Abstract
Ribosome stalling at polyproline stretches is common and fundamental. In bacteria, translation elongation factor P (EF-P) rescues such stalled ribosomes, but only when it is post-translationally activated. In Escherichia coli, activation of EF-P is achieved by (R)-β-lysinylation and hydroxylation of a conserved lysine. Here we have unveiled a markedly different modification strategy in which a conserved arginine of EF-P is rhamnosylated by a glycosyltransferase (EarP) using dTDP-l-rhamnose as a substrate. This is to our knowledge the first report of N-linked protein glycosylation on arginine in bacteria and the first example in which a glycosylated side chain of a translation elongation factor is essential for function. Arginine-rhamnosylation of EF-P also occurs in clinically relevant bacteria such as Pseudomonas aeruginosa. We demonstrate that the modification is needed to develop pathogenicity, making EarP and dTDP-l-rhamnose-biosynthesizing enzymes ideal targets for antibiotic development.
Ribosomes translate an mRNA sequence into a polypeptide chain. During this process, specific X-PP-X tripeptide sequence motifs can induce ribosome stalling1–6. Eukaryotic/archaeal initiation factor 5A (e/aIF5A) and its bacterial ortholog, EF-P, alleviate the stalled ribosomes by binding and stimulating peptide bond formation3,4,7–10. With its three β-barrel domains, the L-shaped EF-P is structurally reminiscent of transfer RNA (tRNA)11 and binds to the ribosome between the sites of peptidyl-tRNA binding (P-site) and tRNA exiting (E-site)12. A positively charged residue at the tip of the loop region in domain I of EF-P protrudes toward the peptidyl-transferase center and can reach into it when elongated by modification12. Accordingly, the conserved lysine in e/aIF5A is extended to hypusine by deoxyhypusine synthase and deoxyhypusine hydroxylase13–15. Analogously, in bacteria such as E. coli the protruding lysine (K34) of EF-P is (R)-β-lysinylated and hydroxylated by the concerted action of EF-P lysyl-transferase (EpmA), lysine amino-mutase (EpmB) and EF-P hydroxylase (EpmC; Fig. 1a)16–20. Here we report the identification of an EF-P subfamily activated by a chemically different modification. Using Shewanella oneidensis as a model organism, we found rhamnosylation of a conserved arginine. We also identified the corresponding glycosyltransferase, EarP. This modification is not only crucial for bacterial fitness but also for pathogenicity in P. aeruginosa, and thus it might be equally important in other clinically relevant species such as Neisseria gonorrhoea or Bordetella pertussis.
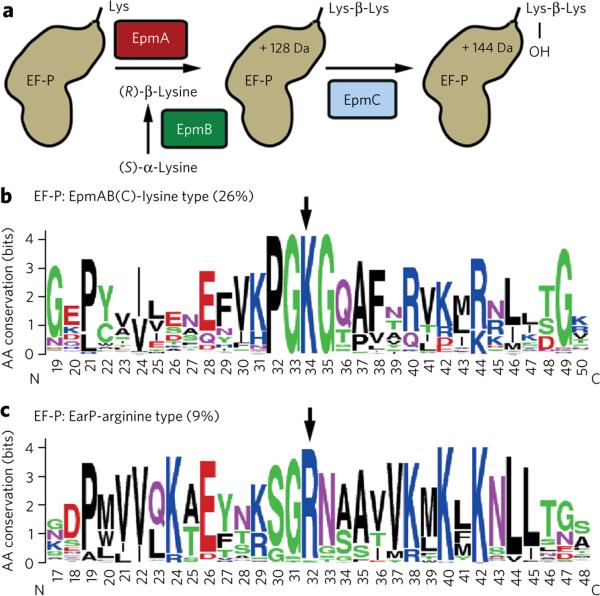
Figure 1. Bioinformatic identification of the EarP-arginine type EF-P subfamily.
(a) (R)-β-lysinylation and hydroxylation of EF-P in E. coli. (b,c) A 31-amino-acid (aa)-long sequence logo of EF-P encompassing a part of domain I, including the loop region. Arrows point to the positively charged residue at the tip of the loop region. Numbering depicts aa in the polypeptide chain. (b) Weblogo generated from EF-P of bacteria encoding EpmA and EpmB. (c) Weblogo generated from EF-P of bacteria derived from the newly identified branch that co-occurs with EarP.
RESULTS
Identification of the EarP-arginine type EF-P subfamily
Whereas the hypusinylation pathway is highly conserved in archaea and eukaryotes, EpmA (also known as YjeA, PoxA, GenX) and EpmB (YjeK) are only found in about 26% of all bacteria20 (Supplementary Results, Supplementary Figs. 1–5 and Supplementary Data Set 1). The third modification enzyme, EpmC (YfcM), co-occurs with EpmA and EpmB but is restricted almost exclusively to γ-proteobacterial genomes (Supplementary Figs. 4,5 and Supplementary Data Set 1) corroborating its minor role in EF-P function3,6–8,21. We hypothesized that the genes encoding EF-P and the associated modification system have coevolved. In a phylogenetic analysis of EF-P sequences, we identified a distinct subfamily, encoded in genomes lacking EpmABC orthologs, that has a strictly conserved arginine (R32) in the position equivalent to K34 in E. coli (Fig. 1b,c, Supplementary Fig. 4 and Supplementary Data Set 1). The members of this subfamily represent about 9% of all EF-Ps, but the distribution deviates from the currently accepted species phylogeny. As the newly identified EF-P branch encompasses all β-proteobacteria, we hypothesize this subdivision as the phylogenetic origin, with subsequent horizontal transfer into several γ-proteobacterial orders (including Pseudomonadales, Aeromonadales and Alteromonadales) as well as some Fusobacteria, Planctomycetes and Spirochetes (Supplementary Data Set 1). We took advantage of the anomalous EF-P phylogeny by searching for putative EF-P modification enzymes associated with this subfamily via gene neighborhood and co-occurrence using STRING22. This led us to identify a protein with a conserved domain of unknown function (DUF 2331), which we designated as EarP. Its distribution strictly coincides with the newly identified EF-P subfamily (Supplementary Fig. 5). Moreover, the corresponding gene, earP, always lies within a four-gene distance to efp; in 94% of cases, both genes are directly adjacent (Supplementary Fig. 6a).
EF-P and EarP are functionally linked
To investigate whether EF-P and EarP are functionally linked, we used the ubiquitous, facultative anaerobic, alteromonadal γ-proteobacterium S. oneidensis. Bacteria of the genus Shewanella are commonly used in microbial fuel cells and have high potential in bioremediation because of their ability to use a wide range of terminal electron acceptors, including heavy metals23,24.
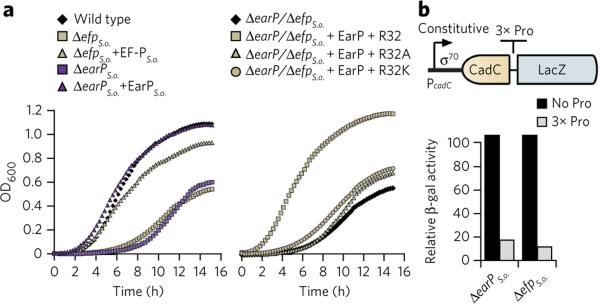
In a first step, we generated markerless in-frame deletions of S. oneidensis efp (locus tag SO_2328) and earP (locus tag SO_2329) and phenotypically characterized the resulting mutant strains, ΔefpS.o. and ΔearPS.o., respectively. Bacteria lacking efp, such as E. coli or Agrobacterium tumefaciens, have diminished growth rates16,25. In line with these results, deleting either efpS.o. or earPS.o. increased the doubling time from 40 min to 110 min and 70 min, respectively, which was reversed by providing the corresponding gene copy in trans (Fig. 2a and Supplementary Fig. 6a). In parallel, we analyzed the growth of the ΔefpS.o. strain, which encodes an EF-P variant where the strictly conserved R32 (Fig. 1c) was substituted by either lysine (R32K) or alanine (R32A). Both strains phenocopied ΔefpS.o., demonstrating the importance of this conserved arginine for EF-PS.o. function (Fig. 2a and Supplementary Fig. 6a). We also investigated whether the synthesis of polyproline-containing proteins is affected in the absence of efpS.o. and earPS.o.. Therefore, we used the reporter plasmid p3LC-TL30-3P, which encodes a LacZ variant that is preceded by a stretch of three proline residues (Fig. 2b)3,7. As expected, the β-galactosidase activities of the ΔefpS.o. or ΔearPS.o. strains were both reduced by about tenfold, providing clear experimental evidence that EarP is required for EF-P activity.
Figure 2. Phenotypic analysis of S. oneidensis MR-1 earP and efp deletion mutants.
(a) Growth of S. oneidensis MR-1 strains. Left, wild-type strains in comparison to ΔefpS.o. and ΔearPS.o. deletion strains and after complementation in trans (+efpS.o., +earPS.o.). Right, the ΔefpS.o. deletion strain and after complementation with plasmids encoding His6 versions of EF-PS.o. (+R32) or the corresponding substitution variants EF-PS.o.R32A (+R32A) and EF-PS.o.R32K (+R32K), respectively. The presented growth curves are average data from three independent data sets, with statistical error below 10%. (b) β-galactosidase (β-gal) activity assay of S. oneidensis MR-1 ΔefpS.o., ΔearPS.o. encoding a constitutively produced LacZ-hybrid without (black bars) or with (gray bars) a polyproline motif (3× Pro). β-galactosidase activity is given in percent and is normalized to the wild-type values. The relative activities are average data from three independent data sets, with statistical error below 10%.
EarP is sufficient to activate EF-P
To test whether EarPS.o. is sufficient for activation of EF-PS.o., we examined the phenotypes of an E. coli efp deletion (ΔefpE.c.) heterologously producing EF-PS.o. and EarPS.o.. As a readout, we used a chromosomal PcadBA-dependent lacZ reporter in which activation of PcadBA strictly depends on the pH-responsive transcriptional activator CadC26. Translation of CadC is impaired in cells lacking active EF-P because of the presence of a polyproline motif (Supplementary Fig. 6b)7. As expected, the E. coli ΔefpE.c. PcadBA::lacZ strain was characterized by low-level β-galactosidase activity, and wild-type–like lacZ expression was restored in the presence of a plasmid expressing EF-PE.c.. Whereas β-galactosidase activity remained low when EF-PS.o. or EarPS.o. were expressed alone, simultaneous production of both proteins complemented for the lack of EF-PE.c.. The diminished growth rate of the ΔefpE.C. mutant18 was consistently eliminated when EF-PS.o. and EarPS.o. were produced together, regardless of the presence of the E. coli EF-P modification enzymes EpmA or EpmB (Supplementary Fig. 6c). Next, we asked whether EpmABC and EarP had adapted to specifically activate their corresponding EF-Ps. Therefore EF-PE.c. K34 and EF-PS.o. R32 substitution variants were produced in ΔefpE.c., and PcadBA activation, growth rate or both were investigated in the resultant strains (Supplementary Fig. 6b,c). Neither EF-PS.oR32K with EpmABC nor the EF-PE.c.K34R with EarP could reverse the mutant phenotype, thus further corroborating our hypothesis for the coevolution of EF-P with its associated modification system.
EarP modifies EF-P at Arg32
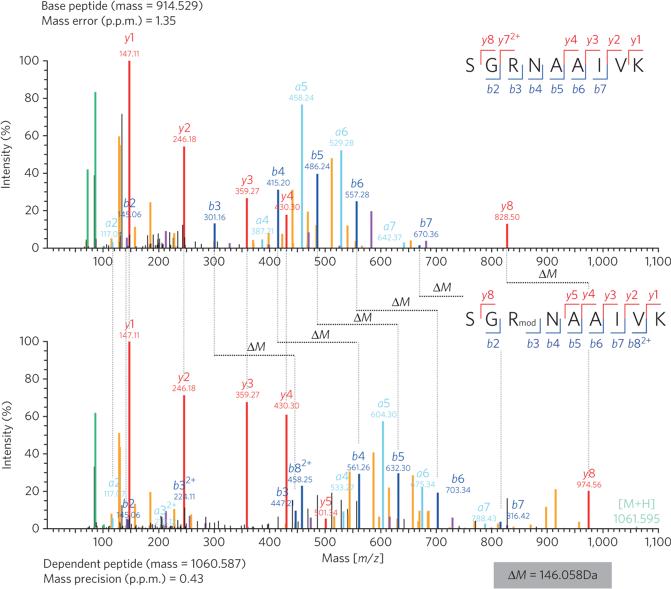
Having demonstrated that EarPS.o. is necessary and sufficient for specific activation of EF-PS.o., we addressed whether EarPS.o. post-translationally modifies the conserved R32 of EF-PS.o.. To that end, we overproduced His6-tagged EF-PS.o. in two S. oneidensis strains, ΔefpS.o. and ΔefpS.o./ΔearPS.o.. Purified EF-PS.o. from these two strains was proteolytically digested, and the resulting peptides were analyzed by high-resolution LC/MS/MS using an unbiased ‘dependent peptide’ search27. We detected eight high-confidence R32-containing peptides that were 146.058 Da heavier than their unmodified counterparts (Fig. 3 and Supplementary Fig. 7). This mass shift only occurred on R32-containing peptides of EF-PS.o. and never when EF-PS.o. was produced in cells lacking EarP (Supplementary Fig. 8). The fragmentation pattern of the modified peptides strongly suggested the modification site to be on R32 (Fig. 3). To further confirm this, we performed a standard variable modification search with a potential arginine mass shift of 146.058 Da, corresponding to a molecular composition of C6H10O4 (146.0579 Da). This second analysis identified the modification to exist exclusively on R32 of EF-P, confirming both the molecular composition and the location of the modification (Supplementary Fig. 9). Notably, no peptides with this modification could be found when two arginine substitution EF-P variants were analyzed (His6-EF-PS.o.R32K and His6-EF-PS.o.R32A; Supplementary Fig. 10). This observation explains the efp-null mutant phenotype of strains encoding His6-EF-PS.o.R32K or His6-EF-PS.o.R32A substitution variants (Fig. 2a and Supplementary Fig. 6) as a consequence of the absence of EF-PS.o. R32 modification by EarPS.o..
Figure 3. Dependent peptide MS analysis of the S. oneidensis MR-1 EF-P modification.
C-terminally His6-tagged EF-PS.o. was analyzed upon homologous overproduction. Modified R32-containing ‘dependent’ peptides (146.058 Da heavier) were identified by a characteristic ΔM mass shift of the precursor and several fragment ions when compared to the unmodified ‘base peptide’. MS/MS spectra of the best-scoring base peptide-dependent peptide pair are shown. Peak colors: red, blue and light blue refer to y, b and a ions (according to Roepstorff-Fohlmann-Biemann nomenclature), respectively; light green, molecular ion; yellow, neutral losses; purple, internal fragments; green, immonium ions, black peaks, unassigned.
EarP is a rhamnosyltransferase
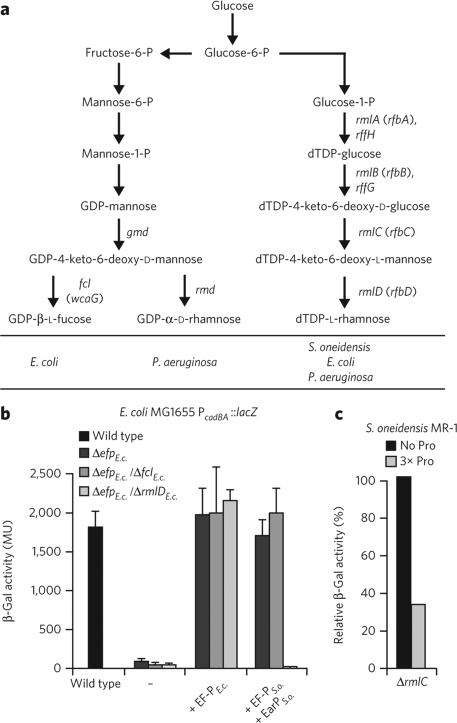
We reasoned that a modification on R32 with a molecular composition of C6H10O4 might be the result of N-glycosylation with an activated deoxyhexose sugar (C6H12O5 – H2O = C6H10O4). E. coli synthesizes two nucleotide diphosphate deoxyhexoses: GDP-l-fucose and dTDP-l-rhamnose (Fig. 4a)28. The biosynthesis genes encoding the latter (represented by rmlD) are strictly conserved in bacteria encoding EarP but are less frequently found in bacteria with EpmABC (Supplementary Fig. 5). To test whether dTDP-l-rhamnose or GDP-l-fucose might act as a substrate for EarPS.o., we interrupted the corresponding synthesis pathways. GDP-l-fucose and dTDP-l-rhamnose are synthesized from fructose-6-phosphate and glucose-l-phosphate, respectively, with each biosynthetic pathway encompassing four specific enzymes (Fig. 4a). To prevent formation of GDP-l-fucose, we deleted fcl, which arrested synthesis at the GDP-4-keto-6-deoxy-d-mannose step, whereas dTDP-l-rhamnose formation was blocked at the intermediate dTDP-4-keto-6-deoxy-l-mannose step by deletion of rmlD (Fig. 4a). The deletions were generated in an E. coli PcadBA::lacZ strain lacking efp. The β-galactosidase activities of both resultant strains deficient either in GDP-l-fucose (ΔefpE.c./ΔfclE.c.) or in dTDP-l-rhamnose (ΔefpE.c./ΔrmlDE.c.) were comparable to wild-type activity when a copy of efpE.c. was provided in trans (Fig. 4b). Similarly, the ΔefpE.c./ΔfclE.c. strain could be complemented with EF-PS.o./EarPS.o., indicating that GDP-l-fucose is not a substrate for EarP. In stark contrast, the coproduction of EF-PS.o./EarPS.o. in the context of ΔefpE.c./ΔrmlDE.c. cells phenocopied the ΔefpE.c. strain, suggesting that dTDP-l-rhamnose is the substrate needed for EarPS.o. to activate EF-PS.o. To further corroborate this result, we deleted rmlC (locus tag SO_3160) in S. oneidensis and analyzed translation of a polyproline-containing LacZ reporter hybrid in the resultant dTDP-l-rhamnose–deficient strain ΔrmlCS.o. (Fig. 4c)3,7. As observed for ΔefpS.o. or ΔearPS.o., β-galactosidase activity was low in the ΔrmlCS.o. strain harboring the p3LC-TL30-3P reporter. Consistent with our previous results, in vivo activation of EF-PS.o. strictly depends on the biosynthesis of dTDP-l-rhamnose, leading us to conclude that dTDP-l-rhamnose acts as the substrate for EF-PS.o. modification and that it was recruited for this role upon the development of the EarP–EF-P phylogenetic relationship (Supplementary Figs. 4,5 and Supplementary Data Set 1).
Figure 4. In vivo analysis of S. oneidensis MR-1 EF-P functionality depending on NDP-deoxyhexose biosyntheses.
(a) Biosynthesis pathways for dTDP-l-rhamnose, GDP-d-rhamnose and GDP-l-fucose. Arrows depict sugar conversion steps. Specific conversion steps are associated with the corresponding biosynthesis gene. Paralogous genes are separated by a comma, and alternative names are given in parentheses. (b) β-galactosidase (β-gal) activity of E. coli PcadBA-lacZ reporter wild-type (WT) and efp deletion strain (ΔefpE.c.) as well as the ΔefpE.c./ΔfclE.c. (GDP-l-fucose deficient) and ΔefpE.c./ΔrmlDE.c. (dTDP-l-rhamnose deficient) double deletion mutants and after complementation either with EF-PE.c. or EF-PS.o. in combination with EarPS.o.. Cells were incubated under cadBA-inducing conditions. Data represent mean values from three independent replicates ± s.d. (c) β-galactosidase activity assay of S. oneidensis MR-1 ΔrmlCS.o. encoding a constitutively produced LacZ hybrid without (black bars) or with (gray bars) a polyproline motif (3× Pro). β-galactosidase activity is given in percent and is normalized to the wild-type values. The relative activities are average data from three independent data sets, with statistical error below 10%.
To directly demonstrate that EarPS.o. can glycosylate EF-PS.o. using dTDP-l-rhamnose as a substrate, we performed in vitro glycosylation reactions with purified components. LC/MS/MS analysis, performed as described above, revealed the presence of R32 rhamnosylation of wild-type EF-PS.o. if and only if all three components were provided (Supplementary Fig. 11). Collectively, our data demonstrate unambiguously both in vivo and in vitro that EarP is an EF-P arginine rhamnosyltransferase essential for post-translational activation.
EF-P stimulates peptide bond formation indirectly
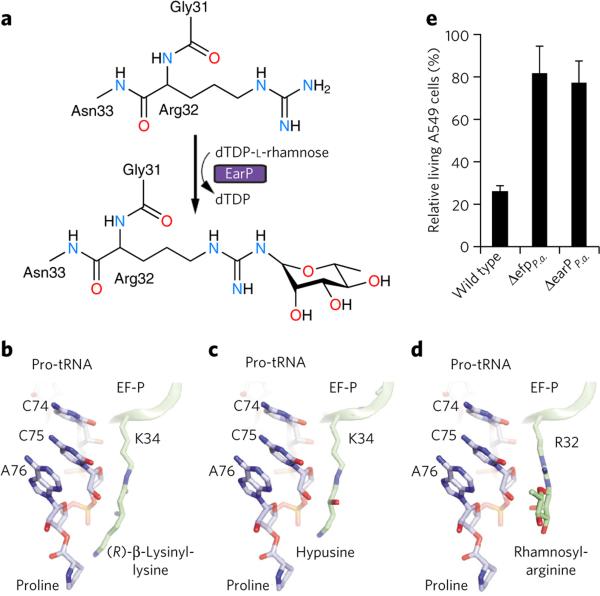
Rhamnosyl-arginine differs substantially from (R)-β-lysinylhydroxylysine and hypusine of EF-PE.c. and a/eIF5A, respectively, raising the question of how this unusual extension protrudes into the peptidyl-transferase center of the ribosome (Fig. 5a–d). To investigate this, we generated molecular models for the different modifications of EF-P orthologs based on the crystal structure of unmodified Thermus thermophilus EF-P bound to the 70S ribosome12. These models suggest that the (R)-β-lysinylation found on EF-PE.c. could reach within 2 Å of the proline attached to the P-site tRNA (Fig. 5b), whereas the hypusine and rhamnose-arginine modifications are shorter and cannot reach the P-site proline (Fig. 5c,d). Therefore, EF-P bearing either hypusine or rhamnosearginine modifications is not likely to stimulate peptide-bond formation by directly influencing the conformation of the polypeptide chain but rather does so indirectly by interacting with and stabilizing the CCA-end of the P-site peptidyl-Pro-tRNA.
Figure 5. EF-P rhamnosylation, mode of action and impact on pathogenicity.
(a) Arginine-rhamnosylation by EarP using dTDP-l-rhamnose as substrate. (b–d) Models of different modified EF-P proteins bound to the ribosome. The CCA-end of P-site bound Pro-tRNA (blue) is shown for reference. Models based on T. thermophilus EF-P-70S structure12. (b) K34 of E. coli EF-P post-translationally modified with (R)-β-lysine. (c) EF-P bearing the K34 hypusine modification. (d) R32 of EF-P modified by l-rhamnose. (e) Effects of ΔefpP.a. and ΔearPP.a. on P. aeruginosa pathogenicity. Cytotoxicity of P. aeruginosa strains was assessed by infecting A549-Gluc cells, which secrete Gaussia luciferase, as a measure of cell integrity. Data represent mean values from three independent replicates ± s.d.
EarP and EF-P are essential for P. aeruginosa pathogenicity
Distinct bacterial strategies to functionalize EF-P may provide a basis for development of customized antibiotics. Deleting EF-P or its modifying enzymes has been shown to reduce bacterial fitness16,25 and lead to a loss of pathogenicity in Salmonella enterica and A. tumefaciens. To test whether rhamnosylated EF-P is also required to develop pathogenicity in the P. aeruginosa strain PAO1, we investigated transposon mutants of efpP.a. (locus tag PA2851) and earPP.a. (locus tag PA2852) in an infection assay using the human cell line A549-Gluc (Fig. 5e). Whereas wild-type P. aeruginosa decreased the number of living cells by about 80%, infection with ΔefpP.a. or ΔearPP.a. mutants had no effect on cell viability. Pathogenicity of P. aeruginosa PAO1 is dependent on a large number of cell-associated and extracellular virulence factors, such as rhamnolipids and pyocyanin, that are important for colonization and invasion during infection29. Bioinformatic analysis on the P. aeruginosa proteome revealed that the synthesis of those virulence factors involves polyproline-containing proteins, suggesting a dependence on EF-P for their translation (Supplementary Table 4). Consistently, efpP.a. or earPP.a. disruption mutants showed a substantial decrease in the production of rhamnolipids and pyocyanin, and production was restored by introducing EarPS.o. and EF-PS.o. but not the substitution mutants EF-PS.o.R32A and EF-PS.o. R32K (Supplementary Fig. 12). Therefore, both EF-PP.a. and the corresponding rhamnosyltransferase EarPP.a. contribute to pathogenicity in P. aeruginosa.
DISCUSSION
Protein glycosylation is a commonly used strategy to alter structural and functional properties of a protein. However, until recently, N-linked glycosylation was almost exclusively associated with asparagine. The only known additions of a sugar to arginine were restricted to two reported examples on eukaryotic proteins. Arginine glycosylation was discovered first in search of a protein primer for starch synthesis in 1995 (ref. 30). Here, the authors identified sweet corn amylogenin to be self-β-glucosylated. Second, in 2013, two independent research groups showed that NleB, an enteropathogenic E. coli type III secretion system effector, antagonizes death receptor signaling by modifying conserved arginines in human death receptor domains with N-acetylglucosamine (GlcNAc)31,32. With S. oneidensis and P. aeruginosa EF-P, we now report what is to our knowledge the first arginine-glycosylated bacterial protein, thus demonstrating that this type of post-translational modification is not restricted to eukaryotic proteins but is common to other domains of life.
ONLINE METHODS
Bioinformatics software
Hidden Markov model (HMM) analyses were carried out using the HMMER3 software package33. Multiple sequence alignments were constructed using the l-ins-i algorithm of the MAFFT version 6.864b software package34. BLASTP searches were performed using the BLAST+ software package version 2.2.26 (ref. 35). All phylogenetic trees were constructed using FastTree with default settings36. Sequences logos were created using the Weblogo server37.
Genome set and domain architecture
1,611 completely sequenced prokaryote genomes from a previously defined set available from 4 April 2012 were collected38. We used a set of 1,004 genomes with reduced redundancy based on the 16S comparison for all sequence analyses. The Pfam26 HMM library39 was used to define domain architecture of all sequences with default gathering thresholds. In the event of domain overlaps, the highest-scoring domain model was chosen for the final architecture.
EarP, EF-P, RmlD and EpmC were identified by collecting sequences that contain the DUF2331, EF-P, RmlD_sub_bind and DUF462 domains, respectively. EpmA consists of a class II tRNA synthetase domain (tRNA-synt_2), which is found in a variety of proteins. All 2,684 sequences containing the tRNA-synt_2 domain were collected and aligned. The core region corresponding to the tRNA-synt_2 domain was extracted from the multiple sequence alignment and used to build a phylogenetic tree. Domain architecture and gene neighborhood analyses identified a conserved clade of 239 sequences in the tree that were determined to be EpmA homologs because of their genomic context association with EF-P homologs (Supplementary Fig. 1). EpmB is composed of a Radical SAM domain (Radical_SAM), which is found in over 15,000 proteins in our sequence set, making a phylogenetics-based approach to EpmB identification challenging. EpmB is often encoded near efp or epmA. We collected all of the sequences with Radical_SAM domains that were encoded within a distance of four genes to efp or epmA homologs (181 sequences) and aligned them. A tree constructed from the alignment revealed two distinct clades, one with many short branches presumed to be true EpmB sequences and another composed of many long branching sequences, which suggest divergence. Furthermore, some sequences in the divergent clade are from genomes represented in the EpmB-associated clade, supporting that they are not true EpmB orthologs (Supplementary Fig. 2). These 36 sequences of the divergent clade were removed from the set, and the remaining sequences were used as queries in BLASTP searches against our representative sequence set. All sequences with an e-value of 0.0001 or less were collected (515 sequences) and aligned. A phylogenetic tree was built from the core region of the multiple sequence alignment corresponding to the Radical_SAM domain. Conserved clades that were associated with EF-P or EpmA on the basis of genome context and phylogenetic distribution were identified (Supplementary Fig. 3). The 236 sequences that are members of these clades were defined as EpmB homologs, and this is consistent with the 239 EpmA homologs we identified that are presumably part of the same pathway. All EarP, EF-P, RmlD, EpmC, EpmA and EpmB homologs in the representative genome set can be found in Supplementary Data Set 1. We further identified all of the EarP homologs in the full 1,611 genome set on the basis of the presence of the DUF2331 domain (Supplementary Data Set 2). We identified all of the EarP-associated EF-Ps in this set using a HMM built from an alignment of the EarP-associated EF-Ps identified in the EF-P phylogenetic analyses. All sequences with a score greater than or equal to that of the lowest-scoring member of the representative set of EarP-associated EF-Ps were identified as homologs (Supplementary Data Set 2).
Phylogenetic analysis of EF-P homologs
A phylogenetic tree was built from the core region of a multiple sequence alignment of EF-P homologs. Conserved clades associated with EarP, EpmA or EpmB on the basis of genome context and phylogenetic distribution data were identified (Supplementary Fig. 4). Sequences from these clades were collected, with the exception of those from an EpmA/EpmB-associated subfamily that includes members of the EF-P-like family (the YeiP subfamily), which lack the conserved lysine. The collected sequences were aligned, and the core region of the alignment was used to construct a phylogenetic tree (Supplementary Fig. 5).
Oligonucleotides, plasmids and bacterial strain construction
Primers, plasmids and strains used in this study are listed in Supplementary Tables 1–3. Of note, transposon mutant P. aeruginosa PAO1 ID 30853 from the Washington Genome Center40 with a transposon insertion in open reading frame (ORF) PA4684 was used as a wild-type control41. Strains BW-Δefp/epmA::npt, BW-Δefp/epmB::cat, MG-CR-efp-fcl and MG-CR-efp-rmlD were constructed by using pRED/ET recombination technology together with rpsL counter-selection42 in accordance to the technical protocol of the Quick and Easy E. coli Gene Deletion Kit of Gene Bridges (http://www.genebridges.com/). Strains ΔefpS.o., ΔearPS.o., ΔefpS.o./ΔearPS.o. and ΔrmlCS.o. were constructed as essentially described in ref. 43, leaving terminal sections of the target gene.
Molecular biology methods
Enzymes and kits were used according to the manufacturer's directions. Genomic DNA was purified according to standard protocols. DNA fragments were purified from agarose gels using a high-yield PCR cleanup and gel extraction kit (Sued-Laborbedarf). Restriction endonucleases were purchased from New England Biolabs. Sequence amplifications by PCR were performed by using the Phusion high-fidelity DNA polymerase from Finnzymes or the Taq DNA polymerase from New England Biolabs, respectively. All efp mutants were constructed by one- or two-step PCR using mismatched primer pairs44.
Growth conditions
E. coli, P. aeruginosa PAO1 and S. oneidensis MR-1 were routinely grown at 37 °C (for E. coli and P. aeruginosa) and 30 °C (S. oneidensis), unless indicated otherwise. According to the NaCl modification of Miller, lysogeny broth (LB)45 was used as complex medium. When indicated, LB was buffered with 100 mM sodium-phosphate to pH 5.8. Microaerobic conditions were achieved by growing cells in closed Eppendorf cups with minimal agitation. Antibiotics were used when necessary with the following concentrations: 100 μg/ml ampicillin sodium salt, 50 μg/ml kanamycin sulfate, 34 μg/ml chloramphenicol, 50 μg/ml streptomycin sulfate or 15 μg/ml tetracycline hydrochloride. For blue-white selection, LB agar plates were additionally supplemented with 80 μM 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal; Sigma Aldrich) and 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG; Sigma Aldrich).
β-Galactosidase activity assay
Cells expressing lacZ under control of the cadC or cadBA promoters were grown in buffered LB medium to mid-exponential growth phase or overnight and harvested by centrifugation. β-Galactosidase activities were determined for at least three independent experiments and are given in Miller units (MU). The significance of the results was determined by applying the two-sided Student's t-test, and results were considered significantly different if P < 0.05.
Overproduction and purification of recombinant proteins
C-terminal His6-tagged EF-PS.o. as well as the corresponding R32A and R32K substitution variants were overproduced either in S. oneidensis MR-1 wild-type, ΔefpS.o. and ΔefpS.o./ΔearPS.o. mutant strains or in E. coli LMG194 and grown in LB overnight at 16 °C after the addition of 0.2% (w/v) l-arabinose to exponentially grow cells. Similarly, C-terminal His6-tagged EarPS.o. was produced in E. coli LMG194. Cells were lysed and purified using Ni-NTA (Qiagen) and 250 mM imidazole. For further MS analysis and in vitro rhamnosylation, proteins were dialyzed against reaction buffer (50 mM HEPES, pH 7.8, 100 mM NaCl, 50 mM KCl, 10 mM MgCl2, 2.5 mM β-mercaptoethanol, 2.0% (v/v) glycerol).
Protein digestion and sample preparation for MS
Purified EF-P was predigested with LysC for 3 h in 8 M urea, 50 mM Tris-HCl, pH 7.5, 1 mM DTT and 5 mM chloroacetamide (CAA). Then, samples were diluted 1:4 with 50 mM Tris HCl, pH 7.5, to decrease the urea concentration to 2 M and digested overnight with trypsin. Peptide mixtures were purified on C18 StageTips46.
LC/MS/MS analysis
Peptides were eluted from the C18 StageTips according to the standard protocol. They were analyzed by reversed-phase LC on an EASY-nLC 1000 system (Thermo Fisher Scientific) directly coupled to a quadrupole Orbitrap mass spectrometer (Q Exactive, Thermo Fisher Scientific). HPLC columns with a length of 50 cm and an inner diameter of 75 μm were packed in-house with ReproSil-Pur 120 C18-AQ 1.9-μm particles (Dr. Maisch GmbH). Peptide mixtures were separated using gradients of either 60 min or 140 min (total run time plus washout) and a two-buffer system: buffer A++ (0.1% formic acid) and buffer B++ (80% acetonitrile in 0.1% formic acid). The flow rate was set to 250 nl/min, and the column was heated to 50 °C using a column oven (Sonation GmbH). Peptides eluting from the column were directly sprayed into the mass spectrometer; spray voltage was set to 2.3–2.4 kV, and the capillary temperature was set to 250 °C. The mass spectrometer was operated in a data-dependent mode with switching between a survey scan and fragmentation scans of the top five most abundant peaks. In the 60-min method, full scans were acquired at a resolution of 140,000 with an AGC target of three E06 ions and a maximum injection time of 20 ms. Precursors were selected with an isolation window of 3 Th, and MS2 scans were acquired at a resolution of 17,500 with an AGC target of one E05 ion and a maximum injection time of 120 ms. In the 140-min method, full scans were acquired at a resolution of 70,000 with an AGC target of three E06 ions and a maximum injection time of 20 ms. Precursors were selected with an isolation window of 2 Th, and MS2 scans were acquired at a resolution of 35,000 with an AGC target of one E05 ion and a maximum injection time of 120 ms. In both cases, peptides were fragmented by higher-energy collisional dissociation (HCD) with a normalized collision energy of 25. To minimize resequencing of peptides, dynamic exclusion was enabled within a time window of 20 s.
MS data analysis
MS raw files were processed using MaxQuant27 version 1.5.0.0. MS/MS spectra were searched using the Andromeda search engine47 against FASTA files obtained from Uniprot adapted to the corresponding sample. For EF-P samples from homologous production in S. oneidensis, raw data were searched against the S. oneidensis reference proteome downloaded from Uniprot on 20 January 2014 and a FASTA file containing the sequence of His6-tagged EF-PS.o.. Depending on the experiment, we added additional FASTA files containing the sequence of His6-tagged EF-PS.o.R32A or EF-PS.o.R32K. Cysteine carbamidomethylation was set as a fixed modification; N-terminal acetylation and methionine oxidation were set as variable modifications. Trypsin was chosen as the specific enzyme, with a maximum of two missed cleavages allowed. Peptide and protein identifications were filtered at a 1% false discovery rate (FDR). The initial mass tolerance was set to 4.5 p.p.m. for the precursor masses and to 20 p.p.m. for the fragment masses. All of the other parameters were left at standard settings. For dependent peptide analysis (essentially as described in ref. 47), the corresponding feature was enabled. For the variable modification searches, l-rhamnose –H2O (C6H10O4 = 146.058 Da) was first defined as a variable modification with specificity for arginine in the Andromeda con figuration and was subsequently added to the other variable modifications in the MaxQuant search.
Bioinformatic analysis of the MaxQuant processed data was performed using the Perseus software (version 1.4.2.35, available in the MaxQuant environment). In brief, for dependent peptide analysis, the ‘all.peptides.txt’ table was loaded and filtered for DP decoy ≠ ‘+’, ‘DP Protein’ = EF-P, ‘DP Base sequence’ containing ‘SGR’, ‘DP Mass Difference’ > 0 and ‘DP Score’ > 80. Remaining hits were further validated in a manual fashion. Spectra were visualized using the Viewer program (version 1.5.0.0, integrated into MaxQuant) and annotated using the Expert System48. For rhamnosylation analysis, the ‘Rhamsites.txt’ table was loaded, and the site table was expanded, logarithmized and then filtered for ‘Protein’ = EF-P, ‘Localization Probability’ = 1 and ‘Score’ > 80.
Enzymatic total synthesis of TDP-l-rhamnose
The synthesis was carried out in two steps. First, TDP-4-keto-6-deoxy-d-glucose was prepared from glucose-1-phosphate using two purified E. coli enzymes (RmlA and RmlB) and TTP. TTP was generated in situ from TMP with a mix of TMP kinase and acetate kinase. TDP-4-keto-6-deoxy-d-glucose is a key intermediate of many 6-deoxyhexoses and can be stored at –80 °C. E. coli BL21(DE3) (EMD 4 Biosciences) was used for the conversion of TDP-4-keto-6-deoxy-d-glucose to TDP-l-rhamnose. This E. coli strain contains naturally TDP-4-ketorhamnose 3,5-epimerase (RmlC) and TDP-4-keto-rhamnose reductase (RmlD). Crude cell lysates of E. coli BL21 (DE3) were added to a solution of TDP-4-keto-6-deoxy-d-glucose in phosphate buffer (50 mM, pH 7.5), MgCl2 (4 mM) and NADH (5 mM). The reaction continued for 3 h at 37 °C. The production of TDP-l-rhamnose was monitored by HPLC49,50.
The resulting solution containing TDP-l-rhamnose was desalted by size-exclusion chromatography (BioGel P2 column) and further purified by HPLC (Waters 600 system consisting of a controller, a Waters 996 photodiode array detector and a Delta 600 pump; a Dionex CarboPac PA1, 4 × 250 mm column was used for 60-min runs at a flow rate of 1.0 ml/min, and UV monitoring absorbance was set at 254 nm). The gradient used was as follows: solvent A, water; solvent B, 0.5 mM ammonium acetate solution. Solvent B was increased from 5% to 20% (0 min to 15 min), from 20% to 60% (15 min to 35 min), and then from 60% to 100% (35 min to 45 min); it was kept at 100% for 5 min before it was decreased back to 5% within 2 min, and it was kept at 5% for the last 8 min49,51. Under these conditions, TDP-l-rhamnose elutes at 34.04 min.
In vitro glycosylation
A total of 10 μM of His6-EF-PS.o. or substitution variants and 10 μM His6-EarPS.o. were incubated in reaction buffer (50 mM HEPES, pH 7.8, 100 mM NaCl, 50 mM KCl, 10 mM MgCl2, 2.5 mM β-mercaptoethanol, 2.0% (v/v) glycerol) with 100 μM of dTDP-l-rhamnose for 60 min at 30 °C.
Cytotoxicity assay
The cytotoxicity of the P. aeruginosa strains was assayed as described earlier52. Briefly, eukaryotic A549-Gluc cells were cultured in completed Dulbecco's modified Eagle's medium at 37 °C with 5% CO2. A549-Gluc cells were generated from A549 by lentiviral gene transfer as described previously53,54. Cytotoxicity of the P. aeruginosa strains was assessed by infecting A549-Gluc cells, which secrete Gaussia luciferase, as a measure of cell integrity. A549-Gluc cells were seeded in 96-well plates at a density of 2.5–5 × 104 cells per well and grown until ~90% confluence. After washing, cells were inoculated with 6-h-old P. aeruginosa LB cultures adjusted to a multiplicity of infection (MOI) of 200 and centrifuged to increase cell-cell contact. 100 μl cell culture super natants were collected after 3 h of incubation, and Gaussia luciferase activity was measured for 0.1 s using an LB 960 Centro XS3 plate luminometer (Berthold Technologies) after the addition of 60 μl of 10 μM coelenterazine (PJK GmbH). Luciferase activities were determined for at least three independent experiments. The significance of the results was determined by applying the two-sided Student's t-test, and results were considered significantly different if P < 0.05.
Assay for pyocyanin production
Pyocyanin was extracted from P. aeruginosa supernatant and measured according to ref. 55. 5 ml of 24-h-old cultures were extracted with 1 volume of chloroform and then re-extracted into 0.2 N HCl to give a pink solution. The aqueous layer was transferred to a fresh tube, and absorbance was measured at 520 nm. Pyocyanin produced per milliliter of culture supernatant was calculated as described elsewhere55.
Quantification of rhamnolipid production
Overnight cultures of P. aeruginosa were freshly diluted to an OD600nm of 0.05 and incubated in LB at 37 °C for 48 h. The colorimetric analysis of the orcinol reaction was adopted from the method described in ref. 56. Briefly, 300 μl of culture super-natant were extracted twice with diethylether. After evaporation of the pooled fraction, the remaining fraction was dissolved in distilled water and incubated with 100 μl 1.6% (w/v) orcinol and 800 μl 60% sulfuric acid. After heating to 80 °C and shaking at 175 r.p.m. for 30 min, the adsorption at 421 nm was determined. In parallel, l-rhamnose at defined concentrations was assayed as described above and used as a standard for determining the l-rhamnose in the culture samples. Rhamnolipid concentrations were then calculated with the assumption that 1 μg of l-rhamnose corresponds to 2.5 μg of rhamnolipid57. Rhamnolipids were quantified from at least three independent experiments. The significance of the results was determined by applying the two-sided Student's t-test, and results were considered significantly different if P < 0.05.
Molecular modeling
The molecular model for modified EF-Ps on the ribosome was generated using the crystal structure of unmodified T. thermophilus EF-P bound to a T. thermophilus 70S ribosome programmed with tRNAfMet at the P-site12. The proline residue was modeled onto the CCA-end of the P-site tRNA by aligning the structure of the 50S subunit with an aminoacylated tRNA substrate in the P-site (PDB1VQN) and by mutagenesis of the amino acid to proline using Coot58. The models for EF-P bearing lysinylation or hypusine modifications were generated by mutagenesis of Arg32 to lysine of ribosome-bound T. thermophilus EF-P and then addition of the required modification moieties to the ε-amino group of the lysine, whereas for EF-P bearing the rhamnosylation modification, an l-rhamnose moiety was added to the η2-amino group of Arg32 of ribosome-bound T. thermophilus EF-P. All of the models were generated and refined in Coot58, and images were produced using the PyMOL Molecular Graphics System (Version 1.5.0.4 Schrödinger, LLC).
Supplementary Material
Acknowledgments
We would like to thank I. Weitl for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft: Center for Integrated Protein Science Munich (CiPSM; Exc114/2 to K.J. and WI3285/4-1 to D.N.W.) and the Max Planck Society (to E.C.K., K.W., L.S.-A. and M.M.). A.L.S. is funded by an AXA Research Fund Postdoctoral Fellowship. The work of S.H. and J.G. was supported by the Helmholtz Association and the Bundesministerium für Bildung und Forschung. J.-M.C. and J.R are funded by the US National Institutes of Health (CA 091901).
Footnotes
Author contributions
J.L. and K.J. designed the experiments and wrote the manuscript. J.L., A.L.S. and D.N.W. discovered EarP and arginine-type EF-P using bioinformatics. K.W., J.L. and L.S.-A. performed phylogenetic analyses. J.L. and M.F. generated S. oneidensis and E. coli deletion mutants as well as all of the plasmids used in the study. All phenotypic analysis was performed by J.L. and M.F. J.L. purified proteins for MS analysis, which was performed by E.C.K. and analyzed by E.C.K. and M.M. TDP-l-rhamnose was synthesized by J.-M.C. and J.R. for in vitro glycosylation performed by J.L. Modeling of modified EF-P to the ribosome was done by D.N.W. Phenotypic characterization of P. aeruginosa Δefp and ΔearP mutants was performed by J.G. and analyzed by J.G. and S.H.
Competing financial interests
The authors declare no competing financial interests.
Additional information
Supplementary information is available in the online version of the paper. Reprints and permissions information is available online at http://www.nature.com/reprints/index.html. Correspondence and requests for materials should be addressed to J.L. or K.J.
References
- 1.Tanner DR, Cariello DA, Woolstenhulme CJ, Broadbent MA, Buskirk AR. Genetic identification of nascent peptides that induce ribosome stalling. J. Biol. Chem. 2009;284:34809–34818. doi: 10.1074/jbc.M109.039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolstenhulme CJ, et al. Nascent peptides that block protein synthesis in bacteria. Proc. Natl. Acad. Sci. USA. 2013;110:E878–E887. doi: 10.1073/pnas.1219536110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peil L, et al. Distinct XPPX sequence motifs induce ribosome stalling, which is rescued by the translation elongation factor EF-P. Proc. Natl. Acad. Sci. USA. 2013;110:15265–15270. doi: 10.1073/pnas.1310642110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersch SJ, et al. Divergent protein motifs direct elongation factor P–mediated translational regulation in Salmonella enterica and Escherichia coli. mBio. 2013;4:e00180–e00113. doi: 10.1128/mBio.00180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elgamal S, et al. EF-P dependent pauses integrate proximal and distal signals during translation. PLoS Genet. 2014;10:e1004553. doi: 10.1371/journal.pgen.1004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starosta AL, et al. Translational stalling at polyproline stretches is modulated by the sequence context upstream of the stall site. Nucleic Acids Res. 2014;42:10711–10719. doi: 10.1093/nar/gku768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ude S, et al. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- 8.Doerfel LK, et al. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez E, et al. eIF5A promotes translation of polyproline motifs. Mol. Cell. 2013;51:35–45. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanawa-Suetsugu K, et al. Crystal structure of elongation factor P from Thermus thermophilus HB8. Proc. Natl. Acad. Sci. USA. 2004;101:9595–9600. doi: 10.1073/pnas.0308667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaha G, Stanley RE, Steitz TA. Formation of the first peptide bond: the structure of EF-P bound to the 70S ribosome. Science. 2009;325:966–970. doi: 10.1126/science.1175800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. USA. 1981;78:2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park MH, Cooper HL, Folk JE. The biosynthesis of protein-bound hypusine (N-ε-(4-amino-2-hydroxybutyl)lysine). Lysine as the amino acid precursor and the intermediate role of deoxyhypusine (N-ε-(4-aminobutyl) lysine). J. Biol. Chem. 1982;257:7217–7222. [PubMed] [Google Scholar]
- 15.Cooper HL, Park MH, Folk JE, Safer B, Braverman R. Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc. Natl. Acad. Sci. USA. 1983;80:1854–1857. doi: 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarre WW, et al. PoxA, YjeK, and elongation factor P coordinately modulate virulence and drug resistance in Salmonella enterica. Mol. Cell. 2010;39:209–221. doi: 10.1016/j.molcel.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilreath MS, et al. β-Lysine discrimination by lysyl-tRNA synthetase. FEBS Lett. 2011;585:3284–3288. doi: 10.1016/j.febslet.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagisawa T, Sumida T, Ishii R, Takemoto C, Yokoyama S. A paralog of lysyl-tRNA synthetase aminoacylates a conserved lysine residue in translation elongation factor P. Nat. Struct. Mol. Biol. 2010;17:1136–1143. doi: 10.1038/nsmb.1889. [DOI] [PubMed] [Google Scholar]
- 19.Peil L, et al. Lys34 of translation elongation factor EF-P is hydroxylated by YfcM. Nat. Chem. Biol. 2012;8:695–697. doi: 10.1038/nchembio.1001. [DOI] [PubMed] [Google Scholar]
- 20.Bailly M, de Crecy-Lagard V. Predicting the pathway involved in post-translational modification of elongation factor P in a subset of bacterial species. Biol. Direct. 2010;5:3. doi: 10.1186/1745-6150-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullwinkle TJ, et al. (R)-β-Lysine–modified elongation factor P functions in translation elongation. J. Biol. Chem. 2013;288:4416–4423. doi: 10.1074/jbc.M112.438879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franceschini A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hau HH, Gralnick JA. Ecology and biotechnology of the genus Shewanella. Annu. Rev. Microbiol. 2007;61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 24.Fredrickson JK, et al. Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 2008;6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 25.Peng WT, Banta LM, Charles TC, Nester EW. The chvH locus of Agrobacterium encodes a homologue of an elongation factor involved in protein synthesis. J. Bacteriol. 2001;183:36–45. doi: 10.1128/JB.183.1.36-45.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haneburger I, Eichinger A, Skerra A, Jung K. New insights into the signaling mechanism of the pH-responsive, membrane-integrated transcriptional activator CadC of Escherichia coli. J. Biol. Chem. 2011;286:10681–10689. doi: 10.1074/jbc.M110.196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 28.Mäki M, Renkonen R. Biosynthesis of 6-deoxyhexose glycans in bacteria. Glycobiology. 2004;14:1R–15R. doi: 10.1093/glycob/cwh040. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez PN, et al. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh DG, et al. β-Glucosylarginine: a new glucose-protein bond in a self-glucosylating protein from sweet corn. FEBS Lett. 1995;376:61–64. doi: 10.1016/0014-5793(95)01247-6. [DOI] [PubMed] [Google Scholar]
- 31.Li S, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–246. doi: 10.1038/nature12436. [DOI] [PubMed] [Google Scholar]
- 32.Pearson JS, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eddy SR. A new generation of homology search tools based on probabilistic inference. Genome. Inform. 2009;23:205–211. [PubMed] [Google Scholar]
- 34.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keilberg D, Wuichet K, Drescher F, Sogaard-Andersen L. A response regulator interfaces between the Frz chemosensory system and the MglA/MglB GTPase/GAP module to regulate polarity in Myxococcus xanthus. PLoS Genet. 2012;8:e1002951. doi: 10.1371/journal.pgen.1002951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Punta M, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt J, et al. The Pseudomonas aeruginosa chemotaxis methyltransferase CheR1 impacts on bacterial surface sampling. PLoS ONE. 2011;6:e18184. doi: 10.1371/journal.pone.0018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heermann R, Zeppenfeld T, Jung K. Simple generation of site-directed point mutations in the Escherichia coli chromosome using Red®/ET® Recombination. Microb. Cell Fact. 2008;7:14. doi: 10.1186/1475-2859-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassak J, Henche AL, Binnenkade L, Thormann KM. ArcS, the cognate sensor kinase in an atypical Arc system of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2010;76:3263–3274. doi: 10.1128/AEM.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 45.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 47.Cox J, et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 48.Neuhauser N, Michalski A, Cox J, Mann M. Expert system for computer-assisted annotation of MS/MS spectra. Mol. Cell. Proteomics. 2012;11:1500–1509. doi: 10.1074/mcp.M112.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kharel MK, Lian H, Rohr J. Characterization of the TDP-d-ravidosamine biosynthetic pathway: one-pot enzymatic synthesis of TDP-d-ravidosamine from thymidine-5-phosphate and glucose-1-phosphate. Org. Biomol. Chem. 2011;9:1799–1808. doi: 10.1039/c0ob00854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang G, et al. Cooperation of two bifunctional enzymes in the biosynthesis and attachment of deoxysugars of the antitumor antibiotic mithramycin. Angew. Chem. Int. Edn. Engl. 2012;51:10638–10642. doi: 10.1002/anie.201205414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang G, Kharel MK, Pahari P, Rohr J. Investigating mithramycin deoxysugar biosynthesis: enzymatic total synthesis of TDP-d-olivose. ChemBioChem. 2011;12:2568–2571. doi: 10.1002/cbic.201100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gödeke J, Pustelny C, Haussler S. Recycling of peptidyl-tRNAs by peptidyl-tRNA hydrolase counteracts azithromycin-mediated effects on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013;57:1617–1624. doi: 10.1128/AAC.02582-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haid S, Windisch MP, Bartenschlager R, Pietschmann T. Mouse-specific residues of claudin-1 limit hepatitis C virus genotype 2a infection in a human hepatocyte cell line. J. Virol. 2010;84:964–975. doi: 10.1128/JVI.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gentzsch J, et al. Hepatitis C virus complete life cycle screen for identification of small molecules with pro- or antiviral activity. Antiviral Res. 2011;89:136–148. doi: 10.1016/j.antiviral.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger KE. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J. Bacteriol. 2007;189:6695–6703. doi: 10.1128/JB.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochsner UA, Koch AK, Fiechter A, Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1994;176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.