Abstract
Objective
Presepsin is highlighted as a diagnostic and prognostic marker of sepsis. Little information is available regarding the accurate association between presepsin levels and the degree of kidney function. We analyzed presepsin levels in patients with a glomerular filtration rate (GFR) in the categories G1 to G5, evaluated via inulin renal clearance test, and receiving hemodialysis (HD).
Methods
Patients who were not receiving HD were included if they had undergone inulin renal clearance measurements for the accurate measurement of GFR (measured GFR), and patients who were receiving hemodialysis (HD) were included if they had anuria. Exclusion criteria were infection, cancer, liver disease, autoimmune disorders, or steroid or immunosuppressant use. GFR category was defined as follows; G1: GFR ≥ 90 ml/min/1.73m2, G2: GFR = 60 to 90 ml/min/1.73m2, G3: GFR = 30 to 60 ml/min/1.73m2, G4: GFR = 15 to 30 ml/min/1.73m2, G5: GFR ≤ 15 ml/min/1.73m2.
Results
Seventy-one patients were included. The median (IQR) presepsin values of patients in each GFR category were as follows: G1 + G2: 69.8 (60.8–85.9) pg/ml; G3: 107.0 (68.7–150.0) pg/ml; G4: 171.0 (117.0–200.0) pg/ml; G5: 251.0 (213.0–297.5) pg/ml; and HD: 1160.0 (1070.0–1400.0) pg/ml. The log-transformed presepsin values, excluding patients receiving HD, inversely correlated with the measured GFR (Pearson’s correlation coefficient = -0.687, P < 0.001). The multivariate analysis revealed that measured GFR and hemoglobin levels significantly correlated with elevated presepsin levels.
Conclusion
Presepsin levels were markedly high in patients receiving HD, similar to values seen in patients with severe sepsis or septic shock. In patients who were not receiving HD, presepsin levels increased as GFR decreased. Thus, the evaluation of presepsin levels in patients with chronic kidney disease requires further consideration, and a different cutoff value is needed for diagnosing sepsis in such patients.
Introduction
Sepsis is a major cause of mortality in patients presenting to the emergency department (ED) or intensive care unit (ICU). Early recognition and treatment initiation are essential for improving the prognosis of patients with sepsis [1,2,3]. Various biomarkers such as procalcitonin (PCT) have been used for diagnosing sepsis [4,5]. Although PCT level often indicates the presence of infection, its levels are also elevated in various conditions that induce systemic inflammatory response syndrome, such as severe trauma, burn injury, or surgical procedures [6,7]. Therefore, it is necessary to develop a biomarker that is more specific and that can be used for the earlier detection of sepsis compared to PCT.
Presepsin is the soluble N-terminal fragment of the cluster of differentiation (CD) marker protein CD14, which is the receptor for lipopolysaccharide (LPS) and LPS-binding protein complexes [8]. Recently, presepsin was reported as valuable for the early diagnosis of sepsis and the evaluation of sepsis severity, and its levels remain unaffected by conditions such as trauma, burn injury, or surgical procedures [8,9]. The diagnostic cutoff levels for sepsis varied among different studies, but most reports suggest approximate levels of 400–600 pg/ml [8,10].
In addition, based on experiments with septic animal models, presepsin levels increase 2 hours after the onset of infection, which is earlier than the elevation of PCT levels [11,12].
Moreover, some studies have reported that the measurement of presepsin levels is useful for predicting the prognosis of septic patients [10,13,14,15]. Because of these characteristics, presepsin is being used in various clinical situations.
However, there is a concern that presepsin level is affected by kidney function. Presepsin is a 13 kDa protein. Although its exact in vivo activity is unclear, it is presumed from its molecular weight that presepsin is filtered by the glomerulus, reabsorbed, and catabolized within proximal tubular cells [16]. Theoretically, it has been proposed that presepsin levels increase as kidney function decreases. Chenevier-Gobeaux et al. [16] measured presepsin levels in patients who presented to the ED with mild illness without acute infection. They showed that presepsin levels were elevated in most patients with a decreased estimated glomerular filtration rate (eGFR; <60 ml/min/1.73m2). Behnes et al. [17] reported that, in an internal ICU, presepsin levels significantly correlated with serum creatinine levels and the number of days on renal replacement therapy, which are both related to kidney function. Nakamura et al. [18] retrospectively analyzed presepsin levels in patients with or without sepsis presenting in the ICU, and found that that presepsin levels were markedly high in patients with renal failure and end-stage kidney disease. Recently, Masson et al. [19] reported that higher serum creatinine was the strongest determinant of presepsin levels in ICU patients. Because these reports studied patients presenting to the ED or ICU, various factors could have affected presepsin levels. Additionally, these studies evaluated kidney function based on eGFR or urine output, and therefore, the accurate association between presepsin levels and GFRs was not elucidated. Considering this, we conducted a cross-sectional study to investigate the effect of GFR, measured precisely, and hemodialysis (HD) dependence on presepsin levels.
Materials and Methods
Patients
Study participants were outpatients who had visited the department of Nephrology at Nagoya University Hospital and Ogaki Municipal Hospital between 2009 and 2013. The study included patients who had undergone inulin renal clearance measurements for the accurate evaluation of GFR and patients with anuria receiving HD with a high-flux dialyzer. All patients receiving HD had dialysis with bicarbonate three times per week. Planned dialysis duration was 240 or 300 minutes, with a blood flow rate of 200 ml/min and a dialysate flow rate of 500 ml/min. Ultrapure dialysate (a bacterial count of < 0.1 colony-forming units /mL and less than 0.03 endotoxin units /ml) was used [20]. Gamma-ray sterilization was performed for all dialyzers and reuse was not permitted. Exclusion criteria were infection, cancer, liver disease, autoimmune disorder, and steroid or immunosuppressant use. The study participants were recruited consecutively from the list of participants who met the study criteria.
The study protocol and consent procedure were approved by the ethics committees of Nagoya University and Ogaki Municipal Hospital according to the Declaration of Helsinki. The approval number was 1135–15. Written informed consent was obtained from every participant.
Data and sample collection
Patients’ clinical characteristics and data were collected retrospectively from their medical records. The eGFR was calculated using the equation generated by the Japanese Society of Nephrology: eGFR (ml/min/1.73 m2) = 194 × Scr-1.094 × Age-0.287 × 0.739 (if female) [21]. The inulin renal clearance (measured GFR) was measured using the simple method previously reported as accurate enough for measuring GFR in clinical practice [22,23].
GFR was categorized according to the KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease [24]. GFR category was defined as follows; G1: GFR ≥ 90 ml/min/1.73m2, G2: GFR = 60 to 90 ml/min/1.73m2, G3: GFR = 30 to 60 ml/min/1.73m2, G4: GFR = 15 to 30 ml/min/1.73m2, G5: GFR ≤ 15 ml/min/1.73m2. High-sensitivity C-reactive protein (CRP) was measured by latex-enhanced immuno-nephelometric assay and the limit of detection level was 0.005 mg/dl. Procalcitonin values were analyzed in patients not receiving HD by electrochemiluminescence immunoassay and the limit of detection level was 0.02 ng/ml.
Plasma samples containing ethylenediaminetetraacetic acid were collected at the time of inulin renal clearance measurement, just before inulin infusion, in a fasting state. In patients receiving HD, plasma samples were collected immediately before an HD session and two days after the prior HD. Plasma samples were stored at -80°C until presepsin measurements were performed.
Presepsin measurement
Plasma presepsin concentrations were measured using a compact automated immunoanalyzer (PATHFAST; LSI Medience Corporation, Tokyo, Japan) based on a chemiluminescent enzyme immunoassay [25,26]. The measurement range of the assay is 20–20,000 pg/ml.
Statistical analysis
Normally distributed variables are expressed as means and standard deviations (SD) and were compared using one-way analysis of variance. Nonparametric variables were expressed as medians and interquartile ranges and compared using Kruskal-Wallis tests with Bonferroni post hoc tests. Categorical variables were expressed as percentages and were compared using Fisher’s exact test. Because the distribution of presepsin values was skewed, values from a logarithmic transformation were used in univariate and multivariate analyses. The univariate analysis for presepsin levels was conducted using Pearson’s or Spearman’s rank correlation coefficients. A multivariate linear regression model for presepsin levels was constructed using a backward stepwise method of selection. All P values were two-tailed, and P < 0.05 was considered statistically significant.
All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 2.13.0) [27].
Results
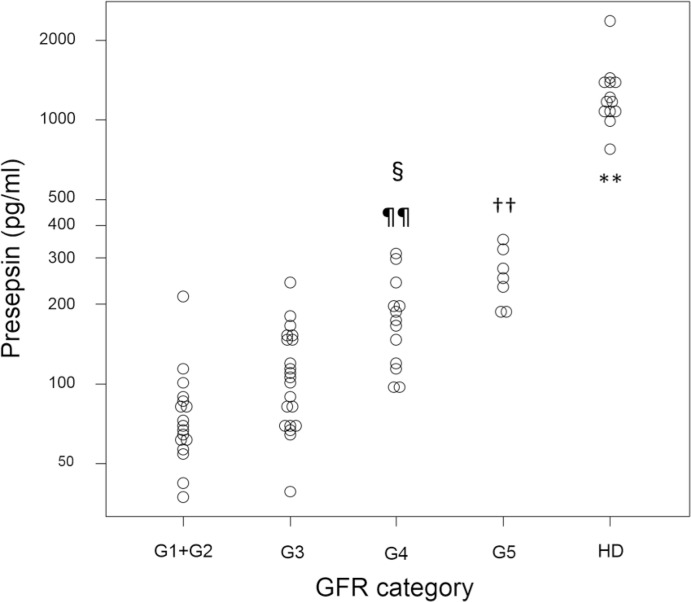
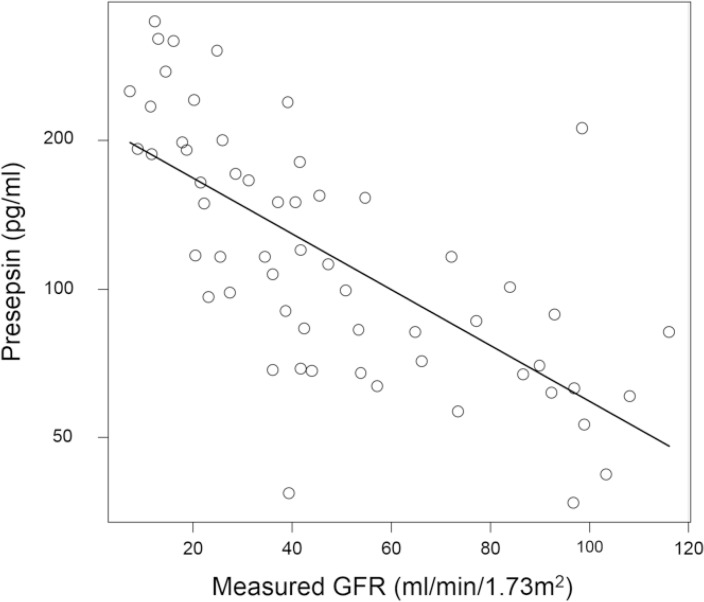
Clinical characteristics of the patient population and subgroups are presented in Table 1. There were 12 kidney transplantation donor candidates in the G1 + G2 group. Patients receiving HD had markedly high presepsin levels compared to those not receiving HD (Fig 1). The median and interquartile range of presepsin values were as follows: G1 + G2: 69.8 (60.8–85.9) pg/ml; G3: 107.0 (68.7–150.0) pg/ml; G4: 171.0 (117.0–200.0) pg/ml; G5: 251.0 (213.0–297.5) pg/ml; and HD: 1160.0 (1070.0–1400.0) pg/ml. Presepsin levels in patients in the G4 or G5 category were significantly higher than those in patients in the G1 + G2 and G3 categories. Presepsin levels were higher in patients in the G3 category than patients in the G1 + G2, and in the G5 than in the G4, but differences in these values were not statistically significant. The log-transformed presepsin values in patients not receiving HD correlated well with measured GFR (Pearson’s test = -0.687, P < 0.001) (Fig 2).
Table 1. Clinical characteristics of the study participants and comparison of patients in the different GFR categories or receiving HD.
| GFR category | |||||||
|---|---|---|---|---|---|---|---|
| Overall | G1+ G2 | G3 | G4 | G5 | HD | P value | |
| Number of patients | 71 | 17 | 21 | 13 | 7 | 13 | |
| Age (years) | 63.4 (11.4) | 60.2 (10.3) | 63.2 (11.9) | 67.2 (8.7) | 60.8 (16.3) | 65.8 (11.7) | 0.46 |
| Gender (male/female) | 42/29 | 7/10 | 16/5 | 11/2 | 4/3 | 4/9 | 0.012 |
| Height (cm) | 160.2 (8.0) | 160.3 (7.7) | 161.7 (6.6) | 165.5 (6.9) | 159.9 (6.1) | 152.3 (7.5) | < 0.001 |
| Weight (kg) | 60.3 (11.4) | 59.9 (11.3) | 63.5 (8.3) | 66.3 (11.0) | 60.6 (14.3) | 49.6 (8.2) | 0.001 |
| Diabetes mellitus, n (%) | 20 (28.2) | 4 (23.5) | 8 (38.1) | 4 (30.8) | 1 (14.3) | 3 (23.1) | 0.776 |
| Total protein (g/dl) | 6.97 (0.53) | 7.1 (0.4) | 7.0 (0.5) | 6.9 (0.6) | 7.1 (0.3) | 6.5 (0.3) | <0.009 |
| Serum albumin (g/dl) | 3.9 (0.3) | 4.2 (0.2) | 3.9 (0.3) | 3.8 (0.4) | 3.8 (0.2) | 3.8 (0.3) | < 0.001 |
| Aspartate aminotransferase (U/l) | 20.6 (6.9) | 22.2 (6.2) | 21.5 (7.6) | 20.2 (6.7) | 20.8 (6.0) | 17.4 (7.3) | 0.421 |
| Alanine aminotransferase (U/l) | 16.6 (7.7) | 18.1 (3.9) | 18.8 (10.0) | 15.5 (6.6) | 17.5 (7.4) | 11.8 (6.5) | 0.101 |
| Uric acid (mg/dl) | 6.89 (1.61) | 5.1 (1.4) | 7.0 (0.8) | 6.9 (0.9) | 7.5 (1.5) | 8.4 (1.3) | < 0.001 |
| Urea nitrogen (mg/dl) | 32.5 (19.8) | 13.2 (3.7) | 22.2 (5.8) | 35.1 (11.1) | 45.8 (12.7) | 64.3 (10.0) | < 0.001 |
| Serum creatinine (mg/dl) | 3.12 (3.75) | 0.68 (0.22) | 1.15 (0.27) | 2.09 (0.43) | 3.20 (0.90) | 10.47 (2.52) | < 0.001 |
| estimated GFR (ml/min/1.73m2) | 50.4 (29.1) | 84.2 (23.9) | 50.1 (12.4) | 25.7 (5.4) | 15.5 (2.6) | NA | < 0.001 |
| measured GFR (ml/min/1.73m2) | 48.2 (30.2) | 89.3 (14.7) | 43.2 (7.2) | 22.5 (3.8) | 11.3 (2.5) | NA | < 0.001 |
| Glucose (mg/dl) | 104 (26) | 100 (25) | 108 (31) | 110 (32) | 95 (12) | 101 (15) | 0.693 |
| C reactive protein (mg/dl) | 0.08 (0.07) | 0.06 (0.05) | 0.09 (0.07) | 0.06 (0.05) | 0.07 (0.08) | 0.12 (0.11) | 0.285 |
| Procalcitonin (ng/ml) | 0.05 (0.03) | 0.04 (0.04) | 0.05 (0.04) | 0.07 (0.04) | 0.08 (0.02) | NA | 0.046 |
| White blood cells (106/l) | 5850 (1632) | 5329 (1546) | 6319 (1675) | 5823 (1146) | 6342 (2310) | 5537 (1636) | 0.339 |
| Hemoglobin (g/dl) | 12.8 (1.6) | 13.8 (1.3) | 13.5 (1.6) | 12.0 (1.4) | 11.0 (1.4) | 12.1 (1.2) | < 0.001 |
| Platelets (1010/l) | 20.4 (5.6) | 21.6 (4.3) | 20.1 (4.5) | 20.8 (5.8) | 22.9 (6.4) | 17.4 (7.5) | 0.212 |
NA: data not available. Continuous variables are expressed as mean (standard deviation). P values were calculated using one-way analysis of variance or Fisher’s exact test. G1: GFR ≥ 90 ml/min/1.73m2, G2: GFR = 60 to 90 ml/min/1.73m2, G3: GFR = 30 to 60 ml/min/1.73m2, G4: GFR = 15 to 30 ml/min/1.73m2, G5: GFR ≤ 15 ml/min/1.73m2, HD: hemodialysis.
Fig 1. Dot plot of presepsin values of patients in the different GFR categories or of patients receiving HD.
G1: GFR ≥ 90 ml/min/1.73m2, G2: GFR = 60 to 90 ml/min/1.73m2, G3: GFR = 30 to 60 ml/min/1.73m2, G4: GFR = 15 to 30 ml/min/1.73m2, G5: GFR ≤ 15 ml/min/1.73m2, HD: hemodialysis. **P <0.01 compared to any other GFR category. ††P <0.01 compared to G3 and G2+G1. ¶¶P <0.01 compared to G1+G2. §P <0.05 compared to G3.
Fig 2. Correlation between the log-transformed presepsin values and measured GFR in patients not receiving hemodialysis.
N = 58, Pearson’s correlation coefficient = -0.687, 95% CI = -0.803 to -0.521, P <0.001.
Univariate analysis found that levels of serum albumin, serum uric acid, serum urea nitrogen, serum creatinine, and hemoglobin, as well as eGFR and measured GFR, significantly correlated with presepsin levels. We excluded levels of serum creatinine, serum urea nitrogen, and eGFR from the multivariate model because the correlation coefficients between those variables and measured GFR were high (0.778, 0.921, and -0.771, respectively; Pearson’s test). Weak correlation was found between measured GFR and serum albumin, uric acid and hemoglobin levels (correlation coefficients were 0.531, 0.663 and 0.437, respectively). In this study, we added all variables with correlation coefficients < 0.7 to the multivariate model. We found that measured GFR and hemoglobin levels were significantly associated with elevated presepsin levels (Table 2).
Table 2. Univariate analysis and multivariate linear regression analysis of the increase in log-transformed presepsin values.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Candidate variables | Correlation coefficient | P value | Beta regression coefficient | Standard error | P value |
| Age | 0.087 | 0.513a | |||
| Gender (female = 1) | -0.110 | 0.404b | |||
| Height (cm) | 0.054 | 0.683a | |||
| Weight (kg) | 0.094 | 0.481a | |||
| Diabetes mellitus (yes = 1) | 0.183 | 0.168b | |||
| Total protein (g/dl) | -0.065 | 0.624a | |||
| Serum albumin (g/dl) | -0.370 | 0.004a | |||
| Aspartate aminotransferase (U/l) | 0.019 | 0.885a | |||
| Alanine aminotransferase (U/l) | -0.033 | 0.803a | |||
| Uric acid (mg/dl) | 0.505 | <0.001a | |||
| Urea nitrogen (mg/dl) | 0.715 | <0.001a | |||
| Serum creatinine (mg/dl) | 0.722 | <0.001a | |||
| estimated GFR (ml/min/1.73m2) | -0.638 | <0.001a | |||
| measured GFR (ml/min/1.73m2) | -0.687 | <0.001a | -0.00483 | 0.00085 | <0.001 |
| Glucose (mg/dl) | 0.191 | 0.151a | |||
| C-reactive protein (mg/dl) | 0.005 | 0.968a | |||
| Procalcitonin (ng/ml) | 0.383 | 0.003a | |||
| White blood cell count (106/l) | 0.117 | 0.381a | |||
| Hemoglobin (g/dl) | -0.482 | <0.001a | -0.032 | 0.014 | 0.035 |
| Platelet count (1010/l) | 0.004 | 0.973a |
a: Pearson’s correlation coefficients
b: Spearman’s correlation coefficients
After obtaining these results in patients receiving HD, we additionally measured presepsin levels in the same patients on another day, immediately before and after HD, two days after the prior HD. Presepsin levels decreased significantly from 1510 (1280–1670) pg/ml before HD to 753 (542–1210) pg/ml after HD (P < 0.001).
Discussion
This study evaluated the clinical impact of kidney function on presepsin levels and found that presepsin level inversely correlated with GFR. In particular, in patients receiving HD, presepsin values were markedly high, at comparable levels to those of severe sepsis or septic shock [8,10]. In patients with chronic kidney disease (CKD) not receiving HD, presepsin levels inversely correlated with the measured GFR. These results suggest that the evaluation of presepsin levels in these patients requires special consideration.
We found that the presepsin values of HD patients without infection were 783–2,360 pg/ml. Liu et al. [14] reported that median presepsin values of in the ED were 787 pg/ml for severe sepsis and 1,084 pg/ml for septic shock. Recently, Nakamura et al. [18] analyzed presepsin levels in patients in the ICU with a loss of kidney function or end-stage renal disease according to RIFLE criteria. Presepsin levels in patients with sepsis ranged from 2,632 to 20,000 pg/ml and those in patients without sepsis ranged from 2,134 to 19,633 pg/ml, suggesting that presepsin measurement could not aid in distinguishing patients with sepsis from those with severe acute kidney injury [18].
However, the reason for its excessive elevation in patients receiving HD is unclear. We measured plasma presepsin concentration before and after the HD session, and found that presepsin levels significantly decreased after the session. The result implies that presepsin could be diffused and filtrated to a certain degree by high-flux HD. However, the values after HD were still high. We presume that elevated presepsin levels in patients receiving HD are due to decreased clearance and/or increased production of presepsin. Further study is needed to clarify this point.
In this study, presepsin levels inversely correlated with GFR (r = -0.68) in patients not receiving HD. The maximal presepsin value was 348 pg/ml (the measured GFR and C-reactive protein level of patients were 21.8 ml/min/1.73m2 and 0.06 mg/dl, respectively), and this values is under the cutoff value for diagnosing sepsis, which was reported to be approximately 400–600 pg/ml. However, this does not imply that the cutoff value is useful in all patients who are not receiving HD, because presepsin levels in patients with CKD who contract even a mild infection could easily surpass the value. Further study is needed to clarify the cutoff value for patients with CKD.
There were two strengths in this study. First, the GFR was measured using inulin renal clearance measurements in each patient. In past reports, the evaluation of GFR was based on eGFR calculated with age, gender, and serum creatinine concentration. However, the calculation of eGFR can be inaccurate because of underlying errors in the formula. Moreover, GFR could fluctuate in patients with sepsis because of potentially coexisting acute kidney injury [28]. Second, this study evaluated the independent effect of kidney function, because patients with infection, cancer, liver disease, autoimmune disorder, and steroid or immunosuppressant use, which could influence the presepsin levels, were carefully excluded.
We then sought to explore variables that correlated with presepsin values. Univariate analysis revealed apart from GFR, levels of serum albumin, serum uric acid, serum urea nitrogen, serum creatinine, and hemoglobin significantly correlated with presepsin levels. All these variables can intrinsically be associated with kidney function. Multivariate linear regression analysis revealed that the measured GFR and hemoglobin levels were independent predictors of presepsin level. It was reported that serum concentrations of inflammatory cytokines, such as interleukin-6 (IL-6) or tumor necrosis factor alpha (TNF-α), were elevated in patients with CKD [29,30,31]. IL-6 affect erythropoiesis through regulation of iron metabolism [32], and TNF-α inhibit erythroid cell development [33,34]. Recently, CKD patients reported to have high levels of circulating endotoxin compared to subjects with normal kidney function because of interstitial bacterial load or gut edema [35]. We supposed that this condition could lead to elevated circulating inflammatory cytokines, anemia and elevated production of presepsin, but further study is needed to elucidate this issue.
This study has limitations. First, this study was preliminary and we did not conduct legitimate sample size estimation. However, we calculated that this study has more than 80% power to detect a between-group difference of 0.8 SD, which corresponds to a large size difference in Cohen's criteria [36]. Second, the cutoff value for diagnosing sepsis in patients with a decreased GFR or in those receiving HD remains uncertain. Further study recruiting a larger number of patients with decreased GFR, including those with infection will be needed.
In conclusion, presepsin levels in patients receiving HD were markedly high, and were comparable to the levels seen in patients with severe sepsis or septic shock. In patients not receiving HD, presepsin levels increased as GFR decreased. Thus, the evaluation of presepsin in patients with CKD warrants special consideration, and a different cutoff value is needed for diagnosing sepsis in such patients.
Supporting Information
(CSV)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Kyowa Hakko Kirin Co., Ltd. Otsuka Pharmaceutical Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., and Mochida Pharmaceutical Co., Ltd. provided support in the form of research grants for authors [TN, YY, MA, TA, TK, SK, NT, SM, SM], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580–637. 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 2. Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD (2010) Critical care and the global burden of critical illness in adults. Lancet 376: 1339–1346. 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369: 840–851. 10.1056/NEJMra1208623 [DOI] [PubMed] [Google Scholar]
- 4. Meisner M (2002) Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta 323: 17–29. [DOI] [PubMed] [Google Scholar]
- 5. Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, et al. (2008) Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother 14: 244–249. 10.1007/s10156-008-0608-1 [DOI] [PubMed] [Google Scholar]
- 6. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P (2013) Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 13: 426–435. 10.1016/S1473-3099(12)70323-7 [DOI] [PubMed] [Google Scholar]
- 7. Henriquez-Camacho C, Losa J (2014) Biomarkers for sepsis. Biomed Res Int 2014: 547818 10.1155/2014/547818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S (2011) Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother 17: 764–769. 10.1007/s10156-011-0254-x [DOI] [PubMed] [Google Scholar]
- 9. Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, et al. (2005) Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother 11: 234–238. [DOI] [PubMed] [Google Scholar]
- 10. Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, et al. (2013) Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care 17: R168 10.1186/cc12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, et al. (2012) Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother 18: 891–897. 10.1007/s10156-012-0435-2 [DOI] [PubMed] [Google Scholar]
- 12. Gilbert DN (2010) Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol 48: 2325–2329. 10.1128/JCM.00655-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, et al. (2014) Presepsin as a powerful monitoring tool for the prognosis and treatment of sepsis: a multicenter prospective study. J Infect Chemother 20: 30–34. 10.1016/j.jiac.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 14. Liu B, Chen YX, Yin Q, Zhao YZ, Li CS (2013) Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care 17: R244 10.1186/cc13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masson S, Caironi P, Spanuth E, Thomae R, Panigada M, Sangiorgi G, et al. (2014) Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: data from the Albumin Italian Outcome Sepsis trial. Crit Care 18: R6 10.1186/cc13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chenevier-Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens YE (2014) Presepsin (sCD14-ST) in emergency department: the need for adapted threshold values? Clin Chim Acta 427: 34–36. 10.1016/j.cca.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 17. Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, et al. (2014) Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care 18: 507 10.1186/s13054-014-0507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura Y, Ishikura H, Nishida T, Kawano Y, Yuge R, Ichiki R, et al. (2014) Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesthesiol 14: 88 10.1186/1471-2253-14-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masson S, Caironi P, Fanizza C, Thomae R, Bernasconi R, Noto A, et al. (2015) Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial. Intensive Care Med 41: 12–20. 10.1007/s00134-014-3514-2 [DOI] [PubMed] [Google Scholar]
- 20. Ledebo I, Nystrand R (1999) Defining the microbiological quality of dialysis fluid. Artif Organs 1: 37–43. [DOI] [PubMed] [Google Scholar]
- 21. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 22. Horio M, Imai E, Yasuda Y, Hishida A, Matsuo S (2009) Simple sampling strategy for measuring inulin renal clearance. Clin Exp Nephrol 13: 50–54. 10.1007/s10157-008-0084-z [DOI] [PubMed] [Google Scholar]
- 23. Horio M, Yasuda Y, Takahara S, Imai E, Watanabe T, Matsuo S (2010) Comparison of a simple and a standard method for inulin renal clearance. Clin Exp Nephrol 14: 427–430. 10.1007/s10157-010-0325-9 [DOI] [PubMed] [Google Scholar]
- 24. KDIGO Working Group (2012) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150. [DOI] [PubMed] [Google Scholar]
- 25. Kurihara T, Yanagida A, Yokoi H, Koyata A, Matsuya T, Ogawa J, et al. (2008) Evaluation of cardiac assays on a benchtop chemiluminescent enzyme immunoassay analyzer, PATHFAST. Anal Biochem 375: 144–146. 10.1016/j.ab.2007.12.030 [DOI] [PubMed] [Google Scholar]
- 26. Okamura Y, Yokoi H (2011) Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin Chim Acta 412: 2157–2161. 10.1016/j.cca.2011.07.024 [DOI] [PubMed] [Google Scholar]
- 27. Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48: 452–458. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. KDIGO Working Group (2012) KDIGO Clinical Practice Guideline for Acute kidney Injury. Kidney Int Suppl 1: 1–138. [Google Scholar]
- 29. Herbelin A, Nguyen AT, Zingraff J, Urena P, Descamps-Latscha B (1990) Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int 37: 116–125. [DOI] [PubMed] [Google Scholar]
- 30. Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B (1991) Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int 39: 954–960. [DOI] [PubMed] [Google Scholar]
- 31. Pecoits-Filho R, Heimbürger O, Bárány P, Suliman M, Fehrman-Ekholm I, Lindholm B, et al. (2003) Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 41: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 32. Raja KB, O Latunde-Dada G, Peters TJ, McKie AT, Simpson RJ (2005) Role of interleukin-6 in hypoxic regulation of intestinal iron absorption. Br J Haematol 131: 656–662. [DOI] [PubMed] [Google Scholar]
- 33. Means RT Jr, Dessypris EN, Krantz SB (1990) Inhibition of human colony-forming-unit erythroid by tumor necrosis factor requires accessory cells. J Clin Invest 86: 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Means RT Jr, Krantz SB (1993) Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. J Clin Invest 91: 416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, et al. (2011) Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141. 10.2215/CJN.04610510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen J (1992) A power primer. Psychol Bull 112: 155–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.