Agitation is excessive restlessness, or non-purposeful physical activity thought to be caused or exacerbated by pain, anxiety, endotracheal tube irritation, or other unpleasant events.1–3 Up to 71% of Intensive Care Unit (ICU) patients have some degree of agitation during their ICU stay.4 Agitation has been shown to extend the length of hospital stay from a median of 5 to 12 days and is associated with adverse clinical outcomes such as a higher rate of self-extubation, unplanned catheter removal, longer duration of mechanical ventilation, excessive sedation, as well as increased utilization of resources, and increased ICU costs.3;5;6 Agitation can be manifested in simple apprehension or anxiety, inappropriate self-removal of indwelling tubes and catheters, and/or to attempted assault of a care provider.7;8
The Society of Critical Care Medicine’s (SCCM) recent sedation and analgesia guidelines highlight the need for prompt identification and treatment of possible underlying causes of agitation.9 Understanding the natural history of agitation may be important as interventions can be implemented to prevent or ameliorate the phenomena and its consequences. Although agitation is associated with deleterious outcomes, there are few data that describe the frequency, onset, and course of agitation in the critical care environment. Therefore, the specific aim of this study was to describe the frequency, onset and patterns of agitation in the adult critically ill population.
METHODS
Subjects and Setting
The study was conducted in an 865-bed academic medical center which offers all critical care specialties in 2010. Approval was obtained from the Institutional Review Board of the university. The study was conducted in 2 adult units (medical-respiratory ICU [MRICU] and surgical trauma ICU [STICU]). The sample included all adult patients, 18 years of age and older, consecutively admitted to the MRICU and STICU over a two month period using a medical record review. Patient exclusion criteria were an ICU length of stay less than 24 hours (to omit those who had a short length of ICU for overnight monitoring), those with medical records that were not available, and patients previously admitted during the study duration. Other exclusion criteria were conditions affecting patient movement interfering with sedation scale scoring including administration of paralytics, patients with neuro-muscular disorders (such as cerebral palsy and Parkinson’s disease), and patients with head trauma or stroke.
Documentation of Agitation
Agitation was identified using the documented Richmond Agitation-Sedation Scale (RASS), a 10 point scale, from +4 (combative) to −5 (unarousable).8 The RASS has demonstrated excellent interrater reliability and criterion, construct, and face validity across a variety of critical care settings.8;10–12 RASS values are routinely obtained every 4 hours in the units and more frequently if needed. A RASS of +1 (restless) through +4 (combative) were used to identify agitation. The +1 RASS was accepted as an indicator for agitation as use of positive numbers in the RASS have been previously documented as agitation.8
Agitation was also documented using the keyword “agitation” (all forms of the word - “agitated”, “agitation”, “agit”) recorded from the medical record using physicians’ and nurses’ notes in the nursing bedside flowsheet, emergency department (ED) documentation, operating room notes, and circle-the-item for reporting agitation in flowsheets.
Procedure
The medical record was used as the primary source of information and data collection was conducted by a single investigator (RSB). A pilot study was performed using subjects not part of the study cohort. Data audits were performed to verify accuracy of information using convenience sampling on approximately 10% of all subjects. The error rate on the data audit was less than 0.03%.
The goal was to obtain an equal number of subjects in each unit that would span the majority of the two month period allowing a broad representation of unit admissions. Data were collected during the first 5 days of ICU stay as agitation onset and duration has been shown to be 3 to 5 days.4;6
For all recurrent data collection, the hour was used as the documentation epoch. Each individual hour was documented as an agitation hour only if the RASS was +1 or above, or the word agitation (and/or its forms) was documented during that hour. Subject demographics were recorded (age, gender, ethnicity, race) as well as admission source (clinic, ED, home, long term care, or outside hospital), admission diagnosis, intubation status, tool scores obtained on admission to the ICU – the Acute Physiology And Chronic Health Evaluation III (APACHE III),13 the Sequential Organ Failure Assessment (SOFA),14 Charlson Comorbidity Index,15 – ICU length of stay (LOS), hospital LOS, and administration of analgesics and sedatives.
Data Analysis
Data were summarized by hour, 4-hour block (block), and day for each subject and categorized as an agitation or non-agitation hour, block, or day. Hourly data were condensed into blocks as the standard ICU flowsheet contained 4-hour blocks with “agitation” available as a circle-the-item. The consolidation of hourly data into blocks reduced documentation redundancy error while smoothing data peaks. If any agitation was documented within the hour, block, or day, or there were multiple documented agitation episodes during the time period, it was considered to be one agitation hour/block/day. Additional data collection included time, ICU day, day of the week, all RASS values, as well as descriptors of agitated behavior. Percent of agitation hours, blocks, and days were based on the number of these divided by the total number of observed hours, blocks and days throughout the 5 day period – these varied based on the subject’s duration of ICU stay. Agitation reported as “any time” included documentation of any agitation at any time during the study period. For agitation onset data, only the first agitation event for hour/block/day for each subject during the study period was evaluated. To investigate agitation temporal patterns, all agitation hours and blocks were grouped by day of the week, day/night intervals, and time of day.
Descriptive data were expressed as counts and percentages for all nominal and categorical data, and mean, range, and standard deviation (SD) for continuous measures. The alpha level for significance was set to 0.05. Univariate analyses were performed between non-agitated and agitated subjects using X2 and Fisher’s Exact Test for categorical data, and Two Sample t-Test for continuous data.
RESULTS
Subjects
Over the two month data collection period, 383 potential subjects were screened (179 MRICU, 204 STICU). The final sample was comprised of 200 subjects (qualified by applying inclusion and exclusion criteria) – 100 from the MRICU and 100 from the STICU. The majority of excluded subjects were in the ICUs less than 24 hours. Data collection for up to 5 days of ICU stay for the 200 subjects resulted in 791 patient-days (17,938 hours of data; 4,621 blocks).
Subjects had a mean age of 55 years and were primarily men, non-Hispanic, and white or African American (Table 1). Characteristics of the sample are shown in Table 1; selected analgesic and sedative medication use are shown in Table 2.
Table 1.
Demographics and other descriptors for entire sample and by presence of agitation (at least one observation of agitation during the study period).
| Entire sample n = 200 | Non-agitated Pts n = 82 (41%) | Agitated Pts n = 118 (59%) | |
|---|---|---|---|
|
| |||
| Variable | n (%) | n (%) | n (%) |
| Gender | |||
| Male | 113 (56.5) | 42 (51) | 73 (62) |
| Female | 87 (43.5) | 40 (49) | 45 (38) |
| Ethnicity | |||
| Hispanic or Latino | 6 (3) | 5 (6) | 1 (1) |
| Not Hispanic or Latino | 194 (97) | 77 (94) | 117 (99) |
| Race | |||
| Asian | 3 (1.5) | 2 (2) | 1 (1) |
| Black or African American | 94 (47) | 39 (48) | 55 (47) |
| White | 103 (51.5) | 41 (50) | 62 (53) |
| ICU Type | |||
| Medical Respiratory ICU | 100 (50) | 36 (44) | 64 (54) |
| Surgical Trauma ICU | 100 (50) | 46 (56) | 54 (46) |
| Admission Source | |||
| Long term care | 3 (1.5) | 2 (1) | 1 (0.5) |
| Home | 16 (8) | 4 (2) | 12 (6) |
| Clinic | 20 (10) | 7 (3.5) | 13 (6.5) |
| Outside hospital | 60 (30) | 23 (11.5) | 37 (18.5) |
| ED | 101 (50.5) | 46 (23) | 55 (27.5) |
| Admitting Diagnosis | |||
| Trauma | 36 (18) | 18 (22) | 18 (15) |
| Sepsis | 35 (17.5) | 17 (21) | 18 (15) |
| Respiratory failure | 27 (13.5) | 6 (7) | 21 (18) |
| Hematologic/oncologic problem | 27 (13.5) | 8 (10) | 20 (17) |
| Other | 22 (11) | 9 (11) | 12 (10) |
| Renal/GI problem/DKA | 28 (14) | 15 (19) | 13 (11) |
| Hepatic problem | 13 (6.5) | 4 (5) | 9 (8) |
| Cardiovascular problem | 8 (4) | 4 (5) | 4 (3) |
| Drug overdose/poisoning | 4 (2) | 1 (1) | 3 (3) |
| Intubated* | 118 (59) | 20 (17) | 98 (83) |
|
| |||
| Variable | Mean (Range, SD) | Mean (Range, SD) | Mean (Range, SD) |
|
| |||
| Age (years) | 55.5 (18–89; +/− 16.4) | 56 (19–87; +/− 16.4) | 55.1 (18–89; +/− 16.5) |
| ICU length of stay (days) | 7.1 (1–99.4; +/− 9.7) | 5.9 (1–99.4; +/− 12.1) | 7.9 (1.5–36.2; +/− 7.6) |
| Hospital length of stay (days) | 16.6 (1–99.5; +/− 15.3) | 15.8 (1–99.5; +/− 16.3) | 17.1 (2.2–79; +/− 14.7) |
| APACHE III score13 | 68 (4–200; +/− 31.9) | 57.7 (4–200; +/− 34.3) | 74.7 (21–170; +/− 28.2) |
| SOFA14 | 6.625 (0–18; +/− 3.8) | 5.39 (1–17; +/− 3.7) | 7.48 (0–18 ; +/− 3.7) |
| Charlson Comorbidity Index15 | 4.69 (0–17; +/− 3.3) | 4.8 (0–13; +/− 3.3) | 4.6 (0–17; +/− 3.4) |
Abbr: intensive care unit (ICU); emergency department (ED); diabetic ketoacidosis (DKA); Acute Physiology and Chronic Health Evaluation III (APACHE III); Sequential Organ Failure Assessment (SOFA)
There was no difference between the numbers of intubated patients in the 2 ICUs
Table 2.
Analgesic and sedative medications received at any time for total sample over 5-day data collection period.
| Medication | Number (%) of pts* |
|---|---|
| Analgesics | |
| Fentanyl | 117 (58.5) |
| Morphine | 83 (41.5) |
| Hydromorphone | 26 (13) |
|
| |
| Sedatives | |
| Midazolam | 95 (47.5) |
| Propofol | 52 (26) |
| Lorazepam | 32 (16) |
| Haloperidol | 24 (12) |
| Diazepam | 2 (1) |
| Dexmedetomidine | 1 (0.5) |
Total is more than 100% as subjects received more than one drug
Agitation Frequency
Of the 200 subjects, 118 (59%) were agitated at any time during the 5 days during 319 (31.9%) patient-days. Approximately one quarter (28.5 %) of the agitation documentation was based on RASS with the balance using an agitation keyword.
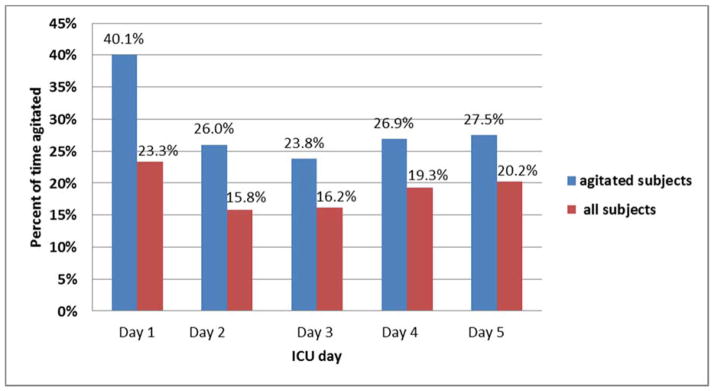
Of 17,938 total data hours, the overall agitation rate was 7.8% of the hourly time (1389 hours) and 19.1% of the block time (883 blocks). Of all 883 agitated blocks, 36.2% occurred on day 1 (n=102), 20.7% on day 2 (n=71), 16.8% on day 3 (n=60), 14.7% on day 4 (n=50), and 11.6% on day 5 (n=36). Figure 1 depicts the percent of agitation in blocks for each day - higher on day 1 than other days.
Figure 1. Percent of block-time agitated per day, for agitated subjects and all subjects.
Agitation Onset
The onset of agitation was investigated. The onset of agitation was a mean of 11.6 hours (SD 22.3; Range 0–114; IQR 0–13.75) from ICU admission. Of those subjects who were agitated at any time during the study, 86% (n=102) had agitation on day 1, the remaining 14% (n=16) had an agitation onset spread out over the next 4 days (Figure 2). The majority of subjects (n=102; 86%) with first-day agitation continued to have agitation across other days, while those with later agitation onset had relatively low agitation frequency.
Figure 2. Number of agitated patients who experienced any agitation per day by agitation onset day.
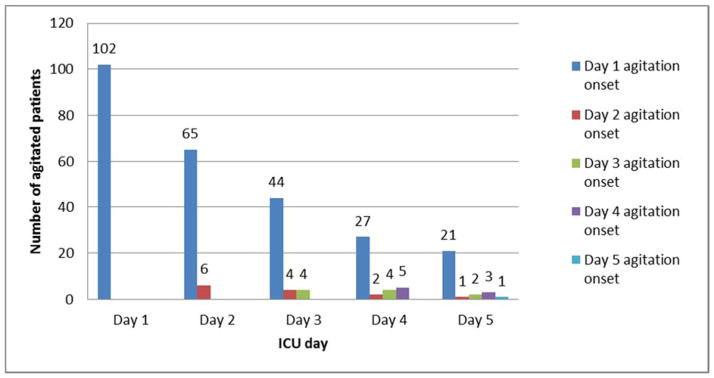
(Day 1 first-time agitated patients [n=102, blue column] were less each succeeding day (65, 44, 27, 21); on day 2 there were only 6 first-time agitated patients [red column] which was also less each succeeding day (4, 2, 1); etc.)
Of the 102 subjects with first-day agitation, 44 (43.1%) had agitation reported on ICU admission; 30 more (another 29.4%) had agitation reported from 1 to 4 hours from ICU admission. The mean onset of agitation for those who had first-day agitation onset was 3.97 hours from admission (SD 6.4; Range 0–24; IQR 0–5).
Patterns of Agitation – Onset and Frequency
Patterns of agitation frequency and onset were investigated for day of the week, day/night intervals, and block of day. For day of the week, Tuesdays had the highest number of agitation hours (253) with the lowest on Friday (157). Considering only first-time agitation hours, Monday was highest (26) closely followed by Tuesday (23), with Sunday the lowest (11). Frequency of agitation during the day (7AM–7PM) versus the night (7PM–7AM) showed a similar number of hours – day (n=679) vs. night (n=710). First-event agitation hours during the day were 63 vs. 55 during the night. For block-time, first agitation onset from 8PM to midnight was higher than others.
Considering the agitation pattern from a day perspective, of the 118 subjects with agitation at any time, 88 (74.6%) had multiple days of agitation including both those with intermittent and consecutive days of agitation (Figure 3).
Figure 3. Day patterns of agitation depicting the significant portion of agitation that occurs on day 1.
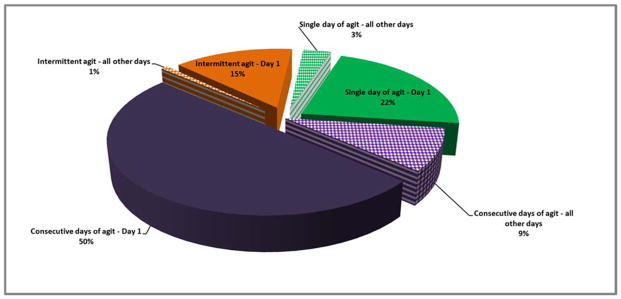
-
●Intermittent agitation – day 1: The percent of total agitation of all patients that had intermittent agitation on day 1
-
●Intermittent agitation – all other days: The percent of total agitation of all patients that had intermittent agitation on any day other than day 1
-
➢Single day of agitation – day 1: The percent of total agitation of all patients that had a single day of agitation on day 1
-
➢Single day of agitation – all other days: The percent of total agitation of all patients that had a single day of agitation on any day other than day 1
-
○Consecutive days of agitation – day 1: The percent of total agitation of all patients that had consecutive days of agitation beginning on day 1
-
○Consecutive days of agitation – all other days: The percent of total agitation of all patients that had consecutive days of agitation beginning on any day other than day 1
Agitation Patterns by Unit
Unit comparisons were conducted to determine differences in agitation between the MRICU and STICU subjects. There was no difference between the MRICU and the STICU in subject age, ethnicity, race, admitting diagnosis, as well as ICU and hospital LOS (Table 3). The MRICU subjects had a greater number of total documentation hours than the STICU although not statistically different. The two units were not different in the number of patients with first day, first hour or first block agitation. APACHE III, SOFA, and Charlson Comorbidity Index scores on admission to the ICUs were significantly higher for MRICU subjects than for STICU subjects. Subjects in the MRICU had a significantly higher percent patient agitation on day 1 and 2 although there was no difference between the two units in mean agitation hour onset.
Table 3.
Comparison of agitated patients between MRICU vs. STICU.
| Unit | MRICU (n =100) | STICU (n =100) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Variable | n (%) | n (%) | |||
| Total documentation hours | 9228 (51.4) | 8710 (48.6) | |||
| Total agitation hours | 931 | 458 | |||
| Total documentation blocks | 2399 (51.9) | 2222 (48.1) | |||
| Total agitation blocks | 560 (23.3) | 323 (14.5) | |||
| * Agitated patients | 64 (54) | 54 (46) | 0.20 | ||
| Agitation hours in first day of admission | 354 | 139 | |||
| Agitation blocks in first day of admission | 207 | 101 | |||
| Patients agitated in first day of admission | 57 (89.1) | 45 (83.3) | 0.12 | ||
| Patients agitated in first hour of admission | 26 (48.1) | 18 (33.3) | 0.23 | ||
| Patients agitated in first block of admission | 36 (56.3) | 24 (44.4) | 0.09 | ||
| Patient Level Data | Mean (SD) | Range | Mean (SD) | Range | p value |
|
| |||||
| Percent agitation by hour | 9.2 (9.5) | 0–43 | 4.7 (6.8) | 0–29 | <.0001 |
| Percent agitation by block | 21.7 (21.4) | 0–68 | 12.6 (16.4) | 0–77 | 0.001 |
| Percent agitation by day | |||||
| Day 1 | 31.9 (32.8) | 0–100 | 15.4 (21.6) | 0–100 | <.0001 |
| Day 2 | 21.4 (26.7) | 0–100 | 9.2 (19.6) | 0–100 | 0.0003 |
| Day 3 | 15.1 (24.2) | 0–100 | 10.3 (24.8) | 0–100 | 0.17 |
| Day 4 | 12.4 (24.6) | 0–100 | 9.5 (19.9) | 0–83 | 0.36 |
| Day 5 | 8.9 (20.6) | 0–83 | 8.5 (22.9) | 0–100 | 0.88 |
| Mean agitation onset hour from admission | 8.4 (17.2) | 0–85 | 15.5 (26.9) | 0–114 | 0.09 |
| Age (years) | 56.7 (16.1) | 18–89 | 53.3 (16.8) | 18–82 | 0.27 |
| ICU length of stay (days) | 7.9 (7.5) | 1.5–36.2 | 7.9 (7.9) | 1.7–34.1 | 0.97 |
| Hospital length of stay (days) | 15.8 (13.1) | 2.2–66.8 | 18.6 (16.3) | 2.8–78.9 | 0.31 |
| APACHE III score on admission to the ICU | 81.2 (27.2) | 23–170 | 66.9 (27.7) | 21–132 | 0.0056 |
| SOFA | 7.6 (3.9) | 0–18 | 5.6 (3.5) | 1–16 | <.0001 |
| Charlson Comorbidity Index | 5.5 (3.3) | 0–17 | 3.8 (3.1) | 0–13 | 0.0084 |
Abbr: intensive care unit (ICU); medical-respiratory intensive care unit (MRICU); surgical-trauma intensive care unit (STICU); Acute Physiology and Chronic Health Evaluation III (APACHE III); Sequential Organ Failure Assessment (SOFA)
Agitated on any ICU study day
DISCUSSION
Agitation Frequency
Agitation is common in the critically ill. In this study the majority of the sample (59%) was agitated at any time during the first 5 days of their ICU stay. This rate is generally similar to previous studies’ agitation rates. Jaber et al.6 studied 182 medical-surgical ICU subjects over 8 months and found an agitation frequency of 52%. Despite differences in inclusion criteria (our study excluded patients in the unit less than 24 hours and Jaber’s did not) and identification of agitation (our RASS and keyword use vs. Ramsay score), the frequency of agitation was similar. Gardner et al.4 studied 83 medical respiratory ICU patients over a 2-month period and found an agitation frequency of 42% using nursing documentation to rate the level of agitation. Fraser et al.16 studied 130 medical and surgical ICU patients over a 4 month period and found an agitation frequency of 70.8%. Their exclusion criteria were similar to ours; however, their identification of agitation differed. They used medical record narratives describing agitated behavior to quantify agitation using the Sedation Agitation Scale (SAS and included “anxious”. Interestingly, using only SAS 6 or 7 data, they reported an any-day agitation frequency of 46.1% – similar to what we report here. Woods et al.5 studied 143 medical ICU patients over a 5 month period and reported any-day agitation of 16.1%. This may be explained by the differences in inclusion criteria (ventilated patients, medical ICU, severity and definition of agitation) as well as the use of a sedative/analgesic protocol.
There were also similar findings between this study and others regarding patient-days of agitation. Ours (31.9%) were generally similar those of Gardner et al.’s4 32% and Fraser et al.’s16 46.1% to 54%. These findings of generally similar agitation frequency suggest that agitation is pervasive. The hourly and block agitation rates for this study are unique in that this level of detail has not been found previously in the literature. The higher rates of block-time may be due to variations in documentation but may also be more accurate due to the ability of documentation at the end of the 4-hour period. Agitation is generally not a quickly resolving issue and the use of the circle-the-item for a 4-hour block may have been used as an efficient indicator. However, due to individual documentation variation in healthcare providers, the per-day agitation rates may allow a more consistent comparison between studies.
Agitation Onset
We found the onset of agitation occurs early in the ICU stay. This is generally consistent with the literature. The vast majority of our sample had agitation onset very early the first day of their ICU stay – considerably earlier than found in previous studies. Fraser et al.15 found the mean onset time from ICU admission to maximum agitation to be 2.4 days. Differences may be due to definition of agitation onset. We computed the mean of all subjects’ agitation onset hours while Fraser reported hours to maximum agitation – however no description of the method for determining maximum agitation was included. Jaber et al.6 found agitation onset to be 4.4 ± 5.6 days, more than four times longer; however they stated that most of the patients became agitated in less than 3 to 5 days.
The causes of such early agitation trends are unclear. Early agitation onset findings in studies have been hypothesized to be linked to sedative use as the majority of agitated subjects received sedatives. Use of sedatives has been associated with agitation in several studies;5;6;15 more studies are needed to determine if or what dose of sedative precipitates agitation or is involved in neurotransmitter imbalance. Severity of disease has also been suggested to be associated with early agitation onset. This appears unlikely as, our scores are generally comparable to other studies.5;6;15
First-day agitation is common in the critically ill. We found significantly higher first-day rates (86%) compared to Woods et al.5 who found 7%. This difference may be attributed partially to the dissimilar populations and outcome measurement discussed earlier. In addition to very high first-day agitation rates, over half of our sample had identified agitation in the first hour of ICU admission. This suggests that subjects were admitted in an agitated state – different from other studies. It is unclear to what this can be attributed. The admission source and location might be thought to influence this finding but no statistical significance or trend was found.
Patterns of Agitation – Onset and Frequency
The hourly patterns of agitation onset and frequency for day of the week, day/night, or block intervals were similar. We found agitation frequency to be higher in the day. Jaber et al.’s6 study did not find a significant difference in day/night agitation. These results suggest that agitation may be heterogeneous with little diurnal fluctuation. It is notable that the majority of patterns of consecutive, intermittent, and single day agitation involved day 1 agitation. Agitation patterns over time revealed that day 1 agitation is implicated in all trends but more significantly in consecutive days.
Agitation Patterns by Unit
In comparing agitated subjects in the MRICU and STICU our findings did not reach statistical significance; however Jaber et al.6 found agitation rates higher in medical subjects. Day 1 and 2 percent patient agitation rates found significantly higher in the MRICU may reflect higher severity of disease. If agitation is a result of dysregulation of neurotransmitters, medical ICU patients may have greater comorbidities and severity of disease that may contribute to the higher agitation rate and earlier onset. To support this, we found the APACHE III and SOFA scores, as well as the Charlson index significantly higher in the MRICU subjects; Jaber and colleagues6 also found a significantly higher Simplified Acute Physiology Score II in medical subjects.
Limitations of this study warrant mention. As this was a retrospective chart review, findings are dependent on data completeness and quality – the data was not originally recorded for research purposes and may lack in quantity and quality. In an effort to mitigate some of these disadvantages, we used a more stringent definition of agitation – documentation of the word “agitation” or the RASS tool – not relying on behavioral cues. Strengths of retrospective reviews exist: they are reflective of usual care and allow investigators to examine processes and outcomes as they occur, void of the Hawthorne effect, and they monitor in real time integrating multiple data sources. We currently lack a continuous method of measuring agitation over time; the best alternative is hourly documentation. Differences in unit samples could be due to differences in unit documentation norms.
Regardless of the evaluation of agitation, whether frequency, onset, or pattern, our data show agitation in the critically ill is a very early phenomenon involving consecutive days. These findings have clinical and resource allocation implications. Focusing efforts, resources, and implementing protocols very early in the ICU stay (or before) may prevent the poor outcomes and dangerous sequellae of agitation as well as reduce ICU costs. In addition, interrupting the trend of consecutive days of agitation may have an equal impact in lowering overall agitation frequency.
CONCLUSION
We found agitation affects over half of ICU patients, largely occurs the first day in the ICU stay, and involves consecutive days. In this study, MRICU patients generally had a higher severity of illness and had higher day 1 and 2 rates of patient agitation than STICU patients. Studies are needed to clarify patient risk factors and identify strategies (both pharmacological and nonpharmacological) to prevent, ameliorate, or treat the condition.
Summary of Key Points.
Agitation was found to affect over half of ICU patients
Agitation largely occurred the first day of the ICU stay
It usually involved consecutive days
Patients in the MRICU generally had a higher severity of illness than the STICU and had higher day 1 and 2 rates of patient agitation
Acknowledgments
This study was supported by the National Institutes of Health; National Institute of Nursing Research, Grant # F31-NR010436
Reference List
- 1.Harvey MA. Managing agitation in critically ill patients. Am J Crit Care. 1996;5:7–16. [PubMed] [Google Scholar]
- 2.Cohen IL, Gallagher TJ, Pohlman AS, Dasta JF, Abraham E, Papadokos PJ. The management of the agitated ICU patient. Crit Care Med. 2002;30:S97–123. [PubMed] [Google Scholar]
- 3.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Gardner K, Sessler CN, Grap MJ. Clinical factors associated with agitation. Am J Crit Care. 2006;15:330–331. [Google Scholar]
- 5.Woods JC, Mion LC, Connor JT, et al. Severe agitation among ventilated medical intensive care unit patients: frequency, characteristics and outcomes. Intensive Care Med. 2004;30:1066–1072. doi: 10.1007/s00134-004-2193-9. [DOI] [PubMed] [Google Scholar]
- 6.Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest. 2005;128:2749–2757. doi: 10.1378/chest.128.4.2749. [DOI] [PubMed] [Google Scholar]
- 7.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 9.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Gautam S, May L, et al. A comparison of different sedation scales in the ICU and validation of the Richmond Agitation-Sedation Scale (RASS)[abstract] In: Ely EW, Gautam S, May L, et al., editors. Am J Respir Crit Care Med. 2001. p. A954. [Google Scholar]
- 11.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 12.Sessler CN, Gosnell MS, Grap MJ, et al. Reliability and validity of a new agitation-sedation scale for intensive care unit patients. Virginia Pulmonary Journal. 1999;5:7. [Google Scholar]
- 13.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. J Hosp Infect. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.Fraser GL, Prato BS, Riker RR, Berthiaume D, Wilkins ML. Frequency, severity, and treatment of agitation in young versus elderly patients in the ICU. Pharmacotherapy. 2000;20:75–82. doi: 10.1592/phco.20.1.75.34663. [DOI] [PubMed] [Google Scholar]


