Abstract
African American/Black and Hispanic persons living with HIV/AIDS (“AABH-PLHA”) are under-represented in HIV/AIDS medical studies (HAMS). This paper evaluates the efficacy of a social/behavioral intervention to increase rates of screening for and enrollment into HAMS in these populations. Participants (N=540) were enrolled into a cluster randomized controlled trial of an intervention designed to overcome multi-level barriers to HAMS. Primary endpoints were rates of screening for and enrollment into therapeutic/treatment-oriented and observational studies. Intervention arm participants were 30 times more likely to be screened than controls (49.3% vs. 3.7%; p < .001). Half (55.5%) of those screened were eligible for HAMS, primarily observational studies. Nine out of ten found eligible enrolled (91.7%), almost all into observational studies (95.2%), compared to no enrollments among controls. Achieving appropriate representation of AABH-PLHA in HAMS necessitates modification of study inclusion criteria to increase the proportion found eligible for therapeutic HAMS, in addition to social/behavioral interventions.
Keywords: Clinical Trials, HIV/AIDS, African American, Black, Hispanic, minority, enrollment, health care disparities
Introduction
African American/Black and Hispanics individuals are under-represented in HIV/AIDS medical studies (HAMS) in the United States compared to Whites, with the greatest disparities found for African-Americans/Blacks [1]. In recent years, African Americans/Blacks have made up approximately 50% of all people living with HIV/AIDS (PLHA) but only 30% of those enrolled in HAMS. Furthermore, Hispanics are under-represented in HAMS in many sites [2, 3]. This low enrollment among these populations raises concerns about the generalizability of research findings to the groups most affected by HIV/AIDS. Further, it denies African American/Black and Hispanic PLHA (referred to as “AABH-PLHA” in the present paper) the opportunity to contribute to medical research [4–6]. In order to enroll in HAMS, patients must first be screened for eligibility, a process in which they are matched to studies based on their medical profiles. Preliminary work by our research team found that a culturally targeted multi-component peer-driven intervention called “ACT2” resulted in large increases in rates of screening for HAMS among AABH-PLHA, where approximately half of those in the intervention arm were screened over the study period compared to less than 5% among controls [7]. The present paper extends this past research to describe rates of enrollment into HAMS in response to the ACT2 intervention. The present study focuses on enrollment into both therapeutic/treatment clinical trials and biomedical observational studies, which evidence similar problematic rates of racial/ethnic under-representation [8]. Although the problem of under-representation of AABH-PLHA is well known, and a number of studies with PLHA have either focused exclusively on or proportionately sampled African American/Black and Latino populations [9–11], no studies have tested intervention strategies to reduce barriers to HAMS for these AABH-PLHA [12, 13].
In past research, we described the constellation of individual, social, organizational, and structural barriers that impede access to HAMS for AABH-PLHA [5, 7, 14, 15], which are reviewed in brief below. At the individual level, AABH-PLHA express mistrust of and fears about medical research [15–17]. Yet, they appear as willing as Whites to join HAMS if actively recruited [18–20]. Thus, AABH-PLHA can be described as “ambivalent” about HAMS. Further, organizational and structural barriers impede their access to studies. AABH-PLHA are less likely than Whites to be referred to HAMS by health care providers [17, 19], often reflecting concerns that patients will not adhere to protocols. Indeed some studies have found that African American/Black PLHA have lower levels of adherence to antiretroviral therapy compared to Whites and Hispanics, even when controlling for other factors [21, 22]. Yet the literature on adherence to HAMS is inconsistent, where AABH-PLHA show worse adherence to and higher drop out from HAMS compared to Whites in some studies [4, 23], but equivalent adherence and retention in others [24, 25], perhaps reflecting both characteristics of the patients who gain access to HAMS, and the clinical trials research unit (CTRU) setting. The ACT2 intervention, described in brief below, was designed to ameliorate these multi-level barriers to HAMS.
The intervention was made up of three main components: 1) six hours of structured activities conducted in small groups and one individual session, 2) the opportunity to independently educate three peers about a set of core messages about ACTs (called “peer education”), while at the same time recruiting participants for the study, and 3) navigation during the screening process for those who chose to pursue screening. Navigation was developed over a decade ago to address racial/ethnic disparities in cancer treatment and has more recently been applied to HIV care [23, 24]. Navigation is an efficacious, low-threshold, individualized approach to identifying and resolving structural and personal barriers that arise in accessing HIV services, such as transportation difficulties, as described in more detail below [26, 27]. The individual intervention session was brief (30 minutes) and was held on the CTRU where later actual screenings took place. Indeed, conducting an intervention session on the CTRU was a strategy designed to reduce fear of and overcome structural barriers to HAMS, such as difficulty finding the unit's physical location, or managing interactions with the CTRU (e.g., how to reschedule appointments). Consistent with the peer-driven intervention model, in this intervention peer education experiences were considered a “dose” of intervention for both the educator and the peer [28]. The intervention's overarching theoretical frame was the Theory of Triadic Influence (TTI) [29], which identifies three “streams of influence” on health behavior: individual, social, and structural. As a social-cognitive theory, the TTI describes the interplay between the environment and individual knowledge, attitudes, and behavioral factors to foster behavior change. Further, we drew on the Theory of Normative Regulation [30], which posits that the behaviors of individuals are amplified through their social groups [30]. Motivational Interviewing (MI) was the intervention's main counseling approach. MI is a method for exploring and resolving ambivalence and fostering decision-making by tapping into an individual's intrinsic motivation to make positive changes, without applying pressure or judgment [31]. Guided by this integrated theoretical model, the following putative barriers to HAMS were directly targeted in the ACT2 intervention: self-efficacy to manage screening, ACT-related knowledge and attitudes (distrust, willingness, readiness, altruism), behavioral skills (e.g., communicating with health care providers), perceived social norms about HAMS, interactions with health care providers, and structural barriers to CTRU access. Because AABH-PLHA have little exposure to HAMS and, at the same time, experience potent barriers to accessing biomedical studies, the ACT2 intervention was designed to build motivation for and facilitate decisions about screening for HAMS - the first low-risk step in the process of accessing biomedical studies. The primary purpose of the present paper is to examine rates of enrollment into HAMS among participants who elected to be screened. A detailed description of the ACT2 intervention components is presented elsewhere [32].
The paper's first aim was to describe rates of screening for, eligibility for, and enrollment into HAMS, comparing intervention and control arms. Because PLHA typically enter HAMS through a screening process, and a past preliminary analysis conducted by this research team showed screening rates were substantial among those in the intervention arm and rare among controls [7], enrollment rates were expected to be much higher in the intervention arm compared to controls. This first aim, therefore, describes the rates at which participants enrolled into HAMS if found eligible. Screening rates for the whole cohort are also presented, in order to update the preliminary findings in screening noted above [7]. The second aim of the present paper was to explore the types of studies for which AABH-PLHA were found eligible and into which they enrolled. To do so, we compared eligibility and enrollment rates for therapeutic/treatment trials and observational biomedical studies. The socio-demographic and health characteristics of those enrolled into HAMS compared with those not enrolled were also described. A third aim was to describe the 30 HAMS open to recruitment during the study period, and numbers of participants enrolled, to identify the specific types of HAMS that AABH-PLHA were most likely to enter, in order to identify gaps and inform future research.
Methods
Sample
A total of 540 PLHA were recruited through respondent-driven sampling (RDS [30]) in New York City between June 2008 and April 2010. RDS is a type of snowball sampling method in which individuals are trained to recruit a small number of their peers into research. Because RDS includes interactions with a small number of peers, it can be integrated with the peer-driven intervention approach. Recruitment began with initial “seeds” nominated by staff at two community-based organizations serving PLHA. Inclusion criteria for initial seeds were: active clients at the two organizations, aged 18 years or older, HIV-infected (confirmed by medical documentation), of African-American or Latino racial/ethnic background, willing to recruit HIV-infected peers, able to conduct research activities in English, and not currently enrolled in an ACT. These seeds were randomly assigned at a 2:1 ratio to an intervention or control arm at the time of enrollment. Because participants in the intervention arm received peer education at the time of recruitment, an intervention activity, the peers recruited into the study were assigned to the same intervention arm as the individual who recruited him/her. Thus, the design is equivalent to a cluster randomized controlled trial, with clusters formed on the basis of initial seeds. Compensation was provided to the recruiter for each peer recruited. Inclusion criteria for peers were similar to those for seeds with two exceptions: racial/ethnic background was not an inclusion criterion, nor were they required to be active clients of the community-based organizations. A total of 49 initial seeds recruited 491 peers over 5 recruitment waves. A total of 351/540 participants were assigned to the intervention arm, and 189/540 to the control arm. Procedures were approved by the IRBs at the collaborating sites. The trial was registered with Clinicaltrials.gov (#NCT00593983), and methods are described in more detail elsewhere [7, 14].
Procedures
Initial seeds and peers presented to the study with a coded recruitment coupon, provided written informed consent, and participated in a 20-minute structured interview to determine study eligibility. Participants received $15 compensation for the brief interview. Those found to be eligible then provided written informed consent for remaining activities, including assessments (baseline, and 16 and 52-weeks post-baseline, each lasting 1–1.5 hours) and intervention sessions. Participants received $25 compensation for each assessment and intervention session. Assessments were administered using laptop computers and included computer-assisted personal interview (CAPI) and audio, computer-assisted self-interviewing (ACASI) segments. Assessments were conducted in a private location at a study field site and intervention activities were conducted by trained and supervised staff members at the field site and hospital site. Figure 1, a Consolidated Standards of Reporting Trials (CONSORT)[33] diagram, provides an overview of study recruitment and retention.
Figure 1.
ACT2 Project CONSORT Flow Diagram
Design and Description of the Intervention
As described above, the intervention was a multi-component social/behavioral program. Participants in the control arm received a time- and attention-matched health education intervention (6 hours of small group activities), which included the current standard of care, namely, information about the purpose and types of HAMS and referrals to local CTRUs. Participants in the control arm were given the opportunity to recruit, but not educate, peers for the study. The ACT2 intervention curriculum is available from the first author.
Procedures to Screen Participants for Studies
Studies Open for Recruitment
A database of HAMS conducted in the local area during the study period was maintained: a total of 30 studies at nine different sites, which comprised the majority of HIV/AIDS CTRUs in the local area. These nine sites included the study's primary collaborating CTRU, which was located in a major medical center and was a former AIDS Clinical Trials Group site. (The AIDS Clinical Trials Group is funded by the U.S. National Institutes of Health through the National Institute of Allergy and Infectious Diseases.) The remaining eight sites included four AIDS Clinical Trials Group network clinical trials research units and sub-units, one community-based organization, two major medical centers, and a local Veteran's Administration Hospital. The 30 HAMS included in the database were sponsored either by the AIDS Clinical Trials Group or the pharmaceutical industry. For each participant, a minimum of 3 and a maximum of 8 HAMS were open at the time the participant was screened.
Description of the Screening Process
Screening for HAMS typically takes place over one to three visits conducted formally or informally by HIV clinics and CTRUs. The core elements of screening generally include a health history interview conducted by a research nurse, review of the characteristics of HAMS for which the participant may be eligible, and medical testing if needed. If a patient is found eligible for a study, additional elements include review of HAMS consent forms and coordination with the patient's primary care provider to harmonize primary care with the study [34]. Rates of eligibility tend to be low for all racial/ethnic groups, due to strict inclusion/exclusion criteria [35], and there is some suggestion that eligibility is lower for minority populations than Whites [35, 36]. Outside of this study, patients are screened for the HAMS being conducted at a single institution. In the next section we describe the steps taken in the present study, where participants were screened for HAMS at the primary collaborating CTRU, as well as for HAMS at eight other CTRUs. This allowed for an examination the ACT2 intervention's effects across a larger and more diverse set of HAMS, compared to if the study had focused on a single CTRU.
The Determination of Eligibility for HAMS Took Place in Three Stages
Step 1: Pre-Screening for HAMS. Participants were first pre-screened by a medically trained study staff member for studies at the main collaborating site. The pre-screening visit was conducted at the primary CTRU and lasted 20–60 minutes. At this stage participants were found either eligible/potentially eligible or not eligible for HAMS at the primary CTRU. If eligible/potentially eligible, participants were referred to the primary CTRU to complete screening with a research nurse. Those not eligible for or not interested in pursuing screening at the primary CTRU moved on to Step 3, described below.
Step 2: Completion of Screening to the Point of Determining Eligibility at the Primary CTRU. As noted above, those potentially eligible for a HAMS at the primary CTRU next met with a research nurse for final determination of eligibility. To address potential structural and individual barriers to completing screening, those found eligible were provided with navigation by the ACT2 study intervention facilitator as needed until enrolled. In this context, navigation entailed brief phone encounters, generally initiated by the interventionist, to remind participants about appointments, answer questions about the studies, and identify and resolve barriers to participation in screening, such as transportation difficulties, needing to change the appointment time, or not being certain where the appointment would be held. As noted above, navigation is one of the ACT2 intervention's three main intervention components.
Step 3: Linking Participants to Alternate Local CTRUs. Participants found ineligible for, or not interested in, HAMS at the primary CTRU, but preliminarily eligible for HAMS at another unit, were linked to a screening appointment there and provided with navigation through the screening and enrollment process, as described above.
Coding and External Verification of the Interim Steps toward Enrollment and Endpoints
First, we assessed whether participants initiated and completed screening to the point of determining eligibility (coded as yes/no), a primary study endpoint. Initiation and completion of screening were assessed first by participant self-report during follow-up interviews, and then externally verified by the relevant CTRU. In this same manner, we assessed whether the participant was found eligible for at least one observational and/or therapeutic study, an interim step in the enrollment process. Finally, we assessed a second endpoint: whether the participant had enrolled in HAMS, which was also verified by the CTRUs. We also obtained the protocol number of the HAMS into which participants enrolled. In most cases participant self-report and CTRUs' reports of screening and enrollment corresponded, an indication of the validity of this endpoint (86% correspondence for screening, 89% correspondence for enrollment). In the small number of cases where participant self-report and CTRUs were not in agreement, the research unit data were used as the final value for analysis.
Self-Report Measures
We used reliable and validated measures to assess socio-demographic characteristics, HIV-related physical health indices (Health Cost and Services Utilization Survey)[37], mental health symptoms (Brief Symptom Inventory)[38], substance use (Risk Factors Survey)[39], and intervention dose, including the number of peers recruited/educated (range 0–3), as peer education is considered one component of the intervention.
Characteristics of HAMS
Using detailed inclusion criteria and study descriptions obtained from study investigators, the 30 HAMS open for recruitment during the study period were coded by senior research staff first as either therapeutic (treatment) trials or biomedical observational studies. HAMS also were coded for the presence/absence of following types of inclusion/exclusion criteria: (1) antiretroviral therapy (ART) – whether use of ART (lifetime or recent) was required or not allowed; (2) Medication Constraints – whether medications other than ART were required or prohibited; (3) Viral Load or CD4 Specification – any requirements for HIV viral load or CD4 cell count to be within a certain range or not within a certain range; (4) Comorbidities – any health conditions other than HIV or AIDS that were required or prohibited; (5) Age – any requirements for participant age to be within a certain range; (6) Gender – any requirements for participants to be either biologically male or female; (7) Pregnancy/Lactation – any requirements for participants to not be pregnant, trying to become pregnant, or lactating; (8) HIV Characteristics – any requirements related to the virus, such as resistance or tropism; (9) AIDS Diagnosis or Defining Condition – whether an AIDS diagnosis or AIDS defining condition was required or prohibited; (10) Participation in Other Trials or Studies – whether participation in other trials or studies concurrently or in the past was prohibited; (11) English speaking required. Three senior members of the research team separately coded these study characteristics. Inter-rater reliability was assessed with Fleiss's formulation of the kappa coefficient for multiple raters [40]. Reliability coefficients ranged from 0.78 to 1.0 (median = .89). All disagreements among these coders were resolved either by discussion or in consultation with the team's AIDS Clinical Trials expert. Other characteristics of HAMS summarized include the number of days open for recruitment and the number of ACT2 intervention participants who pre-screened for and enrolled into each study.
Data Analysis
For the study's first aim, we report the number and percentage of participants, by study arm, who: (1) initiated screening; (2) completed screening to the point of determining eligibility; (3) were found eligible for one or more HAMS; and (4) enrolled in at least one HAMS. Because so few participants in the control arm initiated screening, it was not feasible to compare completion of screening among initiators of screening, eligibility for at least one study among completers of screening, and enrollment among eligible participants using the mixed-effects or cluster-robust regression approaches employed in our prior studies [7, 14] when the focus was screening. Estimation and inference for the enrollment outcome was also made even more challenging by complete separation [41], because no control arm participant enrolled in any HAMS. To overcome these challenges, Fisher's exact test was used to compare intervention and control arms. For the enrollment outcome, we employed Firth's bias reduced logistic regression [42], an approach to estimation in the presence of complete or quasi-complete separation, to estimate the effect of intervention arm on the odds of enrollment and to calculate a p-value using profile likelihood. We also describe socio-demographic and health characteristics of the intervention arm cohort and examine whether there were differences on these factors between those who enrolled and did not enroll into studies. For the second aim, among participants in the intervention arm, we report the number and percentage of participants who enrolled in observational studies and therapeutic trials. An exact version of the McNemar test [43] was used to compare enrollment rates for these two types of HAMS. We include only participants in the intervention arm in this analysis for parsimony and because so few participants in the control arm initiated screening (N=7). For the third aim to explore potential reasons for ineligibility, we describe features of the HAMS that were open to recruitment during the study.
Results
Description of the Cohort at Baseline
More than a third (44.3%) was female and the mean age at baseline was 49.1 years (SD=7.5 years). Two-thirds (64.4%) were African-American/Black, and a quarter was Latino/Hispanic (26.5%), described as “African-American” and “Hispanic” in the present paper. Most were currently taking ART (65.6%), and a quarter (27.0%) had never taken ART in their lifetimes. Two-thirds (65.5%) reported an undetectable viral load (that is, < 50 copies/mL). Almost all (80.1%) had been diagnosed with HIV more than 10 years ago. About a third had injected drugs in their lifetimes (29.3%) and only 3.0% were currently injecting drugs. Less than a third (27.6%) used drugs weekly and less than 10% (6.3%) used alcohol daily. Less than a quarter (19.6%) had been screened for HAMS in the past. As baseline, intervention arm participants were more likely to have screened for HAMS in the past (23%) than control arm participants (13%; Fisher's Exact Test p < .01). There were no significant differences between study arms on any of the other variables described. The cohort (N=540) is described in more detail elsewhere [14].
Screening
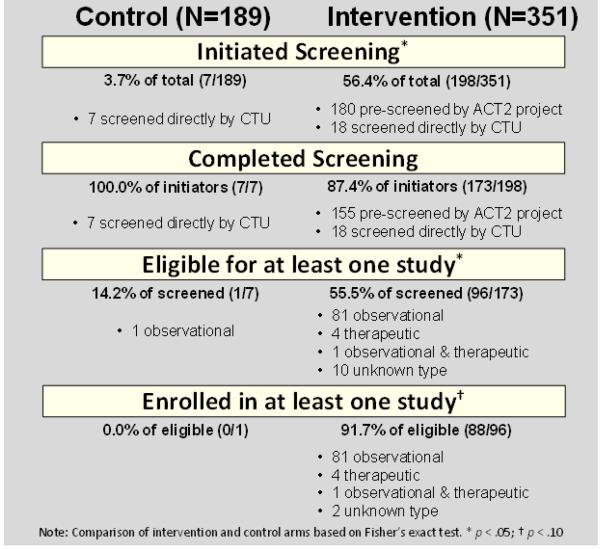
Figure 2 shows the number and percentage of participants who completed each step in the process from initiation of screening to enrollment in HAMS. Participants in the intervention arm were over 30 times more likely to initiate screening than controls (56.4% vs. 3.7%; OR = 33.46; p < .01). Almost all who initiated screening completed screening to the point of determining eligibility (87.4% intervention, 100% control; OR = 1.15, p > .10). Thus 49.3% of those in the intervention arm (173/351), and 3.7% in the control arm (7/189), completed screening (OR = 31.84; p < .001). The difference between arms in completion of screening persisted when past screening for HAMS was included as a covariate (Adjusted OR = 24.29, p < .001).
Figure 2.
Rates of Screening, Eligibility and Enrollment
Eligibility
A total of 96 intervention arm participants were found eligible for at least one HAMS (Fig. 2), 55.5% of those screened (96/173). Most were found eligible only for observational studies (n=81; 84.4%), and a small number were eligible for therapeutic studies (n=4; 4.2%). One individual was eligible for both a therapeutic and observational study (1.0%). Ten (10.4%) were eligible for a study of unknown type because they were screened directly by an alternative, not the primary, CTRU. Only one participant in the control arm was eligible for a study. Among those who completed screening, intervention arm participants were more likely to be found eligible for one or more studies than controls (OR = 7.41; p < .05). Further, an exact version of the McNemar test showed that eligibility for an observational study was far more likely than eligibility for a therapeutic study (OR = 40.50; p < .01).
Enrollment
Among those in the intervention arm, almost all found eligible enrolled in HAMS (91.7% of those found eligible; 88/96), compared to no enrollments among controls. Because only one control arm participant was eligible, it was not possible to estimate the odds ratio for study arm, but Fisher's exact test was not significant (p = .093). We were able to estimate the effect of study arm on the odds of enrollment among eligible participants using Firth's bias reduced logistic regression [42], where the effect was significant (OR = 31.19; p < .05). Participants were more likely to enroll in observational (92% of participants found eligible; 81/88) than therapeutic trials (4.6%; 4/88). Note that some participants enrolled in more than one HAMS, and the total number of enrollments was 105 (14 enrolled in two, and two individuals enrolled in three HAMS). Neither gender nor race/ethnicity was related to eligibility or enrollment.
Description of the 30 HAMS and Numbers Enrolled
Table 1 shows characteristics of the HAMS for which participants were screened. Most studies were therapeutic trials (76.6%). Studies commonly had inclusion/exclusion criteria around ART history (80.0%), other medication constraints (76.6%), HIV viral load or CD4 count (63.3%), comorbidities (90.0%), and pregnancy/lactation (66.7%). Participants enrolled in 9 of the 30 HAMS: 5 observational studies (# 1, 2, 9, 10, 20) and 4 therapeutic (# 3, 4, 24, 26). In Table 2 we note the studies that enrolled at least one study participant, showing that 5 out of 9 were observational, and almost all enrollments were into observational studies (95.2%, 100/105). Table 3 shows the socio-demographic and health characteristics of participants in the intervention arm by enrollment status (enrolled vs. not enrolled). Intervention arm participants who enrolled were more likely to be older, diagnosed with HIV ≥ 10 years ago, daily alcohol users, and experienced with HAMS screening in the past compared with those who did not enroll. Those who enrolled also were more likely to have recruited peers and received the full dose of the intervention than those who did not enroll in HAMS.
Table 1.
Description of trials and studies open to ACT2 participants, number screened, and number enrolled
| Study Number |
ART | Medication Constraints |
VL/CD4 Specifications |
Comorbidity | Age | Gender | Pregnancy/ Lactation |
HIV Characteristics |
AIDS Diagnosis/ Defining Conditions |
Participation in Other Trials/Studies |
English Speaking |
Therapeutic Trial |
Clinicaltrials.gov or Other Identifier |
Brief description | Days Recruiting During ACT2 Project |
Intervention Arm Participants Pre-Screened |
Intervention Arm Participants Enrolled |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | • | • | PHS 398/2590 | Pneumocystis antigen | 443 | 88 | 27 | ||||||||||
| 2 | • | • | • | • | NCT00665561 | POEM: Maraviroc safety | 1033 | 179 | 2 | ||||||||
| 3 | • | • | • | • | NCT00827112 | Novel treatment for treatment naives | 97 | 17 | 1 | ||||||||
| 4 | • | • | • | • | • | • | • | • | • | • | NCT00707733 | Ph3 EVG/r vs RAL | 518 | 110 | 1 | ||
| 5 | • | • | • | NCT01066819 | PROPHESYS 3: HCV, interferon | 457 | 93 | 0 | |||||||||
| 6 | • | • | • | • | • | • | • | • | • | • | Gilead 103/104 | Two arm safety efficacy for treatment naives | 24 | 3 | 0 | ||
| 7 | • | • | • | • | • | • | • | • | • | • | NCT01108510 | Ph 3 Two arm safety efficacy naives | 31 | 5 | 0 | ||
| 8 | • | • | • | • | • | • | NCT00540449 | Two arm safety efficacy naives | 215 | 32 | 0 | ||||||
| 9 | • | • | • | NYU LTNP | Long-term non-progressor for treatment naives | 536 | 121 | 24 | |||||||||
| 10 | • | • | • | NCT00959413 | Oral mucosal disease | 292 | 32 | 9 | |||||||||
| 11 | • | • | • | • | • | • | • | • | NCT00665353 | HCV insulin resistance | 609 | 128 | 0 | ||||
| 12 | • | • | • | • | • | • | • | NCT00537394 | Optimizing treatment | 394 | 37 | 0 | |||||
| 13 | • | • | • | • | • | NCT00604175 | Human papillomavirus vaccine | 729 | 129 | 0 | |||||||
| 14 | • | • | • | • | • | • | • | • | NCT00811954 | 3 NNRTI-sparing HAART regimens for treatment naives | 759 | 129 | 0 | ||||
| 15 | • | • | • | • | • | • | • | NCT00819390 | Chloroquine for reducing immune activation | 624 | 101 | 0 | |||||
| 16 | • | • | • | • | • | • | • | NCT00361257 | Minocycline for decreased mental function | 161 | 34 | 0 | |||||
| 17 | • | • | • | • | • | • | • | • | NCT00090779 | People recently infected and treatment naive | 244 | 60 | 0 | ||||
| 18 | • | • | • | • | • | • | • | NCT00393276 | Vaccines in HIV and HCV infected | 161 | 34 | 0 | |||||
| 19 | • | • | • | • | • | • | • | • | NCT00851786 | Live zoster vaccine | 415 | 39 | 0 | ||||
| 20 | • | • | NCT00933595 | Lung infections and complications | 563 | 99 | 38 | ||||||||||
| 21 | • | • | • | • | NCT00494936 | Effects of HIV, HCV on the brain | 646 | 107 | 0 | ||||||||
| 22 | • | • | • | • | • | • | • | • | • | NCT00963235 | Safety & immunogenicity of 13vPnC | 250 | 55 | 0 | |||
| 23 | • | • | • | • | • | • | NCT00991289 | Treatment for naive HCV genotype 1 | 227 | 52 | 0 | ||||||
| 24 | • | • | • | • | • | • | NCT00130286 | HIV-associated increased abdominal fat | 244 | 60 | 2 | ||||||
| 25 | • | • | • | • | • | • | • | NCT00976404 | Therapeutic intensification plus immunomodulation | 263 | 12 | 0 | |||||
| 26 | • | • | • | • | • | • | • | NCT00547898 | ADVENT: Crofelemer for diarrhea | 195 | 47 | 1 | |||||
| 27 | • | • | • | • | • | • | • | • | • | • | NCT00784147 | Monoclonal antibody | 194 | 46 | 0 | ||
| 28 | • | • | • | • | DHEA Effects on Mood | DHEA effects on mood | 258 | 73 | 0 | ||||||||
| 29 | • | • | • | • | • | • | • | NCT00737204 | Armodafinil for treating fatigue | 258 | 73 | 0 | |||||
| 30 | • | • | NCT00017823 | Acupressure to treat nausea | 309 | 57 | 0 |
Table 2.
Studies with at least one enrollment (N=105 total enrollments)
| # | Clinicaltrials.gov or Other Identifier | Brief description | Therapeutic | Intervention Arm Participants Pre-Screened | Intervention Arm Participants Enrolled | % enrolled |
|---|---|---|---|---|---|---|
| 20 | NCT00933595 | Lung infections and complications | 99 | 38 | 38.4% | |
| 1 | PHS 398/2590 | Pneumocystis antigen | 88 | 27 | 30.7% | |
| 9 | NYU LTNP | Long-term non-progressor for treatment naives | 121 | 24 | 19.8% | |
| 10 | NCT00959413 | Oral mucosal disease | 32 | 9 | 28.1% | |
| 24 | NCT00130286 | HIV-associated increased abdominal fat | • | 60 | 2 | 3.3% |
| 2 | NCT00665561 | POEM: Maraviroc safety | 179 | 2 | 1.1% | |
| 3 | NCT00827112 | Novel treatment for treatment naives | • | 17 | 1 | 0.06% |
| 26 | NCT00547S9S | ADVENT: Crofelemer for diarrhea | • | 47 | 1 | 0.02% |
| 4 | NCT00707733 | Phase 3 EVG/r vs RAL | • | 110 | 1 | 0.01% |
Table 3.
Sociodemographicand health characteristics of intervention arm participants by enrollment status.
| Total (n=351) | Enrolled (n=88) | Not Enrolled (n=263) | Significance | |
|---|---|---|---|---|
| Female | 44.2 | 46.6 | 43.3 | |
| Age 18–40 | 10.5 | 8.0 | 11.4 | |
| Age 41–50 | 45.6 | 37.5 | 48.3 | |
| Age 51+ | 43.9 | 54.5 | 40.3 | * |
| Mean Age (SD) | 49.4 (7.4) | 50.4 (6.9) | 49.1 (7.5) | |
| African-American | 65.8 | 62.5 | 66.9 | |
| Hispanic | 24.8 | 25.0 | 24.7 | |
| Heterosexual | 70.7 | 71.6 | 70.3 | |
| Full Dose of Intervention | 88.3 | 95.5 | 85.9 | |
| Recruited/educated Peers | 56.1 | 67.0 | 52.5 | * |
| At Least Four HIV Medical Appointments Past 12 Months | 88.5 | 87.4 | 91.6 | |
| Current ART | 66.2 | 69.3 | 65.1 | |
| Past ART | 8.6 | 4.5 | 10.0 | |
| ART Naive | 25.2 | 26.1 | 24.9 | |
| CD4 < 350 | 34.7 | 26.4 | 37.6 | |
| CD4 < 350 & No Current ARV | 11.6 | 6.8 | 13.3 | |
| CD4 < 500 | 60.5 | 52.9 | 63.2 | |
| CD4 < 500 & No Current ARV | 19.2 | 18.2 | 19.6 | |
| Uncletectable Viral Load | 64.5 | 63.5 | 64.8 | |
| HIV Diagnosis >= 10 Years | 81.5 | 90.2 | 78.7 | * |
| AIDS Diagnosis | 55.9 | 57.5 | 55.3 | |
| Ever Hepatitis C | 33.9 | 35.2 | 33.5 | |
| Ever Hepatitis B | 19.7 | 23.9 | 18.3 | |
| Mean BSI Global Severity (SD) | 0.49 (0.49) | 0.52 (0.51) | 0.48 (0.48) | |
| Current Alcohol Use | 49.0 | 51.1 | 48.3 | |
| Current Drug Use | 40.5 | 33.0 | 43.0 | |
| Current Alcohol or Drug Use | 61.3 | 55.7 | 63.1 | |
| Ever Injected Drugs | 29.3 | 26.1 | 30.4 | |
| Current Inject Drugs | 2.6 | 2.3 | 2.7 | |
| Weekly Drug Use | 28.8 | 22.7 | 30.8 | |
| Daily Alcohol Use | 6.8 | 12.5 | 4.9 | * |
| Prior HAMS Screening | 23.1 | 31.8 | 20.2 | * |
p < .05 by independent-samples t-test (comparison of means) or Fisher's exact test (comparison of percentages).
Discussion
A previous preliminary study showed that the ACT2 intervention produced a large increase in rates of screening for HAMS among AABH-PLHA [7], but we had not yet assessed whether participants who elected to be screened would enroll into HAMS. The present paper found that nine out of 10 of those screened and found eligible for HAMS did, in fact, enroll in a study. In contrast, screening was rare among those in the control arm, and, as a result, none in that arm enrolled. The present study, therefore, provides strong support for the efficacy of this multi-component peer-driven intervention approach designed to ameliorate individual/attitudinal, social, and structural barriers to HAMS for AABH-PLHA. Moreover, these results support the utility of the intervention's emphasis on boosting motivation for screening, as a means of introducing AABH-PLHA to the possibility of exploring HAMS. Indeed, as we have described above, we found that AABH-PLHA were commonly willing to consider and engage in screening, a low-risk activity, which then led to very high rates of enrollment into HAMS among those found eligible. This pattern of behavior is consistent with Commitment Theory, which articulates how an individual's initial smaller decisions and actions promote more substantial engagement later on [44].
Moreover, these findings suggest that screening for HAMS is itself an important endpoint, even if individuals do not enroll in HAMS. Indeed, we have found in past research that the experience of screening enhances PLHA's access to and positive attitudes toward both CTRUs and HAMS, and may therefore improve the chances of future participation, even if the participant is found ineligible [5, 15, 34]. The screening experience may therefore also promote more positive peer norms about HAMS among the social networks of AABH-PLHA. These screening visits can also benefit CTRUs by increasing the pool of potential patients for future studies.
The Issue Of Low Eligibility Rates For Therapeutic HAMS
Participants were mainly found eligible for and enrolled into biomedical observational studies, although a number entered therapeutic trials. Indeed, participants' high rates of screening provide evidence for their interest in therapeutic studies, as the majority of HAMS are typically therapeutic in nature. However, low eligibility rates were a major impediment to enrolling participants into therapeutic trials. Indeed, awareness is growing about the potential role inclusion and exclusion criteria play in racial/ethnic under-representation in therapeutic clinical trials. In our own past research with AABH-PLHA [45], we found a similar low eligibility rate (13% eligible, half of whom enrolled) which appeared to be due largely to a “mismatch” between participant medical characteristics, such as CD4 and viral load, which were frequently atypical (e.g., high CD4 and high VL), and study inclusion criteria [36], where these medical characteristics and atypical patterns were uncommon among White patients. In cancer clinical trials, Penberthy and colleagues found African-Americans/Blacks were more likely than Whites to be found ineligible, and moreover, to be found ineligible due to non-medical issues such as mental status or perceived noncompliance [46], factors that could potentially be ameliorated. Further, Gandhi and colleagues [35] examined reasons for ineligibility in the largest study of HIV-infected women, including African American/Black and Hispanic women, and found over half would be excluded from key NIH-funded trials based on protocol enrollment criteria. Indeed, they argue this is a very serious concern and recommend modification of broad and/or arbitrary eligibility criteria, as well as criteria requiring `investigator judgment,' in order to increase the proportion of women, including racial/ethnic minority women, in HAMS. Thus while HAMS' very strict study inclusion criteria result in relatively low eligibility rates for all populations of PLHA [35], there is increasing concern that these enrolment criteria exclude AABH-PLHA at disproportionately high rates compared to Whites.
Boosting the Intervention's Effect on Screening
The ACT2 intervention's large effect on screening was notable, given sample heterogeneity with respect to socio-demographic and health characteristics, and participants' ambivalent attitudes toward and limited past experiences with HAMS. Indeed, most participants had not gained access to HAMS screening in the past. Despite these promising findings, the present paper suggests that some PLHA with very serious barriers to HAMS may require repeated opportunities to explore barriers to HAMS and to access screening. Indeed, health issues, such as co-morbid conditions or problems adhering to antiretroviral therapy regimens, contextual factors, such as housing problems, and/or competing priorities such as mental health or substance use problems, may have reduced participants' readiness to screen for HAMS at the time they were enrolled in the intervention study. Yet these types of barriers tend to vary over time, and even resolve, suggesting participants may be more willing or ready to screen for and enroll into HAMS in the future. Addressing barriers to HAMS with intervention programs such as ACT2, and offering screening for HAMS, on a regular and repeated basis may therefore play a role in increasing screening rates.
It is also possible a higher “dose” of intervention, and/or different types of intervention components, are needed for those with the greatest barriers to HAMS or who did not elect to be screened in response to the ACT2 intervention. Despite the considerable formative and theoretical research carried out during the intervention development process, there is growing awareness that “one size fits all” or packaged interventions may be inefficient [47]. Instead, the “adaptive intervention” approach could be applied to address the question of how to boost the ACT2 intervention's efficacy [48]. Adaptive interventions (also known as “adaptive treatment strategies” or “dynamic treatment regimens”) are individually tailored treatments in which a sequence of decision rules specifies how the intensity or type of treatment should change depending on the participant's needs or response to intervention components already received [49]. Adaptive interventions are similar to clinical practice in that those who do not respond sufficiently to an intervention are provided with more options, and sequential multiple assignment randomized trials provide a framework for testing these adaptive interventions in a rigorous fashion. While the ACT2 intervention had a very large impact on the probability of screening for and enrolling into HAMS, in future research we will explore the potential of the adaptive intervention framework to increase the efficacy of the ACT2 intervention.
Characteristics of Those Who Enrolled
Participants were mostly male, African-American, 40 years of age and older, and diagnosed with HIV more than 10 years prior. There were few differences in socio-demographic and health characteristics between those enrolled and those who did not, including no gender or race/ethnic differences in screening, eligibility, or enrollment. In past papers we found that those screened tended to be older, and more likely to have completed the entire intervention and to have recruited/educated peers [7]. The nominal differences between those enrolled and not enrolled in the present paper are similar to these prior findings, suggesting factors contributing to self-selection or that otherwise impede access to HAMS are found mainly at the screening stage.
Generalizability
Recent studies on the HIV treatment cascade highlight the problem of the large numbers of PLHA who are not engaged in care, not taking antiretroviral therapy, and not virally suppressed [50]. In the present study, participants reported engaging in care, taking antiretroviral therapy, and having an undetectable viral load at substantially higher rates than in the underlying population of PLHA nationally and locally [51]. As described above, participants were recruited using respondent-driven sampling, where “seeds” recruited from health care or community-based organizations recruited their peers over multiple waves. It is plausible that the sampling approach tapped into networks of PLHA who tended to be more engaged in care, more likely to be taking antiretroviral therapy, and more likely to have viral load suppression than other PLHA. At the same time, PLHA not engaged in care, not taking antiretroviral therapy, and without viral load suppression may have been less likely to be approached by peers for study recruitment, or may have declined to enroll into the study due to low interest in a study on HAMS.
While these recruitment biases limit generalizability, it is also clear that a large proportion of AABH-PLHA who are well engaged in care are, nonetheless, not accessing HAMS. These patients could potentially benefit from an intervention such as ACT2. On the other hand, PLHA who are less well engaged in care may not be appropriate targets for intervention efforts to engage them in HAMS. Instead, interventions to facilitate progress along the HIV continuum of care should precede efforts to increase access to HAMS.
Thus because the sample of AABH-PLHA in the present study was diverse with respect to background and socio-demographic characteristics, we speculate findings will generalize to similar populations of AABH-PLHA in urban areas in the U.S.
Implications
The present study highlights a number of junctures where AABH-PLHA experience barriers to HAMS, as well as strategies to successfully ameliorate these impediments. First, AABH-PLHA are infrequently recruited for HAMS, and may be more likely than Whites to experience barriers to HAMS when invited. We found the ACT2 intervention greatly reduces these types of barriers through an active recruitment strategy and culturally targeted social/behavioral intervention components. Second, past research has shown that AABH-PLHA evidence difficult negotiating the HAMS system, leading to poor retention. The ACT2 intervention successfully ameliorates that challenge as well. However, the present study found the very low eligibility rates AABHPLHA experience for therapeutic HAMS to be a serious obstacle to enrollment. Therefore, in order to achieve proportional representation of AABH-PLHA, scientists who design HAMS will need to consider the implications of highly restrictive inclusion criteria and other design features on the participation of under-represented groups, while at the same time maintaining study validity. This suggests that social/behavioral interventions such as ACT2 can play a role in boosting AABH-PLHA participation in HAMS, although busy CTRUs will likely need additional partnerships and funding streams to implement such an intervention. Moreover, changes are needed to HAMS study design and inclusion criteria to eliminate this racial/ethnic disparity. Further, we found the strategy of simultaneously pre-screening participants for HAMS at more than one local site was efficient for both participants and CTRUs, and also contributed to the intervention's efficacy. This centralized screening approach can therefore also play a role in reducing these disparities.
Limitations
As noted above, we do not have detailed data on reasons for ineligibility for each HAMS open at the time of screening. Instead, we know whether participants were found eligible for at least one observational study and one therapeutic trial. Further, eligibility was mainly a function of whether participants met study inclusion criteria, but also related to whether the participant elected to pursue a study. Although the study documented low rates of eligibility for therapeutic HAMS, a better understanding of reasons for ineligibility in this population is needed to inform the design of future trials for which more AABH-PLHA will be eligible. Another limitation is the inability to take clustering due to recruitment relationships into account, given the very small number of control arm participants who initiated screening. However, given the sizes of intervention arm effects on eligibility and enrollment, and the fact that almost all eligible participants in the intervention arm enrolled in a HAMS, we are confident the intervention is far more effective in facilitating access to HAMS than current practice. Last, the ACT2 intervention did not extend through the participant's time enrolled in the HAMS, and therefore we do not have information on their adherence to and retention in HAMS.
Summary
CTRUs can eliminate racial/ethnic disparities in HAMS by implementing multi-component interventions such as ACT2 to build motivation and capability to access HAMS, offering repeated access to screening, and centralizing screening efforts where appropriate. Further, modifications to study inclusion criteria will be needed to increase the proportion of AABH-PLHA who enroll in HAMS.
Acknowledgements
This study was supported by a grant from the National Institute of Allergy and Infectious Diseases (R01AI070005) and the Center for Drug Use and HIV Research (P30DA011041) at the New York University College of Nursing. The project is dedicated to the memory of Keith Cylar, MSW, Co-founder and Co-chief Executive Officer of Housing Works, Inc. We would like to thank the men and women who participated in the study, Amy Braksmajer, Ph.D. and Christopher Hilliard, MPH for editorial assistance, Dr. Usha Sharma, the study's Program Officer, and members of the ACT2 Collaborative Research Team: Michael Aguirre, Noreen Boadi, MA, DeShannon Bowens, MA, Patricia Chang, MA, Gwen Costantini, FNP-C, Rebecca de Guzman, Ph.D., Ann Marshak, Sondra Middleton, PA-C, Corinne Munoz-Plaza, MPH, Maya Tharaken, MSSW, Robert Quiles, and Mougeh Yasai, MA.
References
- 1.Castillo-Mancilla J, Cohn S, Krishnan S, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials. 2014;15(1):14–26. doi: 10.1310/hct1501-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Allergy and Infectious Diseases [NIAID] HIV infection in minority populations. 2008 Available from: http://www.niaid.nih.gov/topics/HIVAIDS/Understanding/PopulationSpecificInformation/Pages/minorityPopulations.aspx.
- 3.Ortiz AP, Colon-Lopez V, Girona-Lozada G, et al. Report of the 2012 Capacity building for HIV-HPV clinical trials recruitment among minority underserved populations of Hispanic origin in Puerto Rico. P R Health Sci J. 2012;31(3):185–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Gifford AL, Cunningham WE, Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346(18):1373–82. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 5.Gwadz MV, Colon P, Ritchie AS, et al. Increasing and supporting the participation of persons of color living with HIV/AIDS in AIDS clinical trials. Curr HIV/AIDS Rep. 2010;7(4):194–200. doi: 10.1007/s11904-010-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes P, Eron JJ, Leone PA, Adimora AA, Wohl DA, Miller WC. Recruitment of HIV/AIDS treatment-naive patients to clinical trials in the highly active antiretroviral therapy era: influence of gender, sexual orientation and race. HIV Med. 2011;12(3):183–91. doi: 10.1111/j.1468-1293.2010.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwadz MV, Leonard NR, Cleland CM, et al. The effect of peer-driven intervention on rates of screening for AIDS clinical trials among African Americans and Hispanics. Am J Public Health. 2011;101(6):1096–102. doi: 10.2105/AJPH.2010.196048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196(2):304–12. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 9.Wojna V, Skolasky RL, Hechavarria R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12(5):356–64. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- 10.McLaren PJ, Ripke S, Pelak K, et al. Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum Mol Genet. 2012;21(19):4334–47. doi: 10.1093/hmg/dds226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbie-Smith G, Odeneye E, Banks B, Shandor Miles M, Roman Isler M. Development of a multilevel intervention to increase HIV clinical trial participation among rural minorities. Health Educ Behav. 2013;40(3):274–85. doi: 10.1177/1090198112452124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedberg KA, Sullivan L, Georgakis A, Savetsky J, Stone V, Samet JH. Improving participation in HIV clinical trials: impact of a brief intervention. HIV Clin Trials. 2001;2(3):205–12. doi: 10.1310/PHB6-2EYA-GA06-6BP7. [DOI] [PubMed] [Google Scholar]
- 14.Gwadz M, Cleland CM, Leonard NR, et al. Predictors of screening for AIDS clinical trials among African-Americans and Latino/Hispanics enrolled in an efficacious peer-driven intervention: uncovering socio-demographic, health, and substance use-related factors that promote or impede screening. AIDS Behav. 2013;17(2):801–12. doi: 10.1007/s10461-012-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwadz MV, Leonard NR, Nakagawa A, et al. Gender differences in attitudes toward AIDS clinical trials among urban HIV-infected individuals from racial and ethnic minority backgrounds. AIDS Care. 2006;18(7):786–94. doi: 10.1080/09540120500428952. [DOI] [PubMed] [Google Scholar]
- 16.Russell SL, Katz RV, Wang MQ, et al. Belief in AIDS origin conspiracy theory and willingness to participate in biomedical research studies: findings in whites, blacks, and Hispanics in seven cities across two surveys. HIV Clin Trials. 2011;12(1):37–47. doi: 10.1310/hct1201-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone VE, Mauch MY, Steger K, Janas SF, Craven DE. Race, gender, drug use, and participation in AIDS clinical trials. Lessons from a municipal hospital cohort. J Gen Intern Med. 1997;12(3):150–7. doi: 10.1007/s11606-006-5022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durant RW, Legedza AT, Marcantonio ER, Freeman MB, Landon BE. Different types of distrust in clinical research among Whites and African Americans. J Natl Med Assoc. 2011;103(2):123–30. doi: 10.1016/s0027-9684(15)30261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan PS, McNaghten AD, Begley E, Hutchinson A, Cargill VA. Enrollment of racial/ethnic minorities and women with HIV in clinical research studies of HIV medicines. J Natl Med Assoc. 2007;99(3):242–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simoni JM, Huh D, Wilson IB, et al. Racial/ethnic disparities in ART adherence in the United States: findings from the MACH14 study. J Acquir Immune Defic Syndr. 2012;60(5):466–72. doi: 10.1097/QAI.0b013e31825db0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyser M, Buchacz K, Bush TJ, et al. Factors associated with non-adherence to antiretroviral therapy in the SUN study. AIDS Care. 2011;23(5):601–11. doi: 10.1080/09540121.2010.525603. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg J, Saag M, Squires K, et al. Health-related quality of life in the gender, race, and clinical experience trial. AIDS Res Treat. 2011:349165. doi: 10.1155/2011/349165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anastasi JK, Capili B, Kim GH, Chung A. Clinical trial recruitment and retention of a vulnerable population: HIV patients with chronic diarrhea. Gastroenterol Nurs. 2005;28(6):463–8. doi: 10.1097/00001610-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. J Clin Epidemiol. 1997;50(4):385–91. doi: 10.1016/s0895-4356(97)00041-3. [DOI] [PubMed] [Google Scholar]
- 26.Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139–41. doi: 10.1007/s11524-006-9030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(Suppl 1):S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 28.Broadhead RS, Volkanevsky VL, Rydanova T, et al. Peer-driven HIV interventions for drug injectors in Russia: first year impact results of a field experiment. Intl J Drug Policy. 2006;17(5):379–92. [Google Scholar]
- 29.Flay BR, Snyder F, Petraitis J. The theory of triadic influence. In: DiClimente RJ, Kegler MC, Crosby RA, editors. Emerging theories in health promotion practice and research. Jossey-Bass; New York: 2009. [Google Scholar]
- 30.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99. [Google Scholar]
- 31.Miller W, Rollnick S. Motivational interviewing: preparing people for change. 2nd ed Guilford; New York, NY: 2002. [Google Scholar]
- 32.Leonard NR, Banfield A, Riedel M, et al. Description of an efficacious behavioral peer-driven intervention to reduce racial/ethnic disparities in AIDS clinical trials. Health Educ Res. 2013;28(4):574–90. doi: 10.1093/her/cyt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):834–40. doi: 10.1016/j.jclinepi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Engel S, Cleland CM. The experience of screening for HIV/AIDS medical studies among African-American/Black and Latino/Hispanic persons living with HIV/AIDS: A mixed-methods exploration. J AIDS Clin Res. 2013;4:223. doi: 10.4172/2155-6113.1000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gandhi M, Ameli N, Bacchetti P, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS. 2005;19(16):1885–96. doi: 10.1097/01.aids.0000189866.67182.f7. [DOI] [PubMed] [Google Scholar]
- 36.Marshak A, Costantini G, Middleton S, et al. Screening for AIDS clinical trials in the project ACT cohort of racial/ethnic minorities and women in New York City: substantial interest but low eligibility. 4th International AIDS Society Conference; Sydney, Australia. 2007. [Google Scholar]
- 37.Hays RD, Spritzer KL, McCaffrey D, et al. The HIV cost & services utilization study (HCSUS) measures of health-related quality of life. RAND; Santa Monica, CA: 1998. [Google Scholar]
- 38.Derogatis L, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 39.Des Jarlais DC, Friedman SR, Novick DM, et al. HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;261(7):1008–12. doi: 10.1001/jama.261.7.1008. [DOI] [PubMed] [Google Scholar]
- 40.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–82. [Google Scholar]
- 41.Albert A, Anderson A. On the existence of maximum likelihood estimates in logistic regression models. Biometrika. 1984;71:1–10. [Google Scholar]
- 42.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 43.Fay MP. Two-sided exact tests and matching confidence intervals for discrete data. R Journal. 2010;2(1):53–8. [Google Scholar]
- 44.Burger JM. The foot-in-the-door compliance procedure: a multiple-process analysis and review. Pers Soc Psychol Rev. 1999;3(4):303–25. doi: 10.1207/s15327957pspr0304_2. [DOI] [PubMed] [Google Scholar]
- 45.Gwadz MV, Cylar K, Leonard NR, et al. An exploratory behavioral intervention trial to improve rates of screening for AIDS clinical trials among racial/ethnic minority and female persons living with HIV/AIDS. AIDS Behav. 2010;14(3):639–48. doi: 10.1007/s10461-009-9539-9. [DOI] [PubMed] [Google Scholar]
- 46.Penberthy L, Brown R, Wilson-Genderson M, Dahman B, Ginder G, Siminoff LA. Barriers to therapeutic clinical trials enrollment: differences between African-American and white cancer patients identified at the time of eligibility assessment. Clin Trials. 2012;9(6):788–97. doi: 10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41(2):208–26. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24(10):1455–81. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 49.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy S. A “SMART” design for building individualized treatment sequences. Ann Rev of Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337–44. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 51.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005–2009: do patients who initiate care stay in care? AIDS Patient Care STDS. 2011;25(2):79–88. doi: 10.1089/apc.2010.0151. [DOI] [PubMed] [Google Scholar]