Abstract
RRM2B is a critical ribonucleotide reductase (RR) subunit that exists as p53-inducible and p53-dependent molecule. The p53-independent regulation of RRM2B has been recently studied, and FOXO3 was identified as a novel regulator of RRM2B. However, the p53-independent regulation of RRM2B, particularly under oxidative stress, remains largely unknown. In this study, we investigated the role of RRM2B underoxidative stress-induced DNA damage and further examined the regulation of mitochondrial and inflammatory genes by RRM2B. Our study is the first to report the critical role of RRM2B in mitochondrial homeostasis and the inflammation signaling pathway in a p53-independent manner. Furthermore, our study provides novel insights into the role of the RR in inflammatory diseases.
1. Introduction
Ribonucleotide reductase (RR) catalyzes the conversion of ribonucleoside diphosphates into deoxyribonucleoside diphosphates, playing essential roles in DNA synthesis and repair in humans and influencing vital cellular mechanisms [1–5]. The molecular regulations of the three known RR subunits, RRM1, RRM2, and RRM2B (also called p53R2), have long been studied and reported by our and other groups [6–15]. Previous research has established that RRM2B, a p53-inducible RR subunit, plays vital roles in DNA repair, cell cycle modulation, mitochondrial DNA (mtDNA) synthesis, metastasis suppression, and oxidative stress resistance [1, 6, 7, 9, 16–19]. Mutation or absence of Rrm2b in humans results in defective mtDNA, and severe mtDNA depletion has been observed in Rrm2b−/− animals [9, 16]. In cells, RRM2B can be regulated by p53 and p73, a p53 family member. In addition, RRM2B regulates the p53-dependent cell cycle for DNA damage [6]. RRM2B can suppress the metastasis and proliferation of different cancer cells [20]. A study on a Rrm2b-knockout animal model indicated that the intactness of the RRM2B subunit is critical for maintaining chromosomal stability and that the loss of RRM2B results in plasmacytic neoplasms [21]. Furthermore, RRM2B expression is correlated with improved survival in some cancers, whereas a more progressive phenotype of certain other cancers complicates the RRM2B regulatory pathway [22–24].
Several reports have suggested that RRM2B regulates critical cellular mechanisms irrespective of the p53 status. First, although the p53-mediated RRM2B induction is inhibited in the p53-deficient mouse embryonic fibroblasts cells, it has been observed that RRM2B is expressed at basal levels [16]. Furthermore, a high RRM2B expression has been observed in various p53-deficient cancer cells, and it continues to influence mitochondrial functions irrespective of the p53 status, suggesting that RRM2B-mediated mitochondrial homeostasis is independent of functional p53, and other factors are involved in RRM2B regulation [25, 26]. The findings of our recent study are consistent with those of the aforementioned studies, in which the tumor suppressor FOXO3 binds to the RRM2B promoter and activates RRM2B transcription in a p53-independent manner under physiological conditions [27].
RRM2B is a unique member among the RR subunits that can resist reactive oxygen species (ROS) [8, 19, 25, 28, 29]. However, little is known about the detailed mechanisms and the functional regulations of RRM2B under oxidative stress. We studied the functional regulations of RRM2B in cancer cells under oxidative stress and observed that RRM2B plays a crucial role in the regulation of mitochondrial and inflammation pathways in a p53-independent manner.
2. Materials and Methods
2.1. Cell Lines, Plasmids, and Stable Cell Line Production
Cells from ATCC were cultured in DMEM medium containing fetal bovine serum (10%) and penicillin/streptomycin (1%) incubated at 37°C with 5% CO2. RRM2B overexpression and shRNA plasmids were described before [28]. Stable cell lines were established by infection and selection as described [28].
2.2. Immunofluorescence
The assay was performed as described [30]. In brief, H1299 cells were seeded on coverslips, fixed, permeabilized, stained with primary antibodies, anti-RRM2B (Rockland) and γ-H2AX (Active Motif), and secondary antibodies (Invitrogen), washed, and then mounted with DAPI (Invitrogen) onto the slides. Samples were analyzed by immunofluorescence microscope system, and the scale bars indicate 10 μM.
2.3. Western Blot Analysis and Cytoplasmic/Nuclear Protein Extraction
Cell extracts were prepared and analyzed as described before [31]. Antibodies used in this assay were RRM2B (Rockland), γ-H2AX (Active Motif), GAPDH (Santa Cruz and GeneTex), VDAC1, COX4, p-NFκB, p-p38, and p-IκB (GeneTex). The cytoplasmic/nuclear extraction kit was applied to separate cytoplasmic and nuclear fractions as described in the manufactory protocol (TOOLS) before processing to the western on phospho antibodies. Quantitative protein expression relative to GAPDH was analyzed by Image J where applicable.
2.4. Mitochondrial Mass Measurement
Cells were treated with H2O2, harvested, washed with PBS, and stained with MitoTracker Green probes (Invitrogen) of 50 nM in serum-free medium for 30 mins. Cells were washed with PBS and analyzed by FACSCanto II cytometer. Fluorescent intensity was analyzed by FACSCanto II program.
2.5. Real-Time PCR Analysis
Total RNA was isolated by Trizol, and then RNA was reverse transcribed (Quanta) to obtain cDNA for PCR [32]. cDNA was subjected to real-time PCR using the SYBR Green PCR reagents kit (Stratagene) as described [33]. For quantitation of mtDNA copy number, Nd1 gene level was quantified by q-PCR and normalized to β-Actin. Due to the space limitation, primers are provided upon request.
2.6. Statistical Methods
The statistical analysis was done as described [27]. In brief, Student's t-test was used for P value calculation, and the star ∗ stands for P < 0.05. The results shown in this paper are representative data.
3. Results
3.1. Impact of RRM2B on H2O2-Mediated DNA Damage Resistance in p53-Deficient Cells
Although it is a known fact that RRM2B can resist ROS, little is known about the detailed regulatory mechanisms. We hypothesized that the oxidative resistance of RRM2B is independent of p53. Stable H1299 cell lines without the functional p53 protein containing overexpressed RRM2B or RRM2BshRNA and their respective controls were included in this study, as described previously, [27] and RRM2B expression was evaluated using Western blot analysis (Figure 1(a)) and an immunofluorescence assay (Figure 1(b)).
Figure 1.
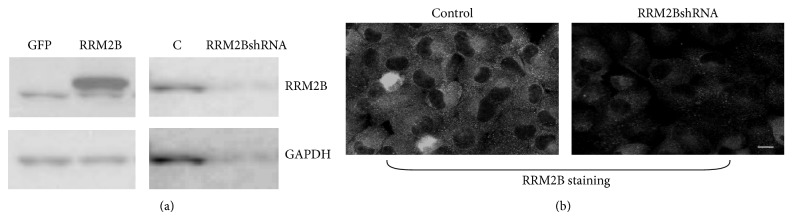
Expression of RRM2B in the H1299 stable cell lines. H1299 stable cell lines were selected as described in the Section 2. Cells were harvested for (a) Western blot analysis or (b) fixed for immunofluorescence assay. Anti-RRM2B and GAPDH were applied in the assays.
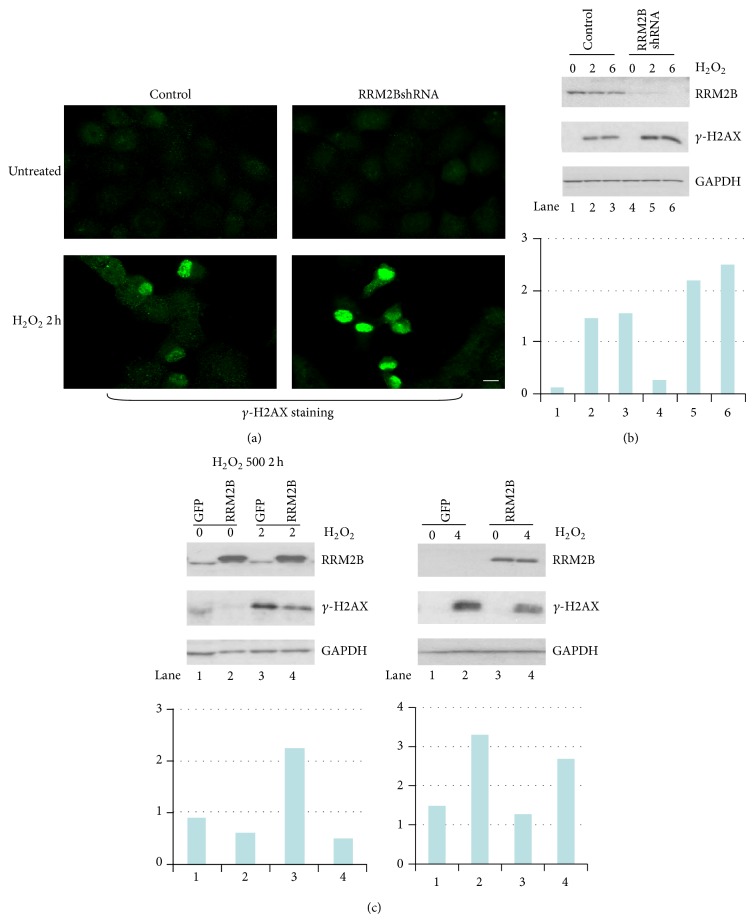
For investigating RRM2B regulation under oxidative stress, hydrogen peroxide (H2O2), a type of oxidative stress-inducing ROS, was used as the source of oxidative stress in this study. Cells were treated with H2O2 for 2 hours and fixed for immunofluorescence staining, in which γ-H2AX foci induction indicated DNA damage. The results suggested that stronger γ-H2AX signals were induced by cells expressing RRM2BshRNA than by the control cells (Figure 2(a)), suggesting that greater DNA damage was associated with lower RRM2B expression in cells.
Figure 2.
RRM2B functions in H2O2-mediated DNA damage resistance in p53-deficient cells. (a) H1299 stable cells were treated with 500 μM H2O2 for 2 hours where applicable for immunofluorescence assay analysis with anti-γ-H2AX antibody. (b)-(c) Two pairs of cell lines expressing control vectors, RRM2BshRNA and RRM2B expressing vector, were treated with H2O2 and harvested at different time points for Western blots analysis with indicated antibodies. (n = 2) Image J was used to normalize γ-H2AX expression to GAPDH.
For the Western blot analysis, we performed the experiment at different time intervals, and the γ-H2AX signals on the blots were quantified relative to the GAPDH activity for quantitative estimation and analysis. Consistent RRM2B depletion was observed, which was correlated with the induction of γ-H2AX signals under H2O2 treatment (Figure 2(b)). Similarly, we observed low γ-H2AX foci signals under H2O2 treatment in RRM2B overexpressed cells (Figure 2(c)). In summary, the results suggested that RRM2B plays a protective role in securing cells against oxidative stress-induced DNA damage in a p53-independent manner.
3.2. RRM2B-Mediated Impact on Mitochondrial Regulation under Oxidative Stress
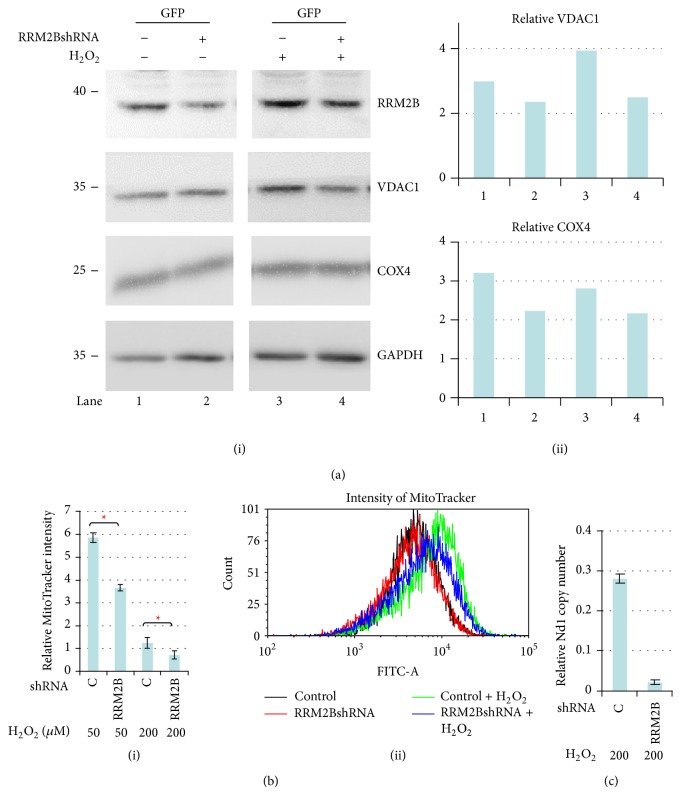
Next, we examined the role of RRM2B in regulating mitochondrial proteins under oxidative stress in p53-deficient H1299 cells. Only stable cells expressing RRM2BshRNA or those acting as control that were subjected to or excluded from H2O2 treatment were included in this study. The cells were then harvested for Western blot analysis, in which the expression of mitochondrial proteins, voltage-dependent anion channel 1 (VDAC1), and cytochrome c oxidase subunit IV (COX4) [34–36] were examined along with RRM2B expression and GAPDH activity (Figure 3(a)(i)). In addition, the relative expression levels of VDAC1 and COX4 were analyzed and are shown in Figure 3(a)(ii). The results suggested that the expression levels of both proteins, particularly VDAC1, were more pronounced under H2O2 treatment, and the expression levels decreased on RRM2B downregulation, confirming that RRM2B regulated the mitochondrial content (Figure 3(a)).
Figure 3.
RRM2B impacts on mitochondrial homeostasis through regulating mitochondrial genes and copy number under oxidative stress. (a) Cells were treated with H2O2 and harvested for Western blot analysis using RRM2B and mitochondrial proteins VDAC1 and COX4 antibodies. GAPDH served as control. Image J was used to normalize VDAC1 and COX4 expression relative to GAPDH, and the normalized figures were shown in 3(a)(ii). (b)(i) H1299 stable cells treated with indicated concentrations of H2O2 were harvested 24 hours later for FACS analysis. The detection of MitoTracker was described in Section 2. The intensity of the MitoTracker Green signal relative to untreated control cells is shown here (means ± SEM, n = 2). (b)(ii) Cells were treated with H2O2 and harvested at 24-hour time point and underwent FACS analysis. MitoTracker Green probe was used for cell staining, and this figure shows the MitoTracker intensity of the representative data from (b)(i). (c) Cells were treated with 200 μM H2O2 and harvested 24 hours later for Q-PCR analysis. Expression of normalized Nd1 relative to untreated control cells is shown (means ± SEM, n = 2).
To further understand the impact of RRM2B on the mitochondria, the mitochondrial mass was measured. FACS was performed using a MitoTracker Green probe for detecting the mitochondrial mass in stable H1299 cells under H2O2 treatment. Under oxidative stress, a significant decrease was observed in the relative mitochondrial mass of the cells expressing RRM2BshRNA, indicating the protective role of RRM2B in mitochondrial homeostasis (Figure 3(b)(i)), which is in agreement with our previous finding [25]. The representative examples of the intensity of MitoTracker on FACS are shown in Figure 3(b)(ii).
Moreover, quantitative PCR was used for measuring the relative mtDNA copy number. The relative copy number of the NADH dehydrogenase subunit 1 (Nd1), a mitochondrial gene [37], under H2O2 treatment was measured using quantitative PCR. Low RRM2B expression resulted in a low Nd1 copy number (Figure 3(c)), suggesting that, under oxidative stress, RRM2B exerts protective effects on the mtDNA content.
The results establish that the RRM2B pathway affects mitochondrial homeostasis under oxidative stress, which is independent of the action of functional p53.
3.3. RRM2B Affects the Inflammation Pathway under Oxidative Stress
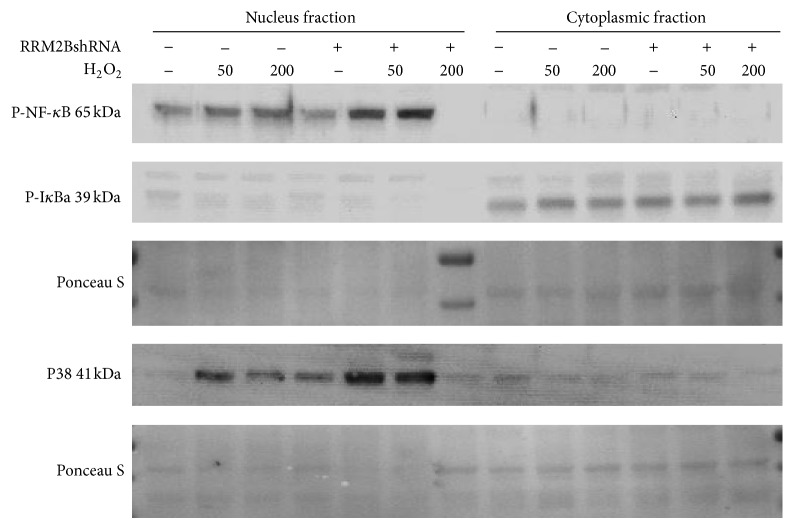
Oxidative stress mediated by H2O2 triggers the inflammation pathway [38, 39], and human inflammatory diseases have long been associated with NF-κB or p38 signaling or both [40–42]. However, the role of RRM2B in the regulation of inflammation has not been investigated. NF-κB and p38 are complex pathways regulating various cellular mechanisms [41, 43–46]. The NF-κB pathway can be activated by various proinflammatory cytokines and is therefore considered a proinflammatory signaling pathway. In addition, extracellular stimuli such as UV light, growth factors, and inflammatory cytokines result in p38 activation. In this study, we investigated the functional role of RRM2B in oxidative stress-mediated NF-κB and p38 signaling. Stable H1299 control or RRM2BshRNA cells were treated with H2O2 and harvested 2 hours later, and the lysates were separated into cytoplasmic and nucleolus fractions. Western blot analysis was performed using phosphorylated NF-κB, phosphorylated IκB, and phosphorylated p38 antibodies for identifying NF-κB and p38 activation in cells (Figure 4).
Figure 4.
RRM2B regulates inflammatory signaling pathway under oxidative stress. (a) Stable cells were treated with 50 or 200 μM H2O2 and harvested 2 hours later, and the lysates were separated into nuclear and cytoplasmic fractions for Western blot analysis. Antibodies against phosphorylated NF-κB, phosphorylated IκB, and phosphorylated p38 were applied, and GAPDH was used as loading control.
The results showed that nuclear NF-κB and p38 signaling on H2O2 treatments were more pronounced in the cells expressing RRM2BshRNA than in the control cells (Figure 4), suggesting that a stronger inflammation signal was induced by RRM2B depletion.
In summary, the findings suggest for the first time that RRM2B can functionally regulate the mitochondrial and inflammatory pathway and that these RRM2B-mediated regulations are independent of p53. Figure 5 illustrates a model summarizing the current findings of RRM2B regulation, suggesting that, under oxidative stress, RRM2B plays critical roles in the upregulation of genes involved in the mitochondrial and inflammation pathways.
Figure 5.
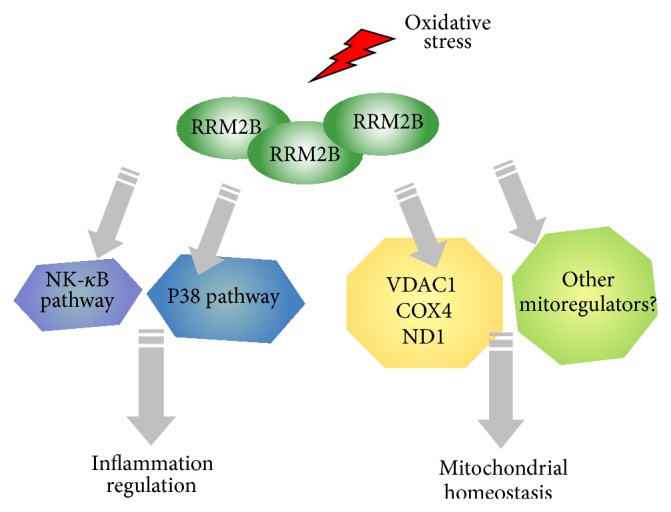
A model of RRM2B-mediated regulation of inflammatory NF-κB and p38 pathways and mitochondrial pathways upon oxidative stress.
4. Conclusion and Discussion
RRM2B is a unique member of the RR enzyme that exhibits anti-ROS potential. It was demonstrated that RRM2B suppressed ROS activation mediated by oxidative stress and is highly induced in a p53-dependent manner during senescence [28]. In our recent study, FOXO3 was observed to be a novel regulator of RRM2B [27]. In this study, the critical role of RR2MB in regulation of mitochondrial and inflammation pathways under oxidative stress in a p53-independent manner was reported for the first time.
RRM2B plays critical roles in vital cellular mechanisms such as DNA replication, and low RRM2B expression sensitizes cancer cells under various stresses, and therefore studies have suggested that low RRM2B expression can potentially be considered a chemosensitizer for cancer treatment [2, 47, 48]. In this study, H1299 cells were subjected to oxidative stress, and the induced γH2AX foci signals were stronger in RRM2BshRNA cells, indicating that the chemosensitivity may generate equal outcomes in p53-deficient cancer cells. Additional therapeutical applications remain to be uncovered.
As mentioned, RRM2B mutation results in severe mtDNA depletion [9]. In this study, we further demonstrated that the presence of RRM2B affects mitochondrial protein expression. Mitochondrial mass was damaged under low RRM2B expression and was further destroyed under oxidative stress. Findings of previous studies and the data in the present study indicate that the intactness of RRM2B is critical for complete functioning of the mitochondria, despite the presence of functional p53.
Oxidative stress has been shown to have a strong association with inflammation pathways. Moreover, our previous study using RRM2B-knockout animal models suggested that RRM2B is critical in maintaining chromosomal stability and preventing chronic inflammation-associated tumorigenesis [21]. In this study, we demonstrated that the NF-κB and p38 signaling pathways were upregulated by oxidative stress, particularly under low RRM2B conditions, which is in agreement with our previous finding, suggesting that RRM2B is crucial in preventing chronic inflammation and acts by inhibiting the NF-κB and p38 pathways.
Both NF-κB and p38 signaling pathways affect vital cellular regulatory mechanisms that include inflammation and apoptosis [46, 49]. Therefore, accompany with our data, the RRM2BshRNA cells could potentially trigger stronger inflammation and apoptosis signals under DNA damage conditions. Recently, more complex roles of NF-κB have been suggested in NF-κB activation in pro- and anti-inflammation processes and pro- and antiapoptosis [49, 50], which may depend on the nature of the model systems. The status of p53 further increased the complexity. Our current study provides new insights into the role of RR in inflammatory diseases, and the intriguing regulation mechanisms of RRM2B under oxidative stress in inflammation remain to be explored.
Acknowledgments
The authors are grateful to the Ministry of Science and Technology (MOST 103-2320-B-038-006-MY2), Taipei Medical University (TMUTOP103004-2), and Comprehensive Cancer Center of Taipei Medical University (funding from Health and Welfare Surcharge of Tobacco Products, MOHW104-TDU-B-212-124-001), Taiwan, for supporting this study.
Conflict of Interests
The authors have no conflict of interests to declare.
References
- 1.Tanaka H., Arakawa H., Yamaguchi T., et al. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404(6773):42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 2.Shao J., Zhou B., Chu B., Yen Y. Ribonucleotide reductase inhibitors and future drug design. Current Cancer Drug Targets. 2006;6(5):409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- 3.Cerqueira N. M. F. S. A., Fernandes P. A., Ramos M. J. Ribonucleotide reductase: a critical enzyme for cancer chemotherapy and antiviral agents. Recent Patents on Anti-Cancer Drug Discovery. 2007;2(1):11–29. doi: 10.2174/157489207779561408. [DOI] [PubMed] [Google Scholar]
- 4.Aye Y., Li M., Long M. J. C., Weiss R. S. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2014 doi: 10.1038/onc.2014.155. [DOI] [PubMed] [Google Scholar]
- 5.Guarino E., Salguero I., Kearsey S. E. Cellular regulation of ribonucleotide reductase in eukaryotes. Seminars in Cell and Developmental Biology. 2014;30:97–103. doi: 10.1016/j.semcdb.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Nakano K., Balint E., Ashcroft M., Vousden K. H. A ribonucleotide reductase gene is a transcriptional target of p53 and p73. Oncogene. 2000;19(37):4283–4289. doi: 10.1038/sj.onc.1203774. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B., Liu X., Mo X., et al. The human ribonucleotide reductase subunit hRRM2 complements p53R2 in response to UV-induced DNA repair in cells with mutant p53. Cancer Research. 2003;63(20):6583–6594. [PubMed] [Google Scholar]
- 8.Xue L., Zhou B., Liu X., et al. Structurally dependent redox property of ribonucleotide reductase subunit p53R2. Cancer Research. 2006;66(4):1900–1905. doi: 10.1158/0008-5472.can-05-2656. [DOI] [PubMed] [Google Scholar]
- 9.Bourdon A., Minai L., Serre V., et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nature Genetics. 2007;39(6):776–780. doi: 10.1038/ng2040. [DOI] [PubMed] [Google Scholar]
- 10.Smith P., Zhou B., Ho N., et al. 2.6 Å X-ray crystal structure of human p53R2, a p53-inducible ribonucleotide reductase. Biochemistry. 2009;48(46):11134–11141. doi: 10.1021/bi9001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie M., Yen Y., Owonikoko T. K., et al. Bcl2 induces DNA replication stress by inhibiting ribonucleotide reductase. Cancer Research. 2014;74(1):212–223. doi: 10.1158/0008-5472.can-13-1536-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian H., Ge C., Li H., et al. Ribonucleotide reductase M2B inhibits cell migration and spreading by early growth response protein 1-mediated phosphatase and tensin homolog/Akt1 pathway in hepatocellular carcinoma. Hepatology. 2014;59(4):1459–1470. doi: 10.1002/hep.26929. [DOI] [PubMed] [Google Scholar]
- 13.Aird K. M., Li H., Xin F., Konstantinopoulos P. A., Zhang R. Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer. Cell Cycle. 2014;13(2):199–207. doi: 10.4161/cc.26953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H. Y., Kim C. W. Ribonucleotide reductase subunit m2 can be new molecular target and prognostic biomarker of hepatocellular carcinoma. Gut and Liver. 2014;8(6):580–581. doi: 10.5009/gnl14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee B., Ha S. Y., Song D. H., Lee H. W., Cho S. Y., Park C. High expression of ribonucleotide reductase subunit m2 correlates with poor prognosis of hepatocellular carcinoma. Gut and Liver. 2014;8(6):662–668. doi: 10.5009/gnl13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura T., Takeda S., Sagiya Y., Gotoh M., Nakamura Y., Arakawa H. Impaired function of p53R2 in Rrm2b-null mice causes severe renal failure through attenuation of dNTP pools. Nature Genetics. 2003;34(4):440–445. doi: 10.1038/ng1212. [DOI] [PubMed] [Google Scholar]
- 17.Xue L., Zhou B., Liu X., Qiu W., Jin Z., Yen Y. Wild-type p53 regulates human ribonucleotide reductase by protein-protein interaction with p53R2 as well as hRRM2 subunits. Cancer Research. 2003;63(5):980–986. [PubMed] [Google Scholar]
- 18.Xue L., Zhou B., Liu X., et al. Ribonucleotide reductase small subunit p53R2 facilitates p21 induction of G1 arrest under UV irradiation. Cancer Research. 2007;67(1):16–21. doi: 10.1158/0008-5472.CAN-06-3200. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Xue L., Yen Y. Redox property of ribonucleotide reductase small subunit M2 and p53R2. Methods in Molecular Biology. 2008;477:195–206. doi: 10.1007/978-1-60327-517-0_15. [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Zhou B., Xue L., et al. Metastasis-suppressing potential of ribonucleotide reductase small subunit p53R2 in human cancer cells. Clinical Cancer Research. 2006;12(21):6337–6344. doi: 10.1158/1078-0432.CCR-06-0799. [DOI] [PubMed] [Google Scholar]
- 21.Chang L., Guo R., Huang Q., Yen Y. Chromosomal instability triggered by Rrm2b loss leads to IL-6 secretion and plasmacytic neoplasms. Cell Reports. 2013;3(5):1389–1397. doi: 10.1016/j.celrep.2013.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanamoto S., Kawasaki G., Yoshitomi I., Mizuno A. Expression of p53R2, newly p53 target in oral normal epithelium, epithelial dysplasia and squamous cell carcinoma. Cancer Letters. 2003;190(2):233–243. doi: 10.1016/S0304-3835(02)00588-8. [DOI] [PubMed] [Google Scholar]
- 23.Hsu N.-Y., Wu J.-Y., Liu X., et al. Expression status of ribonucleotide reductase small subunits hRRM2/p53R2 as prognostic biomarkers in stage I and II non-small cell lung cancer. Anticancer Research. 2011;31(10):3475–3481. [PubMed] [Google Scholar]
- 24.Liu X., Lai L., Wang X., et al. Ribonucleotide reductase small subunit M2B prognoses better survival in colorectal cancer. Cancer Research. 2011;71(9):3202–3213. doi: 10.1158/0008-5472.CAN-11-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Liu X., Xue L., et al. Ribonucleotide reductase subunit p53R2 regulates mitochondria homeostasis and function in KB and PC-3 cancer cells. Biochemical and Biophysical Research Communications. 2011;410(1):102–107. doi: 10.1016/j.bbrc.2011.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K., Wu J., Wu X., et al. p53R2 inhibits the proliferation of human cancer cells in association with cell-cycle arrest. Molecular Cancer Therapeutics. 2011;10(2):269–278. doi: 10.1158/1535-7163.MCT-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho E. C., Kuo M. L., Liu X., et al. Tumor suppressor FOXO3 regulates ribonucleotide reductase subunit RRM2B and impacts on survival of cancer patients. Oncotarget. 2014;5(13):4834–4844. doi: 10.18632/oncotarget.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo M.-L., Sy A. J., Xue L., et al. RRM2B suppresses activation of the oxidative stress pathway and is Up-regulated by P53 during senescence. Scientific Reports. 2012;2, article 822 doi: 10.1038/srep00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang M. Y., Kim H.-B., Piao C., et al. The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death and Differentiation. 2013;20(1):117–129. doi: 10.1038/cdd.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho E.-C., Zheng S., Munro S., et al. Arginine methylation controls growth regulation by E2F-1. The EMBO Journal. 2012;31(7):1785–1797. doi: 10.1038/emboj.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansson M., Durant S. T., Cho E.-C., et al. Arginine methylation regulates the p53 response. Nature Cell Biology. 2008;10(12):1431–1439. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- 32.Sandoval S., Kraus C., Cho E.-C., et al. Sox4 cooperates with CREB in myeloid transformation. Blood. 2012;120(1):155–165. doi: 10.1182/blood-2011-05-357418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pigazzi M., Manara E., Bresolin S., et al. MicroRNA-34b promoter hypermethylation induces CREB overexpression and contributes to myeloid transformation. Haematologica. 2013;98(4):602–610. doi: 10.3324/haematol.2012.070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S., Sampson M. J., Decker W. K., Craigen W. J. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochimica et Biophysica Acta. 1999;1452(1):68–78. doi: 10.1016/s0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 35.Roman I., Figys J., Steurs G., Zizi M. In vitro interactions between the two mitochondrial membrane proteins VDAC and cytochrome c oxidase. Biochemistry. 2005;44(39):13192–13201. doi: 10.1021/bi050674s. [DOI] [PubMed] [Google Scholar]
- 36.Siletsky S. A., Konstantinov A. A. Cytochrome c oxidase: charge translocation coupled to single-electron partial steps of the catalytic cycle. Biochimica et Biophysica Acta. 2012;1817(4):476–488. doi: 10.1016/j.bbabio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Boer P. H., Gray M. W. Genes encoding a subunit of respiratory NADH dehydrogenase (ND1) and a reverse transcriptase-like protein (RTL) are linked to ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. The EMBO Journal. 1988;7(11):3501–3508. doi: 10.1002/j.1460-2075.1988.tb03226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roebuck K. A. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review) International Journal of Molecular Medicine. 1999;4(3):223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 39.Yanagisawa S., Koarai A., Sugiura H., et al. Oxidative stress augments toll-like receptor 8 mediated neutrophilic responses in healthy subjects. Respiratory Research. 2009;10, article 50 doi: 10.1186/1465-9921-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laveti D., Kumar M., Hemalatha R., et al. Anti-inflammatory treatments for chronic diseases: a review. Inflammation & Allergy-Drug Targets. 2013;12(5):349–361. doi: 10.2174/18715281113129990053. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez A., Tripathy D., Yin X., et al. p38 MAPK: a mediator of hypoxia-induced cerebrovascular inflammation. Journal of Alzheimer's Disease. 2012;32(3):587–597. doi: 10.3233/jad-2012-120829. [DOI] [PubMed] [Google Scholar]
- 42.Cheriyan J., Webb A. J., Sarov-Blat L., et al. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011;123(5):515–523. doi: 10.1161/CIRCULATIONAHA.110.971986. [DOI] [PubMed] [Google Scholar]
- 43.Bauer J., Namineni S., Reisinger F., Zller J., Yuan D., Heikenwlder M. Lymphotoxin, NF-κB, and cancer: the dark side of cytokines. Digestive Diseases. 2012;30(5):453–468. doi: 10.1159/000341690. [DOI] [PubMed] [Google Scholar]
- 44.Osorio F. G., López-Otín C., Freije J. M. P. NF-κB in premature aging. Aging. 2012;4(11):726–727. doi: 10.18632/aging.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobon-Velasco J., Cuevas E., Torres-Ramos M. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS & Neurological Disorders—Drug Targets. 2014;13(9):1615–1626. doi: 10.2174/1871527313666140806144831. [DOI] [PubMed] [Google Scholar]
- 46.Zarubin T., Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Research. 2005;15(1):11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 47.Wang X., Zhenchuk A., Wiman K. G., Albertioni F. Regulation of p53R2 and its role as potential target for cancer therapy. Cancer Letters. 2009;276(1):1–7. doi: 10.1016/j.canlet.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Tsai M. H., Chen X., Chandramouli G. V. R., et al. Transcriptional responses to ionizing radiation reveal that p53R2 protects against radiation-induced mutagenesis in human lymphoblastoid cells. Oncogene. 2006;25(4):622–632. doi: 10.1038/sj.onc.1209082. [DOI] [PubMed] [Google Scholar]
- 49.Vousden K. H. Partners in death: a role for p73 and NF-κB in promoting apoptosis. Aging. 2009;1(3):275–277. doi: 10.18632/aging.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology. 2009;1(6) doi: 10.1101/cshperspect.a001651.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]