Abstract
The forkhead box (Fox) superfamily of transcription factors plays essential roles in organogenesis and tissue differentiation. Foxa1 and Foxa2 are expressed during prostate budding and ductal morphogenesis, while Foxa1 expression is retained in adult prostate epithelium. Previous characterization of prostatic tissue rescued from embryonic Foxa1 knockout mice revealed Foxa1 to be essential for ductal morphogenesis and epithelial maturation. However, it is unknown whether Foxa1 is required to maintain the differentiated status in adult prostate epithelium. Here, we employed the PBCre4 transgenic system and determined the impact of prostate-specific Foxa1 deletion in adult murine epithelium. PBCre4/Foxa1loxp/loxp mouse prostates showed progressive florid hyperplasia with extensive cribriform patterning, with the anterior prostate being most affected. Immunohistochemistry studies show mosaic Foxa1 KO consistent with PBCre4 activity, with Foxa1 KO epithelial cells specifically exhibiting altered cell morphology, increased proliferation, and elevated expression of basal cell markers. Castration studies showed that while PBCre4/Foxa1loxp/loxp prostates did not exhibit altered sensitivity in response to hormone ablation compared with control prostates, the number of Foxa1 positive cells in mosaic Foxa1 KO prostates were significantly reduced compared to Foxa1 negative cells following castration. Unexpectedly, gene expression profile analyses revealed that Foxa1 deletion caused abnormal expression of seminal vesicle associated genes in KO prostates. In summary, these results indicate Foxa1 expression is required for the maintenance of prostatic cellular differentiation.
Keywords: Androgen, AR, castration, prostate, prostate cancer, prostate-specific, FOXA
The Forkhead Box (Fox in mice, FOX in human) family of transcription factors consists of 43 proteins, which are organized into 19 subfamilies based on amino acid sequence conservation (1). Foxa1 (originally termed hepatocyte nuclear factor one alpha; HNF1α) was originally discovered in mammals as a transcription factor in the liver (2). Importantly, the structure of the DNA binding domain of Foxa proteins contains a variant of the helix-loop-helix motif, giving it the appearance of a “winged helix.” This structure closely resembles that of a linker histone (3), and enables Foxa1 to directly influence chromatin structure. In addition, the C-terminus of Foxa1 binds to core histones and is required for chromatin opening in vitro (4). For these reasons, Foxa proteins make DNA sequences accessible for the binding of additional transcriptional activators and/or repressors, and have accordingly been described as “pioneer factors.” The “pioneering” function of Foxa proteins has led to the suggestion that these proteins enable cells to respond quickly to changes in their environment by altering transcriptional regulation of genes. The ability of Foxa transcription factors to impart “competence” for the rapid initiation of gene expression has been suggested as central to their function. In addition, the observation that Fox family members are retained on mitotic chromosomes (5, 6), suggests an important role for Foxa1 in the maintenance of cell fate.
Foxa1 and Foxa2 expression is restricted to the epithelial compartment during embryonic prostate development (7). While Foxa2 expression is extinguished following budding morphogenesis, Foxa1 expression is maintained in the adult prostate epithelium. Our laboratory previously showed that Foxa1 expression is required for normal prostate organogenesis (8, 9). In fact, expression of Foxa proteins play an essential role during the development of several organs, and Foxa1 and Foxa2 have been shown to act in a cooperative manner during the normal development of the liver, lungs, dopaminergic neurons and pancreas (10). Our laboratory was the first to report the direct physical interaction of Foxa1 with the androgen receptor (AR), and the importance of Foxa1 expression for the regulation of prostate-specific gene expression (11). While altered levels of Foxa1 and Foxa2 expression have been implicated in prostate cancer (12–17), the importance of Foxa1 expression in the adult, non-neoplastic prostate is unknown. Therefore, we undertook this study to determine the impact of prostate-specific genetic ablation of Foxa1in adult mice.
Materials and Methods
Mouse lines and breeding
Foxa1loxp mice have been previously described (18). PBCre4 (19) activity was detected 14 days after birth, and increases with rising testicular androgen production and the onset of sexual maturity in mice. PBCre4 has been previously reported to result in prostate-specific Cre recombinase activity, and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (Jackson Laboratories, Bar Harbor, MA) express red fluorescence prior to, and green fluorescence following Cre-mediated recombination (20). To identify specific cell populations targeted by Cre-mediated recombination in the PBCre4 mouse line, PBCre4 mice were bred with Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, resulting in PBCre4/Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (hereafter designated PBCre4/Tomato mice). Prostate-specific Foxa1 knockout was achieved by breeding PBCre4 mice to Foxa1loxp and Foxa1loxp/loxp mice, resulting in PBCre4/Foxa1loxp and PBCre4/Foxa1loxp/loxp mice, respectively. PBCre4/Foxa1loxp and PBCre4/Foxa1loxp/loxp mice were aged up to 40 weeks. Following sacrifice, individual prostate lobes, seminal vesicle and bladders were dissected, formalin fixed and processed for paraffin embedding according to standard procedures.
Immunohistochemistry and immunofluorescence
Slides were deparaffinized, rehydrated through a series of graded alcohols and washed in double deionized water for 5 minutes. Tissues were then placed in antigen unmasking solution (Vector Labs, Burlingame, CA) and antigen retrieval was performed by microwaving samples for 20 minutes at 30% power in a 900 W microwave oven. Slides were then cooled to room temperature, and then washed 3 times for 10 minutes in PBS (pH 7.4). For immunohistochemistry, all incubations were performed at room temperature unless otherwise stated. Endogenous peroxidase activity was blocked with the use of Peroxidase blocking reagent (Dako North America, Carpinteria, CA) for 20 minutes, after which sections were again washed in PBS 3 times for 10 minutes. Prepared slides were incubated in horse serum for 30 min to reduce non-specific antibody binding. Slides where then incubated overnight at 4°C in a humidified chamber with primary antibody. After 3 10 min washes in PBS, slides were then incubated overnight in the presence of primary antibody. On the following day, slides were then washed 3 times for 10 min with PBS and biotinylated secondary antibody diluted in PBS was then added. Primary antibody was visualized using the Vectastain Elite ABC Peroxidase kit (Vector Labs) according to the manufacturer’s protocol with DAB in substrate buffer as chromogen (Thermo Scientific, Fremont CA). For immunofluorescence studies, the above described procedure was followed on the first day, but after washing in PBS, slides were incubated for 1 hr in the dark with secondary antibody (1:1000 in blocking solution). Following PBS wash, slides were mounted in DAPI cotaining Vector medium. Primary antibodies used included Krt 8/18, Krt 14 (Dako), Foxa1, AR, GFP, p63 (Santa Cruz) (8,21,22), and Foxc1 (Sigma; http://www.proteinatlas.org/). Ki67 staining was performed by the Vanderbilt Experimental Pathology Core, as previously reported (8). Slides were visualized on a Zeiss Axio Imager.M1 microscope with Axiovision image capture software (Carl Zeiss Microimaging, Thornwood NY).
Quantitative RT-PCR
RNA was extracted using the RNeasy kit from Qiagen (Valencia, CA, USA). Reverse transcription was conducted using SuperScriptII from Invitrogen (Carlsbad, CA, USA). Real-time PCR was performed on iCycler using iQ SYBR Green Supermix from Bio-Rad (Hercules, CA, USA). PCR was performed as follows: 95 °C for 4 min, followed by 40 cycles of 95 °C/30 s, 58 °C/30 s and 72 °C/30s. All samples were normalized to GAPDH. Results were expressed as fold change of each sample versus control using the 2(−ΔΔCt) method (23). Primer sequences used in this study are as follows: Foxc1 (CCAATGCTTCCTTAAGCGGC, TGGCTAAGGCGGCCAAATAA); Svs3b (GGATGTGCTACTGTCCAAGAG, TGCAAGGTGTTAAGGACTACAGG); Svs6 (CCACCAGCTTCTTTCTCCTTACA, CCCATCTTCACCATTGACCAT); Fam3b (AAGGGCTTTGAGCTCCCTTC, CCTTCGATCTGGATCTCCGC); Trpv6 (ACCAGCCTTCCACCCCAT, TCAGAGCCTGGACATCGTTT); Prom2 (CGGGCTGGAGAAAATCCACT, GCTTCGAAGTTCTCTGGCCT) Usp18 (GACAGTCGACAGAAAGCCGT, CAAATCAGGGACTCCTGCGT).
Transient transfection and co-immunoprecipitation
Co-immunoprecipitation of both endogenously expressed Foxc1 and transiently overexpressed Foxc1 with AR were performed, using the prostate myofibroblast cell line WPMY-AR, which was previously engineered to stably overexpress AR (24). Transient transfection of WPMY-AR cells with Foxc1 expression plasmid was performed using lipofectamine 2000 (Invitrogen) according to manufacturer recommendation, and essentially as previously reported (25). Transfected WPMY-AR cells expressing Foxc1 were washed three times with cold PBS and lysed with 1 ml of nondenaturing lysis buffer (50 mM Tris, 150 mM NaCl, 10 mM EDTA, 0.02% NaN3, 50 mM NaF, 1 mM Na3VO4, 1% NP-40, 1 mM PMSF, 0.5 mM dithiothreitol, and 1X concentration of complete protease inhibitor cocktail (Roche, Nutley, NJ). After sonication, centrifugation, and pre-clearing, 1 mg of total cell lysate for each reaction was incubated at 4 C overnight with 20 μl (dry volume) protein G-Sepharose beads (Amersham Biotech, Piscataway, NJ), which were conjugated with 1 μg experimental antibody or IgG antibody. Following overnight incubation, samples were centrifuged and the pelleted protein G-Sepharose beads were washed four times with lysis buffer and once with PBS followed by protein dissociation and Western blotting analysis. The affinity purified rabbit polyclonal antibody (HPA040670, Sigma-Aldrich) was used to immunoprecipitate Foxc1. Western blotting to identify interactions between Foxc1 and AR were subsequently performed with anti-AR (N20; Santa Cruz Biotechnology, Santa Cruz, CA). IgG was used as a negative control.
Microarray analysis
Microarray analysis on RNA extracted from anterior prostates (AP) dissected from control and experimental mice, as well as the seminal vesicles dissected from control mice was performed using the GeneTitan1.1.ST mouse array (Affymetrix Corp; Santa Clara, CA) by the Vanderbilt Technologies for Advanced Genomics (VANTAGE) core. The GeneTitan1.1.ST mouse array chip contains probes for 26,166 coding transcripts (RefSeq), and approximately 7,000 non-coding transcripts, including 2,000 long intergenic non-coding transcripts. Instrument control and data acquisition was performed using the Affymetrix GeneChip Command Console. Fold change was calculated in a log base 2 space, using the following formula (Fold Change = 2^(experimental − control)), followed by inverting and negating ratios less than 1. Microarray analysis was performed both to identify increases in seminal vesical associated gene expression in the AP of Foxa1 KO mice, as well as to identify genes whose expression was altered coordinately following both castration and Foxa1 KO. See supplementary data for gene lists.
Results
Foxa1 expression is restricted to luminal epithelium; a subpopulation of cells which are efficiently targeted by PBCre4
As FOXA1 expression in the human prostate is restricted to luminal epithelium, while basal prostate epithelium fails to exhibit positive FOXA1 immunostaining (26), we performed dual immunofluorescence microscopy to determine the extent of Foxa1 colocalization with cytokeratin (Krt) 8/18 and Krt14, which are markers of luminal and basal epithelium, respectively (Figure 1). We performed this staining on the anterior prostates (AP), as this lobe of the murine prostate has the largest population of Krt14 positive basal cells. As is the case in humans (26), results show that Foxa1 expression is restricted to Krt 8/18 positive epithelium (Figure 1D, inset) in the adult mouse, and is not detected in the Krt14 positive basal cell layer (Figure 1H, inset). Promoters vary in their penetrance, which is the primary determinant in their ability to efficiently drive transgene expression in specific cell types. Therefore, selecting the proper promoter to drive Cre recombinase is essential. Initial immunofluorescence studies required that we utilize a promoter specifically expressed in luminal epithelium to achieve genetic ablation of Foxa1 in the prostate. In order to verify the suitability of the PBCre4 transgenic line for the targeting of Foxa1 positive luminal prostate epithelium, we bred PBCre4 mice to the Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter line (Figure 2). Dual immunofluorescence studies (2A; DAPI, 2B; Foxa1, 2C; GFP, 2D Merge) on the AP of an adult mouse revealed that PBCre4 targeted a subset of cytokeratin-8/18 positive luminal epithelium (Figure 2C). A range of GFP positive cells in which Cre was activated was detected in the anterior prostate (AP), with the distal tips and base of the AP being the most affected. A similar range of GFP positive cells in the lateral (LP), dorsal (DP) and ventral (VP) prostates were GFP positive (see supplementary data). Dual immunofluorescence for GFP and Foxa1 revealed that Foxa1 positive murine prostate epithelium was efficiently targeted by Cre recombinase (Figure 2D) in the adult AP. In summary, these data suggest PBCre4 is suitable to achieve conditional Foxa1 knockout in the murine prostatic luminal cells.
Figure 1. Foxa1 expression in murine prostate epithelium co-localizes with cytokeratin 8/18 expression, but is absent in cytokeratin 14 positive basal prostate epithelium.
Panels A (DAPI), B (Cytokeratin 8/18), C (Foxa1) and D (merge) show co-localization of Foxa1 with cytokeratin 8/18 positive prostate epithelium in the anterior prostate. Panels E (DAPI), F (cytokeratin 14), G (Foxa1) and H (merge) show cytokeratin 14 positive basal epithelium does not express Foxa1 in the mouse prostate anterior prostate (see annotation arrows in H inset).
Figure 2. PBCre4 efficiently targets Foxa1 positive prostate epithelium.
Immunofluorescence on anterior prostate tissue dissected from PBCre4/Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (PBCre4/Tomato mice) was performed to determine the extent to which PBCre4 targets Foxa1 positive prostate epithelium. The presence of membrane bound GFP in PBCre4/Tomato mice is indicative of a cre-mediated recombination event. Panels A (DAPI), B (Foxa1), C (GFP) and D (merge) show that Foxa1 positive prostate epithelium in the anterior prostate are efficiently targeted by PBCre4 in the AP. In terms of percentage, a range of GFP positive cells where Cre was activated was detected in the anterior prostate (AP), with the distal tips and base of the AP being the most affected. (see supplementary data for analysis of other prostate lobes).
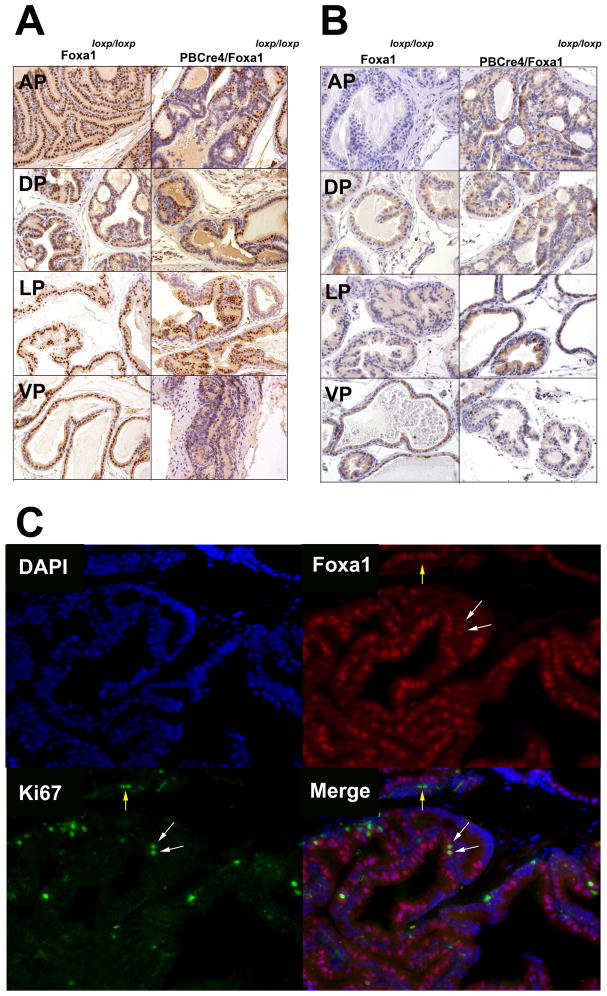
Prostate-specific mosaic Foxa1 knockout results in florid hyperplasia
To determine the impact of Foxa1 ablation on tissue morphology, PBCre4/Foxa1loxp and PBCre4/Foxa1loxp/loxp mice (and control Foxa1loxp and Foxa1loxp/loxp mice) were aged and sacrificed at 7, 24, and 33 weeks of age. Histological analysis (Figure 3; top panel Foxa1loxp/loxp control mice; bottom panel PBCre4/Foxa1loxp/loxp mice) showed changes as early as 7 weeks of age in the AP and DP, which progressed to florid hyperplasia complete with cribriform patterning (Figure 3; bottom panel). Progressive hyperplasia was present in all lobes by 24 weeks. We did not observe epithelial cell invasion into the prostatic stroma. In keeping with PBCre4/Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J cre mapping studies, Foxa1 knockout was mosaic in nature in all lobes of the prostate (Figure 4; top left). Ki67 staining revealed a increase in the number of proliferating cells (Figure 4; top right), with the largest proportion of Ki67 staining present in the AP and DP (Figure 4; bottom panel; D is depiction of AP). We previously reported that FOXA1 loss coincided with increased Ki67 staining in human bladder cancer specimens, suggesting that Foxa1 negative murine prostate epithelium would be Ki67 positive (21). However, this did not appear to be the case, as increases in Ki67 labeling were not restricted to Foxa1 negative cells as shown by dual immunofluorescence of the AP dissected from 24 week-old PBCre4/Foxa1loxp/loxp mice (Figure 4; bottom panel; D). These results indicate PBCre4-mediated Foxa1 knockout increases the proliferation of prostate epithelium in a manner that is not simply restricted to Foxa1 knockout epithelium.
Figure 3. Prostates dissected from PBCre4/Foxa1loxp/loxp mice exhibit progressive hyperplasia with cribriform patterning.
Top panel: Anterior, dorsal, ventral and lateral prostatic tissue dissected from 7 week, 24 week, and 33 week old Foxa1loxp/loxp control mice, which exhibit normal histomorphology following H&E stain. Bottom panel: H&E stain of anterior, dorsal, ventral and lateral prostatic tissue dissected from age-matched PBCre4/Foxa1loxp/loxp mice reveals largely progressive hyperplasia with extensive cribriform patterning. This phenotype was most pronounced in the anterior prostate.
Figure 4. PBCre4-mediated Foxa1 knockout is mosaic in nature, and results in increased epithelial proliferation.
Panel A: Foxa1 immunostaining of anterior, dorsal, lateral and ventral prostate tissue dissected from from 24 week old control Foxa1loxp/loxp mice and age-matched PBCre4/Foxa1loxp/loxp mice reveal Foxa1 knockout is mosaic in nature, in keeping with PBCre4/Tomato mice cre mapping studies. Panel B: Ki67 staining reveals an increase in the number of proliferating cells following Foxa1 knockout in 24 week old PBCre4/Foxa1loxp/loxp mice (see supplementary data for graphical representation of Ki67 index). Panel C: Dual immunofluorescence for Foxa1 (red) and Ki67 (green) shows that detection of Ki67 staining is not limited to Foxa1 negative cells in the anterior prostate (see merged panel in D). White arrows point to Foxa1 negative, Ki67 positive epithelium, while yellow arrows point to Foxa1 negative epithelium that is Ki67 positive.
PBCre4/Foxa1loxp/loxp mice exhibit altered localization of p63 positive basal cells
Embryonic Foxa1 knockout was previously reported to result in an expansion of the basal cell population (8). Immunohistochemistry for p63 in 24 week old PBCre4/Foxa1loxp/loxp mice revealed a similar increase in the number of basal epithelial cells (Figure 5, A). In addition to being expanded, the p63 positive cell population was not limited to the basal compartment of the epithelium (see PBCre4/Foxa1loxp/loxp Figure 5A). We performed dual immunofluorescence on the AP of 24 week old PBCre4/Foxa1loxp/loxp mice to determine if cells with PBCre4-mediated recombination and Foxa1 knockout were also activating p63 expression. Dual immunofluorescence clearly showed p63 is not expressed in Foxa1-null luminal epithelium (Figure 5B). Increases in p63 could also be mediated by an expansion of the intermediate cell population, indicating a failure of Foxa1 negative cells to fully differentiate. Dual immunofluorescence of 24 week old PBCre4/Foxa1loxp/loxp mice demonstrated that Krt8/18 and p63 expression were mutually exclusive in the AP, indicating the lack of an expanding intermediary cell population in response to Foxa1 knockout (Figure 5C).
Figure 5. Foxa1 knockout results in a non-cell autonomous expansion of p63 positive basal epithelium.
A: Immunostaining for the basal cell marker p63 in prostate tissue dissected from 24 week old control Foxa1loxp/loxp mice reveals a normal staining pattern limited to basal epithelium adjacent to the prostatic stroma. Immunostaining for p63 in age-matched PBCre4/Foxa1loxp/loxp mice reveal an expansion of the basal cell population. In addition, p63 positive epithelium in PBCre4/Foxa1loxp/loxp mice are not limited to the basal cell population, and are detected throughout the prostatic epithelium. B: Dual immunofluorescence for Foxa1 (red) and p63 (green) reveal that Foxa1 negative prostate epithelium exhibits a lack of p63 expression, indicating that absence of Foxa1 does not increase p63 expression in a cell autonomous manner. C: Intermediate prostate epithelial cells are a rare population typified by dual positivity for the luminal marker cytokeratin 8 and the basal marker p63. In order to determine if increases in the number of p63 positive cells following Foxa1 knockout is the result of an increase in the number of intermediate prostate cells, we performed dual immunofluorescence for cytokeratin 8 (red) and p63 (green). Results show a mutual exclusivity between cytokeratin 8 and p63 staining, indicating the absence of an increased population of intermediary prostate epithelial cells in PBCre4/Foxa1loxp/loxp mice.
While Foxa1 knockout prostates retain androgen sensitivity, Foxa1 positive cells appear more sensitive to castration
Examination of the weights of anterior prostates dissected from the intact Foxa1loxp/loxp and PBCre4/Foxa1loxp/loxp mice revealed significant differences (p=0.013), which may be attributed to decreased secretory activity of Foxa1 KO prostates (8). PBCre4/Foxa1loxp/loxp mice retain AR expression (see supplementary data), but it was unclear how Foxa1 knockout would influence prostate regression following castration. We therefore examined the prostates of 33 week old PBCre4/Foxa1loxp/loxp mice and control Foxa1loxp and Foxa1loxp/loxp mice 2 weeks after castration. Both Foxa1loxp/loxp and PBCre4/Foxa1loxp/loxp mice showed prostatic atrophy after castration, as indicated by significant decreases in prostate weight (Figure 6A; Student’s t test; p<0.0005), decreased Ki67 immuno positivity, as well as histological alterations (regression of glandular structure, increased expansion of smooth muscle, and altered nuclear to cytoplasmic ratio; data not shown). Comparison of tissue dissected from intact and castrated 33 week-old PBCre4/Foxa1loxp/loxp mice revealed a significant decrease in the number of Foxa1 positive cells following castration. (Figure 6C; Mann-Whitney test; p=0.0003). No significant changes in Foxa1 immunostaining were detected in the following the comparison of intact and castrated tissue dissected from 33 week-old Foxa1loxp/loxp mice (Figure 6C; Mann-Whitney test; p=0.7). These results potentially indicate that in the subpopulation of prostate luminal epithelium not targeted by PBCre4, and therefore retain Foxa1, are especially sensitive to androgen deprivation while the Foxa1-null cells are more resistant to castration.
Figure 6. Castration increases the number of Foxa1-negative prostate epithelial cells in PBCre4/Foxa1loxp/loxp mice.
Panel A: Analysis of wet weights of the anterior prostates dissected from intact and castrated 33-week old Foxa1loxp/loxp and PBCre4/Foxa1loxp/loxp prostates revealed a significant decrease in weight following castration in both control and Foxa1 knockout mice. Panel B: Comparison of intact 33-week old Foxa1loxp/loxp and PBCre4/Foxa1loxp/loxp prostates revealed significant differences in weight, which is in keeping with reduced cytoplasm of Foxa1 negative epithelium (see annotation arrows in B), further suggesting a role for Foxa1 in the maintenance of a differentiated phenotype. Panel C: Inspection of H&E staining from both Foxa1loxp/loxp and PBCre4/Foxa1loxp/loxp prostates revealed atrophy of the gland following castration in both groups indicative of androgen sensitivity and consistent with decreased prostatic weight following castration (H&E stain). Although PBCre4-mediated Foxa1 knockout is mosaic in nature, castration results in a decrease in the number of Foxa1 positive cells in castrated PBCre4/Foxa1loxp/loxp prostates compared to control Foxa1loxp/loxp mice Panel D: Graphical representation of the % of Foxa1 positive cells in intact and castrated PBCre4/Foxa1loxp/loxp prostates. Comparison of intact and castrated Foxa1loxp/loxp anterior prostates revealed no significant differences in the number of Foxa1 positive cells after castration in genetic controls (Mann-Whitney test; p=0.7), whereas comparison of intact and castrated PBCre4/Foxa1loxp/loxp anterior prostates did reveal significant differences in the number of Foxa1 positive cells following castration in Foxa1 KO mice (Mann-Whitney test; p=0.0003).
PBCre4-Foxa1 KO prostates express markers of seminal vesicle differentiation
We performed microarray analysis to gain further insights into changes in gene expression following Foxa1 knockout. Microarray analysis on the Foxa1 KO AP dissected from a 33 week-old mouse, as well as age-matched controls revealed that three of the six major Seminal Vesicle Secretions (SVS) genes were up regulated in this lobe of the murine prostate. Therefore, microarray analysis was performed on the seminal vesicles and a comparison of genes expressed in intact seminal vesicles was made to the Foxa1 KO AP. This analysis revealed that 57 genes normally specific to the seminal vesicles were now expressed by the Foxa1 KO AP (see supplementary data for gene list). Because Foxa1 activity is so intricately tied to AR function in the prostate, Foxa1 KO could alter pathways that are associated with response to circulating androgens. Therefore, we performed microarray analysis to identify the number of genes (if any) that were coordinately regulated by both castration and Foxa1 KO (see supplementary data). This analysis revealed of 240 genes whose expression decreased in response to castration, 47 (19.6%) of the same genes changed following Foxa1 KO. Of 271 genes whose expression increased following castration of a control prostate, 30 (11.1%) of the same genes changed following Foxa1 KO.
In regard to the increased expression of genes associated with seminal vesicle, qRT-PCR and subsequent statistical analysis of cDNA samples prepared from AP dissected from 33 week-old Foxa1loxp/loxp mice and aged-matched PBCre4/Foxa1loxp/loxp mice revealed significant increases (Student’s t test) in the expression of Foxc1, as well as the seminal vesicle marker genes Svs3b and Svs6 (Figure 7A;). In addition, we were able to detect significant increases in the expression of Fam3b, Trpv6, and Prom2, all of which are normally expressed in a relatively seminal vesicle-specific manner (Figure 7A). Immunohistochemistry of murine seminal vesicle showed that Foxc1 is expressed at high levels in this androgen target tissue (Figure 7B; left panel). Furthermore, while we failed to detect Foxc1 expression in AP prostates dissected from 40 week-old Foxa1loxp/loxp (Figure 7B; middle panel), we detected increased Foxc1 expression in AP epithelial cells dissected from age-matched PBCre4/Foxa1loxp/loxp mice (Figure 7B; right panel). Since we have previously shown that Foxa1 interacts with the AR in human prostate epithelial cells, we postulated that Foxc1 may also physically interact with AR in tissues where these transcription factors are co-expressed. To test this hypothesis, we performed co-immunoprecipitation experiments on cells expressing both AR and Foxc1. The WPMY-AR cell line, which are a myofibroblastic cell line engineered to stably overexpress AR (24), was chosen because Foxc1 expression was previously reported to be increased in prostatic stroma (27). Immunoprecipitation of Foxc1, followed by western blotting analysis, showed that AR co-precipitated indicating a physical interaction with endogenously expressed Foxc1 in WPMY-AR cells (Figure 7C). Our ability to detect this interaction was enhanced following overexpression of Foxc1 in WPMY-AR cells (Figure 7D). These results indicate Foxc1 interacts with AR, and that this complex may be important in the regulation of AR target genes in Foxa1-negative cells.
Figure 7. PBCre4-mediated Foxa1 knockout results in the increased expression of the novel AR-interacting protein Foxc1 and additional seminal vesicle-associated genes.
A: Q-RT-PCR analysis shows increases in the expression of Foxc1, and the seminal-vesicle associated genes Svs3b, Svs6, Fam3b, Trpv6, and Prom2. B: Immunohistochemistry for Foxc1 in murine (left panel) seminal vesicle, (middle panel) Foxa1loxp/loxp anterior prostate and (right panel) PBCre4/Foxa1loxp/loxp anterior prostate shows an increase in Foxc1 expression following Foxa1 knockout. WPMY-AR cells were used for co-immunoprecipitation experiments to determine if Foxc1 physically interacts with AR. Western blotting results show that AR interacts with both endogenous (C) Foxc1 and (D) exogenously expressed Foxc1 following transient transfection.
Discussion
We had previously reported an essential role for Foxa1 expression during normal prostate development (8), where knockout of Foxa1 during embryonic development resulted in the failure of prostate morphogenesis, an expansion of a p63 positive basal cell population, and the retention of embryonic markers of prostate development, including Foxa2. However, embryonic knockout of Foxa1 made it impossible to determine the importance of this transcription factor for the maintenance of normal prostatic differentiation that occurs with sexual maturation. In the current study, we used the PBCre4 system to ablate Foxa1 in a prostate-specific manner during sexual maturation. We show that the PBCre4 efficiently targets the luminal prostate epithelium that expresses Foxa1 (See Figures 1 & 2, as well as supplementary data), and that PBCre4-mediated Foxa1 knockout results in progressive and florid hyperplasia with cribriform patterning, which is most pronounced in the AP (See Figure 3). These studies revealed an important role for Foxa1 expression in the adult prostate for the maintenance of a differentiated phenotype.
PBCre4 targets luminal prostate epithelium, resulting in a “mosaic” knockout of Foxa1. This phenomenon revealed differences in the relative androgen sensitivity of Foxa1 positive and negative cells. These results indicate that Foxa1 positive cells are more sensitive to castration (or that Foxa1 negative cells are more resistant to castration), enabling a relative expansion of the Foxa1 negative epithelial cell population (Figure 6). It is impossible to know if these results are a direct influence of decreased AR action on the epithelial cell compartment (which is now heterogenous in regard to Foxa1 expression), or if they are a reflection of differences in which FOXA1 positive and negative epithelium responds to androgen-regulated paracrine signals from the stroma (28). Put differently, in the epithelium our observation may be the result of the physical interaction between Foxa1 and AR, as well as the fact that these transcription factors act in a cooperative manner to regulate gene expression in response to paracrine stromal signals. Further studies are planned to identify the exact mechanism by which Foxa1 status influences sensitivity to androgens. It is also interesting to note that PBCre4 induced recombination in the majority of prostate epithelium as revealed by our reporter mouse studies (see Figure 2 and supplementary data), which would predict a more complete knockout of Foxa1 then was revealed in our studies (see Figure 4). This apparent discrepancy may indicate that Foxa1 knockout is initially toxic in a subset of prostate epithelium, while additional populations in which Foxa1 is successfully ablated survive this genetic change. Nonetheless, Foxa1 ablation with the PBCre4 did result in a striking phenotype (see Figure 3). Further study is required to determine the short-term impact of Foxa1 knockout.
AR expression is widely distributed among tissues derived from the developing germ layers (endoderm, ectoderm, and mesoderm); yet, precise genes associated with differentiation are regulated by AR in specific cell populations (29). The cell-specific expression of AR target genes is explained via the combinational control of gene expression model. This paradigm predicts that the presence and activity of a unique combination of different regulatory proteins during development and in adult tissues dictates cell fate (29, 30). The family of forkhead proteins is particularly interesting since their expression is temporally restricted during development and specific to adult cell populations. We reported that the AR and Foxa1 protein formed a complex via their DNA binding domains on multiple prostate specific genes and that this complex is critical for differentiation of the prostate gland (8, 9, 11). It is now widely recognized that the AR/FOXA1 complex is fundamental in androgen action in the prostate and the function of FOXA1 has been extended to include estrogen receptor action in human breast (31). Recently, ChIP-Seq experiments on the glucocorticoid receptor showed that binding sites for this steroid receptor were enriched for forkhead binding sites—the precise forkhead remain unidentified (32). Here we report that the conditional knock out of Foxa1 in the adult mouse prostate results in the loss of prostate-specific gene expression and the gain of at least 57 genes normally expressed in the seminal vesicle. Within this group of genes, we see expression of the androgen regulated seminal vesicle secretion (SVS) genes. SVS2 is expressed in both the prostate and seminal vesicles (33, 34) but SVSI and SVS3, SVS4, SVS5, and SVS6 are major androgen regulated secretory proteins specific to the seminal vesicles (35). In keeping with this observation, we discovered that Foxa1 KO resulted in the increased expression of Nr4a3 and Foxc1 (see supplementary data), both of which are associated with mesodermal development (36, 37). This switch of gene expression demonstrates that the prostate, which originates from endoderm, can be reprogrammed by the loss of Foxa1 to express androgen target gene that are specific to the seminal vesicle, which originates from mesoderm. This reprogramming is consistent with other studies which have demonstrated that not all AR binding sites depend upon FOXA1 binding, and in fact, loss of FOXA1 can reprogram AR to bind alternative promoters, and result in expression of AR-regulated genes that are normally repressed in the presence of FOXA1 (38). In the mouse prostate, loss of FOXA1 appears to mediate the expression of seminal vesicle genes.
In order to determine possible alternative forkhead binding partners for AR, we looked both in our microarray analysis of the PB-Foxa1 mouse and the literature. Foxc1 was the most up regulated forkhead family member by microarray analysis, and analysis of Fox family members in prostate cancer cell lines had previously revealed that FOXA1 and FOXC1 where inversely expressed in androgen-dependent and independent cell lines (27). Although Foxa1 expression is largely restricted to endoderm, Foxa1 is detected in some mesodermally and ectodermally derived tissues (39, 40). However, IHC staining of the seminal vesicle shows that the epithelial cells are Foxa1 negative but Foxc1 positive. Also, IHC staining of the prostate shows that the control prostates are Foxc1 negative but the conditional knock out of Foxa1 results in expression of Foxc1 in the prostate epithelium. Further, we demonstrate that the AR and Foxc1 can directly interact suggesting that an AR/Foxc1 complex is important in androgen action in the seminal vesicle. Unfortunately, no seminal vesicle cell lines have been developed that would allow us to confirm the AR/Foxc1 complex regulates seminal vesicle genes that are enriched following Foxa1 knockout. However, it is interesting to note that approximately 40% of primary prostate tumors are FOXA1 negative, and these negative prostate tumors exhibit relatively indolent behavior (41). It would be interesting to determine the extent to which FOXC1 and seminal vesicle-related genes are upregulated in this tumor cohort, as well as the ramifications of these potential gene expression changes in regard to tumor biology.
Forkhead transcription factors are grouped into subfamilies (such as the Foxa subfamily) based on the extent of sequence conservation within the DNA binding domain. Of the 43 forkhead proteins, Foxb1, Foxb2, and Foxc1 have the next most conserved DNA binding motifs to Foxa1 (1). Taken together, this suggests a model where some of the organ-specific target genes induced by the AR can, in part, be due to specific complexes between this receptor and a forkhead protein. Likely, this model would apply to other steroid receptors that would partner with specific forkhead proteins to control steroid receptor action in multiple organs.
Supplementary Material
Acknowledgments
Grant support:
5R01-DK055748 to RJM
5T32 DK007563-21 and 1T32 CA119925 to DJD
American Cancer Society Great Lakes Division-Michigan Cancer Research Fund Postdoctoral Fellowship ( to DJD
1T32-CA119925 to MMG
5R01-DK067049 to SWH
5K01-DK085194 to NG
Abbreviations
- AP
anterior prostate
- DAPI
4′,6-diamidino-2-phenylindole
- DP
dorsal prostate
- EDTA
ethyldiamine tetra acetic acid
- Fam3b
Family With Sequence Similarity 3, Member B
- FOX/Fox
human/mouse forkhead box superfamily of transcription factors
- Foxa1
forkhead box a1
- Foxa2
forkhead box a2
- Foxc1
forkhead box c1
- GFP
green fluorescent protein
- Gt(ROSA)26Sortm4(ACTB-tdTomato-EGFP)Luo/J
a reporter mouse strain; expresses red fluorescent protein prior to recombination, green fluorescent protein after recombination
- HNF1α
hepatocyte nuclear factor 1 alpha; original name for FOXA1
- KO
knockout
- Krt
cytokeratin
- LNCaP
human prostate cancer cell line
- LP
lateral prostate
- PBCre4
probasin-promoter driving Cre recombinase; a prostate-specific promoter
- PBCre4/Tomato
PBCre4 mouse strain bread to Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; enables identification of cells targeted by PBCre4 in prostate
- Prom2
Prominin 2
- SV
seminal vesicle
- SVS
seminal vesicle secretion; a family of seminal vesicle-specific genes
- Svs3b
seminal vesicle secretion 3b
- Svs6
seminal vesicle secretion 6
- Trpv6
transient receptor potential cation channel, subfamily V, member 6
- Usp18
ubiquitin specific peptidase 18
- VP
ventral prostate
- WPMY-AR
human prostate stromal cell line, engineered to stably express the androgen receptor
Footnotes
Disclosure/duality of interests:
The authors have nothing to disclose.
References
- 1.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nature reviews Genetics. 2009;10(4):233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai E, Prezioso VR, Smith E, Litvin O, Costa RH, Darnell JE., Jr HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev. 1990;4(8):1427–1436. doi: 10.1101/gad.4.8.1427. [DOI] [PubMed] [Google Scholar]
- 3.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 4.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9(2):279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 5.Yan J, Xu L, Crawford G, Wang Z, Burgess SM. The forkhead transcription factor FoxI1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol Cell Biol. 2006;26(1):155–168. doi: 10.1128/MCB.26.1.155-168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25(21):2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62(4):339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- 8.Gao N, Ishii K, Mirosevich J, et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132(15):3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 9.DeGraff DJ, Yu X, Sun Q, et al. The role of Foxa proteins in the regulation of androgen receptor activity. In: Tindall DJ, Mohler JL, editors. Androgen Action in Prostate Cancer. 2008. [Google Scholar]
- 10.Bernardo GM, Keri RA. FOXA1: a transcription factor with parallel functions in development and cancer. Biosci Rep. 2012;32(2):113–130. doi: 10.1042/BSR20110046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao N, Zhang J, Rao MA, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17(8):1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Yu X, Case T, et al. Mash1 expression is induced in neuroendocrine prostate cancer upon the loss of Foxa2. Prostate. 2013;73(6):582–589. doi: 10.1002/pros.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-Catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30(16):1868–1879. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbieri CE, Bangma CH, Bjartell A, et al. The Mutational Landscape of Prostate Cancer. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44(6):685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. 2006;66(10):1013–1028. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 18.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22(24):3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101(1–2):61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 20.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 21.DeGraff DJ, Clark PE, Cates JM, et al. Loss of the urothelial differentiation marker FOXA1 is associated with high grade, late stage bladder cancer and increased tumor proliferation. PloS one. 2012;7(5):e36669. doi: 10.1371/journal.pone.0036669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30(16):1868–1879. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner MJ, Welliver RC, Jr, Chen M, et al. Effects of androgen receptor and androgen on gene expression in prostate stromal fibroblasts and paracrine signaling to prostate cancer cells. PLoS One. 2011;6(1):e16027. doi: 10.1371/journal.pone.0016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Gao N, DeGraff DJ, et al. Characterization of cis elements of the probasin promoter necessary for prostate-specific gene expression. Prostate. 2010;70(9):934–951. doi: 10.1002/pros.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerhardt J, Montani M, Wild P, et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am J Pathol. 2012;180(2):848–861. doi: 10.1016/j.ajpath.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 27.van der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103(11):1574–1580. doi: 10.1111/j.1464-410X.2009.08351.x. [DOI] [PubMed] [Google Scholar]
- 28.Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000;62(4):831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- 29.Matusik RJ, Jin RJ, Sun Q, et al. Prostate epithelial cell fate. Differentiation. 2008;76(6):682–698. doi: 10.1111/j.1432-0436.2008.00276.x. [DOI] [PubMed] [Google Scholar]
- 30.Gierer A. Molecular models and combinatorial principles in cell differentiation and morphogenesis. Cold Spring Harb Symp Quant Biol. 1974;38:951–961. doi: 10.1101/sqb.1974.038.01.097. [DOI] [PubMed] [Google Scholar]
- 31.Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13(7):482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 32.Sahu B, Laakso M, Pihlajamaa P, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73(5):1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 33.Dodd JG, Kreis C, Sheppard PC, Hamel A, Matusik RJ. Effect of androgens on mRNA for a secretory protein of rat dorsolateral prostate and seminal vesicles. Mol Cell Endocrinol. 1986;47(3):191–200. doi: 10.1016/0303-7207(86)90112-7. [DOI] [PubMed] [Google Scholar]
- 34.Harris SE, Harris MA, Johnson CM, et al. Structural characterization of the rat seminal vesicle secretion II protein and gene. J Biol Chem. 1990;265(17):9896–9903. [PubMed] [Google Scholar]
- 35.Ostrowski MC, Kistler MK, Kistler WS. Purification and cell-free synthesis of a major protein from rat seminal vesicle secretion. A potential marker for androgen action. J Biol Chem. 1979;254(2):383–390. [PubMed] [Google Scholar]
- 36.Wilm B, James RG, Schultheiss TM, Hogan BL. The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev Biol. 2004;271(1):176–189. doi: 10.1016/j.ydbio.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 37.DeYoung RA, Baker JC, Cado D, Winoto A. The orphan steroid receptor Nur77 family member Nor-1 is essential for early mouse embryogenesis. J Biol Chem. 2003;278(47):47104–47109. doi: 10.1074/jbc.M307496200. [DOI] [PubMed] [Google Scholar]
- 38.Sahu B, Laakso M, Ovaska K, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. Embo J. 2011;30(19):3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118(1):47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 40.Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5(2):193–208. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Imamura Y, Sakamoto S, Endo T, et al. FOXA1 promotes tumor progression in prostate cancer via the insulin-like growth factor binding protein 3 pathway. PLoS One. 2012;7(8):e42456. doi: 10.1371/journal.pone.0042456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.