Abstract
The application of nanomaterial in cancer treatment is promising and intriguing. Anti-tumor immunotherapy has the potential to significantly improve the prognosis of cancer treatment, though the efficacy of immunotherapy generally needs further improvement. One way to improve the efficacy is using immune adjuvants, but the adjuvants for anticancer immunotherapy have to be more potent than for prophylactic vaccines. Here, we report that compared to conventional alum adjuvant, aluminum oxide nanoparticles (nano-alum) may further enhance the anticancer effects of an immunotherapy that employs tumor cell vaccine (TCV). The average tumor size tends to be lower in animals that receive the combinational treatment of nano-alum and TCV. The anticancer cytotoxicity by the lymphocytes was also significantly higher in the treatment group that received both TCV and nano-alum. These results suggest that nano-alum may potentially serve as a potent immune adjuvant and have prospective applications in anticancer immunotherapy.
Keywords: Aluminum, Nanoparticle, Anticancer, Immune adjuvant
Introduction
Nanotechnology may have important applications in multiple biomedical fields (Williams et al. 2002; Heller et al. 2006; Elder et al. 2008; Jain 2008; Cai et al. 2005; Fenske and Cullis 2008; Bianco et al. 2005a; Martin and Kohli 2003; Liu et al. 2005; Ni et al. 2005; Sun et al. 2002; Zanello et al. 2006; Bianco et al. 2005b; Hartman et al. 2008), and the use of nanomaterial in cancer treatment is particularly intriguing (Fifis et al. 2004; Farokhzad et al. 2006). One important issue in cancer immunology is the search of novel immune adjuvants that can enhance the efficacy of anti-tumor immunotherapy (Mesa and Fernandez 2004). Immunotherapy is an important form of adjunctive cancer treatment that may significantly improve the prognosis (Vermorken et al. 1999; Schirrmacher 2005; Takayama et al. 2000). Prior clinical trials of immunotherapy have achieved promising results in treating malignancies such as melanoma, malignant glioma, or renal cell carcinoma (Berd et al. 2004; Yu et al. 2004; Rosenberg et al. 1994), which tend to respond poorly to chemotherapies. Nevertheless, the efficacy of current anticancer immunotherapy generally needs further improvement (Emens 2006). Because tumor antigens are usually self-derived and are, therefore, poorly immunogenic, the adjuvants for anticancer immunotherapy have to be more potent than for prophylactic vaccines (Mesa and Fernandez 2004). One way to increase the efficacy is using immune adjuvants, which are defined as products that increase the immune response toward antigens. The identification of novel and more efficacious adjuvants is thus of practical importance for anticancer immunotherapy (Mesa and Fernandez 2004; Emens 2006).
Aluminum nanoparticle (nano-alum) may serve as a candidate of prospective adjuvant for anticancer immunotherapy, which needs to be both safe and efficacious. Alum (aluminum hydroxide, phosphate, or hydroxyphosphate) has an excellent safety record for systemic vaccination. It has been used as adjuvant for more than 60 years and is at present the most widely used adjuvant in both veterinary and human vaccines (Mesa and Fernandez 2004; Lindblad 2004). However, alum is mostly a Th2 stimulator of the immune system and primarily enhances the antibody-mediated reaction (Lindblad 2004). It has limited influence on the cytotoxic T cell-mediated response, which is considered the main mechanism of anticancer immune effects (Mesa and Fernandez 2004; Berd et al. 2004; Lindblad 2004). Since nanosized materials often exhibit unique properties that may offer significant advantage for biomedical applications (Jain 2008; Martin and Kohli 2003), it is sensible to investigate if nano-alum will be more efficacious in enhancing anticancer immune response than conventional alum. Here, we studied whether nano-alum would influence the efficacy of an immunotherapy that employs tumor cell vaccine (TCV), to evaluate nano-alum’s applicability as an adjuvant in a commonly used anticancer immune regimen.
Materials and methods
Compounds
Nano-alum (Al2O3, MW 101.96) was purchased from Degussa Nanotechnology of Evonik Industries. Scanning electron microscopy (SEM) was performed to evaluate the size of the nanoparticles. Conventional alum in the form of Al(OH)3 (analytical grade, MW 78.0) was purchased from Beijing Chemical Reagents Company.
Animal
BALB/c mice were purchased from the Experimental Animal Institute of the Chinese Academy of Medical Sciences (Beijing, China), bred and maintained under defined flora conditions in individually ventilated (high-efficiency particle-arresting filtered air) sterile microisolator cages (Techniplast, Milan, Italy). All animal handling and experimental procedures were approved by the Animal Care and Use Committee of the Chinese Academy of Medical Sciences.
Preparations of alum or nano-alum adjuvants
Conventional alum in the form of Al(OH)3 was mixed with D-Hanks solution at a concentration of 500 µg per ml (6.4 mM/l) and fully sonicated, yielding a suspension ready for further use. Nano-alum was mixed with D-Hanks solution at the concentration of 326.9 µg per ml, to ensure that the final molar concentration of nano-alum (6.4 mM/l) is the same as that of the conventional alum adjuvant. The nano-alum mixture was then sonicated immediately prior to application, yielding a semi-clear solution ready for immune studies.
Preparations of tumor cell vaccine
TCV was prepared by incubating H22 liver cancer cells in D-Hanks solutioin containing Mitomycin C (80 mg/l) for 60 min, followed by thorough wash with D-Hanks for five times.
Experiments of immunotherapy
Immunotherapy experiments were conducted using female adult BALB/c mice, 8 weeks of age, weighing 18 to 22 g. Twenty-eight mice were randomly divided into four groups: (1) the control group, (2) the TCV treatment group, (3) the TCV plus conventional alum (TCV + alum) treatment group, and (4) the TCV plus nano-alum (TCV + nano-alum) treatment group. On day zero, every mouse was inoculated with 2 × 106 live H22 cells subcutaneously in the right hind leg. On day 7 and 14, the treatment groups also received two subcutaneous doses of designed immune treatment agents in the left hind leg for triggering anti-tumor reactions. Specifically, the TCV group received 2 × 106 TCV cells in 200 µl D-Hanks; the TCV + alum group received 2 × 106 TCV cells in 100 µl D-Hanks mixed with 100 µl of alum adjuvant; and the TCV + nano-alum group received 2 × 106 TCV cells in 100 µl D-Hanks mixed with 100 µl of nano-alum adjuvant. On day 7 and day 14, the control group also received injections of 200 µl D-Hanks that did not contain TCV or adjuvant. Tumor dimensions were monitored using calipers at right angles every 3 to 4 days. The tumor size was measured using the product of the two longest dimensions perpendicular to each other.
Immune cytotoxicity studies
Animals randomly picked from all groups were terminated on day 30, and peripheral blood lymphocytes were extracted and co-incubated with the H22 tumor cells. Ten thousand H22 tumor cells (target cells) were seeded on the bottom of 96-well plates (Falcon) at a density of 2 × 108/L. Periphery murine blood monocytes/lymphocytes (effector cells) were obtained through standard Ficoll gradient centrifugation. Effector cells (1 × 105 in 50 μL) were added to the target cells, keeping the effector-to-target ratio at 10:1. The final combined volume per well was 100 μL. Tissue culture plate was centrifuged at 250 rpm for 5 min to ensure cell–cell contact, then incubated at 37°C with 5% CO2 for 4 h. The lactate dehydrogenase (LDH) assay reagents were added per manufacturer’s (Promega) instruction. The optical density of the supernatant was measured using an ELISA plate reader with a 490 nm filter. The values of effector cells’ spontaneous LDH release, target cells’ spontaneous LDH release, target cells’ maximum LDH release, and culture medium background reading were also measured. The percentage of target cell killed was then calculated per manufacturer’s protocol. The percent tumor cell killed was calculated according to the following equation:
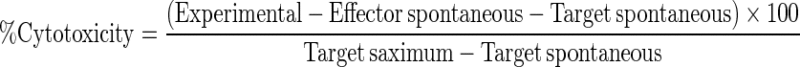 |
Histological studies of the tumor tissue
Tumor tissues were fixed and imbedded in paraffin. Tumor sections of 5 μm were cut from the embedded tissue and stained by standard hematoxylin and eosin procedure (HE stain), for evaluation of tumor necrosis and lymphocyte infiltration.
Statistics
Statistical analysis was performed with the statistical SPSS 13.0 software. The nonparametric test was used to calculate the probability of significant differences among the groups. Statistical significance was defined as p < 0.05.
Results
Characterization of the nano-alum
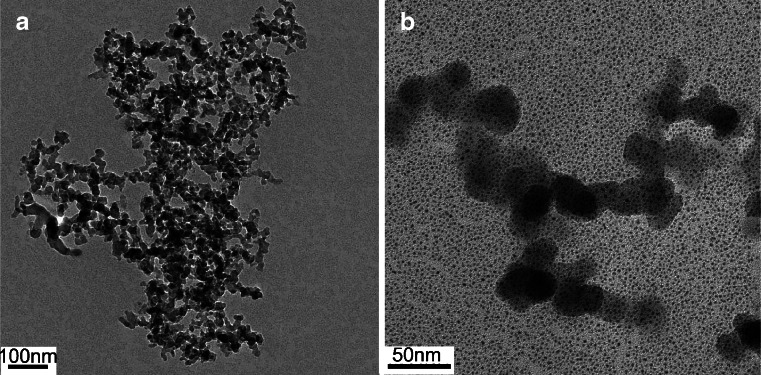
SEM of the nano-alum was carried out to evaluate the size of the nanoparticles. The average diameter of the aluminum nanoparticles was 20–30 nm (Fig. 1a and b). For immune experiments, nano-alum adjuvant was freshly made through mixing with D-Hanks solution, and sonicated immediately prior to the injection, yielding semi-clear solution that was stable and without precipitation for the entire duration of the experiment.
Fig. 1.
Scanning electron microscopy images of the aluminum nanoparticle at low (a) and high resolutions (b). The average particle size was about 20–30 nm
Tumor size study
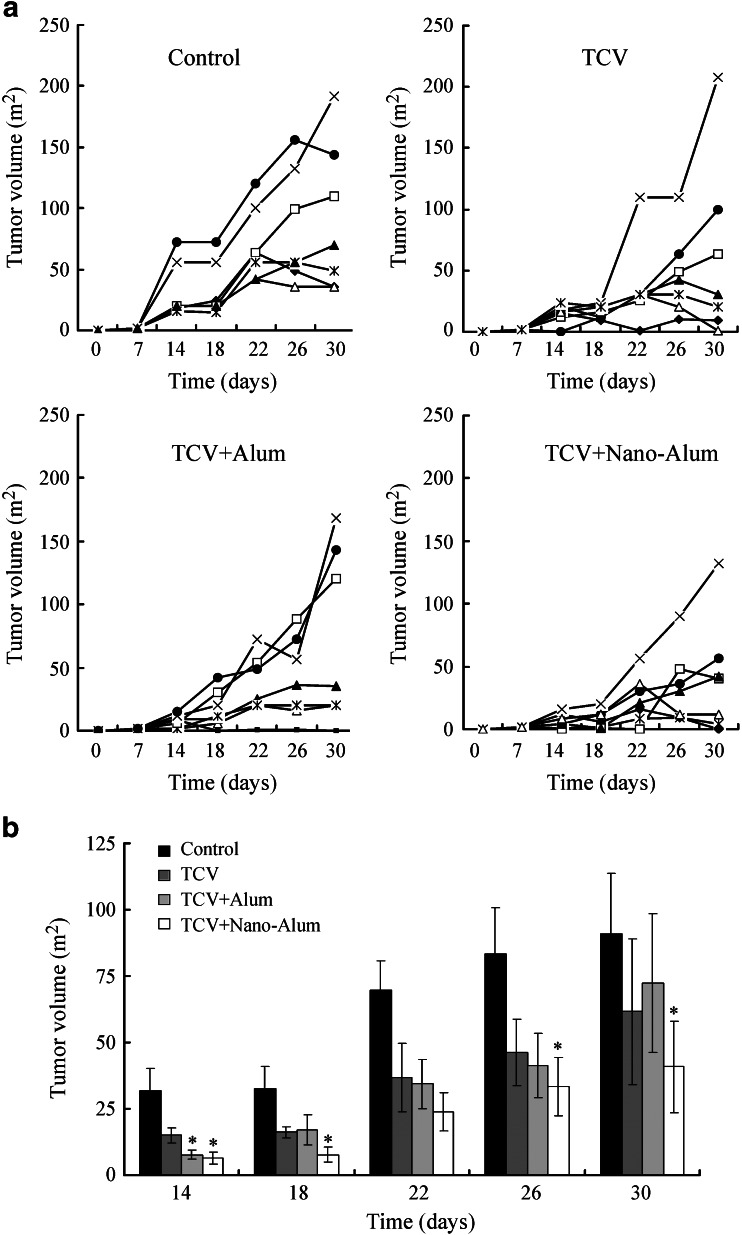
The animal study employed the H22 murine hepatoma because it was a mature tumor model that had been frequently used in immunotherapy research (Luo et al. 2006). The outcome of the immune treatment was assayed directly by the tumor size. The individual tumor growth curve of each animal was provided in Fig. 2a. After inoculation with H22 cells, most animals gradually developed detectable subcutaneous tumor mass in 5 to 10 days. In the control group, most tumors steadily increased in size over the entire duration of the experiment, whereas in the treatment groups, some tumors tended to grow at slower rate. Moreover, in some animals that received treatments, the tumors stopped growing and gradually shrank in size after about 20 days (Fig. 2a). The averaged tumor size of each group was shown in Fig. 2b: all the treatment groups tended to have lower average tumor size than the control group, with the lowest average tumor size belonging to the TCV + nano-alum group. On day 14, the tumor size of the control group, the TCV group, the TCV + alum group, and the TCV + nano-alum group was 31.71 ± 8.53 mm2, 15 ± 2.88 mm2, 7.71 ± 1.83 mm2, and 6.42 ± 2.22 mm2, respectively. On this day, both the TCV + alum and the TCV + nano-alum groups had significantly smaller average tumor size compared to the control group (p < 0.05). From day 18 to day 30, the differences in average tumor size among the groups gradually became more prominent. On day 30, the tumor size was 91 ± 22.69 mm2 for the control group, 61.27 ± 27.63 mm2 for the TCV group, 72.28 ± 26.05 mm2 for the TCV + alum group, and 40.85 ± 17.18 m2 for the TCV + nano-alum group. The difference between the TCV + nano-alum group and the control group was statistically significant (p < 0.05) on days 18, 22, 26, and 30, whereas none of the other treatment groups showed a significant difference from the control group. The results suggested that nano-alum probably enhanced the anticancer reaction more efficiently, resulting in the lower average tumor size observed in the TCV + nano-alum group.
Fig. 2.
Tumor growth studies with the tumor size measured every 4 days. a Individual tumor growth curves of each experiment group. b Average tumor size of each experiment group (±SE, n = 7 for each group). The star indicates a statistically significant difference (p < 0.05) from the control group
Immune cytotoxicity studies
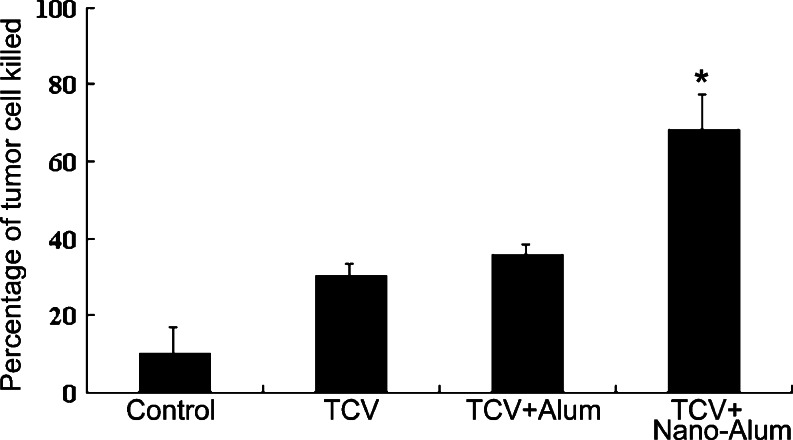
To explore the mechanism of the improved outcome observed in the treatment groups, cellular immunological experiments were conducted. Specifically, the lymphocytes’ anti-tumor cytotoxicity was evaluated and compared among the groups. Animals randomly picked from all groups were terminated on day 30, and peripheral blood lymphocytes were extracted and co-incubated with the H22 tumor cells. The cytotoxic effects of the lymphocytes against the H22 cells were then measured using a standard LDH assay. As shown in Fig. 3: the percentage of tumor cell killed was 9.9 ± 6.9 for the control group, 30.1 ± 3.09 for the TCV group, 35.63 ± 2.8 for the TCV + alum group, and 67.96 ± 9.54 for the TCV + nano-alum group. Thus, the TCV + nano-alum group generated significantly higher anticancer cytotoxicity compared to the other treatment groups (p < 0.05). The results suggested that nano-alum had a more potent adjuvant effect than conventional alum, and better enhanced the anti-tumor response induced by TCV.
Fig. 3.
In vitro anti-tumor cytotoxicity studies. The lymphocytes’ cytotoxicity against the H22 tumor cells were measured using a standard LDH assay at day 30 of the experiment. The most potent anti-tumor cytotoxicity was observed in the TCV + nano-alum group (*p < 0.05, ±SE, n = 6)
Histological studies of the tumor tissue
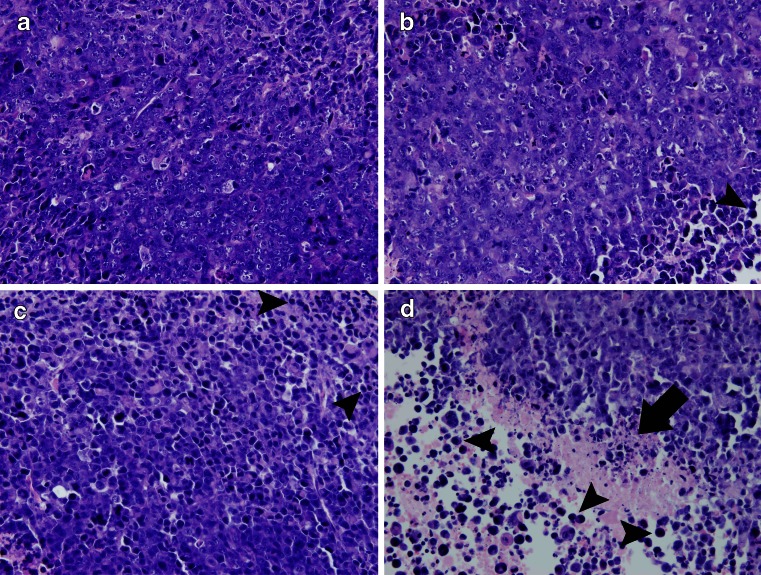
Histological studies of the tumor tissue were also performed to evaluate the treatment outcome. The tumor tissue of the control group was found relatively well maintained with minimal lymphocyte infiltration (Fig. 4a). In the treatment groups, however, the tumor tissues were infiltrated with more lymphocytes (Fig. 4b, c, d). Moreover, the tumor tissue of the TCV + nano-alum group developed obvious necrosis (Fig. 4d), suggesting that anti-tumor immune reaction was actively in process.
Fig. 4.
Histological slides of the tumor tissues stained with hematoxylin and eosin. a Control group. b TCV group. c TCV + alum group. d TCV + nano-alum group. Lymphocyte infiltrations were found in all the treatment groups (b, c, d, arrowheads). Tumor necrosis was observed in the TCV + nano-alum group (d, straight arrow)
Discussion
The aim of this study was to investigate if nano-alum would be a more potent adjuvant than conventional alum in an anti-tumor immunotherapy employing TCV. The result showed that nano-alum enhanced lymphocytes’ cytotoxicity against the H22 liver cancer cells, whereas conventional alum failed to generate a positive immune stimulating effect (Fig. 3). The average tumor size in the TCV + nano-alum group also tended to be lower (Fig. 2), presumably because of the enhanced anti-tumor immune cytotoxicity in this group. In addition, histological study also revealed increased lymphocyte infiltration and tumor necrosis in the TCV + nano-alum group (Fig. 4), again suggesting that nano-alum boosted the anti-tumor response.
Frey et al. reported that aluminum nanoparticles chemically conjugated to the C4 domain of the gp120 HIV protein can generate relatively high antibody response toward gp120 (Frey et al. 1999). Here, we showed that nano-alum alone, without conjugation to antigen, could enhance the TCV-induced anticancer immune response, and that conventional alum failed to generate a similar effect (Fig. 3). Both studies suggest that nano-alum is a more potent immune adjuvant than conventional alum. This presumably could be attributed to the unique physical and chemical properties of nano-alum, which might be more favorable for immune stimulation. It should be noted, however, that the detailed mechanisms of both conventional alum and nano-alum adjuvants remain to be unveiled to date (Lindblad 2004), and much further research is still warranted.
TCV was used in this study for triggering immune response. TCV carries a mixture of tumor proteins and is multivalent in terms of tumor antigens (Copier and Dalgleish 2006). The major advantage of TCV is that it may be applied in treatment of multiple malignancies, irrespective of the genetic makeup of the cancer. However, the lack of clearly defined tumor antigens in TCV therapy renders the antibody responses difficult to assess. Because the nature of the immunogens was not known, the efficacy of TCV therapy was generally measured by tumor growth retardation or prolonged survival, and not by antibody production (Copier and Dalgleish 2006; Levy and Colombetti 2006). Nevertheless, some clinical studies based on such vaccines have generated promising results (Vermorken et al. 1999; Schirrmacher 2005). In this study, nano-alum was found to enhance the anti-tumor immunity induced by TCV. Since TCV can often be made from surgically resected tumors, nano-alum thus may have potential application in immune treatment of multiple types of tumors that are surgically accessible.
In summary, this study showed that nano-alum enhanced the anticancer immune response induced by TCV. The results suggest that nano-alum may potentially serve as an effective adjuvant in anticancer immunotherapies.
Acknowledgment
The authors thank Professors Hui Li and Yan Shen of the Chinese Academy of Medical Sciences for insightful suggestions. This work was made possible by the funding support from the China Medical Board, the Ministry of Science and Technology (2006CB933204), and Natural Science Foundation of Beijing (Z0005190043511).
Footnotes
Zhao Sun and Wei Wang contributed equally to this paper.
Contributor Information
Haiyan Xu, Phone: +86-10-65296437, FAX: +86-10-65296437, Email: xuhy@pumc.edu.cn.
Chen Wang, Phone: +86-10-65296437, FAX: +86-10-65296437, Email: wangch@iccas.ac.cn.
Xian-Da Yang, Phone: +86-10-65296437, FAX: +86-10-65296437, Email: ayangmd@gmail.com.
References
- Berd D, Sato T, Maguire HC, Jr, Kairys J, Mastrangelo MJ. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol. 2004;22:403–415. doi: 10.1200/JCO.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Bianco A, Kostarelos K, Partidos CD, Prato M. Biomedical applications of functionalised carbon nanotubes. Chem Commun (Camb) 2005;5:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Cai D, Mataraza JM, Qin ZH, Huang Z, Huang J, Chiles TC, Carnahan D, Kempa K, Ren Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods. 2005;2:449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- Copier J, Dalgleish A. Overview of tumor cell-based vaccines. Int Rev Immunol. 2006;25:297–319. doi: 10.1080/08830180600992472. [DOI] [PubMed] [Google Scholar]
- Elder JB, Liu CY, Apuzzo ML. Neurosurgery in the realm of 10(-9), part 2: applications of nanotechnology to neurosurgery—present and future. Neurosurgery. 2008;62:269–284. doi: 10.1227/01.neu.0000315995.73269.c3. [DOI] [PubMed] [Google Scholar]
- Emens LA. Roadmap to a better therapeutic tumor vaccine. Int Rev Immunol. 2006;25:415–443. doi: 10.1080/08830180600992423. [DOI] [PubMed] [Google Scholar]
- Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5:25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- Frey A, Mantis N, Kozlowski PA, Quayle AJ, Bajardi A, Perdomo JJ, Robey FA, Neutra MR. Immunization of mice with peptomers covalently coupled to aluminum oxide nanoparticles. Vaccine. 1999;17:3007–3019. doi: 10.1016/S0264-410X(99)00163-2. [DOI] [PubMed] [Google Scholar]
- Hartman KB, Wilson LJ, Rosenblum MG. Detecting and treating cancer with nanotechnology. Mol Diagn Ther. 2008;12:1–14. doi: 10.1007/BF03256264. [DOI] [PubMed] [Google Scholar]
- Heller DA, Jeng ES, Yeung TK, Martinez BM, Moll AE, Gastala JB, Strano MS. Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science. 2006;311:508–511. doi: 10.1126/science.1120792. [DOI] [PubMed] [Google Scholar]
- Jain KK. Nanomedicine: application of nanobiotechnology in medical practice. Med Princ Pract. 2008;17:89–101. doi: 10.1159/000112961. [DOI] [PubMed] [Google Scholar]
- Levy F, Colombetti S. Promises and limitations of murine models in the development of anticancer T-cell vaccines. Int Rev Immunol. 2006;25:269–295. doi: 10.1080/08830180600992407. [DOI] [PubMed] [Google Scholar]
- Lindblad EB. Aluminium adjuvants—in retrospect and prospect. Vaccine. 2004;22:3658–3668. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu DC, Zhang WD, Jiang X, He CB, Chung TS, Goh SH, Leong KW. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew Chem Int Ed Engl. 2005;44:4782–4785. doi: 10.1002/anie.200500042. [DOI] [PubMed] [Google Scholar]
- Luo Y, Wen YJ, Ding ZY, Fu CH, Wu Y, Liu JY, Li Q, He QM, Zhao X, Jiang Y, Li J, Deng HX, Kang B, Mao YQ, Wei YQ. Immunotherapy of tumors with protein vaccine based on chicken homologous Tie-2. Clin Cancer Res. 2006;12:1813–1819. doi: 10.1158/1078-0432.CCR-05-1990. [DOI] [PubMed] [Google Scholar]
- Martin CR, Kohli P. The emerging field of nanotube biotechnology. Nat Rev Drug Discov. 2003;2:29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- Mesa C, Fernandez LE. Challenges facing adjuvants for cancer immunotherapy. Immunol Cell Biol. 2004;82:644–650. doi: 10.1111/j.0818-9641.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- Ni Y, Hu H, Malarkey EB, Zhao B, Montana V, Haddon RC, Parpura V. Chemically functionalized water soluble single-walled carbon nanotubes modulate neurite outgrowth. J Nanosci Nanotechnol. 2005;5:1707–1712. doi: 10.1166/jnn.2005.189. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. Jama. 1994;271:907–913. doi: 10.1001/jama.271.12.907. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol Immunother. 2005;54:587–598. doi: 10.1007/s00262-004-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Fu K, Lin Y, Huang W. Functionalized carbon nanotubes: properties and applications. Acc Chem Res. 2002;35:1096–1104. doi: 10.1021/ar010160v. [DOI] [PubMed] [Google Scholar]
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG, Jr, Pinedo HM. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- Williams KA, Veenhuizen PT, de la Torre BG, Eritja R, Dekker C. Nanotechnology: carbon nanotubes with DNA recognition. Nature. 2002;420:761. doi: 10.1038/420761a. [DOI] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6:562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]