Abstract
Chitinases are pathogensis-related proteins, which play an important role in plant defense mechanisms. The role of the sugarcane chitinase family genes remains unclear due to the highly heterozygous and aneuploidy chromosome genetic background of sugarcane. Ten differentially expressed chitinase genes (belonging to class I~VII) were obtained from RNA-seq analysis of both incompatible and compatible sugarcane genotypes during Sporisorium scitamineum challenge. Their structural properties and expression patterns were analyzed. Seven chitinases (ScChiI1, ScChiI2, ScChiI3, ScChiIII1, ScChiIII2, ScChiIV1 and ScChiVI1) showed more positive with early response and maintained increased transcripts in the incompatible interaction than those in the compatible one. Three (ScChiII1, ScChiV1 and ScChiVII1) seemed to have no significant difference in expression patterns between incompatible and compatible interactions. The ten chitinases were expressed differentially in response to hormone treatment as well as having distinct tissue specificity. ScChiI1, ScChiIV1 and ScChiVII1 were induced by various abiotic stresses (NaCl, CuCl2, PEG and 4 °C) and their involvement in plant immunity was demonstrated by over-expression in Nicotiana benthamiana. The results suggest that sugarcane chitinase family exhibit differential responses to biotic and abiotic stress, providing new insights into their function.
Sugarcane, Saccharum spp., is an established source of sugar and has become the current benchmark renewable feedstock for efficient biofuel production. Plant disease is an important factor that affects the sugarcane yield and quality. Sugarcane smut (Sporisorium scitamineum) is one of the main diseases in sugarcane production areas1. Commercial sugarcane cultivars are poly-aneuploid interspecific hybrids with ploidy level ranging from 5X to 16X2 and contain in excess of a hundred chromosomes3. Pyramiding resistance using cross breeding, in combination to the important agronomic traits of stalk yield, sucrose content and disease resistance in a specific variety is very difficult. It is generally believed that the cultivation of disease-resistant cultivars is the most economic and effective measure to prevent smut4. Characterising sugarcane disease resistance genes will benefit to resistance breeding by providing excellent genetic resources and provide the basis for development of related molecular markers for sugarcane breeding.
Research on sugarcane pathology has focused on the cytology5, morphology6, physiology and biochemistry7, as well as genetics of sugarcane smut, to explore resistant mechanisms. Although the genome sequencing of S. scitamineum should provide new insights into the pathogenic mechanisms of sugarcane smut8, the studies on the interaction between sugarcane and S. scitamineum at the molecular level focuses mainly on the cloning and quantification of resistance-related genes, such as flavonoid pathway transcription factors X1, serine/threonine protein kinase, auxin-binding protein and 1-aminocyclopropane-1-carboxylate oxidase, in sugarcane genotypes after inoculation with S. scitamineum9,10. Transcriptomics can help to reveal the impact of S. scitamineum on sugarcane, such as the metabolic pathways and networks of molecular regulation to explore key resistant genes responding to S. scitamineum attack11.
Pathogenesis-related (PR) proteins are specific proteins induced by pathological conditions and play an important role in the plant disease resistance reaction12. Chitinases (EC 3.2.2.14), are one category of PR protein, and can catalyze chitin in the main components of the pathogen cell wall, then inhibit the growth of fungi and help to improve plant defense against fungi13,14,15. Plant chitinase genes have been grouped into seven classes (class I~VII), suggesting that chitinase isozymes were encoded by a multi-gene family16,17,18. A variety of chitinase family genes involved in pathogen attack19,20, hormone application21, temperature change22, high salt content22, metal and wounding stresses23, have been cloned and characterized from various plants such as Arabidopsis thaliana24, Nicotiana tabacum25, Oryza sativa26, Triticum aestivum17 and Sorghum bicolor27. A class I chitinase isolated from Hordeum vulgare has been reported to demonstrate antifungal activity28. A pathogen-inducible acidic class III chitinase protein, was purified from N. tabacum infected with tobacco mosaic virus (TMV)20. Genomic sequences of chitinases in Vitis vinifera were isolated by PCR walking29. Two of these belong to the class I chitinases with a putative vacuolar (Vvchit1a) and extracellular (Vvchit1b) localization, while the third one belongs to class III (VvchitIII)29. Singh et al.17 characterized an acidic form of class VII chitinase (glycosyl hydrolase family 19) from T. aestivum and demonstrated that the purified chitinase exerted a broad-spectrum antifungal activity against Alternaria sp., Colletotrichum falcatum, Fusarium sp., Rhizoctonia solani, Sarocladium oryzae and Pestalotia theae. Liu et al.21 described a chitinase gene Mmchi1 in Mikania micrantha and demonstrated that its transcripts were up-regulated after challenged with Cuscuta campestris. The expression levels of Mmchi1 gene were also increased in response to abscisic acid (ABA), salicylic acid (SA), zinc sulfate (ZnSO4) and wounding. Davis et al.30 tested the expression of various chitinases (class I, II and IV) of Ananas comosus in response to pathogen-associated signals, including necrotrophic pathogen Fusarium subglutinans f. sp. pini, SA and jasmonic acid (JA) stresses.
Plant chitinases distribute in plant roots, stems, leaves, and flowers31. Many chitinase genes are developmentally regulated and may play a part in the specific physiological processes32. The expression of AtchitIV gene was analyzed in Arabidopsis exposed to abiotic stress by Gerhardt et al.23. Transcripts accumulation was detected in leaves in response to UV light exposure, exogenous SA administration and wounding. The AtchitIV expression was also analyzed during Arabidopsis embryo development23.
In sugarcane, chitinase genes have been cloned and identified by their individual classes22,33,34. Ten nucleotide sequences of sugarcane chitinases are currently found in the GenBank database (GU219846.1, KF664180.1, KF279662.1, KF279661.1, EF123043.1, EU914816.1, EU914815.1, EF120468.1, EF113913.1, AF402937.1). Among these, only three full-length chitinase genes, ScChi (KF664180), ScChiVII1 (KF279662) and ScChiB1 (EU914815.1) have been functionally analyzed22,33,34. A class III chitinase gene ScChi, highly expressed in sugarcane leaf and stem epidermal tissues, has been cloned and characterized22. Its transcript was more abundant and maintained higher level for longer in a resistance cultivar challenged with S. scitamineum. Overexpression of ScChi in N. benthamiana suggested a close relationship between the expression of ScChi and plant immunity. Wang et al.34 reported a class VII chitinase gene ScChiVII1 from sugarcane. Differential gene expression pattern of ScChiVII1 in the smut resistant genotype Yacheng05-179 compared with the susceptible genotype Liucheng03-182 was found when challenged with S. scitamineum. Expression of ScChiVII1 in buds was significantly higher than that in roots, stems, leaves and epidermis. Rahul et al.33 obtained a full-length class IV chitinase gene ScChiB1 from the red rot resistant cultivar Co93009 with a high expression level in the incompatible interaction during challenge with Colletotrichum falcatum compared with the compatible interaction. In addition, a partial chitinase sequence from chewing cane cultivar Fuan was up-regulated after challenged with C. falcatum35.
In this study, chitinase genes in sugarcane responding to S. scitamineum were surveyed and comparative analysis was conducted. The transcriptomes of Yacheng05-179 (smut resistant) and ROC22 (smut susceptible) challenged with S. scitamineum at 24 h, 48 h and 120 h post infection (hpi) were used to identify differentially expressed genes encoding chitinases. The structure and the classification of 10 sugarcane chitinase family genes (class I~VII), as well as their response to pathogens, defense signal compounds and their expression in different sugarcane tissues were investigated using qRT-PCR (quantitative real-time polymerase chain reaction). In addition, the full-length cDNA sequences of three chitinase genes, ScChiI1, ScChiIV1 and ScChiVII1, were isolated and further analyzed for subcellular localization, transient expression in N. benthamiana, and gene expression profile under various abiotic stresses.
Results
Expression profile of sugarcane chitinase genes after S. scitamineum inoculation
The transcriptome analysis of sugarcane induced by S. scitamineum at 24 hpi, 48 hpi and 120 hpi was conducted, using the S. scitamineum-resistant and -susceptible genotypes (Yacheng05-179 and ROC22). An unigene library containing a total of 99,824 unigenes was constructed11.
We previously reported identification and functional analysis of two sugarcane chitinase genes, ScChi (KF664180)22 and ScChiVII1 (KF279662)34. To identify more chitinase genes, from the transcriptome analysis, 17 unigenes differentially expressed during sugarcane-S. scitamineum interaction were identified as possibly encoding for chitinase genes (Table 1). Transcripts of chitinase genes (gi34957207, Sugarcane_Unigene_BMK.68059, gi35992663, Sugarcane_Unigene_BMK.60821, Sugarcane_Unigene_BMK.56580, Sugarcane_Unigene_BMK.64954, Sugarcane_Unigene_BMK.48857, gi36021860, Sugarcane_Unigene_BMK.60821) were all up-regulated in the resistant genotype Yacheng05-179. However, 12 genes displayed a mixed expression pattern (10 up-regulated and 2 down-regulated) in the susceptible genotype ROC22. Furthermore, the expression of chitinase genes in Yacheng05-179 (24 hpi ~ 48 hpi) occurred earlier than that in ROC22 (48 hpi ~ 120 hpi), suggesting resistance specificity and early timing of these genes in the incompatible interaction between sugarcane and S. scitamineum.
Table 1. A list of differentially expressed chitinase genes in Yacheng05-179 and ROC22 after challenged with Sporisorium scitamineum for 24 h, 48 h and 120 h, respectively.
| Unigene ID |
Yacheng05-179 log2 fold change (T/CK)* |
ROC22 log2 fold change (T/CK)* |
BLAST annotation | ||||
|---|---|---|---|---|---|---|---|
| 24 hpi | 48 hpi | 120 hpi | 24 hpi | 48 hpi | 120 hpi | ||
| gi32815041 | — | — | — | — | — | 1.64 | Chitinase 1 [Oryza sativa] |
| gi34957207 | — | 2.38 | — | — | −2.14 | −1.88 | Chitinase 12 [Oryza sativa] |
| Sugarcane_Unigene_BMK.68059 | 1.49 | — | — | — | — | — | Chitinase 12 [Oryza sativa] |
| gi35992663 | 1.47 | — | 1.22 | — | 1.68 | 4.23 | Chitinase 11 [Oryza sativa] |
| Sugarcane_Unigene_BMK.51590 | — | — | — | −1.55 | — | — | Acidic endochitinase [Vitis vinifera] |
| Sugarcane_Unigene_BMK.60821 | 1.37 | — | — | — | — | 2.34 | Acidic endochitinase [Vitis vinifera] |
| Sugarcane_Unigene_BMK.56580 | 1.97 | 2.10 | — | — | — | 1.55 | Endochitinase [Zea mays] |
| Sugarcane_Unigene_BMK.64954 | — | 1.70 | — | — | — | — | Chitinase 2 [Tulipa bakeri] |
| Sugarcane_Unigene_BMK.48857 | 1.61 | 1.68 | — | — | — | — | Chitinase 10 [Oryza sativa] |
| gi36021860 | 1.61 | 1.68 | — | — | — | — | Endochitinase A [Zea mays] |
| Sugarcane_Unigene_BMK.60821 | 1.37 | — | — | — | — | — | Acidic endochitinase [Vitis vinifera] |
| gi35081719 | — | — | — | — | 1.33 | — | Chitinase 6 [Oryza sativa] |
| Sugarcane_Unigene_BMK.49423 | — | — | — | — | — | 1.64 | Chitinase 1 [Oryza sativa] |
| gi36003099 | — | — | — | — | — | 3.60 | Chitinase 1 [Tulipa bakeri] |
| gi35980761 | — | — | — | — | — | 2.08 | Chitinase 2 [Oryza sativa] |
| Sugarcane_Unigene_BMK.60969 | — | — | — | — | — | 1.87 | Chitinase 2 [Oryza sativa] |
| gi36066432 | — | — | — | — | — | 1.96 | Chitinase 8 [Oryza sativa] |
*T indicated the transcriptome of sugarcane challenged with S. scitamineum. CK mean the transcriptome of the mock material.
Phylogenetic analysis of chitinase gene family
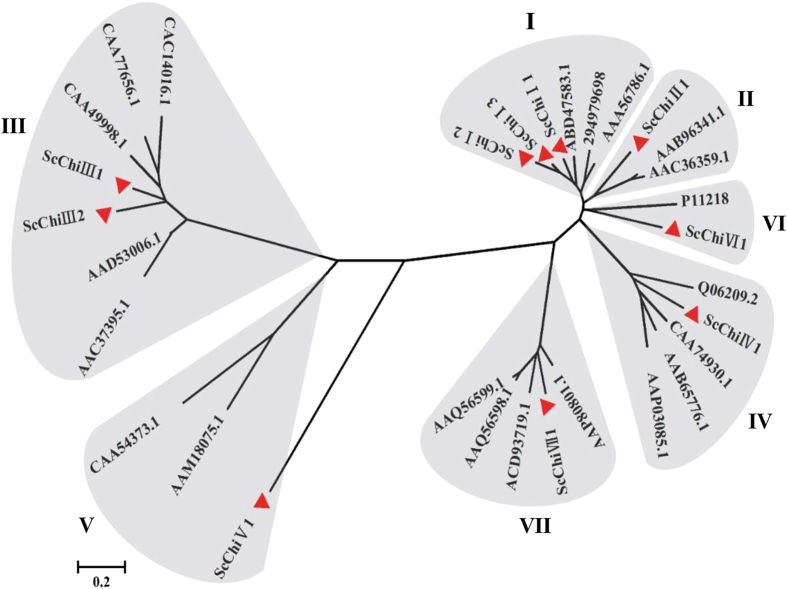
Among the 17 differentially expressed chitinase unigenes, a total of 9 members was predicted to have full-length sequences with open reading frames (ORFs). The assembled sequence of ScChiVII1 based on homologous cloning method according to the predicted S. bicolor chitinase gene (XM_002460419.1) was added. To study the phylogenetic relationships of the chitinase family genes in sugarcane, a multiple alignment analysis was performed. The 10 genes with ORF structures were classified into seven types (class I ~ VII) based on the similarity of their amino acid sequences with 21 biotic stress resistance-related chitinases of other plant species from NCBI17,18. As shown in Fig. 1, they were segregated into two branches, one comprising classes III and V, and the other one including the classes I, II, IV, VI and VII. The 10 sugarcane chitinase genes were named by classification system of the chitinase in the phylogenetic tree and described as ScChiI1 (gi32815041), ScChiI2 (gi34957207), ScChiI3 (Sugarcane_Unigene_BMK.68059), ScChiII1 (gi35992663), ScChiIII1 (Sugarcane_Unigene_BMK.51590), ScChiIII2 (Sugarcane_Unigene_BMK.60821), ScChiIV1 (Sugarcane_Unigene_BMK.56580), ScChiV1 (Sugarcane_Unigene_BMK.64954), ScChiVI1 (Sugarcane_Unigene_BMK.48857) and ScChiVII1. The nucleotide and protein sequences of these chitinases are provided in Supplementary Table S1.
Figure 1. The phylogenic tree of chitinase family genes in sugarcane and other plant species.
The tree was constructed using the neighbor-joining method and diagrams drawn with MEGA 5.05. The red triangle represents the 10 sugarcane chitinase family genes.
Sequence analysis of chitinase gene family
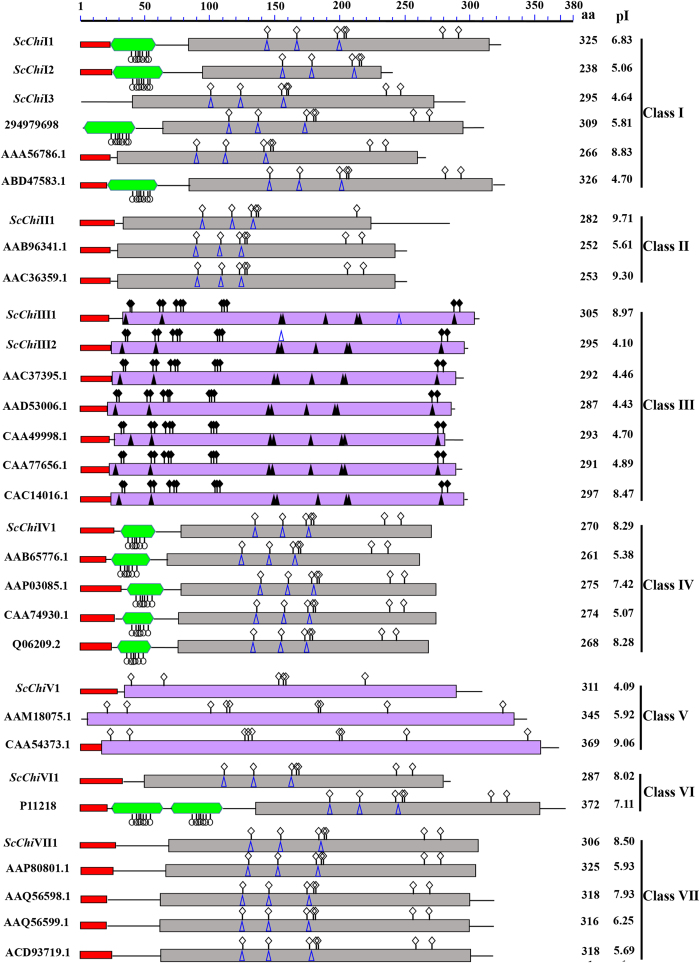
In order to gain insight into the diversification among the above 10 chitinases from sugarcane and 21 from other plant species, several features of the predicted proteins were analyzed. The typical domains of chitinase, including chitin binding domain (CBD), chitinase domains of glycoside hydrolase family 18 and family 19, were shown in Fig. 2. In addition, the signal peptide, isoelectric point (pI) and the number of amino acids (aa) were also presented in Fig. 2. We found that not all chitinases contained signal peptide at their N-termini, such as ScChiI3 and O. Sativa chitinase (294979698) in class I and Momordica charantia chitinase (AAM18075.1) in class V. The length of the ORFs in sugarcane chitinases ranged from 238 aa to 325 aa. The average ORF length was 291 aa. The isoelectric point (pI) in different members was not identical in the same class, as some were acidic and others were basic. The sugarcane chitinases, including classes I, II, IV, VI and VII members, have a lysozyme-like domain in their structures which may exhibit lysozyme activity.
Figure 2. Domain architecture of chitinases classes I~VII in sugarcane and other plant species.
aa: the number of amino acids; pI: isoelectric point;  : signal peptide;
: signal peptide;  : chitin binding domain (CBD);
: chitin binding domain (CBD);  : glycoside hydrolase family 19 chitinase domain;
: glycoside hydrolase family 19 chitinase domain;  : glycoside hydrolase family 18 chitinase domain;
: glycoside hydrolase family 18 chitinase domain;  : carbohydrate binding site;
: carbohydrate binding site;  : catalytic residues;
: catalytic residues;  : sugar binding site;
: sugar binding site;  : active site;
: active site;  : substrate-binding cleft.
: substrate-binding cleft.
Class I members ScChiI1 and ScChiI2 both contained the N-terminal signal peptide, following the chitin binding domain (CBD) which was rich in cysteines (9) and a glycoside hydrolase family 19 chitinase domain. Though the protein domain of ScChiI3 lacked a signal peptide and the CBD structure and was different from those of ScChiI1 and ScChiI2, they sharing 63.96% amino acid sequence identity. A spacer hinge region, rich in proline (3, 12 and 13) and glycine (5, 6 and 5) residues, was found between the CBD and the glycoside hydrolase family 19 chitinase domains of ScChiI1, ScChiI2 and ScChiI3. Class II chitinase ScChiII1 lacked the CBD and the hinge region, but contained a N-terminal signal peptide and a glycoside hydrolase family 19 chitinase domain (amino acids 34 ~ 223), sharing a high degree of homology (70.82%) with class I members. Like class I protein, class IV chitinase ScChiIV1 consisted of the CBD, hinge region and glycoside hydrolase family 19 chitinase domain. However, there was only 59% identity in the catalytic domain among class I and class IV. The class VI chitinase ScChiVI1, which lacked the duplicated CBDs in its N-terminal region which was different from chitinase (P11218) in Urtica dioica endochitinase36, had a signal peptide, a hinge region (1 prolines and 6 glycines) and a glycoside hydrolase family 19 chitinase domain. Class VII chitinase ScChiVII1 lacked the CBD and the hinge region, and its amino acid sequences were 47.57% homology to class I and Class II chitinases. Unlike other sugarcane chitinase family members, the catalytic domain in class III (ScChiIII1 and ScChiIII2) and class V (ScChiV1) chitinases was glycosyl hydrolase family 18 but not glycoside hydrolase family 19. These results suggest that all ORFs in sugarcane chitinases contained at least one typical domain.
Tissue-specific expression of chitinase family genes in sugarcane
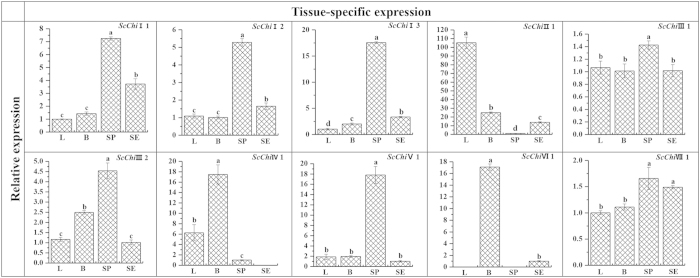
qRT-PCR was performed to determine the expression patterns of these putative chitinase genes in different sugarcane above-ground tissues. As shown in Fig. 3, the expression of chitinase genes belonging to classes I, II, III, V and VII was detected in all of the four sugarcane tissues including leaf, bud, stem pith and stem epidermis. Compared with the other three tissues, the chitinase genes with the highest expression levels in stem pith were ScChiI1, ScChiI2, ScChiI3, ScChiIII1, ScChiIII2, ScChiV1 and ScChiVII1. ScChiII1 showed the highest level of transcripts in sugarcane tissues with transcripts most abundant in leaf. Transcripts of ScChiIV1 and ScChiVI1 accumulated to the highest level in bud tissues. These results showed a certain degree of tissue specificity in sugarcane chitinase family genes (Fig. 3).
Figure 3. Tissue-specific expression analysis of the 10 chitinase family genes in different tissues of sugarcane genotype Yacheng05-179 by qRT-PCR.
Data was normalized to the GAPDH expression level. All data points are the means ± SE (n = 3). Different lowercase letters indicate a significant difference, as determined by the least-significant difference test (p-value < 0.05). L: Leaf; B: Bud; SP: Stem pith; SE: Stem epidermis.
Accumulation of chitinase gene mRNAs in sugarcane post inoculation with S. scitamineum
qRT-PCR was used to examine the expression patterns of the 10 sugarcane chitinase family genes during sugarcane-smut interaction (Fig. 4). It was seen that all 10 transcripts were induced by infection of S. scitamineum but different patterns were evident.
Figure 4. Expression analysis of the 10 chitinase family genes during sugarcane-smut (Sporisorium scitamineum) interaction by qRT-PCR.
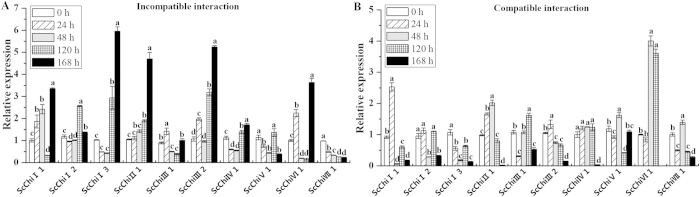
Data was normalized to the GAPDH expression level. All data points (with the deduction of their mocks) were the means ± SE (n = 3). Different lowercase letters indicate a significant difference, as determined by the least-significant difference test (p-value < 0.05). Incompatible interaction: the interaction between sugarcane resistant genotype Yacheng05-179 and S. scitamineum; Compatible interaction: the interaction between sugarcane susceptible genotype ROC22 and S. scitamineum.
During the incompatible interaction using Yacheng05-179, one smut resistant sugarcane genotype, early transcriptional elevation of ScChiI1, ScChiIII1, ScChiIII2 and ScChiVI1 was observed at 24 hpi (Fig. 4A). The transcript of ScChiIII1 reached the maximum at 24 hpi, while the maximal accumulation of the other 3 genes was observed at 168 hpi. ScChiI2 and ScChiV1 transcripts decreased at 24 hpi and 48 hpi, but increased to the peak at 120 hpi and again reduced at 168 hpi. Although the ScChiI3 and ScChiIV1 accumulation decreased at initial stage (from 0 hpi to 48 hpi), they gradually elevated at the later stage (from 120 hpi to 168 hpi). ScChiII1 was up-regulated from 48 hpi to 168 hpi. In contrast, ScChiVII1 demonstrated a down-regulation during the incompatible interaction.
During the compatible interaction using ROC22, a popular genotype which is susceptible to S. scitamineum, transcripts of ScChiI1, ScChiII1 and ScChiIII2 were observed to be elevated as early as 24 hpi, suggesting rapid response to the infection of smut pathogen (Fig. 4B). Their expression values were accumulated to the maximal levels at either 24 hpi or 48 hpi. Transcripts of ScChiI2 and ScChiIV1 maintained almost at the same level after inoculation. ScChiI3 was down-regulated compared with that at 0 hpi. The transcripts of ScChiIII1, ScChiV1, ScChiVI1 and ScChiVII1 peaked at 48 hpi. The data indicated that all genes except ScChiVI1 had the lowest expression level at 168 hpi during the compatible interaction.
Gene expression in response to different defense-related signal compounds
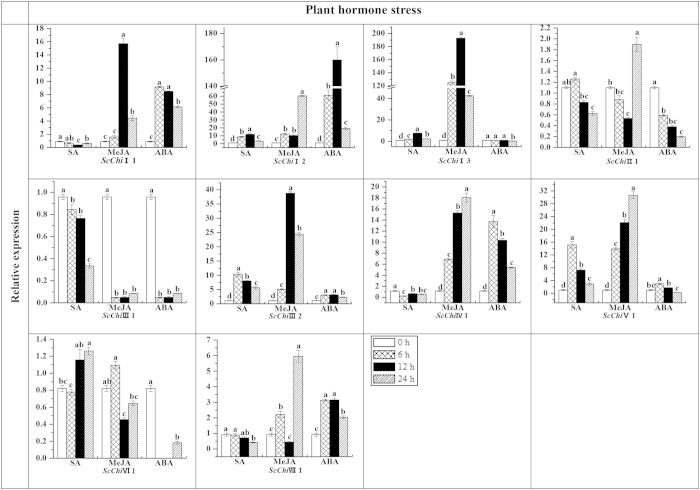
Transcript accumulation of chitinase genes in sugarcane plantlets under different phytohormone treatments, including SA, MeJA (methyl jasmonate) and ABA stresses, were examined by qRT-PCR (Fig. 5). The results revealed that all three signal compounds up-regulated ScChiI2, ScChiIII2 and ScChiV1, while ScChiIII1 was down-regulated. ScChiI1, ScChiIV1 and ScChiVII1 were up-regulated by MeJA and ABA but down-regulated by SA. In addition ScChiVI1 was down-regulated by MeJA and ABA but up-regulated by SA. ScChiI3 was up-regulated by SA and MeJA but suppressed by ABA. ABA treatment down-regulated ScChiII1 while ScChiVII1 was up-regulated. These results suggest that the transcription of individual chitinase genes respond differently to SA, MeJA and ABA.
Figure 5. Expression analysis by qRT-PCR of the 10 chitinase family genes in Yacheng 05-179 plantlets after treatment with 5 mM SA, 25 μM MeJA and 100 μM ABA.
Data was normalized to the GAPDH expression level. All data points were the means ± SE (n = 3). Different lowercase letters indicate a significant difference, as determined by the least-significant difference test (p-value < 0.05). SA, salicylic acid; MeJA, methyl jasmonate; ABA, abscisic acid.
Functional characterization of three chitinase genes, ScChiI1, ScChiIV1 and ScChiVII1, during pathogen infection
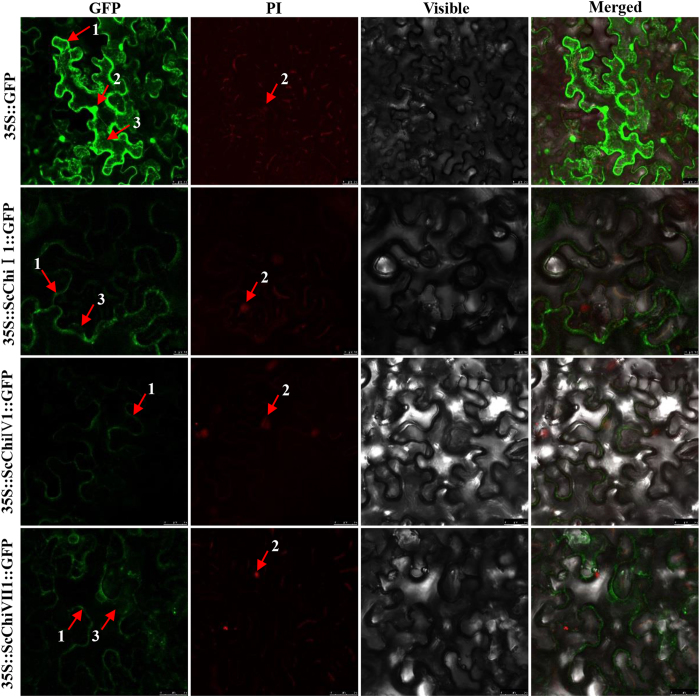
Based on the information of differentially expressed chitinase genes post S. scitamineum infection, the full-length cDNA sequences of three chitinase genes, ScChiI1, ScChiIV1 and ScChiVII1, were isolated from sugarcane. The sequence data of ScChiI1, ScChiIV1 and ScChiVII1 were submitted to GenBank under accession number of KF664182, KF664178 and KF664179, respectively. The ORF fragment was recombined into the plant expression vector of pCAMBIA 2300 containing the 35S promoter and the GFP reporter gene. Their subcellular localization was characterized by transient expression of the target gene and GFP in N. benthamiana leaves with Agrobacterium-mediated transformation method37. Infiltrated leaves observed under a confocal laser scanning microscope showed that 35S::ScChiI1::GFP, 35S::ScChiIV1::GFP and 35S::ScChiVII1::GFP fusion proteins were located in cytoplasm and plasma membrane, plasma membrane, cytoplasm and plasma membrane, respectively (Fig. 6). In addition, the mock of 35S::GFP was shown in the nucleus, cytoplasm and plasma membrane cells.
Figure 6. Subcellular localization analysis of ScChiI1, ScChiIV1 and ScChiVII1 in Nicotiana benthamiana leaves 48 h after infiltration.
PI images indicate nuclear staining. The epidermal cells were used for taking images of green fluorescence, red fluorescence, visible light and merged light. Read Arrows 1, 2 and 3 indicated plasma membrane, nucleus and cytoplasm, respectively. Bar = 25 μm.
Chitinase genes have been reported to be induced not only by biotic but also by abiotic stress12,38. The expression patterns of ScChiI1, ScChiIV1 and ScChiVII1 in Yacheng05-179 plantlets were investigated after treatment with 25% PEG (polyethylene glycol), 250 mM NaCl (sodium chloride), 100 μM CuCl2 (copper chloride), and low temperature (4 °C) (Fig. 7). This showed induction of high levels of ScChiIV1 transcripts with all four abiotic treatments. PEG, NaCl and CuCl2 appeared to cause an increase of accumulated ScChiI1 transcripts post stress, while low temperature caused slightly decrease at 12 h. The expression of ScChiVII1 was up-regulated by low temperature and down-regulated by NaCl. In response to PEG and NaCl stresses, the level of ScChiVII1 transcript reduced slightly at 6 h and 12 h, but increased at 12 h and 24 h, respectively.
Figure 7. Expression analysis by qRT-PCR of ScChiI1, ScChiIV1 and ScChiVII1 genes in Yacheng05-179 plantlets after treatment with 25% PEG, 250 mM NaCl, 100 μM CuCl2 and low temperature (4 °C).
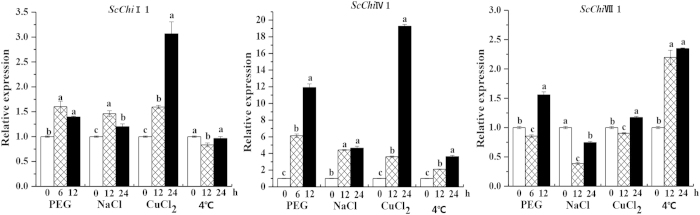
Data were normalized to the GAPDH expression level. All data points were the means ± SE (n = 3). Different lowercase letters indicated a significant difference, as determined by the least-significant difference test (p-value < 0.05). PEG, polyethylene glycol; NaCl, sodium chloride; CuCl2, copper chloride.
Transient expression of ScChiI1, ScChiIV1 and ScChiVII1 induces a defense response in N. benthamiana
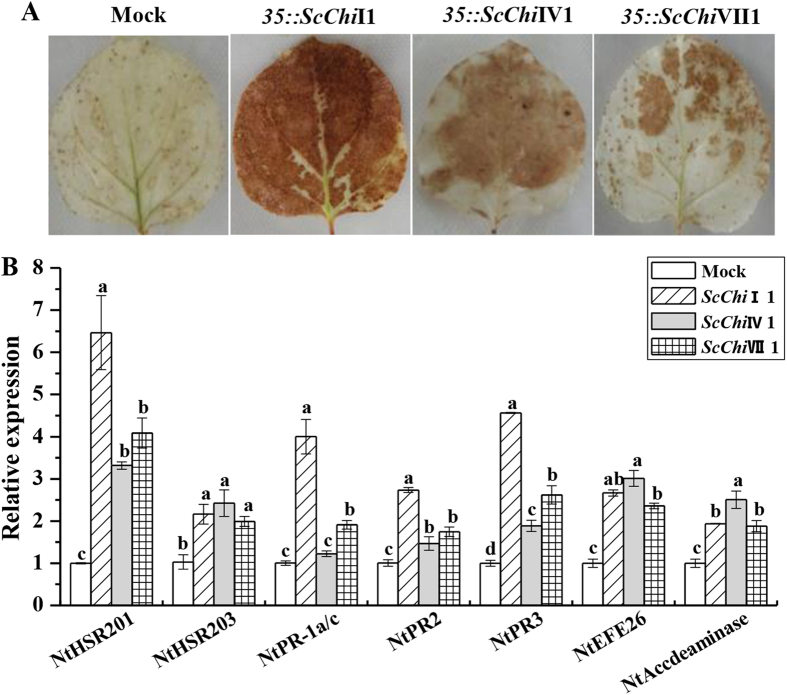
To test whether the target genes can induce hypersensitive response (HR) and immunity in plant, ScChiI1, ScChiIV1 and ScChiVII1 genes were transiently over-expressed in N. benthamiana leaves. After 48 h post infiltration, a typical HR symptom with deeper DAB staining was found in the leaves expressing 35S::ScChiI1, 35S::ScChiIV1 and 35S::ScChiVII1, respectively (Fig. 8). The bronzing color after over-expressing ScChiI1 was the darkest. Furthermore, the expression levels of seven immunity associated marker genes including the HR marker genes NtHSR201 and NtHSR203, the JA- associated genes NtPR-1a/c and NtPR2 and NtPR3, and the ethylene synthesis depended genes NtEFE26 and NtAccdeaminase, were increased post 24 h infiltration. These results suggest that ScChiI1, ScChiIV1 and ScChiVII1 were involved in cell death responses.
Figure 8. The transient expression of ScChiI1, ScChiIV1 and ScChiVII1 and qRT-PCR analysis of the immunity associated marker genes in Nicotiana benthamiana leaves.
(A) DAB (3,3’-diaminobenzidinesolution) staining with N. benthamiana leaves 48 h after Agrobacterium strain infiltration. (B) The transcripts analysis of the immunity associated marker genes, including the hypersensitive response marker genes NtHSR201 and NtHSR203, the jasmonate associated genes NtPR-1a/c and NtPR2 and NtPR3, and the ethylene synthesis depended genes NtEFE26 and NtAccdeaminase. NtEF1-α was used to normalize the transcript levels. Mock: the Agrobacterium strain carrying 35S::00. All data points are the means ± SE (n = 3). Different lowercase letters indicate a significant difference, as determined by the least-significant difference test (p-value < 0.05).
Discussion
Many plants contain multiple chitinase isozymes. They have been categorized into seven classes (class I ~ VII) based on their primary structure, substrate specificity, mechanisms of catalysis and sensitivity to inhibitors17,18. On the basis of the annotations of O. sativa and Arabidopsis genomic sequences, 37 and 24 chitinases were found in O. sativa and Arabidopsis, respectively24. Analysis revealed that each cluster had distinct amino acid characteristics. Krishnaveni et al.39 had observed three antifungal chitinases, CH1, CH2 and CH3, from S. bicolor. Four cDNAs encoding acidic and basic isoforms of chitinases were isolated from Cladosporium fulvum-infected tomato leaves40. We have previously reported cloning and identification of one class III and one class VII chitinases from sugarcane post S. scitamineum inoculation22,34. The current study of the sugarcane chitinase family indicated the presence of at least 17 expressed genes induced by smut pathogen.
Chitinase isozymes are a diverse group of enzymes with different characteristics, such as enzymatic activities, primary sequence, pI and cellular localization41. Based on the domain architecture of chitinases classes I~VII in sugarcane and other plants, not all chitinases contained a signal peptide, and the CBD structure was absent in ScChi VI1 (class VI). According to the most popular classification system described earlier17,18,42, class I chitinases contain three domains: a cysteine-rich N-terminal CBD, a proline- and glycine-rich hinge region and a highly conserved C-terminal catalytic domain. Class II chitinases are generally extracellular which lack the CBD and the hinge region, but their amino acid sequences in the catalytic domain are nearly identical to class I chitinases (more than 65%). Class III lacks CBD and has little sequence identity to the class I and class II catalytic domain, while Class IV contains the CBD, hinge region and catalytic domain, but displays deletion in the catalytic domain. Class V chitinases has little sequence similarity with the other chitinases, but more similar to bacterial chitinases, such as those from Bacillus circulans and Serratia marcescens. Class VI chitinases possess the duplicated CBDs in their N-terminal regions, while Class VII chitinases lack the CBD and the hinge region. In this study, seven types of sugarcane chitinases coincided with the former classification17,18,42. In Fig. 1, although ScChiV1 and the chitinase proteins from Momordica charantia (AAM18075.1) and N. tabacum (CAA54373.1) were not at the consistent branch of the phylogenic tree, it was assigned to the class V subfamily containing the same domain of glycoside hydrolase family 18. Nearly all sugarcane chitinases, except ScChiI 3, contained the N-terminal targeting domain which may involve in directing them to either the vacuole or the apoplast (Fig. 2). Like other plant species43, sugarcane chitinases of classes I, II, IV, VI and VII have the glycoside hydrolase family 19 domain belong to class PR-3 family, and class III and class V possess the glycoside hydrolase family 18 domain belong to PR-8 and PR-11 families, respectively. Chitinases including class I, II, IV, VI and VII were predicted to contain a lysozyme like domain44, suggesting that most sugarcane chitinases possess lysozyme activity.
According to previous reports, the only route of invasion of the smut pathogen is via sugarcane buds45. Previous studies also revealed that plant chitinases are developmentally regulated, indicating a role in the specific physiological processes18,46. In this study, transcripts of sugarcane chitinase genes differently accumulated in the noninfected sugarcane above-ground tissues (Fig. 3). Seven chitinase genes expressed at high expression levels in stem pith, suggesting specific roles in stem pith. ScChiII1 showed the highest expression level in sugarcane and its transcript was most abundant in leaf. Considering the significantly higher expression of ScChiIV1 and ScChiVI1 in sugarcane buds than in other tissues, it suggests that ScChiIV1 and ScChiVI1 may play a positive role in sugarcane smut resistance.
In the present study, during S. scitamineum infection (0 hpi ~ 168 hpi), the expression of at least 10 sugarcane chitinases was induced. However they showed different expression patterns in the incompatible/compatible interactions. In Yacheng05-179, four chitinase genes, ScChiI1, ScChiIII1, ScChiIII2 and ScChiVI1, rapidly responded to smut pathogen inoculation at initial stage (from 0 hpi~24 hpi) (Fig. 4), and reached maximal accumulation at 168 hpi. Conversely, in ROC22, almost all the target genes (except ScChiVI1) had lower expression levels at 168 hpi (Fig. 4). These results suggest that sugarcane chitinase genes are pathogen-inducible and are involved in disease resistance. Previously, a class III sugarcane chitinase gene ScChi was shown to be induced after challenge in the incompatible interaction (Yacheng05-179 vs. S. scitamineum) and its expression remained higher than that in a compatible interaction (Liucheng03-182 vs. S. scitamineum)22.
In plants, levels of chitinases are regulated by biotic and abiotic stress, such as pathogen infection, cold, drought, heavy metals, salt, and plant hormones12,22,38. As reported, SA, JA and ethylene are considered as the defense signal compounds for systemic acquired resistance (SAR) and induced systemic resistance (ISR), two types of plant induced resistance21. In plant responses to environmental stress, the reaction of the signaling molecule JA is the fastest, and plays an important part in resistance reaction. JA-related gene expression has been reported to be up-regulated and cause JA accumulation under biotic and abiotic stress47. Previous studies suggested that ABA affects plant response to biotic stress mainly via interaction with other stress responsive pathways48. In our study, the expression levels of sugarcane chitinase genes could be differentially modulated by SA, MeJA and ABA (Fig. 5). Exogenously applied SA resulted in an increase accumulation of ScChiI2, ScChiI3, ScChiIII2, ScChiV1 and ScChiVI1 transcripts. Application of MeJA increased the expressions of ScChiI1, ScChiI2, ScChiI3, ScChiII1, ScChiIII2, ScChiIV1, ScChiV1 and ScChiVII1. The exogenous application of ABA increased the levels of ScChiI1, ScChiI2, ScChiIII2, ScChiIV1, ScChiV1 and ScChiVII1 transcripts.
Full-length cDNA sequences of three sugarcane chitinase genes, each one of class I chitinase ScChiI1, class IV chitinase ScChiIV1 and class VII chitinase ScChiVII1, were isolated from smut resistant genotype Yacheng05-179. These three genes were pathogen-inducible post S. scitamineum infection (Fig. 4), and were up-regulated by MeJA and ABA but down-regulated by SA (Fig. 5). Protein localization revealed that 35S::ScChiI1::GFP and 35S::ScChiVII1::GFP fusion proteins were located in cytoplasm and plasma membrane, while 35S::ScChiIV1::GFP was located in plasma membrane (Fig. 6). ScChiI1 and ScChiIV1 were up-regulated by PEG, NaCl and CuCl2 stresses, while ScChiVII1 was not (Fig. 7). ScChiIV1 and ScChiVII1 transcripts were increased under 4 °C low temperature stress, but ScChiI1 was not (Fig. 7). However, all these genes induced defense responses in N. benthamiana by transient expression (Fig. 8). These results suggest that the different sugarcane chitinases have individual functions in response to various environmental stresses.
Although functions of sugarcane chitinases genes are not fully understood, some chitinases in plant species have been shown to inhibit the growth of chitin-containing fungi, both in vitro49 and in vivo14,15. When compared with wild-type plants, in many cases, transgenic plants constitutively expressing chitinases showed enhanced resistance to fungal infection or delayed development of disease symptoms50,51. The transgenic Musa acuminata expressing the O. sativa chitinase gene exhibited resistance to black leaf streak disease caused by the pathogenic fungus, Mycosphaerella fijiensis26. In our previous work, a close relationship between the expression of sugarcane class III chitinase gene ScChi (KF664180) and plant immunity was demonstrated from inoculation experiments and the validation of in vitro antibacterial activity. There was also a report of smut resistance improvement in sugarcane varieties ROC22 and ROC10 by introduction of a β-1,3 glucanase together with the modified class I chitinase gene from N. tabacum52. From the characteristics of the 10 sugarcane chitinase genes obtained here, the possible contribution of all these genes for plant defense against pathogen attack is suggested. However, the conclusive validation and precise functional determination of these genes by genetic transformation into sugarcane is still in progress.
Methods
Plant materials and inoculation with S. scitamineum
Sugarcane varieties, Yacheng05-179 and ROC22, as well as smut whips, were obtained from the Key Laboratory of Sugarcane Biology and Genetic Breeding, Ministry of Agriculture (Fuzhou, China). Two-bud sets of both sugarcane genotypes (Yacheng05-179 and ROC22), were grown at 28 °C in condition of 12 h light/12 h dark photoperiod, then inoculated with 0.5 μL suspension containing 5 × 106 spores·mL−1 of S. scitamineum in 0.01% (v/v) Tween-20. The controls were mock inoculated with 0.01% (v/v) Tween-20 in sterile distilled water instead of spores37 to eliminate the effect of wounding. At 0 hpi, 24 hpi, 48 hpi, 120 hpi and 168 hpi, one biological replicate consisting of five buds for each group were excised, immediately frozen in liquid nitrogen and then stored at −80 °C.
Tissue distribution study
For tissue distribution study, one biological replicate with six healthy 10 month old plant of Yacheng05-179 was selected. The samples were collected from the youngest fully expanded leaf (+1 leaf) with a visible dewlap (the collar between the leaf blade and sheath), buds, stem pith and stem epidermis. These samples were fixed in liquid nitrogen and kept at −80 °C until RNA extraction37.
Abiotic stress treatments
To investigate the expression of sugarcane chitinase family genes in response to stress factors, 4 month old tissue cultured plantlets of Yacheng05-179 were grown in water for one week and then exposed to various chemical stimuli37. The plantlets were treated with 5 mM SA solution, 25 μM MeJA in 0.1% (v/v) ethanol and 0.05% (v/v) Tween-20, 100 μM ABA, or 25% PEG8000, respectively. Plantlets were kept in conical tubes at 28 °C in condition of 16 h light/8 h dark photoperiod and harvested at 0 h, 6 h, 12 h and 24 h. Another group of sugarcane plantlets were separately treated with 250 mM NaCl, 100 μM CuCl2 and 4 °C low temperature for 0 h, 12 h, 24 h and 48 h. All of the above treatments were carried out in three biologic replicates.
RNA extraction
Total RNA was extracted with Trizol reagent (Invitrogen, China) according to the manufacturer’s protocol. RNA was treated with DNase I (Promega, USA) to remove DNA contamination. The first-strand cDNA synthesis was completed by the Prime-ScriptTM RT Reagent Kit (TaKaRa, China).
Isolation of chitinase genes in sugarcane challenged with S. scitamineum
To isolate chitinase family genes in sugarcane post S. scitamineum infection, 26 unigenes which were differently expressed in Yacheng05-179 and ROC22 after inoculation with S. scitamineum, were annotated to chitinase genes11. Further BLAST by amino acid sequences, 17 unigenes predicted to encode for chitinase protein were analyzed. These unigenes ID were gi32815041, gi34957207, Sugarcane_Unigene_BMK.68059, gi35992663, Sugarcane_Unigene_BMK.51590, Sugarcane_Unigene_BMK.60821, Sugarcane_Unigene_BMK.56580, Sugarcane_Unigene_BMK.64954, Sugarcane_Unigene_BMK.48857, gi36021860, Sugarcane_Unigene_BMK.60821, gi35081719, Sugarcane_Unigene_BMK.49423, gi36003099, gi35980761, Sugarcane_Unigene_BMK.60969, gi36066432. A ScChiVII1 sequence was isolated by homology-based cloning method according to the predicted chitinase gene (XM_002460419.1) from S. bicolor genome database.
According to the results of previous researches by Kirubakaran et al.28, Rahul et al.33 and Singh et al.17, along with the information from bioinformatic analysis and their expression profile under the stresses of MeJA, ABA and SA indicated in this study, three out of ten chitinase genes, ScChiI1, ScChiIV1 and ScChiVII1 were chosen for further study. Based on the sequences of the above predicted chitinase genes, the primers used to clone the target genes were designed. Amplification of ScChiI1 (gi32815041) was performed with primers ScChiI1: FW-ACATACATAGTTGCTTGCYTTGC and RV-CCTTTTGCTTTATTCATTGCTC on first-strand cDNA template of Yacheng05-179 under 4 °C low temperature treatment for 24 h. ScChiIV1 (Sugarcane_Unigene_BMK.56580) and ScChiVII1 were amplified with primers ScChiIV1: FW-GCACCGCAGCAACGAA and RV-CGGAGCCATGCAAGGAG, ScChiVII1: FW-AAGATGAAGCGGAAGACG and RV-GCTAAAACAGACCCATTGTG, on first-strand cDNA template of Yacheng05-179 post 48 h S. scitamineum inoculation. These PCR products were gel-purified, cloned into the pMD18-T vector (TaKaRa, China) and sequenced (Shenggong, China).
Sequence analysis of chitinase genes
ORF analysis was performed with the ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The pI was calculated with the ProtParam tool (http://www.expasy.ch/tools/protparam.html). SignalP 4.0 Server (http://www.cbs.dtu.dk/services/SignalP/), NCBI Conserved Domains (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and SMART (http://smart.embl-heidelberg.de/) programs were employed to scan for the signal peptides and the motifs on the primary structure of the deduced protein sequences. Subcellular location of the putative proteins was predicted with PSORT Prediction (http://psort.hgc.jp/form.html). ClustalW software was used to perform multiple alignment of sugarcane chitinases with other previously published plant chitinases17,18. Based on this alignment, a phylogenetic tree was constructed according to the neighbor-joining (NJ) method (1,000 bootstrap replicates) using the MEGA 5.05 program.
Transcript level analysis
Expression patterns of sugarcane chitinase family genes in different tissues and their response to biotic and abiotic stress were analyzed by qRT-PCR, which followed the instructions of the SYBR Green Master (ROX) (Roche, China) on a 7500 real time PCR system (Applied Biosystems, USA). The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene (Table 2) was used as an internal control. According to sequences of ScChiI1 ~ ScChiVII1, the specific primers (Table 2) were designed using the Beacon Designer 8.12 program. The qRT-PCR reaction system (20 μL) contained 10 μL FastStart Universal SYBR Green PCR Master (ROX), 1.0 μL of first-strand cDNA (10 × diluted) and 0.5 μM of each primer. PCR with distilled water as template was performed as control. The qRT-PCR reaction condition was held at 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. At the end of the PCR reaction, a melting curve was established. Each qRT-PCR was conducted in triplicate. The 2−ΔΔCt method was adopted to analyze the qRT-PCR results53. For calculating gene expression level during developmental stages, the tissue exhibiting the lowest expression level was served as control. For the abiotic stress treatments, unstressed sample was used as control. During the biotic stress, gene expression profile was calculated by the expression level of the inoculated sample of S. scitamineum minus the level of the mock at each corresponding time point to eliminate any effect of wounding. Data points in qRT-PCR time course were plotted as means ± SE of three replicates.
Table 2. qRT-PCR primers used in this study.
| Primer | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| ScChiI1 | TTGCTTTGCTTCCCTCACGA | AGAGGGACTCGGAGATGATGGA |
| ScChiI2 | CCTGTTCAACCAGATGCT | AAGGCGGAGTAGGTGTAG |
| ScChiI3 | GTCCTCACCAACATCATCA | GCTGTAGCAGTCCAAGTT |
| ScChiII1 | TGACCACCAACATCATCAA | ATTCCAAGCATATCGCAGTA |
| ScChiIII1 | CATCAAGGTCCTGCTCTC | CCGAGGTAGTTGTTCCAG |
| ScChiIII2 | ATGGCGGCTAATCTCAAG | GATGACGTAGGCGTAGAG |
| ScChiIV1 | CGAGACCGGACATTTCTGCTAC | CGAACCCCTGCGACATCAC |
| ScChiV1 | GACGAGCAGATGGTGAACT | CCAGATGAAGATGCCGTAGAG |
| ScChiVI1 | GTGGTCGCTTTCCTCGTTGC | TGGATGAAGGAGGCGTAGGTGT |
| ScChiVII1 | TCAAGAAGAACCAGCCGTCAGC | TGCTGCTTCTGGTCATCCGTTG |
| GAPDH | CACGGCCACTGGAAGCA | TCCTCAGGGTTCCTGATGCC |
| NtHSR201 | CAGCAGTCCTTTGGCGTTGTC | GCTCAGTTTAGCCGCAGTTGTG |
| NtHSR203 | TGGCTCAACGATTACGCA | GCACGAAACCTGGATGG |
| NtPR-1a/c | AACCTTTGACCTGGGACGAC | GCACATCCAACACGAACCGA |
| NtPR2 | TGATGCCCTTTTGGATTCTATG | AGTTCCTGCCCCGCTTT |
| NtPR3 | CAGGAGGGTATTGCTTTGTTAGG | CGTGGGAAGATGGCTTGTTGTC |
| NtEFE26 | CGGACGCTGGTGGCATAAT | CAACAAGAGCTGGTGCTGGATA |
| NtAccdeaminase | TCTGAGGTTACTGATTTGGATTGG | TGGACATGGTGGATAGTTGCT |
| NtEF1-α | TGCTGCTGTAACAAGATGGATGC | GAGATGGGGACAAAGGGGATT |
The role of three chitinase genes in response to pathogen infection
Subcellular location assay with Agrobacterium-mediated transformation was followed from Su et al.22. ORF fragments of ScChiI1, ScChiIV1 and ScChiVII1 were inserted into the vector of pCAMBIA 2300-GFP and transformed into the competent cells of A. tumefaciens strain EHA105, respectively. The subcellular localization of the fusion protein was visualized using a confocal laser scanning microscope Leica TCS SP5 (Germany) equipped with 10 × lense.
As reported, cell death presented at the infected site is the most efficient method to restrict pathogen growth and development54. The stimulation of reactive oxygen species (ROS) and defense-related hormones, induction of R gene expression and ion fluxes are the common response of cell death55,56. For the transient expression of the target gene in N. benthamiana, overexpression vectors pCAMBIA 1301-ScChiI1, pCAMBIA 1301-ScChiIV1 and pCAMBIA 1301-ScChiVII1 were constructed to analyze their defense responses. Agrobacterium strain EHA105 carrying the recombinant vector was transiently expressed in N. benthamiana leaves. Each treatment was carried out in three replicates. DAB (3,3’-diaminobenzidinesolution) was used to stain H2O2 produced in agroinfiltrated leaves22. The leaves were incubated in 1.0 mg/mL DAB-HCl solution in the dark overnight and destained by boiling in 95% ethanol for 5 min. The bronzing color of the leaves for H2O2 detection was photographed. qRT-PCR analysis of the expression of seven immunity associated marker genes were conducted post 24 infiltration, including the hypersensitive response marker genes NtHSR201 and NtHSR203, the jasmonate associated genes NtPR-1a/c, NtPR2 and NtPR3, and the ethylene synthesis depended genes NtEFE26 and NtAccdeaminase (Table 2)22. NtEF1-α (Table 2) was used to normalize the transcript levels.
Additional Information
How to cite this article: Su, Y. et al. Identification, Phylogeny, and Transcript of Chitinase Family Genes in Sugarcane. Sci. Rep. 5, 10708; doi: 10.1038/srep10708 (2015).
Supplementary Material
Acknowledgments
We thank Andrew C Allan for critical reading of the manuscript and useful advice. This work was funded by Natural Science Foundation of Fujian province, China (2015J06006 and 2015J05055), the Program for New Century Excellent Talents in Fujian Province University (JA14095), the earmarked fund for the Modern Agriculture Technology of China (CARS-20) and Research Funds for Distinguished Young Scientists in Fujian Provincial Department of Education (JA13090).
Footnotes
Author Contributions Conceived and designed the experiments: Y.S., L.X. and Y.Q. Performed the experiments: Y.S., S.W. and Z.W. Analyzed the data: Y.Y. and Y.C. Wrote the paper: Y.S., L.X. and Y.Q. Revised the final version of the paper: L.X. and Y.Q. Approved the final version of the paper: L.X. and Y.Q.
References
- Que Y. X., Xu L. P., Lin J. W., Chen R. K. & Grisham M. P. Molecular variation of Sporisorium scitamineum in mainland China revealed by RAPD and SRAP markers. Plant Dis. 96, 1519–1525 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang J. S. et al. Genome size variation in three Saccharum species. Euphytica 185, 511–519 (2012). [Google Scholar]
- Manners J. M. Functional genomics of sugarcane. Adv. Bot. Res. 60, 89–168 (2011). [Google Scholar]
- Xu L. P., Chen R. K. & Chen P. H. Analysis on infection index of smut caused by Ustilago scitaminea in sugarcane segregated population. Chinese J. Trop. Crop. 25, 33–36 (2003). [Google Scholar]
- Solas M. T., Pinon D., Vicente C. & Legaz M. E. Ultrastructural aspects of sugarcane bud infection by Ustilago scitaminea teliospores. Sugar Cane (United Kingdom) 2, 14–18 (1999). [Google Scholar]
- Waller J. M. Sugarcane smut (Ustilago scitaminea) in Kenya: II. Infection and resistance. T. Brit. Mycol. Soc. 54, 405–414 (1970). [Google Scholar]
- Da-Gloria B. A., Capote Albernas, M. C. & Amorim, L. Structural characteristics of buds of sugarcane cultivars with different levels for resistance to smut. J. Plant Dis. Protect. 102, 502–508 (1995). [Google Scholar]
- Que Y. X. et al. Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genomics 15, 996 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze B. S., Thokoane L. N., Williams N. J., Barnes J. M. & Rutherford R. S. The smut-sugarcane interaction as a model system for the integration of marker discovery and gene isolation. Proc. S. Afr. Sug. Technol. Ass. 75, 88–93 (2001). [Google Scholar]
- Borrás-Hidalgo O. et al. Identification of sugarcane genes induced in disease-resistant somaclones upon inoculation with Ustilago scitaminea or Bipolaris sacchari. Plant Physiol. Biochem. 43, 1115–1121 (2005). [DOI] [PubMed] [Google Scholar]
- Que Y. X., Su Y. C., Guo J. L., Wu Q. B. & Xu L. P. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-seq. PloS One 9, e106476 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters D., Walsh D., Newton A. & Lyon G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95, 1368–1373 (2005). [DOI] [PubMed] [Google Scholar]
- Liu J. J., Ekramoddoullah A. K. M. & Zamani A. A class IV chitinase is up-regulated by fungal infection and abiotic stresses and associated with slow-canker-growth resistance to Cronartium ribicola in western white pine (Pinus monticola). Phytopathology 95, 284–291 (2005). [DOI] [PubMed] [Google Scholar]
- Maximova S. N. et al. Over-expression of a cacao class I chitinase gene in Theobroma cacao L. enhances resistance against the pathogen, Colletotrichum gloeosporioides. Planta 224, 740–749 (2006). [DOI] [PubMed] [Google Scholar]
- Xiao Y. H. et al. Cloning and characterization of a balsam pear class I chitinase gene (Mcchit1) and its ectopic expression enhances fungal resistance in transgenic plants. Biosci., biotechnol., Biochem. 71, 1211–1219 (2007). [DOI] [PubMed] [Google Scholar]
- Neuhaus J. M. et al. A revised nomenclature for chitinase genes. Plant Mol. Biol. Rep. 14, 102–104 (1996). [Google Scholar]
- Singh A., Isaac-Kirubakaran S. & Sakthivel N. Heterologous expression of new antifungal chitinase from wheat. Protein Expres. Purif. 56, 100–109 (2007). [DOI] [PubMed] [Google Scholar]
- Ahmed N. U. et al. Identification and expression analysis of chitinase genes related to biotic stress resistance in Brassica. Mol. Biol. Rep. 39, 3649–3657 (2012). [DOI] [PubMed] [Google Scholar]
- Mauch F., Mauch-Mani B. & Boller T. Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 88, 936–942 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K., Ward E., Payne G., Moyer M. & Ryals J. Acidic and basic class III chitinase mRNA accumulation in response to TMV infection of tobacco. Plant Mol. Biol. 19, 735–743 (1992). [DOI] [PubMed] [Google Scholar]
- Liu B. et al. Cloning and characterization of a wheat β-1, 3-glucanase gene induced by the stripe rust pathogen Puccinia striiformis f. sp. tritici. Mol. Biol. Rep. 37, 1045–1052 (2010). [DOI] [PubMed] [Google Scholar]
- Su Y. C. et al. ScChi, encoding an acidic class III chitinase of sugarcane, confers positive responses to biotic and abiotic stresses in sugarcane. Int. J. Mol. Sci. 15, 2738–2760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt L. B. d. A. et al. AtchitIV gene expression is stimulated under abiotic stresses and is spatially and temporally regulated during embryo development. Genet. Mol. Biol. 27, 118–123 (2004). [Google Scholar]
- Xu F. H., Fan C. M. & He Y. Q. Chitinases in Oryza sativa ssp. japonica and Arabidopsis thaliana. J. Genet. Genomics 34, 138–150 (2007). [DOI] [PubMed] [Google Scholar]
- Melchers L. S. et al. A new class of tobacco chitinases homologous to bacterial exo-chitinases displays antifungal activity. Plant J. 5, 469–480 (1994). [DOI] [PubMed] [Google Scholar]
- Kovács G. et al. Expression of a rice chitinase gene in transgenic banana (‘Gros Michel’, AAA genome group) confers resistance to black leaf streak disease. Transgenic Res. 22, 117–130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincoff P. C., Garcia Cortez D. A., Ueda-Nakamura T., Nakamura C. V. & Dias Filho B. P. Isolation and characterization of a 30 kD antifungal protein from seeds of Sorghum bicolor. Res. Microbiol. 157, 326–332 (2006). [DOI] [PubMed] [Google Scholar]
- Kirubakaran S. I. & Sakthivel N. Cloning and overexpression of antifungal barley chitinase gene in Escherichia coli. Protein Expres. Purif. 52, 159–166 (2007). [DOI] [PubMed] [Google Scholar]
- Robert N. et al. Expression of grapevine chitinase genes in berries and leaves infected by fungal or bacterial pathogens. Plant Sci. 162, 389–400 (2002). [Google Scholar]
- Davis J. M. et al. Pathogen challenge, salicylic acid, and jasmonic acid regulate expression of chitinase gene homologs in pine. Mol. Plant Microbe In. 15, 380–387 (2002). [DOI] [PubMed] [Google Scholar]
- Libantová J., Kämäräinen T., Moravčíková J., Matušíková I. & Salaj J. Detection of chitinolytic enzymes with different substrate specificity in tissues of intact sundew (Drosera rotundifolia L.). Mol. Biol. Rep. 36, 851–856 (2009). [DOI] [PubMed] [Google Scholar]
- Porat R., Vinokur V., Holland D., Gregory McCollum T. & Droby S. Isolation of a citrus chitinase cDNA and characterization of its expression in response to elicitation of fruit pathogen resistance. J. plant physiol. 158, 1585–1590 (2001). [Google Scholar]
- Rahul P. R. et al. Characterization and 3D structure prediction of chitinase induced in sugarcane during pathogenesis of Colletotrichum falcatum. J. Plant Biochem. Biot. 24, 1–8 (2015). [Google Scholar]
- Wang S. S., Su Y. C., Yang Y. T., Guo J. L. & Xu L. P. Molecular cloning and expression analysis of chitinase gene ScChiVII1 in sugarcane. Chinese J. Trop. Crop. 35, 289–298 (2014). [Google Scholar]
- Lin S. et al. Molecular responses to the fungal pathogen Gibberella fujikuroi in the leaves of chewing cane (Saccharum officinarum L.). Sugar tech 12, 36–46 (2010). [Google Scholar]
- Lerner D. R. & Raikhel N. V. The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase. J. Biol. Chem. 267, 11085–11091 (1992). [PubMed] [Google Scholar]
- Su Y. C. et al. Isolation of a novel peroxisomal catalase gene from sugarcane, which is responsive to biotic and abiotic stresses. PloS One 9, e84426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan R. & Samiyappan R. Antifungal activity of chitinases produced by some fluorescent pseudomonads against Colletotrichum falcatum Went causing red rot disease in sugarcane. Microbiol. Res. 155, 309–314 (2001). [DOI] [PubMed] [Google Scholar]
- Krishnaveni S., Liang G. H., Muthukrishnan S. & Manickam A. Purification and partial characterization of chitinases from sorghum seeds. Plant Sci. 144, 1–7 (1999). [Google Scholar]
- Danhash N., Wagemakers C. A., van Kan J. A. & de Wit P. J. Molecular characterization of four chitinase cDNAs obtained from Cladosporium fulvum-infected tomato. Plant Mol. biology 22, 1017–1029 (1993). [DOI] [PubMed] [Google Scholar]
- Meins F. et al. Plant chitinase genes. Plant Mol. Biol. Rep. 12, S22–S28 (1994). [Google Scholar]
- Sun Y. L. & Hong S. K. Effect of chitinase on resistance to fungal pathogens in sea buckthorn, Hippophae rhamnoides, and cloning of Class I and III chitinase genes. Biochem. Genet. 50, 600–615 (2012). [DOI] [PubMed] [Google Scholar]
- Arie M., Hikichi K., Takahashi K. & Esaka M. Characterization of a basic chitinase which is secreted by cultured pumpkin cells. Physiol. Plantarum 110, 232–239 (2000). [Google Scholar]
- Li D. M., Staehelin C., Wang W. T. & Peng S. L. Molecular cloning and characterization of a chitinase-homologous gene from Mikania micrantha infected by Cuscuta campestris. Plant Mol. Biol. Rep. 28, 90–101 (2010). [Google Scholar]
- Xu L. P., Lin Y. Q. & Fu H. Y. Evaluation of smut resistance in sugarcane and identification of resistance in sugarcane varieties. Fujian J. Agr. Sci. 29, 292–295 (2000). [Google Scholar]
- Passarinho P. A., Van Hengel A. J., Fransz P. F. & de-Vries S. C. Expression pattern of the Arabidopsis thaliana AtEP3/AtchitIV endochitinase gene. Planta 212, 556–567 (2001). [DOI] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot.-London 100, 681–697 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B. & Mauch F. The role of abscisic acid in plant-pathogen interactions. Curr. Opin. Plant Biol. 8, 409–414 (2005). [DOI] [PubMed] [Google Scholar]
- Schlumbaum A., Mauch F., Vögeli U. & Boller T. Plant chitinases are potent inhibitors of fungal growth. Macmillan J. Ltd. 324, 365–367 (1986). [Google Scholar]
- Datta K. et al. Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci. 160, 405–414 (2001). [DOI] [PubMed] [Google Scholar]
- Yamamoto T. et al. Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep. 19, 639–646 (2000). [DOI] [PubMed] [Google Scholar]
- Gu L. H. et al. Introduction of chitin and β-1,3-glucan into sugarcane. Mol. Plant Breed. 6, 277–280 (2008). [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Choi D. S., Hwang I. S. & Hwang B. K. Requirement of the cytosolic interaction between pathogenesis-related PROTEIN10 and leucine-rich repeat PROTEIN1 for cell death and defense signaling in pepper. Plant Cell 24, 1675–1690 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Z., Tessaro M. J., Li X. & Zhang Y. L. Regulation of the expression of plant resistance gene SNC1 by a protein with a conserved BAT2 domain. Plant physiol. 153, 1425–1434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melech-Bonfil S. & Sessa G. Tomato MAPKKKε is a positive regulator of cell-death signaling networks associated with plant immunity. Plant J. 64, 379–391 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.