Abstract
Partner-age difference is an HIV-risk factor among young women in Africa, but the underlying mechanisms are poorly understood. We used nationally representative data among black South Africans (men: 3530; women: 3946) to examine the proportion of women in partnerships involving male partner concurrency by age of female partners and by age-disparate (≥5 years) partnerships. Of all partners reported by men, 35% of young (16–24) women were in partnerships involving male partner concurrency of four weeks or longer during the past 12 months. Young women in age-disparate partnerships were more likely to be in partnerships with men who had other concurrent partners (9%; OR 1.88 p<0.01) and more likely to be connected to an older sexual network. Our results suggest that the relationship between male concurrency and age-disparate relationships may increase HIV risk for young women by connecting them to larger and older sexual networks.
Keywords: partnership age-gaps, HIV risk, concurrent sexual partnerships, women, Southern Africa
Introduction
In sub-Saharan Africa, the global epicentre of the HIV/AIDS epidemic, a greater burden of the disease falls on women [1]. Young women face particularly high risk, with HIV prevalence two and a half to three times higher than that of their male peers [2,3]. Marked partner age differences have been identified as one factor that increases HIV risk significantly among young women [4–7]. However, there is still much that we do not yet understand about how age-disparate partnerships increase HIV risk. This information is necessary in order to develop interventions to reduce the role that these partnerships play in the spread of HIV.
Age-disparate partnerships are defined in this paper as heterosexual partnerships in which the woman is five years or more younger than her male partner. The underlying mechanism through which age-disparate partnerships increase HIV risk for women is generally hypothesized to be the HIV-age profiles among men and women. As HIV prevalence peaks five years later in men than among women [2,8], a young woman whose partner is older than she is more likely to have sex with an HIV positive man than a young woman with a sex partner of the same age.
The HIV infection risk for women attributed to sex with older men will be further exacerbated if age-disparate partnerships are associated with sexual behaviours that facilitate HIV transmission. Condom use appears to be one compounding factor, with unprotected sex being more common among women in age-disparate partnerships compared to women having sex within their peer group [9–13]. Our understanding of how other sexual risk factors like concurrency differ by partner age-gaps is limited [14,15].
Concurrent sexual partnerships among men – any temporal overlap of one or more sexual partnerships – have the potential to further compound the HIV risk to women in age-disparate partnerships if the partners of women in age-disparate partnerships are more likely to have other partners than the partners of women in similar age partnerships. An emerging body of evidence indicates that concurrency levels among certain younger, male populations in sub-Saharan Africa are relatively high [16–21]. While different definitions and measures of concurrency in these studies make comparisons difficult, estimates of concurrency among young male samples (all under 30 years old) ranged between 25% and 44%. As men generally have partners that are younger than they are [14,18,22,23], these findings indicate that any interplay between partnership age-gaps and concurrency may have practical significance for large proportions of young women.
Who men select as concurrent partners will determine whether there is any interplay between age-disparate partnerships and male concurrency. Concurrent and age-disparate partnerships would be positively correlated if both (or all) partners that men select to have concurrently tend to be significantly younger than the partners of monogamous men. Evidence to support this was not found in previous research [18,24]. These studies were conducted among 14–25 year old South African men and women and it is unclear whether the findings generalise to older populations. Age-disparate partnerships could also increase the likelihood that young women are connected to a broader sexual network if men in concurrent relationships tend to select one partner of similar age to the partners of men in monogamous relationships and another, much younger women. Some evidence was found for this in a study of 15–49 year old men in Zambia: in univariate analyses, no relationship was found between concurrency and age-gap with primary partners, but a positive relationship was found between concurrency and age-gaps with the most recent non-cohabiting partners [25]. However, it remains unclear whether confounding factors influenced these results.
This paper uses data on partnerships reported by men in a nationally representative survey in South Africa to examine whether the interplay between concurrency and age-disparate partnerships influences whether women are connected to a broader sexual network. Specifically, we assess whether young women in age-disparate partnerships are more likely to be connected to a broader sexual network than young women in partnerships with their peers.
Methods
Data
We used data from the second National HIV Communication Survey (NCS) conducted in South Africa between June and August 2009 and designed to be representative of 16-to-55 year olds. A multi-stage, sampling approach was used with stratification by province, district councils and area type. Primary sampling units (2001 census small areas) were selected based on probability proportional to size techniques; thereafter households, followed by individuals, were randomly selected within primary sampling units [26]. Of note, individual response rates were low with 58% of selected individuals interviewed. Non-responders included ineligible households, households where nobody was at home and refusals. The questionnaire covered socio-demographic characteristics, exposure to HIV programmes, and indicators of HIV knowledge, attitudes and behaviour. The University of the Witwatersrand’s Human Research Ethics Committee and the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health granted ethical approval for the NCS.
Relevant to the current study, data were collected on up to five of participants’ most recent sexual partners, including dates of first and last sex, and anticipation about future sex (see Partner History Table, Supplemental Digital Content 1, for a list of relevant questions). We restricted the data to 16–49 year old participants and to the three most recent partners reported by respondents in order, as closely as possible, to follow the UNAIDS recommended protocol of measuring concurrency [27]. We also restricted the data to black African respondents (n= 7926; men: 3704; women: 4222) given far higher prevalence rates of HIV [2,28], and concurrency [19,29], among this population group in South Africa.
Measures
Binary variables were created to identify partnerships with a five-year or greater age disparity and those with an age difference of ten or more years. Indicators of individual concurrency were calculated according to the protocols recommended by the panel of experts convened by UNAIDS [27]. A binary variable was created to indicate individual point prevalence of concurrency for participants who reported partnerships with overlapping dates at the point exactly 6 months prior to the survey interview. A binary variable was also created to indicate individual cumulative concurrency for participants who reported any partnerships with overlapping dates during the 12 months prior to the survey interview.
We then created a partnership roster including all female partners (up to three in the past 12 months) reported by men. The roster included the measures of the age of both partners, the age difference between partners, whether the man reported another partnership with overlapping dates, and measures of partnership duration and the duration of overlap with another partnership. In addition, we created an indicator of whether the female partner was a ‘primary’ or ‘additional’ partner. All partners that did not overlap with another partner (i.e. the man was monogamous) were considered ‘primary’ partners. For overlapping partnerships, the self-perceived nature of each relationship was compared. Women in partnerships described as “married”, “living together” or “main partner” were coded as the ‘primary’ partner. If the relationship with the other women was reported as “a friend”, “someone I’ve known for a while”, “someone I just met”, or “a one night encounter,” then the other women in these concurrent partnerships were defined as “additional partners.” In cases where the relationships of both (all) overlapping partners were given the same description, the women in the longer partnership was considered the ‘primary’ partner and the other women the ‘additional’ partner.
Analysis
We first present descriptive statistics on sample characteristics, age-gaps between partners and the prevalence of concurrent sexual partnerships among men and women. We then examine how the prevalence of concurrency among men varies by age. Using the partnership roster we created, we next assess, after controlling for men’s age, whether age-differentials between partners are associated with partnership type.
Thereafter we assess whether patterns of male concurrency and partner age differences interact in a manner that increases the likelihood that young women with age-disparate partners are connected to a broader sexual network. We examined the proportion of 16-to-49 year old women in partnerships in which the man reported having another partner. This analysis was conducted by age of the female partner and by age-disparate partnerships. This novel approach helps overcome an important obstacle in research on concurrency: it is the sexual behaviour of one’s partner(s) outside of an individual’s partnership dyad that is important for individual risk of sexually transmitted infections, but perceptions about what one’s partner is doing and with whom are unreliable at best [30,31]. In this analysis we excluded partnerships of less than four weeks duration. The analysis therefore focuses on partnerships that pose higher HIV risk to women as the likelihood of acquiring HIV from once-off, or a few, unprotected sexual encounters is low [32], and partners are more likely to use condoms when they have these short term partnerships [33].
Multivariate logistic regression modelling was employed to check the robustness of the relationship between age-gaps and partner concurrency while controlling for partnership characteristics (duration), household characteristics reported by the male partner (urban versus rural and assets), and individual characteristics of the male partner (age, marital status, highest level of education completed, employment status).
Finally, within all partnerships involving partner concurrency, we examined the average age of the man’s other partner by the age of the women in each reported partnership and by whether the partnership age-gap was five years or more. This analysis provides an indication of who women may be connected to in a sexual network. Where relevant, the statistical significance of differences between groups was derived using two-sample differences in means and proportions tests. All analyses were conducted with Stata 12.0 (Stata Corporation, College Station, TX, USA). All analyses were adjusted to account for the survey design and non-response.
Results
Descriptive statistics for men (n=3530) and women (3946) are displayed in Table 1. The majority of participants were under 35 with a mean age of 28 for men and 29.5 for women. Participants had, on average, completed 9.7 grades of schooling and the large differences in education between older and younger participants is consistent with other nationally representative South African data [34]. Almost half the sample resided in rural areas and, consistent with other data, rates of marriage were relatively low [35]. In terms of age differences in the most recent partnerships reported by women, a significant proportion (43%) of 16-to-49 year old women were in partnerships with a man five or more years their senior. Among young women (16-to-24) their last sexual encounter in roughly a third of cases was with a man five or more years older than they were and 7% with a man 10 or more years older than they were.
Table I.
Sample characteristics
| Men | Women | |||
|---|---|---|---|---|
| Mean/median/% | n | Mean/median/% | n | |
| Demographics | ||||
| Mean age (years) | 28.2 (0.24) | 3530 | 29.5 (0.18) | 3946 |
| Median age (years) | 25 | 3530 | 27 | 3946 |
| Age groups: 16-to-24 | 40% | 3530 | 35% | 3946 |
| 25-to-34 | 35% | 3530 | 34% | 3946 |
| 35-to-44 | 17% | 3530 | 23% | 3946 |
| 45-to-49 | 7% | 3530 | 8% | 3946 |
| Mean highest grade of schooling completeda | 9.74 (0.11) | 2637 | 9.7 (0.08) | 3186 |
| 25-to-30 year olds | 10.44 (0.11) | 691 | 10.5 (0.09) | 811 |
| 45-to-49 year olds | 7.62 (0.42) | 243 | 7.15 (0.26) | 267 |
| Urban formal | 37% | 3528 | 36% | 3945 |
| Urban informal | 20% | 3528 | 20% | 3945 |
| Rural | 43% | 3528 | 44% | 3945 |
| Currently Married | 17% | 3530 | 22% | 3946 |
| Had sex in the past 12 months | 85% | 3010 | 80% | 3522 |
| Mean number sex partners in past 12 months | 1.4 (0.03) | 2546 | 1.04 (0.01) | 2816 |
| Median number of sex partners in past 12 months | 1 | 2546 | 1 | 2816 |
| More than 1 sex partner in past 12 months | 24% | 2546 | 4% | 2816 |
| Age-gaps | ||||
| Last partner 5 or more years younger (for men)/older (for women) | ||||
| 16-to-49 years old | 39% | 2533 | 43% | 2796 |
| 16-to-24 years old | 8% | 964 | 36% | 1002 |
| Last partner 10 or more years younger (for men)/older (for women) | ||||
| 16-to-49 years old | 11% | 2533 | 12% | 2796 |
| 16-to-24 years old | 0% | 964 | 7% | 1002 |
| Concurrency | ||||
| Point concurrency (16-to-49 years old) | 12% | 2024 | 0.7% | 2322 |
| Concurrency at time of interview (16-to-49 years old) | 14% | 2202 | 1% | 2365 |
| Cumulative concurrency (16-to-49 years old) | 21% | 2452 | 2.0% | 2690 |
Notes:
Standard error in parentheses.
Highest grade of education excludes individuals who were studying at the time of the interview. All respondents who reported some tertiary education (men, n=125; women, n=114) were coded as having completed grade 12. Concurrency measures: The point prevalence of concurrency indicates participants who reported partnerships with overlapping dates at the point exactly 6 months prior to the survey interview. Concurrency at the time of the interview identifies participants who expected to have sex again with two or more recent sexual partners. The cumulative prevalence of concurrency indicates participants who reported any partnerships with overlapping dates during the 12 months prior to the survey interview.
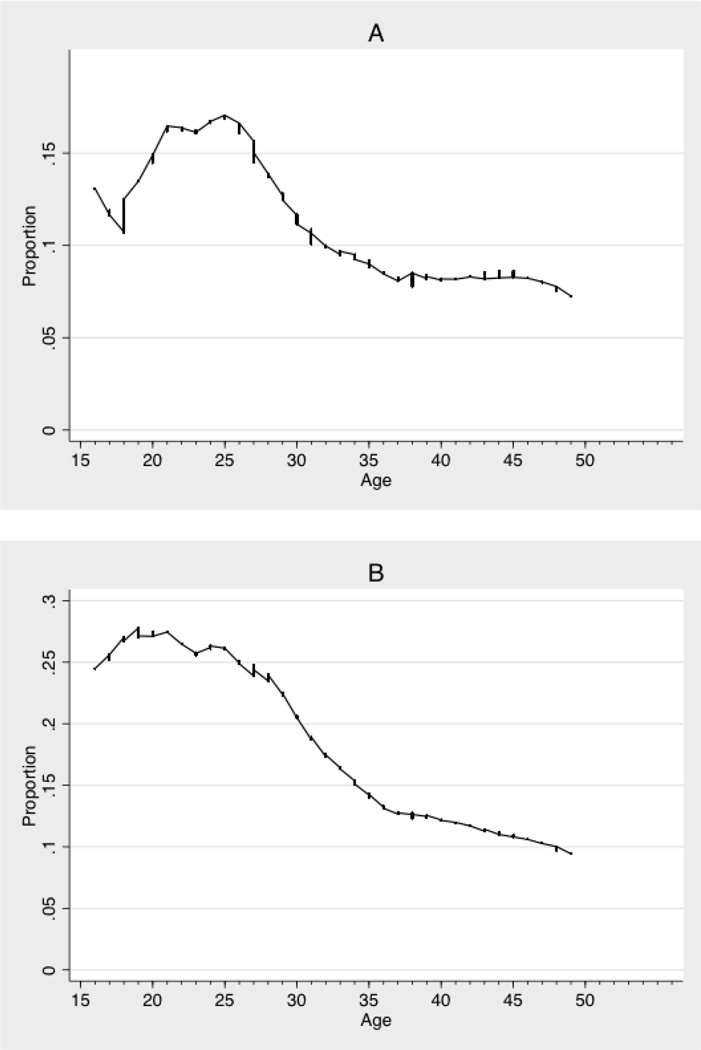
The point prevalence of concurrency among 16-to-49 year old men was 12% and 0.7% among women, and the cumulative prevalence of concurrency roughly double these figures. However, as shown in Figures 1A and 1B, there was significant variation in concurrency among men by age, with the prevalence of concurrency greater among younger men. Among 16-to-30 year old men, the point prevalence of concurrency was 6 percentage points greater (p<0.01) and the prevalence of cumulative concurrency 14 percentage points greater (p<0.01) compared to men 31-to-49 years old.
Figure 1.
Point and cumulative prevalence of concurrency among men by age. Figure 1A: Point prevalence of concurrency (n=2024). Figure 1B: Cumulative prevalence of concurrency (n=2452). Indicators of individual concurrency were calculated according to the protocols recommended by the panel of experts convened by UNAIDS [27]. The point prevalence of concurrency indicates participants who reported partnerships with overlapping dates at the point exactly 6 months prior to the survey interview. The cumulative prevalence of concurrency indicates participants who reported any partnerships with overlapping dates during the 12 months prior to the survey interview. Values smoothed using lowess, bandwidth 0.3.
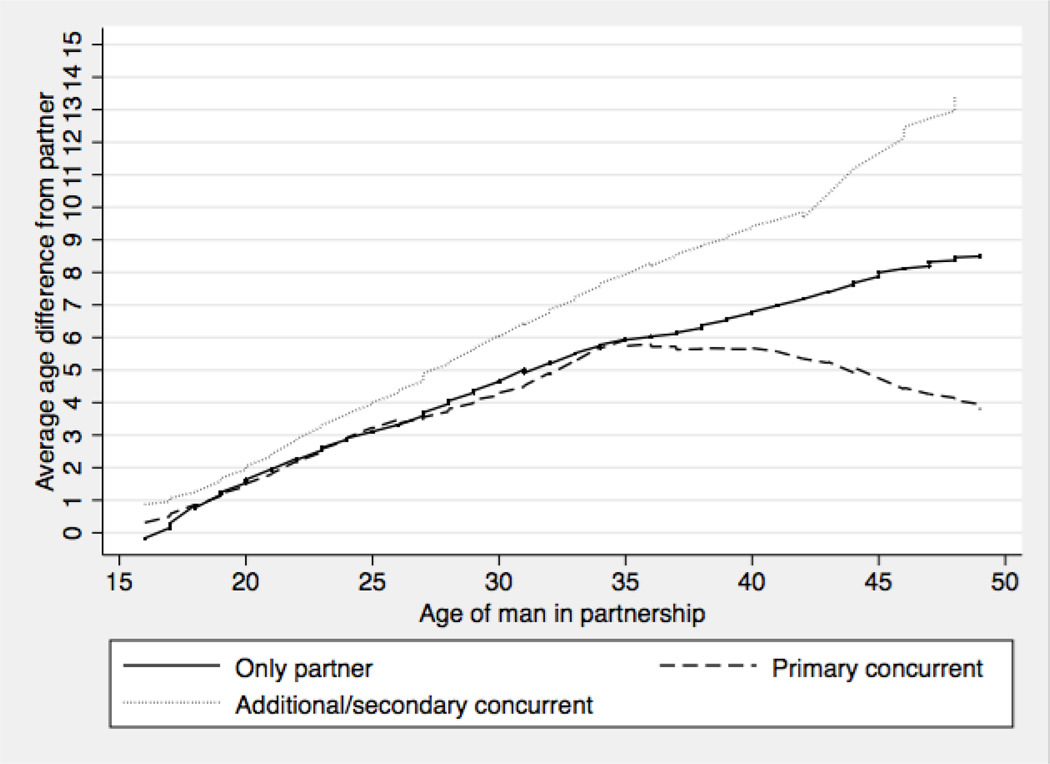
A total of 3217 partnerships were reported by men for which valid dates were given for initial and most recent sex. The data on age differences indicate that men were five or more years older in 40% of partnerships involving 15-to-49 year old women, and 36% involving young women (16-to-24). Sixty-five percent of all partnerships in the past year reported by 16–49 year old men involved only one female partner. The remaining partnerships were characterised by overlapping partnerships at some point, with 16% of these partnerships with a women categorised as the primary partner and 19% with the ‘additional’ partners in concurrent partnerships. Figure 2 shows the average age difference between men and their partners by partnership type and age. Results indicate that, on average, the age gap between men and their primary partners was similar for men up to the age of about 35, regardless of whether these partners are men’s only partners or one of their concurrent partners. In contrast, the partners men selected as their additional partners in concurrent partnerships were generally younger than their primary partners, with the difference increasing as men get older.
Figure 2.
Average age difference between men and their partners by partnership type and age (n=3217). Three types of partnerships was identified: (1) the female partner was the man’s only partner (i.e. the man did not report an overlapping partner in past 12 months); (2) the female partner was the man’s ‘primary’ concurrent partner; (3) the female partner was the man’s ‘additional’ partner. Women in partnerships described as “married”, “living together” or “main partner” were coded as the ‘primary’ partner. If the relationship with the other women was reported as “a friend”, “someone I’ve known for a while”, “someone I just met”, or “a one night encounter,” then the other women in these concurrent partnerships were defined as “additional partners.” In cases where the relationships of both (all) overlapping partners were given the same description, the women in the longer partnership was considered the ‘primary’ partner and the other women the ‘additional’ partner. Values smoothed using lowess, bandwidth 0.3.
Consequently, the proportion of age-disparate partnerships (five years or more age-gap) were roughly 11 percentage points higher (p<0.01) in partnerships in which the woman was the additional partner in the man’s concurrent partnership compared to partnerships involving the primary partner. Furthermore, there was more than twice the proportion of partnerships (41% vs 16%; p<0.01) with an age-gap of ten years or more among 31–49 year old men involving additional partners compared to primary partners. Focusing on concurrent partnerships reported by men, these patterns result in the man’s other partner being significantly older than the women in age-disparate partnerships compared to women in similar age partnerships. For example, for 16-to-24 year old women in partnerships involving male partner concurrency, the man’s other partner was, on average, 4.0 years older (p<0.01) if the age-gap between partners was five years or more compared to partnerships with an age-gap of less than five years (see Figure, Supplemental Digital Content File 2, for a graphical illustration).
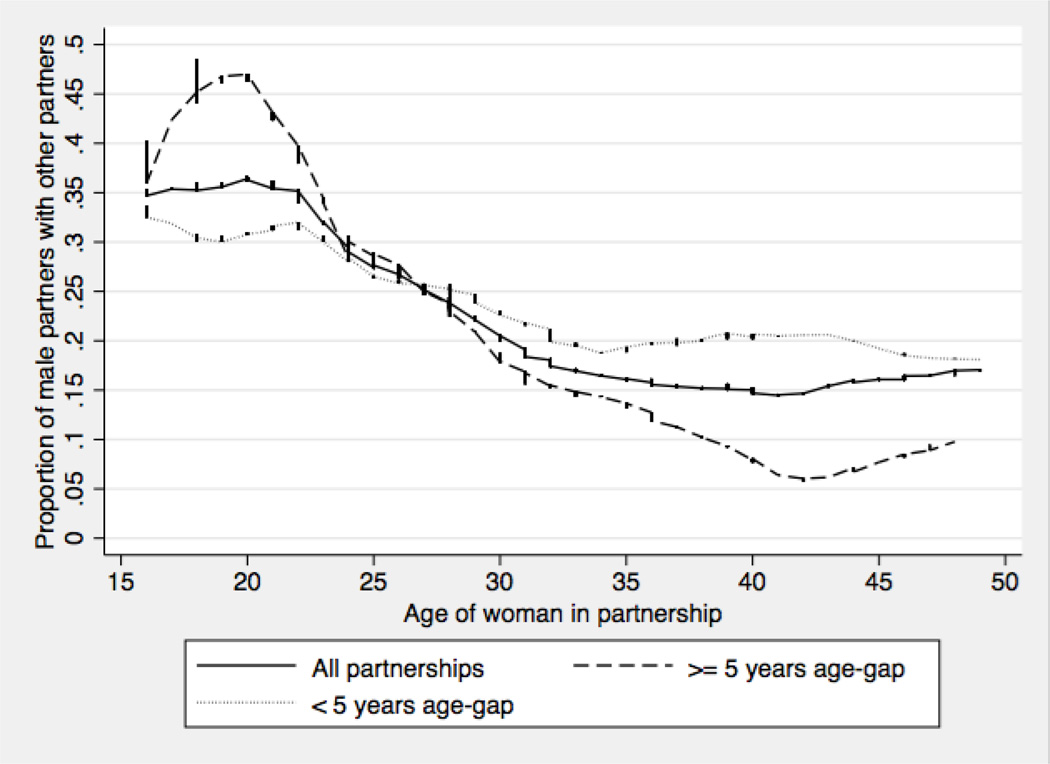
Figure 3 displays the proportion of women, by age, in partnerships reported by men during which the man had another sexual partner for longer than a month. The solid line (all partnerships) shows that the proportion of women whose partners have other partners varied widely by age. More than 30% of women younger than 25 were reported to have been in partnerships involving partner concurrency, with a steady decline after 24 years of age until roughly 15% of women older than 35 were reported to have been in such partnerships. Differences in the proportion of women reported to have been in partnerships involving male partner concurrency were observed by partnership age-gaps (dashed versus dotted lines), and this relationship varied by age. On average, 16-to-24 year old women were nine percentage points more likely to have a partner with other partners if their partner was five or more years older than them (p<0.01). This relationship held in the multivariate logistic regression that showed that women in partnerships reported by men had greater odds (1.88; p<0.01; 95% CI: 1.25–2.83) of having a male partner who had other partners if the partnership age-gap was five years or more (see Logistic Regression Model, Supplemental Digital Content 3, for details about this analysis). In contrast the difference by partner age-gap in the proportion of women 25 years and older in partnerships in which the male partner reported partner concurrency was small and not statistically significant (4%, p=0.204).
Figure 3.
Male partner concurrency (partnerships in the past 12 months in which the man reported having another partnership with overlapping start and end dates) for all women in partnerships reported by men (n=2963), women in age-disparate partnerships (n=1132) and women in partnerships with < 5-year age difference (n=1831). Partnerships of less than four weeks duration were excluded. Values smoothed using lowess, bandwidth 0.3.
Discussion
Understanding how age-disparate partnerships increase HIV risk for young women is critical for the development of interventions to reduce the role that these partnerships play in the spread of HIV [4–6]. Using nationally representative data from South Africa, we found that more than a third of 16-to-24 year old women reported partners five or more years their senior. These data are consistent with other national survey data on age-mixing among 15-to-24 year old South African women which found that 32.6% of partnerships had an age-gap of at least five years [36]. Our findings show that the odds of younger women (aged 16-to-24) being connected to a broader sexual network (i.e. being in partnerships with men who have other concurrent partners) were significantly greater for women with partners five years or more older than they were compared to women having sex with their peers. Furthermore, for young women whose partner had another partner, those in age-disparate partnerships were more likely to be connected to an older sexual network.
The implications of these results for HIV-infection risk among young women is illustrated using nationally representative South African data from around the time of our study data collection. HIV-prevalence was 5.1% and 21% among 20-to-24 year old men and women respectively, and 15.7% and 33% among 25-to-29 year old men and women respectively [2]. A 20-to-24 year old woman who has a partner five or more years older than herself, as opposed to her own age, is therefore roughly three times more likely to have sex with an HIV-positive man. Our data suggest that this woman’s risk of HIV-infection may be increased further by two compounding factors. First, she is more likely to be in a partnership that involves partner concurrency (i.e. the male partner has another partner). Secondly, her male partner is more likely to be having sex with another women in the 25-to-29 year age bracket in which HIV prevalence is 36% higher than that of women 20-to-24 years old.
We also found significant variation in concurrency by age with levels of concurrency peaking among younger men (more than a fifth of men younger than 30 reported a concurrent partnership in the past 12 months) and then declining steadily among men older than 30 until roughly 10% of men older than 45 reported having had concurrent sexual partnerships in the past 12 months. This pattern may explain why studies among 15-to-49 year old men in sub-Saharan Africa find lower levels of concurrency (less than 11% point concurrency in many countries) [37], than studies among younger sub-populations in the region [16–21,38]. In addition, we found that when men acquire additional concurrent sexual partners they tend to select secondary partners who are much younger than the women who are their primary partners. As a result of these factors, significantly greater proportions of young women appeared to be connected to a broader sexual network than older women, with 35% of 16-to-24 year old women in all partnerships reported by men involving male partner concurrency of four weeks or longer during the past 12 months.
These patterns of concurrency have implications for how we think about the relationship between concurrent sexual partnerships and HIV-infection risk. The UNAIDS recommended protocol for measuring concurrent sexual partnerships stipulates that the prevalence of concurrency be measured among 15–49 year olds [27]. Consequently, a large proportion of concurrency research, and hence of the implications drawn, has been focused on concurrency within general adult population samples. For example, Sawers’s (2013) reviewed the prevalence of concurrency in 15-to-49 year olds in Demographic and Health Surveys samples from sub-Saharan Africa [37]. He found the prevalence was generally low and concluded that concurrency cannot play a significant role in the HIV epidemic and, therefore, that HIV-prevention efforts should focus attention elsewhere. Our results indicate that the point prevalence of male concurrency is greater in those under 30 years old. This combined with the patterns of age-mixing may generate densely interconnected sexual networks in subpopulations within the total 15 to 49 year old population. These findings point to the need to refine our thinking away from concurrency’s impact on HIV transmission in the general population and towards the risk that different patterns of concurrency may pose to different populations and the mechanisms through which this may occur. In particular further work is needed to assess the role that concurrency plays in HIV infection risk among young women, and in particular young women with older partners.
There are several limitations to our study. As with all data on sexual behaviour collected via face-to-face survey interviews, social desirability bias may have influenced measures of concurrency [39]. Measures of concurrency derived from partnership history tables and dates of first and last sex may also be influenced by the censoring of information on partners [40,41], and recall bias [42]. Importantly, we had to use data on partnerships reported by men to assess male partner concurrency in female partnerships. We do not know how accurately these partnerships represent partnerships involving women in South Africa. In addition, non-response in the NCS was high and we did not have data to conduct an analysis of non-response. We therefore do not know whether non-response was random and we cannot be sure that results are representative of the South African population.
A randomised experiment conducted in Kenya found that information given to teenage school girls about the relative risk of HIV infection by partner’s age resulted in a decrease in teen pregnancy and a substitution away from older partners towards same-age partners [43]. Results from our study point towards an additional mechanism for changes in relative risk by partner-age. Further research is required to determine whether patterns of concurrency in other populations support our findings and ultimately whether the relationship between partner concurrency and age-gaps does increase HIV-infection risk for young women. This information could prove useful for message framing in future prevention efforts that employ messaging about relative risk by partner’s age.
Supplementary Material
Footnotes
List of Supplemental Digital Content
Supplemental Digital Content 1.doc: Table: Partner History Table from the Second National Communication Survey, 2009
Supplemental digital content 2.doc: Figure, Average age of partner’s other partner by age of women in all concurrent partnerships reported by men
Supplemental digital content 3.doc: Logistic Regression Model
References
- 1.UNAIDS. UNAIDS global report 2012. Geneva: UNAIDS; 2012. [Google Scholar]
- 2.Shisana O, Rehle T, Simbayi L, et al. South African National Prevalence, Incidence, Behaviour and Communication Survey, 2008: A Turning Tide Among Teenagers? Cape Town: Human Sciences Research Council Press; 2009. [Google Scholar]
- 3.Nattrass N, Maughan-Brown B, Seekings J, Whiteside A. Poverty, sexual behaviour, gender and HIV infection among young black men and women in Cape Town, South Africa. Afr J AIDS Res. 2012;11:307–317. doi: 10.2989/16085906.2012.754830. [DOI] [PubMed] [Google Scholar]
- 4.Katz I, Low-Beer D. Why Has HIV Stabilized in South Africa, Yet Not Declined Further? Age and Sexual Behavior Patterns Among Youth. Sex Transm Dis. 2008;35:837–842. doi: 10.1097/OLQ.0b013e31817c0be5. [DOI] [PubMed] [Google Scholar]
- 5.Kelly RJ, Gray RH, Sewankambo NK, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr. 2003;32:446–451. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Gregson S, Nyamukapa C, Garnett G, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet. 2002;359:1896–1903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 7.Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual activity classes. Philos Trans R Soc Lond B Biol Sci. 1993;342:137–159. doi: 10.1098/rstb.1993.0143. [DOI] [PubMed] [Google Scholar]
- 8.Bärnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell M-L. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008;22:139–144. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 9.Bankole A, Ahmed FH, Neema S, Ouedraogo C, Konyani S. Knowledge of correct condom use and consistency of use among adolescents in four countries in sub-Saharan Africa. Afr. J. Reprod. Health. 2007;11:197–220. [PMC free article] [PubMed] [Google Scholar]
- 10.Longfield K, Glick A, Waithaka M, Berman J. Relationships between older men and younger women: implications for STIs/HIV in Kenya. Stud Family Plann. 2004;35:125–134. doi: 10.1111/j.1728-4465.2004.00014.x. [DOI] [PubMed] [Google Scholar]
- 11.Glynn J, Carael M, Auvert B, et al. Why do young women have a much higher prevalence of HIV then young men? A study in Kisumu, Kenya and Ndola, Zambia. AIDS. 2001;15(supple 4):S51–S60. doi: 10.1097/00002030-200108004-00006. [DOI] [PubMed] [Google Scholar]
- 12.Luke N. Confronting the “sugar daddy” stereotype: age and economic asymmetries and risky sexual behavior in urban Kenya. Int Fam Plan Perspect. 2005;31(1):6–14. doi: 10.1363/3100605. [DOI] [PubMed] [Google Scholar]
- 13.Langeni T. Contextual factors associated with treatment-seeking and higher-risk sexual behaviour in Botswana among men with symptoms of sexually transmitted infections. Afr J AIDS Res. 2007;6:261–269. doi: 10.2989/16085900709490422. [DOI] [PubMed] [Google Scholar]
- 14.Ott MQ, Bärnighausen T, Tanser F, Lurie MN, Newell M-L. Age-gaps in sexual partnerships: seeing beyond “sugar daddies”. AIDS. 2011;25:861–863. doi: 10.1097/QAD.0b013e32834344c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beauclair R, Kassanjee R, Temmerman M, Welte A, Delva W. Age-disparate relationships and implications for STI transmission among young adults in Cape Town, South Africa. Eur J Contracept Reprod Health Care. 2012;17:30–39. doi: 10.3109/13625187.2011.644841. [DOI] [PubMed] [Google Scholar]
- 16.Carter MW, Kraft JM, Koppenhaver T, et al. “A Bull Cannot be Contained in a Single Kraal”: Concurrent Sexual Partnerships in Botswana. AIDS Behav. 2007;11:822–830. doi: 10.1007/s10461-006-9203-6. [DOI] [PubMed] [Google Scholar]
- 17.Harrison A, Cleland J, Frohlich J. Young People's Sexual Partnerships in KwaZulu-Natal, South Africa: Patterns, Contextual Influences, and HIV Risk. Stud Family Plann. 2008;39:295–308. doi: 10.1111/j.1728-4465.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffenson AE, Pettifor AE, Seage GR, Rees HV, Cleary PD. Concurrent sexual partnerships and human immunodeficiency virus risk among South African youth. Sex Transm Dis. 2011;38:459–466. doi: 10.1097/OLQ.0b013e3182080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maughan-Brown B. Concurrent sexual partnerships among young adults in Cape Town, South Africa: how is concurrency changing? Sex Health. 2013;10:246–252. doi: 10.1071/SH12148. [DOI] [PubMed] [Google Scholar]
- 20.Westercamp N, Mattson CL, Bailey RC. Measuring Prevalence and Correlates of Concurrent Sexual Partnerships Among Young Sexually Active Men in Kisumu, Kenya. AIDS Behav. 2013;17:3124–32. doi: 10.1007/s10461-013-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanis TJ, Doherty IA, Weir SS, et al. From Coitus to Concurrency: Sexual Partnership Characteristics and Risk Behaviors of 15–19 Year Old Men Recruited from Urban Venues in Tanzania. AIDS Behav. 2013;17:2405–2415. doi: 10.1007/s10461-012-0312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon C, Dlamini S, Boulle A, White R, Badri M. A network-level explanation for the differences in HIV prevalence in South Africa's racial groups. Afr J AIDS Res. 2010;8:243–254. doi: 10.2989/AJAR.2009.8.3.1.922. [DOI] [PubMed] [Google Scholar]
- 23.Auvert B, Buvé A, Ferry B, et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS. 2001;15(Suppl 4):S15–S30. doi: 10.1097/00002030-200108004-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kenyon C, Boulle A, Badri M, Asselman V. “I don‘t use a condom (with my regular partner) because I know that I’m faithful, but with everyone else I do”: The cultural and socioeconomic determinants of sexual partner concurrency in young South Africans. SAHARA J. 2010;7:35–43. doi: 10.1080/17290376.2010.9724967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandøy IF, Dzekedzeke K, Fylkesnes K. Prevalence and Correlates of Concurrent Sexual Partnerships in Zambia. AIDS Behav. 2010;14:59–71. doi: 10.1007/s10461-008-9472-3. [DOI] [PubMed] [Google Scholar]
- 26.Johnson S, Kincaid L, Laurence S, Chikwava F, Delate R, Mahlasela L. The Second National HIV Communication Survey, 2009. Pretoria: JHHESA; 2010. Available at: http://www.uj.ac.za/EN/CorporateServices/ioha/surveys/Documents/ncs_report.pdf. [Google Scholar]
- 27.Consultation on Concurrent Sexual Partnerships. UNAIDS. Nairobi, Kenya: UNAIDS; 2009. UNAIDS Reference Group on Estimates, Modelling and Projections. [Google Scholar]
- 28.Actuarial Society of South Africa. ASSA 2008 HIV/AIDS projection model. 2011 Available at: http://aids.actuarialsociety.org.za/ASSA2008-Model-3480.htm. [Google Scholar]
- 29.Kenyon C. Association of HIV prevalence and concurrency of sexual partnerships in South Africa’s language groups: An ecological analysis. S Afr J HIV Med. 2013;14:25–28. [Google Scholar]
- 30.Drumright LN, Gorbach PM, Holmes KK. Do people really know their sex partners? Concurrency, knowledge of partner behavior, and sexually transmitted infections within partnerships. Sex Transm Dis. 2004;31:437–442. doi: 10.1097/01.olq.0000129949.30114.37. [DOI] [PubMed] [Google Scholar]
- 31.Helleringer S, Kohler H-P. Role of concurrency in generalised HIV epidemics. Lancet. 2011;378:1844–1845. doi: 10.1016/S0140-6736(11)61805-9. [DOI] [PubMed] [Google Scholar]
- 32.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 33.Manning WD, Flanigan CM, Giordano PC, Longmore MA. Relationship dynamics and consistency of condom use among adolescents. Perspect Sex Reprod Health. 2009;41:181–190. doi: 10.1363/4118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branson N, Lam D. Education inequality in South Africa: Evidence from the National Income Dynamics Study. Studies in Economics and Econometrics. 2010;34:85–109. [Google Scholar]
- 35.Posel D, Casale D. The relationship between sex ratios and marriage rates in South Africa. Applied Economics. 2013;45:663–676. [Google Scholar]
- 36.Pettifor A, Rees H, Kleinschmidt I, et al. Young people's sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19:1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 37.Sawers L. Measuring and modelling concurrency. J. Int. AIDS Soc. 2013;16(1) doi: 10.7448/IAS.16.1.17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mah T. Prevalence and correlates of concurrent sexual partnerships among young people in South Africa. Sex Transm Dis. 2010;37:105–108. doi: 10.1097/OLQ.0b013e3181bcdf75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauclair R, Meng F, Health DO, et al. Evaluating audio computer assisted self-interviews in urban south African communities: evidence for good suitability and reduced social desirability bias of a cross-sectional survey on sexual behaviour. BMC Medical Research Methodology. 2013;13(11) doi: 10.1186/1471-2288-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maughan-Brown B, Venkataramani A. Measuring concurrent partnerships: potential for underestimation in UNAIDS recommended method. AIDS. 2011;25:1549–1551. doi: 10.1097/QAD.0b013e32834905c4. [DOI] [PubMed] [Google Scholar]
- 41.Helleringer S, Kohler H-P, Kalilani-Phiri L, Mkandawire J, Armbruster B. The reliability of sexual partnership histories: implications for the measurement of partnership concurrency during surveys. AIDS. 2011;25:503–511. doi: 10.1097/QAD.0b013e3283434485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warnecke RB, Sudman S, Johnson TP, O'Rourke D, Davis AM, Jobe JB. Cognitive aspects of recalling and reporting health-related events: Papanicolaou smears, clinical breast examinations, and mammograms. Am J Epidemiol. 1997;146:982–992. doi: 10.1093/oxfordjournals.aje.a009226. [DOI] [PubMed] [Google Scholar]
- 43.Dupas P. Do teenagers respond to HIV risk information? Evidence from a field experiment in Kenya. Am. Econ. J. Appl. Econ. 2009;3:1–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.