfusion with the effluent collected from a preconditioned isolated rabbit heart alleviated I/R injury in the non-preconditioned isolated heart and transfusion of blood from a preconditioned rabbit into a non-preconditioned rabbit reduced I/R injury [10]. Serum derived from patients after LIPC reduced hypoxia-induced cell damage in cultured human intestinal cells via inhibition of matrixmetalloproteinase -2 and -9 [4]. Upregulation of heme oxygenase-1 expression and antioxidative effect was suggested responsible for LIPC-induced protection on injured organs including heart [5, 11] and liver [6]. These studies suggest that the protective substances are produced upon LIPC, released to serum and transported through blood to convey the protection on the remote jeopardized parenchymal cells. It was suggested that the humoral factors (hydrophobic and less than 15 kDa) were responsible for the protection which is transferable across species [12].
Repair, rejuvenation and regeneration of injured parenchymal cells depend on the local blood supply. Blood vessels and vascular cells play an irreplaceable role in mediating and/or translating effects of released bioactive substances on the remote injured cells. Endothelial cells are especially important, because they serve as a paracrine system in regulating other cells both in vasculature and in the parenchymal cells via cross-talking mechanisms. Moreover, the endothelium itself may be both a vital target and amplifier for biologic responses to circumstance changes including I/R. Therefore, it can be supposed that the responses of vascular cells to LIPC may, at least partially, mediate LIPC-induced protection.
Effectiveness of LIPC in improving endothelial functions has been proved in human volunteer of I/R subjects [1–3], healthy and hypertensive subjects [13], intensive-exercise subjects [14] and subjects receiving percutaneous coronary intervention [15]. These studies mainly demonstrated that LIPC improves endothelium-dependent vasodilation, but little attention has been paid to how LIPC affects the vascular endothelial cells biochemically and biophysically. A better understanding of the underlying mechanisms is a prerequisite for proper clinic uses of LIPC. The present study was designed to investigate whether LIPC can prevent endothelial cells from oxidative stress injury, and if so, what are the mechanisms underlying the protection.
Materials and Methods
Animals
Male Sprague-Dawley rats (250–300 g, 8 weeks old, provided by Animal Facility Center of Shanxi Medical University, China) were used. The protocols and procedures described in the present experiments were approved by the Animal Care and Use Committee of Shanxi Medical University and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011).
Animal groups, LIPC induction and serum collection
Twenty four male Sprague-Dawley rats were randomly divided into sham preconditioning, early preconditioning and delayed preconditioning groups. The rats were housed in constant condition (temperature 22 ± 2°C and humidity 50%-60%) in a 12 h-light/12 h-dark cycle for 2 weeks as an acclimatization period before the experiments. A modified noninvasive blood pressure radiometer cuff was placed around the right hind-limb of the rats. LIPC was performed by three cycles of 5 min ischemia (inflated the cuff around the arteria femoralis to 200 mmHg) followed by 5 min reperfusion (deflated the cuff). Sera were derived from rats with sham LIPC (non-preconditioning rat serum, NPS), 20 min after LIPC (early preconditioning serum, EPS) and 24 h after LIPC (delayed preconditioning serum, DPS). Animals were anesthetized with intraperitoneal administration of sodium pentobarbital (40 mg/kg) before the blood was collected from abdominal aorta. Sera were obtained by centrifuging the blood at 4°C, aliquoted to 50 μl each tube and kept at -80°C before addition to the culture medium. Blood gases and electrolytes were measured to make sure that the rats were in good conditions without either acidosis or hyperkalaemia.
Cell culture
The human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (Manassas, VA, USA). HUVECs were cultured in medium composed of Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies, Carlsbad, CA, USA) containing 4.5 g/L D-glucose, 2.5 mM L-glutamine, 110 mg/L sodium pyruvate, 100 U/ml penicillin/streptomycin, 0.125 mg/L amphotericin B, and 10% heat-inactivated fetal calf serum (Invitrogen Life Technologies, Carlsbad, CA, USA) in a humidified incubator with 5% CO2 at 37°C. HUVECs were not used in the experiments until they had reached 80% confluence.
The HUVECs were divided into 5 groups. Control: cultured with normal medium without any intervention throughout the experiment. Model: cultured with normal medium for 12 h and then incubated with 1mM H2O2 for 2 h. NPS, EPS and DPS: cultured with normal medium containing 5% (v/v) NPS, 5% EPS or 5% DPS respectively for 12 h and followed by 2 h incubation with 1mM H2O2. In the experiments with kinase inhibitors, U0126 (Cell Signaling Technology, Boston, MA, USA) or LY294002 (Cell Signaling Technology, Boston, MA, USA) was added 1 h before the addition of the sera.
MTT method
Cells’ viability was determined by a MTT (4,5- dimethyl- 2- thiazolyl)- 2,5- diphenyl- 2- H- tetrazolium bromide, Sigma-Aldrich, St. Louis, MO) assay. The MTT powder was dissolved (5 mg/ml) into phosphate buffered saline (PBS) and sterilized through a 0.22-μm filter before use. HUVECs were seeded in a 96-well plate (5,000 cells/well, total 100 μl) and cultured overnight. At the end of the treatments, 10μl of the MTT solution was added to each well and incubated with 5% CO2 at 37°C for 4 h. Finally, the MTT solution was replaced by 150 μl dimethyl sulfoxide and the plate was incubated at room temperature for 15 min. The absorbance was measured at 570 nm by a microplate reader. Cells’ viability was expressed as the ratio of optical density (OD) with OD value of the control as 100%. All experiments were performed in six wells and repeated for three times.
Measurements of malondialdehyde and activities of antioxidases
The medium level of malondialdehyde (MDA), as an indicator of lipid peroxidation, was assessed by the thiobarbituric acid reactive substances (TBARS) method using commercial kit (Nanjing Jiancheng Biotechnology Institute, China) according to the manufacturer’s instructions. The absorbance was measured at 532 nm using spectrophotometer.
The activities of total superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) in cell lysate were measured using kits (Nanjing Jiancheng Biotechnology Institute, China). Total SOD activity was spectrophotometrically quantified at 550 nm according manufacturer’s instruction. CAT activity was measured at 240 nm by analyzing the rate at which it caused the decomposition of H2O2 at 25°C. GSH-Px activity was determined based on its catalyzation by the oxidation of reduced glutathione in the presence of cumene hydroperoxide. The generation of nicotinamide adenine dinucleotide phosphate was measured spectrophotometrically at 340 nm.
Measurement of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) was evaluated by measuring changes in the fluorescence intensity in HUVECs preloaded with a ROS probe dihydroethidium (DHE, Invitrogen Life Technologies, Carlsbad, CA, USA). Three visual areas were randomly selected in each sample and the images of fluorescence were recorded by Nikon TES-2000s microscope. The software Image Pro Plus 5.0 was used to analyze the OD values. The data were expressed as the OD ratio with the OD values of the control as 100%.
Immunofluorescence staining
HUVECs in logarithmic phase were seeded into 24-well chamber slides. After the treatments for the indicated times, cells were fixed with 4% (w/v) paraformaldehyde for 20 min, rehydrated in PBS for 15 min, and permeabilized in 0.1% (w/v) Triton X-100 at room temperature for 10 min. After rinse with PBS, the cells were blocked with 5% BSA in PBS for 30 min at room temperature. The cells were incubated with primary antibody (Abcam, Cambridge, UK) at 4°C overnight followed by FITC-conjugated secondary antibody for 1 h at room temperature. The images of Nrf2 with FITC staining were captured using a confocal microscope (OLYMPUS plus confocal system fluoview Ver 3.0).
Real-time reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent according to the manufacturer's instructions (Invitrogen Life Technologies, Carlsbad, CA, USA) and treated with DNase I before cDNA synthesis to remove DNA contamination. First-strand cDNA was generated using SuperScript III first-strand synthesis system as recommended by the manufacturer and stored at -20°C until use. Subsequently, real-time PCR was performed to determine the mRNA expression of CAT, SOD-1, SOD-2 and GSH-Px-1 using the LightCycler 2.0 system. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. Primer 3 software was used to design the primer sequences. The primer sequences are listed in Table 1. The final volume of the PCR reaction mixture was 25 μl, which consisted of 12.5 μl 2×SYBR premixture, 0.5 μl 10 μM forward primer and reverse primer, 1μl cDNA, and 10.5 μl sterilized deionized water. The PCR cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. Data were collected during each cycle at the 60°C extension step. The amplification efficiency was tested in standard curves using serial cDNA dilutions. Amplification specificity was checked using melting curves. For the comparison between the two groups, the software calculated the variation in the cycle threshold (Ct) in the treated group compared to the cycle threshold (Ct) of the control group, and this was expressed as a logarithmic base (2) value based on the formula 2-ΔΔCt, which was calculated as follows: 2-ΔΔCt with ΔΔCt = (Ctantioxidases −CtGAPDH) treat groups-(Ctantioxidases −CtGAPDH) control group.
Table 1. List of primers for qPCR analysis.
| Gene | Up primer | Down primer | Product size |
|---|---|---|---|
| GAPDH | tccctgagctgaacgggaag | ggaggagtgggtgtcgctgt | 217bp |
| SOD-1 | cgagcagaaggaaagtaatgga | cacaccatctttgtcagcagtc | 223bp |
| SOD-2 | cgtgactttggttcctttgac | atttgtaagtgtccccgttcc | 116bp |
| CAT | gcctttggctactttgaggtc | gatgaagaaaatgggggtgtta | 225bp |
| GSH-Px-1 | agtcggtgtatgccttctcg | tcgttcatctgggtgtagtcc | 142bp |
Statistical analysis
All values are expressed as means ± SEM. All date were first tested normality and homogeneity of variance. If these tests were passed, one-way ANOVA followed by Scheffe’s post-hoc test was performed to determine whether there were significant differences (P < 0.05) among groups and between two groups respectively. Otherwise, non-parametric analysis (Kruskal-Wallis test followed by mann-whitney U test) was performed. For all statistical comparisons, a P value < 0.05 was accepted to indicate significant differences.
Results
Rat LIPC sera protected H2O2–injured HUVECs
In pilot experiments searching for the optimal concentration of rat serum to be added to the culture medium, MTT assays were performed to evaluate the effects of different concentrations (0%, 5%, 10%, 20%) of rat serum on HUVECs proliferation with fetal calf serum as control. The maximum concentration (supplementation with 5%) of rat serum that did not induce cell proliferation was chosen.
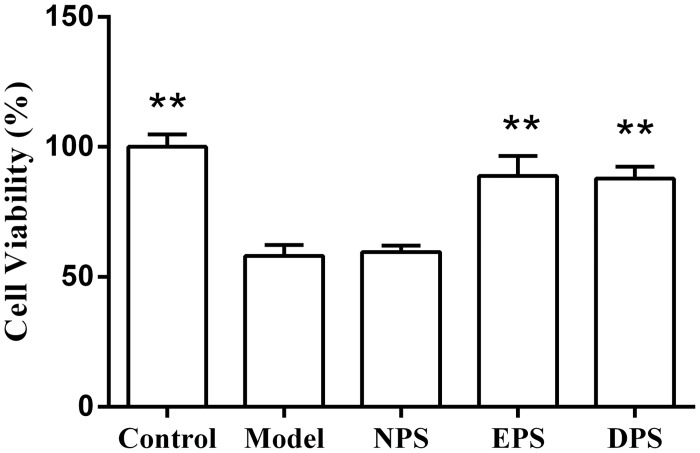
The cells incubated with 1mM H2O2 were found rounding, shriveling and detached from the growth surface. The cells’ viability in H2O2 model was significant reduced to 58.10 ± 4.27% (P < 0.01, Fig 1). The cells’ viabilities of EPS- and DPS-treated HUVECs were 88.76 ± 7.78% and 87.76 ± 4.65%, respectively, significantly higher than the model (P < 0.01). The cells’ viability of NPS-treated HUVECs was not significantly affected as compared with H2O2 model (59.56 ± 2.51% vs 58.10 ± 4.27%, P > 0.05).
Fig 1. Rat LIPC sera increased the cells’ viability of H2O2-injured HUVECs.
HUVECs were pretreated with 5% different sera for 12 h and followed by 2 h incubation with 1 mMH2O2. Cells’ viability was measured by MTT assay and expressed as the ratio of optical density (OD) with OD value of the control as 100%. The data (mean ± SEM) were obtained from at least three independent experiments, **P < 0.01 vs model. Control: cultured with normal medium without any intervention throughout the experiment. Model: cultured with normal medium for 12 h and then incubated with 1mM H2O2 for 2 h. NPS, EPS and DPS: cultured with normal medium containing either 5% NPS (serum derived from rats after sham LIPC), 5% EPS (serum derived from rats 20 min after LIPC) or 5% DPS (serum derived from rats 24 h after LIPC) respectively for 12 h and followed by 2 h incubation with 1mM H2O2. The figure legends are same in following figures unless illustrated elsewhere.
Rat LIPC sera reduced ROS in H2O2-injured HUVECs
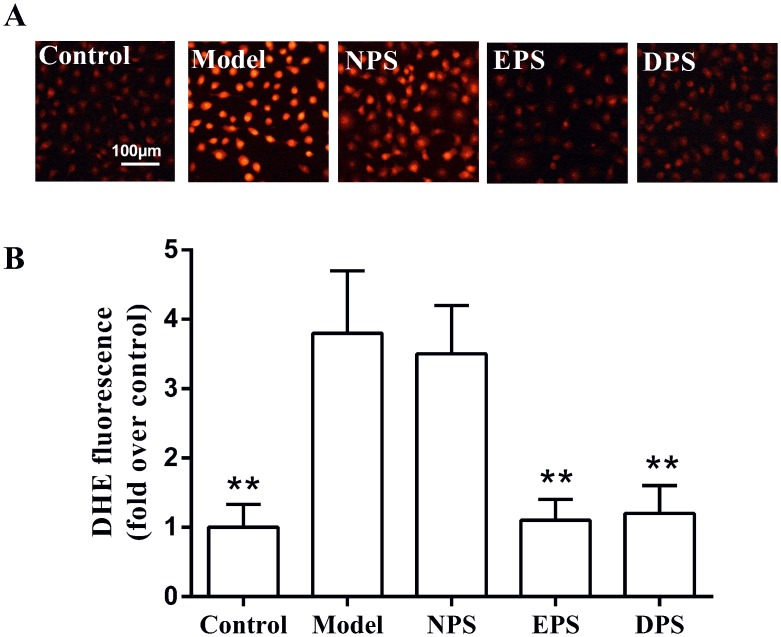
Incubation with H2O2 for 2 h strikingly increased DHE fluorescence intensity (3.8 ± 0.9 fold of control, P < 0.01, Fig 2). Compared with H2O2 model, pretreatment with 5% EPS or 5% DPS for 12 h significantly reduced DHE fluorescence intensity to 1.1 ± 0.3 and 1.2 ± 0.4 fold of control (P < 0.01), respectively. However, NPS had no significant effect on the fluorescence intensification induced by H2O2 in HUVECs (3.5 ± 0.7 vs 3.8 ± 0.9 fold of control, P > 0.05).
Fig 2. Rat LIPC sera decreased ROS in H2O2-injured HUVECs.
ROS level was determined by measuring the intensity of dihydroethidium (DHE) fluorescence. The relative fluorescence intensities of DHE were analyzed by Image-Pro Plus software taking the fluorescence intensity of the control as 100%. A: original images of the cells preloaded with DHE. B: pooled data are expressed as mean ± SEM, n = 5, **P < 0.01 vs model.
Rat LIPC sera decreased MDA and increased activities of antioxidases in H2O2-injured HUVECs
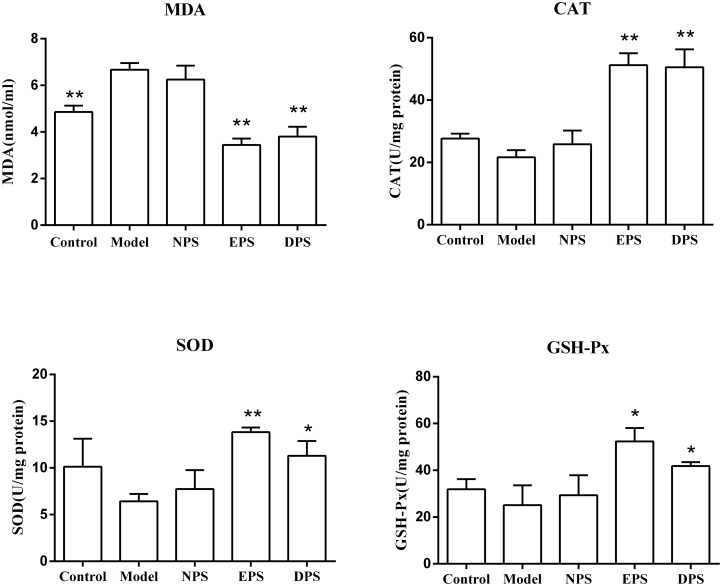
The medium level of MDA is routinely used to reflect the extents of cell membrane damage attacked by H2O2 [16, 17]. Fig 3 shows that incubation with 1 mM H2O2 for 2 h increased the medium MDA concentration from 4.86 ± 0.28 nmol/ml to 6.67 ± 0.29 nmol/ml (P < 0.01). Compared with H2O2 model, MDA levels in EPS and DPS were reduced to 3.44 ± 0.27 nmol/ml (P < 0.01) and 3.80 ± 0.41 nmol/ml (P < 0.01). NPS had no significant effect on MDA level in H2O2-injured HUVECs (6.25 ± 0.59 nmol/ml vs 6.67 ± 0.29 nmol/ml, P > 0.05). Enzymatic study showed that H2O2 incubation did not significantly affected the endothelial intracellular activities of CAT, GSH-Px and SOD, while preincubation with EPS or DPS, but not NPS, significantly elevated the activities of the antioxidases (P < 0.01 vs model).
Fig 3. Rat LIPC sera reduced MDA and increased the activities of antioxidases in H2O2-injured HUVECs.
Medium MDA concentration was measured spectrophotometrically at 532 nm. The activities of CAT, GSH-Px and total SOD were analyzed spectrophotometrically at 240 nm, 340 nm and 550 nm respectively. All data are expressed as mean ± SEM, n = 6, *P < 0.05, **P < 0.01 vs model.
Rat LIPC sera upregulated the expression of antioxidases
Real-time PCR study showed that incubation with either 5% EPS or 5% DPS, but not 5% NPS, significantly increased the mRNA levels of the antioxidases (CAT, SOD-1, SOD-2, GSH-Px-1), compared with model (Fig 4). Statistical analysis revealed that EPS and DPS were almost equipotent on the expression of CAT, SOD-1, SOD-2, but EPS on GSH-Px-1 was weaker than DPS (1.97 ± 0.17 vs 4.66 ± 0.71, P < 0.01).
Fig 4. Rat LIPC sera upregulated the mRNA expression of antioxidases in HUVECs.
CAT, SOD-1, SOD-2 and GSH-Px-1 mRNA levels in HUVECs were detected by real-time PCR. The mRAN expression of antioxidases was presented by normalizing the antioxidases expression with GAPDH and taking control as 100%. All data are expressed as mean ± SEM, n = 6, *P < 0.05, **P < 0.01 vs model.
Rat LIPC sera enhanced nuclear factor E2-related factor 2 (Nrf2) translocation in H2O2-injured HUVECs
Nrf2 is a transcription factor that regulates the expression of cytoprotective genes in response to oxidative stress [18]. As the translocation of Nrf2 into the nucleus is critical for its activation, the distribution of Nrf2 in HUVECs was examined by immunofluorescence in the present study. Fig 5 shows that the pattern of Nrf2 distribution between nucleus and cytoplasm was not significantly different in control, model and NPS. Pretreatment with EPS or DPS significantly intensified the positive signals of Nrf2 in the nucleus.
Fig 5. Rat LIPC sera enhanced Nrf2 localization into nucleus inH2O2-injured HUVECs.
Localization of Nrf2 was performed by immunofluorescence and confocal microscopy. Nrf2 was stained with an anti-Nrf2 antibody and visualized with a secondary antibody conjugated with FITC (green). The nuclei were counterstained with DAPI (4’,-diamidino-2-phenylindole) staining indicating the location of the nucleus (blue). The merged image showed the nuclear location of Nrf2 protein.
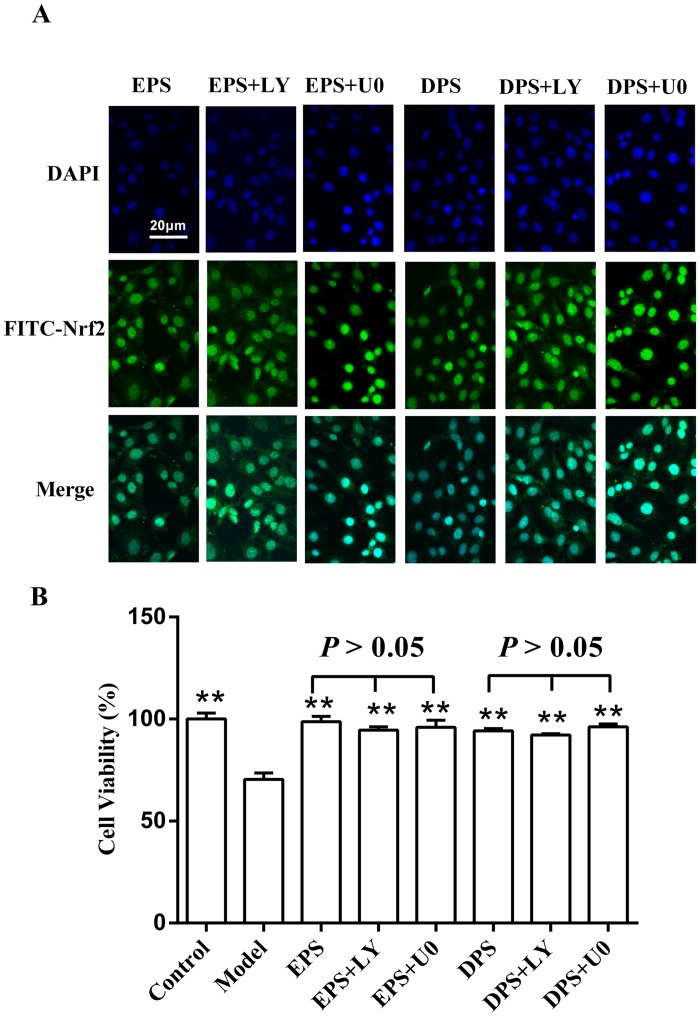
Effects of rat LIPC sera on Nrf2 translocation and cells’ viability were affected by neither PI3K/Akt inhibitor nor MEK/ERK inhibitor in H2O2-injured HUVECs
Since it has been reported that ischemic preconditioning activates the MEK/ERK and PI3K/AKT signaling pathway [6, 19], we studied whether PI3K/Akt and MEK/ERK signaling pathways were involved in LIPC-induced protection. The cells were pretreated with a MEK/ERK inhibitor U0126 (26 μM) or a PI3K/Akt inhibitor LY294002 (25 μM) for 1 h prior to the addition of the sera. The results showed that neither LY294002 nor U0126 significantly affected the effects of rat LIPC sera on Nrf2 translocation (Fig 6A) and cells’ viability in H2O2-injured HUVECs (Fig 6B).
Fig 6. Effects of rat LIPC sera on Nrf2 translocation and cellular viability were affected by neither PI3K/Akt inhibitor nor MEK/ERK inhibitor in H2O2-injured HUVECs.
The cells were cultured in medium for 24 h, after which the inhibitors U0126 (U0, 26 μM) and LY294002 (LY, 25 μM) were added 1 h before the especial serum and H2O2 treatments. A: nucleus localization of Nrf2 determined by immunofluorescence and confocal microscopy. B: cells’ viability detected using MTT method. The data (mean ± SEM) were obtained from at least three independent experiments, **P < 0.01 vs model.
Discussion
The major findings of the present study are (1) LIPC protects the HUVECs from the oxidative stress injury induced by H2O2; (2) the Nrf2 translocation and upregulation of antioxidases including CAT, SOD-1, SOD-2 and GSH-Px-1 are involved in the protection.
Vascular endothelial cells widely distribute in the body and play an irreplaceable role in vascular homeostasis. Endothelium dysfunction is, in most cases, preluded with production of excessive amounts of ROS [20, 21], which appears inevitably in hypertension [22], hyperlipidemia, obesity [23], diabetes [24], ischemia, atherosclerosis and other vascular disorders [25]. Protection of endothelium from oxidative stress with antioxidant drugs is suggested as a promising treatment for cardiovascular diseases [20]. Antioxidative effects were suggested responsible, at least partially, for LIPC-induced protection on injured parenchymal cells of the heart [5, 11] and liver [6]. It has also demonstrated that LIPC is beneficial to endothelium-dependent vasodilation in a number of conditions [1–3, 13, 15]. However, how LIPC affects redox state of endothelium remains largely unknown. The present experiments mimicked oxidative stress by applying H2O2 in cultured HUVECs and studied the protective effects of LIPC. The present study demonstrated that the rat EPS and DPS protected the HUVECs from oxidant stress injury. These results were in consistence with reported results that the cardioprotection of LIPC was transferable across species [12] and that LIPC prevented endothelial I/R injury in conduit vessels with two temporally distinct phases of protection in human in vivo [2].
Based on the fact that inhibition of excessive production of ROS and reinforcement of cellular antioxidant capability protect the cells from the oxidative stress injury, we supposed that LIPC may protect HUVECs by upregulating antioxidases. Nrf2 is a key transcription factor that regulates intracellular redox balance and inflammation through activation of its targeting genes encoding antioxidant and detoxifying molecules [26, 27]. Under normal conditions, Nrf2 binds to Kelch-like erythroid cell-derived protein 1 (Keap1), a cytosolic repressor protein, that disenables Nrf2 translocation from cytoplasm to the nucleus. Upon activation, the Keap1/Nrf2 complex is disaggregated and Nrf2 is released from Keap1 and translocates to the nucleus, where it binds to antioxidant response element (ARE) to induce expression of antioxidant enzymes, which include heme oxygenase-1, SOD, GSH-Px, CAT, NAD(P)H: quinine oxidoreductase 1, thioredoxin-1, etc [28, 29]. Many studies have showed that the activation of Nrf2 was involved in the protection of vascular endothelium [30–32]. The present study demonstrated that pretreatment of HUVECs with EPS or DPS enhanced the translocation of Nrf2 from cytoplasm to nucleus, and markedly increased mRNA levels of antioxidases including CAT, SOD-1, SOD-2, GSH-Px-1. These results suggested that LIPC protected HUVECs from H2O2-induced injury via the Nrf2-ARE axis.
Although the detailed mechanisms via which LIPC confers the cellular protection remains unclarified, it has been suggested that LIPC activated the MEK/ERK in liver cells [6] and PI3K/AKT in cardiac cells [33]. Interestingly, a recent study showed that human LIPC plasma protects HUVECs from hypoxia-induced cell damage with increased phosphorylation of ERK-1/2 [34]. However, in the present study, neither MEK/ERK inhibitor U0126 nor PI3K/AKT inhibitor LY294002 significantly affected the LIPC-induced protective effects and enhancement of Nrf2 translocation. The differences among the reported and our results may be presumably due to following factors: (1) used kinase inhibitors have limited specificity towards the oriented target kinase [35]; (2) observed biologic phenomena may, in almost every case, be results of intricate net regulation rather than of just one signaling pathway; (3) LIPC may be pleiotropic. Further studies are needed to explore the molecular mechanisms underlying LIPC-induced cytoprotection.
In conclusion, the present study demonstrates that LIPC protects HUVECs from H2O2-induced damage and suggests that Nrf2 translocation and the upregulation of its downstream antioxidase expression may be involved in the cytoprotection.
Acknowledgments
This work was funded by the Key Discipline Construction Funds of Shanxi Province, Shanxi Province Nature Science Fund No 2011011034–3 and the Shanxi Medical University Research Fund for the Doctoral Program No 03201310.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by the Key Discipline Construction Funds of Shanxi Province, Shanxi Province Nature Science Fund, No. 2011011034-3, and the Shanxi Medial University Research Fund for the Doctoral Program, No. 03201310.
References
- 1. Contractor H, Stottrup NB, Cunnington C, Manlhiot C, Diesch J, Ormerod JO, et al. Aldehyde dehydrogenase-2 inhibition blocks remote preconditioning in experimental and human models. Basic research in cardiology. 2013;108(3):343 10.1007/s00395-013-0343-3 . [DOI] [PubMed] [Google Scholar]
- 2. Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. Journal of the American College of Cardiology. 2005;46(3):450–6. 10.1016/j.jacc.2005.04.044 . [DOI] [PubMed] [Google Scholar]
- 3. Luca MC, Liuni A, McLaughlin K, Gori T, Parker JD. Daily ischemic preconditioning provides sustained protection from ischemia-reperfusion induced endothelial dysfunction: a human study. Journal of the American Heart Association. 2013;2(1):e000075 10.1161/JAHA.112.000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zitta K, Meybohm P, Bein B, Heinrich C, Renner J, Cremer J, et al. Serum from patients undergoing remote ischemic preconditioning protects cultured human intestinal cells from hypoxia-induced damage: involvement of matrixmetalloproteinase-2 and -9. Molecular medicine. 2012;18:29–37. 10.2119/molmed.2011.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou C, Li H, Yao Y, Li L. Delayed remote ischemic preconditioning produces an additive cardioprotection to sevoflurane postconditioning through an enhanced heme oxygenase 1 level partly via nuclear factor erythroid 2-related factor 2 nuclear translocation. Journal of cardiovascular pharmacology and therapeutics. 2014;19(6):558–66. 10.1177/1074248414524479 . [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, et al. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PloS one. 2014;9(6):e98834 10.1371/journal.pone.0098834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Randhawa PK, Bali A, Jaggi AS. RIPC for multiorgan salvage in clinical settings: evolution of concept, evidences and mechanisms. European journal of pharmacology. 2015;746:317–32. 10.1016/j.ejphar.2014.08.016 . [DOI] [PubMed] [Google Scholar]
- 8. Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circulation research. 2014;114(10):1601–10. 10.1161/CIRCRESAHA.114.303822 . [DOI] [PubMed] [Google Scholar]
- 9. Bell RM, Yellon DM. Conditioning the whole heart—not just the cardiomyocyte. Journal of molecular and cellular cardiology. 2012;53(1):24–32. 10.1016/j.yjmcc.2012.04.001 . [DOI] [PubMed] [Google Scholar]
- 10. Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, et al. Rabbit heart can be "preconditioned" via transfer of coronary effluent. The American journal of physiology. 1999;277(6 Pt 2):H2451–7. . [DOI] [PubMed] [Google Scholar]
- 11. Zhou C, Li L, Li H, Gong J, Fang N. Delayed remote preconditioning induces cardioprotection: role of heme oxygenase-1. The Journal of surgical research. 2014;191(1):51–7. 10.1016/j.jss.2014.03.054 . [DOI] [PubMed] [Google Scholar]
- 12. Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clinical science. 2009;117(5):191–200. 10.1042/CS20080523 . [DOI] [PubMed] [Google Scholar]
- 13. Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. American journal of hypertension. 2014;27(7):918–25. 10.1093/ajh/hpu004 . [DOI] [PubMed] [Google Scholar]
- 14. Bailey TG, Birk GK, Cable NT, Atkinson G, Green DJ, Jones H, et al. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. American journal of physiology Heart and circulatory physiology. 2012;303(5):H533–8. 10.1152/ajpheart.00272.2012 . [DOI] [PubMed] [Google Scholar]
- 15. Manchurov V, Ryazankina N, Khmara T, Skrypnik D, Reztsov R, Vasilieva E, et al. Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. The American journal of medicine. 2014;127(7):670–3. 10.1016/j.amjmed.2014.02.012 . [DOI] [PubMed] [Google Scholar]
- 16. Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chemical reviews. 2011;111(10):5944–72. 10.1021/cr200084z . [DOI] [PubMed] [Google Scholar]
- 17. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine. 1991;11(1):81–128. . [DOI] [PubMed] [Google Scholar]
- 18. Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochemical pharmacology. 2013;85(6):705–17. 10.1016/j.bcp.2012.11.016 . [DOI] [PubMed] [Google Scholar]
- 19. Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circulation research. 2000;87(4):309–15. . [DOI] [PubMed] [Google Scholar]
- 20. Weseler AR, Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments. Current hypertension reports. 2010;12(3):154–61. 10.1007/s11906-010-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxidants & redox signaling. 2014;20(17):2794–814. 10.1089/ars.2013.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Dong J, Liu P, Lau CW, Gao Z, Zhou D, et al. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. British journal of pharmacology. 2014;171(13):3171–81. 10.1111/bph.12660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. da Cunha NV, Pinge-Filho P, Panis C, Silva BR, Pernomian L, Grando MD, et al. Decreased endothelial nitric oxide, systemic oxidative stress, and increased sympathetic modulation contribute to hypertension in obese rats. American journal of physiology Heart and circulatory physiology. 2014;306(10):H1472–80. 10.1152/ajpheart.00520.2013 . [DOI] [PubMed] [Google Scholar]
- 24. Joshi M, Kotha SR, Malireddy S, Selvaraju V, Satoskar AR, Palesty A, et al. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Molecular and cellular biochemistry. 2014;386(1–2):233–49. 10.1007/s11010-013-1861-x . [DOI] [PubMed] [Google Scholar]
- 25. Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed). 2011;16:1733–45. . [DOI] [PubMed] [Google Scholar]
- 26. Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicology and applied pharmacology. 2010;244(1):57–65. 10.1016/j.taap.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants & redox signaling. 2005;7(3–4):385–94. 10.1089/ars.2005.7.385 . [DOI] [PubMed] [Google Scholar]
- 28. Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free radical biology & medicine. 2009;47(9):1304–9. 10.1016/j.freeradbiomed.2009.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. The Journal of biological chemistry. 2009;284(20):13291–5. 10.1074/jbc.R900010200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pecorelli A, Bocci V, Acquaviva A, Belmonte G, Gardi C, Virgili F, et al. NRF2 activation is involved in ozonated human serum upregulation of HO-1 in endothelial cells. Toxicology and applied pharmacology. 2013;267(1):30–40. 10.1016/j.taap.2012.12.001 . [DOI] [PubMed] [Google Scholar]
- 31. Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. American journal of physiology Heart and circulatory physiology. 2011;300(4):H1133–40.: 10.1152/ajpheart.00402.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2011;21(4):277–85. 10.1016/j.numecd.2009.12.008 . [DOI] [PubMed] [Google Scholar]
- 33. Li J, Xuan W, Yan R, Tropak MB, Jean-St-Michel E, Liang W, et al. Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of beta-catenin. Clinical science. 2011;120(10):451–62. 10.1042/CS20100466 . [DOI] [PubMed] [Google Scholar]
- 34. Weber NC, Riedemann I, Smit KF, Zitta K, van de Vondervoort D, Zuurbier CJ, et al. Plasma from human volunteers subjected to remote ischemic preconditioning protects human endothelial cells from hypoxia-induced cell damage. Basic research in cardiology. 2015;110(2):17: 10.1007/s00395-015-0474-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saponara S, Fusi F, Sgaragli G, Cavalli M, Hopkins B, Bova S. Effects of commonly used protein kinase inhibitors on vascular contraction and L-type Ca(2+) current. Biochemical pharmacology. 2012;84(8):1055–61. 10.1016/j.bcp.2012.07.025 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.