Abstract
Tens of millions of patients diagnosed with vivax malaria cannot safely receive primaquine therapy against repeated attacks caused by activation of dormant liver stages called hypnozoites. Most of these patients lack access to screening for glucose-6-phosphate dehydrogenase (G6PD) deficiency, a highly prevalent disorder causing serious acute hemolytic anemia with primaquine therapy. We optimized CuCl inhibition of G6PD in normal red blood cells (RBCs) to assess G6PD diagnostic technologies suited to point of care in the impoverished rural tropics. The most widely applied technology for G6PD screening—the fluorescent spot test (FST)—is impractical in that setting. We evaluated a new point-of-care G6PD screening kit (CareStart G6PD, CSG) against FST using graded CuCl treatments to simulate variable hemizygous states, and varying proportions of CuCl-treated RBC suspensions to simulate variable heterozygous states of G6PD deficiency. In experiments double-blinded to CuCl treatment, technicians reading FST and CSG test (n = 269) classified results as positive or negative for deficiency. At G6PD activity ≤40% of normal (n = 112), CSG test was not inferior to FST in detecting G6PD deficiency (P = 0.003), with 96% vs 90% (P = 0.19) sensitivity and 75% and 87% (P = 0.01) specificity, respectively. The CSG test costs less, requires no specialized equipment, laboratory skills, or cold chain for successful application, and performs as well as the FST standard of care for G6PD screening. Such a device may vastly expand access to primaquine therapy and aid in mitigating the very substantial burden of morbidity and mortality imposed by the hypnozoite reservoir of vivax malaria.
Abbreviations: ACD, acid citrate dextrose; CSG, CareStart G6PD deficiency test; FST, fluorescent spot test; G6PD, glucose-6-phosphate dehydrogenase; NADP, nicotinamide adenine diphosphate; RBC, red blood cell
Introduction
At A Glance Commentary.
Baird JK, et al.
Background
Plasmodium vivax threatens 2.5 billion and causes >100 million clinical attacks, most originating from untreated forms in the liver. These are rarely treated because the only drug, primaquine, causes threatening acute hemolytic in patients having an inborn deficiency in glucose-6-phosphate dehydrogenase (G6PD).
Translational Significance
-
•
We affirm noninferiority of a potentially important new clinical instrument—a G6PD deficiency test suitable for use where most patients with malaria live—compared with the laboratory standard test.
-
•
We detail a novel laboratory technique for such evaluations—copper inhibition of G6PD in normal red blood cell modeling the full range of phenotype heterogeneity among hemizygotes and heterozygotes.
More than 2.5 billion people live at risk of infection by the blood parasite Plasmodium vivax, and more than a hundred million suffer clinical attacks every year.1, 2 Although long viewed as a relatively benign infection, reports and studies from endemic areas and in travelers over the past decade reveal an often pernicious and sometime fatal course associated with a diagnosis of vivax malaria.3, 4 This understanding has focused renewed emphasis and interest on long-neglected clinical and public health issues regarding this infection, especially the very difficult problem of glucose-6-phosphate dehydrogenase (G6PD) deficiency and primaquine therapy.5
The main clinical and public health problem is the ability of P. vivax to place dormant forms in the liver called hypnozoites. These parasites typically cause 3 or more clinical attacks in relatively quick succession in the months after the primary attack, or may do so up to 1 or 2 years later.6 Some heavily exposed patients suffer up to 20 distinct hypnozoite-borne attacks of vivax malaria within 2 years.7 Among cohorts in Thailand and Indonesia, the incidence density of first relapse in the 2 months after a primary attack was about 5/person-year.8, 9, 10 Such attack rates approximate those of Plasmodium falciparum in the highest risk zones of sub-Saharan Africa.11 Failure to prevent relapse in vivax malaria results in very high risk of debilitating illness of deepening seriousness and opportunities for onward transmission to others. Nonetheless, most patients diagnosed with vivax malaria do not receive therapy against relapse as a consequence of the rational fear of causing serious harm with primaquine among unscreened patients with G6PD deficiency.5
Among the many drugs available to treat the acute attack of vivax malaria, none affect the latent hypnozoites.12 The only drug registered as safe and effective in preventing relapses is primaquine, and it has been in continuous use since 1952. At therapeutic dosing against relapse, primaquine causes a mild to severe acute hemolytic anemia in patients having an inborn deficiency of G6PD.13, 14 This extraordinarily diverse and complex X-linked trait occurs most frequently where there is endemic malaria transmission, as it may confer some protection against the onset of severe and threatening malaria.15 About 400 million people are affected, with an average prevalence of G6PD deficiency in malaria endemic nations of about 8%.16 The blind administration of primaquine to patients diagnosed with vivax malaria is often rationally considered unacceptably hazardous or reckless by providers of malaria treatment services. In impoverished rural settings, patients very often are not provided primaquine therapy as a direct consequence of a lack of access to G6PD screening.
G6PD deficiency as the basis of hemolytic sensitivity to primaquine was described in 1956,17 and a variety of diagnostic tests for the disorder appeared within a decade. One of the most widely recommended and used has been the fluorescent spot test (FST) described in 1966 by hematologist and pioneering G6PD scientist Ernest Beutler.18 It has seen several decades of practical and safe use in the developed world, but finds almost no routine application where most patients with malaria live. The reasons include cost, specialized equipment, laboratory skills, temperature sensitivity, and a cold chain for the reagents. Any one of those pitfalls may suffice to prohibit routine use in impoverished tropical settings. The combination of them explains more than 50 years without access to G6PD screening, which in turn accounts for the lack of access to primaquine therapy against vivax malaria for almost all those patients. We consider this deceptively simple problem the likely basis of most clinical attacks of vivax malaria and attendant burdens of morbidity and mortality.
In the present study, we conducted a laboratory-based evaluation of the performance of a new G6PD screening device that appears to have overcome the obstacles to practical use where most patients with malaria live. The CareStart G6PD kit (CSG; AccessBio Inc, New Jersey) requires no specialized training, equipment, cold chain, or controlled temperature setting. A result is rendered within 10 minutes. The kit sells for $1.50 per test. We reasoned that practical point-of-care qualitative screening for G6PD by CSG should be noninferior to the FST in red blood cells (RBCs) exhibiting variable levels of residual G6PD activity after being incubated with the G6PD inhibitor CuCl. After optimizing that inhibition, we designed and executed a series of double-blinded experiments to assess the noninferiority of CSG to FST using simulated G6PD-deficient RBCs for both hemizygous and heterozygous states. We aimed this work at generating the evidence needed to inform decisions for investment in more ambitious evaluations in patients in rural tropical settings.
Materials and Methods
G6PD quantitative assay
The quantitative assay for erythrocytic G6PD activity in hemolysate was performed using the commercial kit from Trinity Biotech (Ireland) as catalog number (cat#) 345-B. The manufacturer's instructions were followed. In brief, substrate of glucose-6-phosphate and cofactor nicotinamide adenine diphosphate, NADP+, was reconstituted with sterile double-distilled water and 2 mL added to 1 mL of hemolysate reaction buffer (provided by the manufacturer). Then, 10 μL of whole blood collected in acid citrate dextrose (ACD) tubes (BD Vacutainer ACD Solution A; Becton-Dickinson) was added to the 3 mL mixture. The tube was incubated at 30°C for 5 minutes and its absorbance at 340 nm wavelength was measured on an ultraviolet spectrophotometer (Biowave II; Biochrome) and recorded as “initial” absorbance optical density. An additional 5 minutes in the 30°C water bath was followed by another absorbance measurement recorded as “final.” Hemoglobin levels on all venous blood samples were measured using a clinical blood analyzer (Abbott Cell Dyne CD1700). These values were applied to calculate the international units of enzyme activity per gram hemoglobin as per the manufacturer's instructions.
In accordance with the Code of Ethics of the World Medical Association expressed in the Declaration of Helsinki, each collection of blood in these experiments was done with the signed, informed consent of the 2 G6PD normal subjects involved under a protocol for such collections that was reviewed and approved by the Ethics Review Board of the Eijkman Institute for Molecular Biology.
Optimizing CuCl inhibition of RBC G6PD activity
Copper inhibits G6PD activity,19 but no work yet described optimized inhibition in intact RBC suspensions. We evaluated quantitative G6PD activity in hemolysate prepared from intact RBCs incubated with copper under a variety of conditions, including anticoagulant, RBC preparation (whole blood, packed RBCs, or hemolysate), incubation time (in a 37°C water bath for 1, 2, 3, or 24 hours), copper ion (CuCl from Fluka cat# 00664 vs CuSO4), and wide ranges of copper concentration (1 nM–0.1 M). We found whole blood collected with ACD anticoagulant and incubated with final concentrations of 0.2–1.0 mM CuCl (1:9 vol/vol CuCl solution in water to whole blood) for 24 hours at 37°C consistently inhibited G6PD activity in a dose-dependent manner by up to 95%. The concentrations of CuCl reported represent those in the final suspension of whole blood with CuCl. These conditions of CuCl treatment represent the experiments detailed in this report.
Modeling variation in hemizygous males or mosaicism in heterozygous females
As an X-linked trait, G6PD deficiency occurs in males only in the hemizygous state, that is, the lone X chromosome is either G6PD wild type or mutant, and all RBCs will express either normal or deficient phenotypes. The heterogeneity of G6PD activity among hemizygotes ranges from nearly normal to barely detectable.20 We modeled this heterogeneity among male hemizygotes by treating RBCs with variable concentrations of CuCl, where all RBCs in the suspension had impaired G6PD activity.
Females, in contrast, possess 2 X chromosomes that may be wild type:wild type, wild type:mutant, or mutant:mutant (wild type, heterozygous, and homozygous, respectively). The heterozygotes pose a particular diagnostic problem because of the lyonization of the trait during random inactivation of 1 X chromosome during embryonic development.21 This results in RBCs of individual females expressing either fully normal or fully deficient phenotypes in a mosaic of fixed proportions ranging between 0% and 100%. We modeled this mosaicism among female heterozygotes by mixing variable proportions of untreated and 1.0 mM CuCl-treated RBCs for diagnostic evaluation. Homozygous females have 100% deficient RBC populations and were effectively represented by the hemizygous model.
G6PD qualitative assays
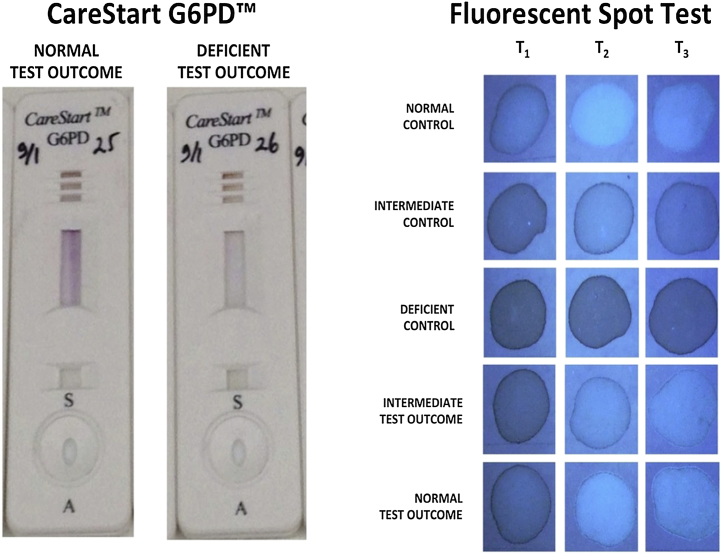
Two commercially available qualitative G6PD deficiency screening kits were used in the experiments: (1) G-6-PDH, cat# 203-A from Trinity Biotech, Bray, Ireland and (2) CareStart G6PD, cat# G0221 from AccessBio (Somerset, New Jersey). Henceforth, these kits will be referred to as FST and CSG, respectively, throughout this report. The kits have been used as per manufacturer's instructions. The FST was always executed with 3 G6PD controls sold separately by the manufacturer (Trinity Biotech): (1) G6PD normal control (cat# G6888); (2) G6PD intermediate control (cat# G5029); and (3) deficient control (cat# G5888). In brief, the FST involved placing 10 μL whole blood into the manufacturer's hemolyzing (0.2% saponin) buffer containing NADP+ cofactor and glucose-6-phosphate substrate and placed into a 37°C water bath. Aliquots of 20 μL were taken and placed onto filter paper at designated intervals. The dried filters (about 30 minutes) were read under ultraviolet light within a few minutes in a dark room. G6PD normal hemolysate on filter paper fluoresced brightly (by the dominance of nicotinamide adenine diphosphate), whereas G6PD-deficient hemolysate remained dark (by the dominance of NADP+). Fig 1 illustrates this distinction in color development.
Fig 1.
Photographs illustrating the visual read of the G6PD screening devices according to the intensity of color development.
The CSG involved placing 2–3 μL whole blood into a receptacle within a plastic cassette, followed by a few drops of hemolyzing reaction buffer provided with the kit. The cassette was visually read after standing 10 minutes at room temperature. Development of a distinct purple color in the cassette window represented a negative test outcome, whereas development of no color, or color distinctly lighter than most others, constituted evidence of a positive test outcome, that is, positive for G6PD deficiency. Fig 1 illustrates this distinction in color development. The CSG is composed of a cellulose strip impregnated with the G6P substrate of G6PD and a colorless tetrazolium compound salt (patent pending). Reduction of that compound yields a purple formazan dye. In the strip containing hemolysate and G6P substrate, the extent of reduction depends on G6PD activity. The package insert for this product specifies that a tested concentration of 0.156 mM (2.5 mg/dL) CuCl did not impact with the assay system. The highest final concentration of CuCl in the G6PD activity assays did not exceed 0.04 mM (after dilution of RBC suspension in lysates). We thus considered CuCl interference in the assays by direct redox disturbance (as opposed to its known G6PD enzyme inhibitory properties) very unlikely.
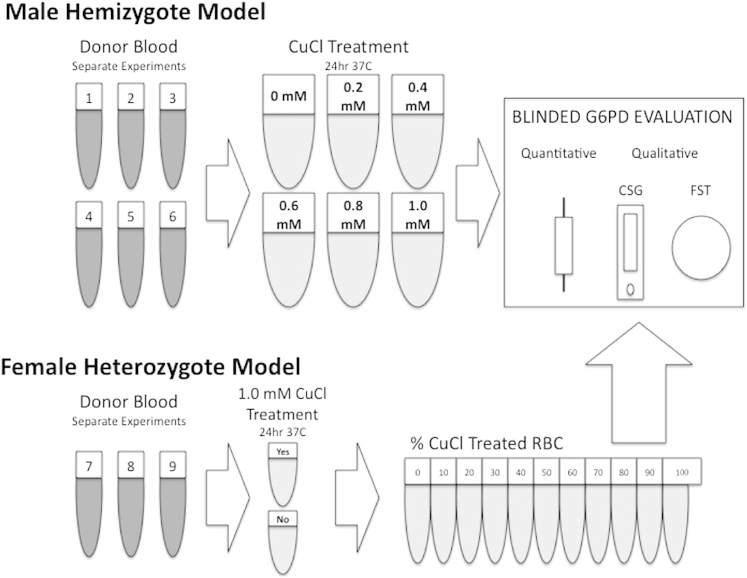
A total of 9 separate experiments over the course of several months using 2 known G6PD normal blood donors were conducted (see Fig 2). On each occasion a suspension of 0.45 mL whole blood mixed with 0.05 mL water served as the normal (no CuCl) G6PD activity control. In the case of the hemizygote model, 5 other tubes contained the same except with the addition of CuCl to water to provide final whole blood suspension of CuCl concentrations of 0.2, 0.4, 0.6, 0.8, and 1.0 mM. In the case of the heterozygote model, blood was incubated with 1.0 mM CuCl in water or water only. These were placed in a 37°C water bath and incubated for 24 hours.
Fig 2.
Diagram illustrating 9 separate experiments and the course of treatment with copper chloride before blinded diagnostic evaluation by 3 distinct devices; quantitative, and 2 qualitative tests, the CareStart G6PD (CSG) and fluorescent spot test (FST). The representations of tubes under donor blood indicate experiments on different days. The tubes showing CuCl treatments from 0 to 1.0 mM (top) represent a set of 5 replicates each for each separate experiment (#1–#6). The tubes showing %CuCl treated from 1% to 100% (bottom) each represent a set of triplicates for each of the experiments conducted (#7–#9).
After gentle mixing, these tubes were immediately treated essentially as whole blood in the conduct of the quantitative and qualitative G6PD assays as outlined previously in accordance with the standard instructions. In case of the hemizygote model, single tubes representing each of the inhibition treatments were aliquoted into 5 tubes. Each of those tubes was then used for all 3 of the G6PD assays that immediately followed: quantitative, FST, and CSG. Each of these 6 experiments thus generated 30 measurements of G6PD activity, 30 FST readings, and 30 CSG readings, or a total of 180 each. In the heterozygote model, each of the 10 distinct CuCl treatments (see Fig 2) were aliquoted into 3 vials, each generating a separate G6PD assessment, or 30 for each of the 3 separate experiments for a total of 90 assessments. In all, 270 separate assessments were conducted for each of the 3 distinct G6PD assays in both models. A single treatment tube evaluated by all 3 tests was rejected as flawed: a G6PD activity value of 0.5 U/gHb was read as negative for G6PD deficiency by both FST and CSG, and we considered this lone set an error of treatment or labeling in excluding it from the analyses reported here. Thus, the total sample evaluated was 269 for each of the 3 methods of G6PD assessment.
Double-blinded reading of qualitative assays
Assay of quantitative and qualitative G6PD in the blood treatments was carried out immediately after the 24 hours of incubation with CuCl or water. A technician not involved in the assays removed the tubes from the water bath and covered them with opaque tape, recording an identity unrelated to CuCl treatment. All results were recorded by that identity. The blinded tubes were taken to the laboratory for carrying out the G6PD quantitative assays and required aliquots were removed, followed by the same for the 2 separate laboratories doing the FST and CSG screening. These 2 laboratories alternated conduct of the FST and CSG on each of the separate days of experiments represented in this report.
All the 6 technicians involved in the qualitative test analysis were trained in doing so beforehand. The training included prohibition on classifying a test outcome as intermediate or indeterminate based on partial color development alone. The demand was made to decide on “positive” or “negative” (deficient or normal), with clear instructions to consider noticeably diminished color development relative to normal control as positive. We considered this approach appropriate for the intended use of the kits, that is, in guiding a decision to apply primaquine therapy, in which a classification of an “intermediate” as positive for deficiency errs in favor of the safety of the patient. Further, instruction to consider the development of color of any intensity as negative likely leads to underestimation of the sensitivity of G6PD deficiency screening.22
Assessment of CSG noninferiority to FST
The statistical analysis of this study applied the methods of testing equivalence or noninferiority essentially as described by da Silva et al.23 The conventional analyses of sensitivity and specificity for diagnostic devices suffer the drawback imposed by broad heterogeneity of G6PD activity (both in the experimental model and in patients). There is uncertainty of the threshold of that activity for safety with a decision to proceed with primaquine therapy. In other words, the simple dichotomy of positive or negative test outcomes underpinning the mathematical treatment of sensitivity and specificity estimates imposes real uncertainty in the context of G6PD deficiency and primaquine safety. Statistical testing for noninferiority largely solved these problems.
Conventional hypothesis testing statistics evaluate differences between groups. Typically, P value estimates <0.05 reflect statistical significance of difference, and those >0.05 indicate a lack of difference, or statistical sameness. The test of noninferiority does not rely on P values >0.05, largely because such values may simply be a product of insufficient power to discern real difference. The statistical treatment for noninferiority averts such possible ambiguity by instead assigning P values <0.05, where there is genuine, adequately powered equivalence or noninferiority. The noninferiority testing reported here applied an alpha of 5%, a 90% confidence interval, and a noninferiority margin of 5%.
The statistical analysis of noninferiority combined the screening test outcomes from both the male hemizygous and female heterozygous models. We reasoned that the percent of normal G6PD activity in the RBC suspension as a whole, regardless of the means of its compromise, represented the key determinant of diagnostic performance. Noninferiority of CSG to FST was assessed at discrete levels of residual G6PD activity, a key advantage of the CuCl model over naturally G6PD-deficient RBCs for this purpose.
Assessment of sensitivity and specificity of FST and CSG
We examined the diagnostic performance of the FST and CSG relative to quantitative G6PD activity using standard estimates of sensitivity, specificity, positive predictive value, and negative predictive value for each. This approach required establishing a firm threshold of G6PD activity for positivity for G6PD deficiency. We chose ≤40% of normal values as the threshold. We reasoned that hemizygotes having higher levels than this threshold could safely receive primaquine therapy. However, it is acknowledged that such a threshold is not grounded in sufficient clinical evidence, and it may not apply to heterozygous females for the complex reasons explained. Nonetheless, these diagnostic performance characteristics provide a useful, even if strictly limited, metric of diagnostic performance in the context of screening for primaquine therapy.
Results
G6PD quantitative activity with CuCl treatment
The 5 treatments with CuCl (0.2, 0.4, 0.6, 0.8, and 1.0 mM) modeling hemizygous states resulted in levels of G6PD inhibition ranging from slight with 0.2 mM CuCl to as much as 95% with 1.0 mM CuCl. These values represented a continuum between 138% (9.6 U/gHb) and 5% (0.4 U/gHb) of the mean value from samples not exposed to CuCl (6.9 U/gHb) in that series.
A similar continuum of G6PD activity occurred among the variable proportions of CuCl-treated (1.0 mM) RBC modeling heterozygous states. The mean-untreated G6PD activity was 7.8 U/gHb in that series and ranged between 10.8 and 0.8 U/gHb (138%–10% of normal, respectively).
Noninferiority analysis of CSG to FST
The analysis of noninferiority of CSG to FST treated G6PD activity as a continuous variable rather than according to groups of CuCl treatment. Table I lists the results of this analysis. Qualitative test outcomes were pooled into groups according to percent of normal G6PD activity in increments of 10 percentiles and each statistically analyzed for noninferiority. At all levels of G6PD activity except the lowest and highest increments (which were inconclusive because of inadequate sample size), the diagnostic performance of the CSG was not inferior to the FST, with P values ranging from 0.006 to <0.001.
Table I.
Table lists G6PD screening test outcomes at various thresholds of G6PD inhibition and the statistical noninferiority statistics of CSG vs FST at each level
| % Normal G6PD activity | Test type | Test positive for deficiency | Test negative for deficiency | Number of tests | % Positive for deficiency | P value | Result |
|---|---|---|---|---|---|---|---|
| ≤20% | FST | 30 | 1 | 31 | 97% | 0.058 | Inconclusive |
| CSG | 31 | 0 | 31 | 100% | |||
| ≤30% | FST | 75 | 3 | 78 | 96% | 0.006 | Not inferior |
| CSG | 77 | 1 | 78 | 99% | |||
| ≤40% | FST | 101 | 11 | 112 | 90% | 0.003 | Not inferior |
| CSG | 107 | 5 | 112 | 96% | |||
| ≤50% | FST | 117 | 33 | 150 | 78% | 0.001 | Not inferior |
| CSG | 131 | 19 | 150 | 87% | |||
| ≤60% | FST | 119 | 60 | 179 | 67% | 0.001 | Not inferior |
| CSG | 137 | 42 | 179 | 77% | |||
| ≤70% | FST | 121 | 81 | 202 | 60% | 0.001 | Not inferior |
| CSG | 142 | 60 | 202 | 70% | |||
| ≤80% | FST | 121 | 97 | 218 | 56% | <0.001 | Not inferior |
| CSG | 145 | 73 | 218 | 67% | |||
| ≤90% | FST | 122 | 110 | 232 | 53% | 0.001 | Not inferior |
| CSG | 146 | 86 | 232 | 63% | |||
| ≤100% | FST | 122 | 124 | 246 | 50% | 0.001 | Not inferior |
| CSG | 146 | 100 | 246 | 59% | |||
| >100% | FST | 0 | 23 | 23 | 0% | 0.443 | Inconclusive |
| CSG | 0 | 23 | 23 | 0% |
Abbreviations: CSG, CareStart G6PD; FST, fluorescent spot test; G6PD, glucose-6-phosphate dehydrogenase.
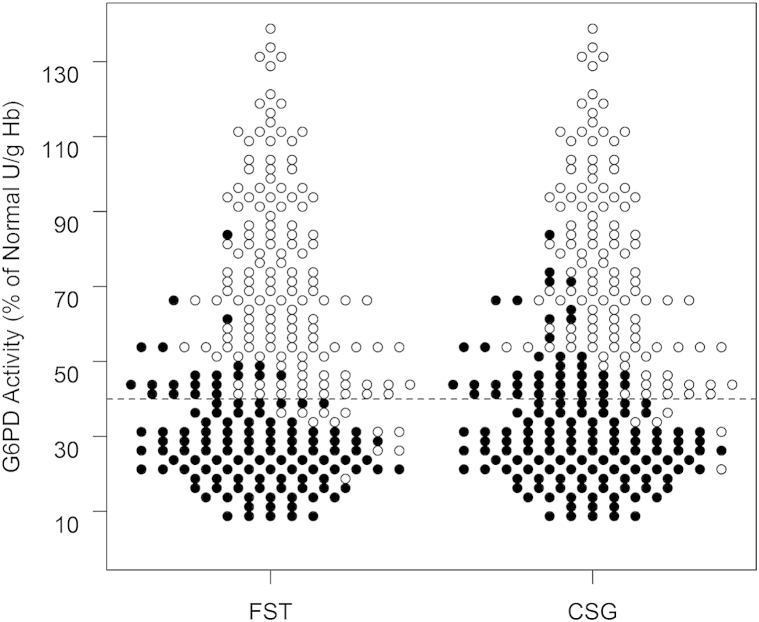
Fig 3 illustrates all these test outcomes grouped in increments of 0.25 U/gHb. The graphic visualizes the general equality in G6PD detection across the range of G6PD activity levels. The lateral dotted line in the graphic represents the cutoff of 40% of normal G6PD activity applied to separate those positive or negative for G6PD deficiency. The graphic also shows the slightly lower frequency of false negatives among CSG, and the higher frequency of false positives, especially at levels immediately higher than 40% of normal G6PD activity.
Fig 3.
Scatter plot illustrates individual screening test outcomes by qualitative fluorescent spot test (FST, to left) or CareStart G6PD (CSG, to right) at 2.5% of normal increments of G6PD activity (y-axis) among all tests conducted (n = 269 each), where black circles indicate a reading as positive for G6PD deficiency, and the white circles indicate a negative reading. The horizontal dashed line across the graph represents the 40% cutoff adopted in this study for separating G6PD-deficient and normal activity levels.
Sensitivity and specificity of the FST and CSG
Table II lists the test outcomes and statistics for the sensitivity and specificity of the FST and CSG when using ≤40% of normal G6PD activity as the threshold of positivity for G6PD deficiency. The analysis tends to affirm the trends seen in the scatter plot of Fig 3, that is, equality of sensitivity in the FST and CSG (90% vs 96%; P = 0.19) and lesser specificity in the CSG (89% vs 75%; P = 0.01). In brief, the CSG performed as well as the FST in detecting G6PD deficiency at ≤40% of normal, but more often misclassified higher levels of activity as positive for deficiency.
Table II.
Summary of the diagnostic performance of FST and CSG when considering <40% of normal G6PD activity as the threshold for positivity for G6PD deficiency
| Test | Outcome | G6PD activity (% of normal U/gHb) |
Diagnostic performance |
||||
|---|---|---|---|---|---|---|---|
| ≤40% | >40% | % Sensitivity (95% CI) | % Specificity (95% CI) | % Positive predictive value (95% CI) | % Negative predictive value (95% CI) | ||
| FST | Positive | 101 | 21 | 90 (83–95) | 87 (80–92) | 83 (75–89) | 93 (87–96) |
| Negative | 11 | 136 | |||||
| CSG | Positive | 107 | 39 | 96 (90–99) | 75 (68–82) | 73 (65–80) | 96 (91–99) |
| Negative | 5 | 118 | |||||
| P value | 0.19 | 0.01 | 0.08 | 0.31 | |||
Abbreviations: CI, confidence interval; CSG, CareStart G6PD; FST, fluorescent spot test; G6PD, glucose-6-phosphate dehydrogenase.
CSG and FST positivity in simulated hemizygote and heterozygote states
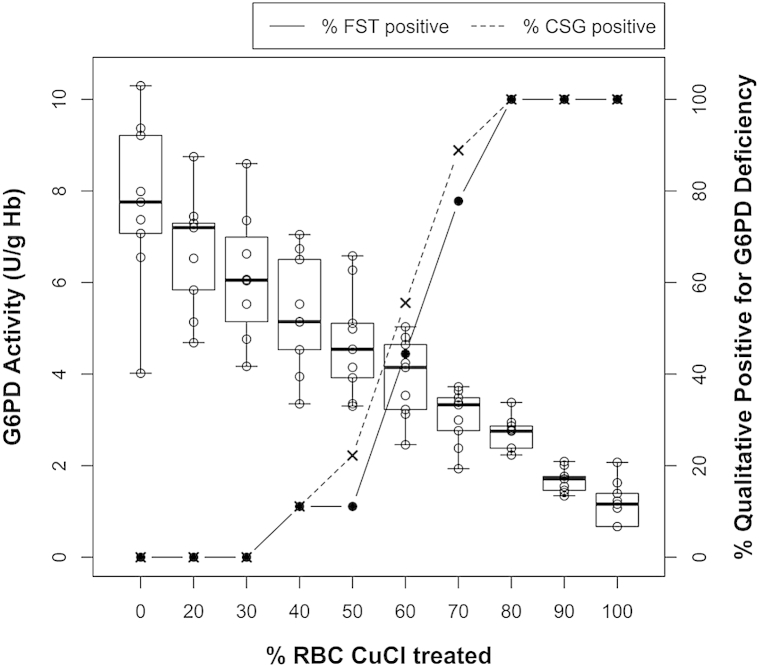
Fig 4, Fig 5 illustrate FST and CSG positivity across the range of G6PD activity levels that naturally occur among patients in both the hemizygous and heterozygous states. The essentially similar findings across CuCl treatments (either variable concentrations or variable proportions of treated RBCs) affirm the dependence of qualitative diagnostic outcomes on net G6PD activity in RBC suspensions. In other words, the presence of uninhibited G6PD enzyme did not overcome the effects of variable proportions of CuCl-inhibited G6PD enzyme. The model suggests that hemizygotes and heterozygotes will test as G6PD deficient depending on the same net G6PD activity level, whether because of all RBCs being inhibited or some proportion of them.
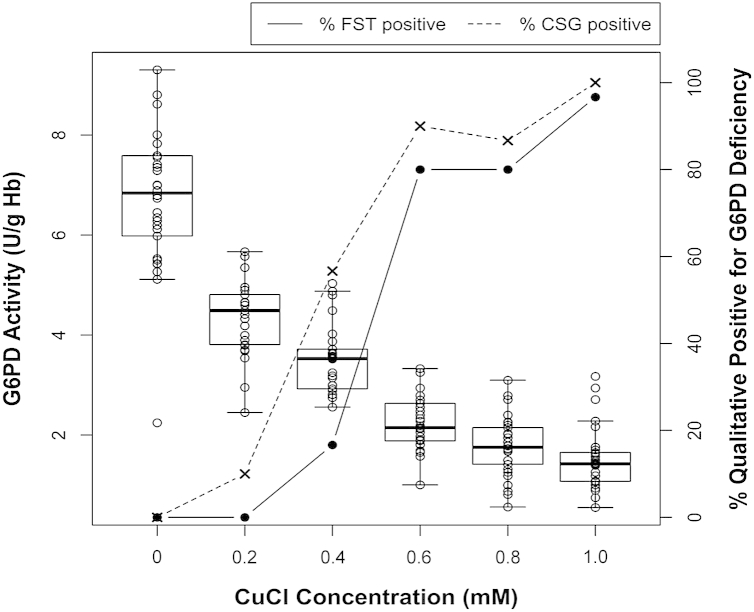
Fig 4.
Graph illustrates diminishing quantitative G6PD activity (left y-axis) with increasing concentrations of CuCl treatment (x-axis) and the proportion of qualitative tests (fluorescent spot test [FST], solid line and filled circles or CareStart G6PD [CSG], dashed line and × marks) positive for G6PD deficiency (right y-axis). As in hemizygous states of G6PD deficiency, all red blood cells (RBCs) experience the effects of CuCl inhibition of G6PD. Hollow circles indicate quantitative G6PD measures defining a mean (solid lateral line) within boxes of 95% confidence intervals in the observed range of values (T lines at either end of the boxes).
Fig 5.
Graph illustrates diminishing quantitative G6PD activity (left y-axis) with increasing proportions of 1.0 mM CuCl-treated red blood cells (RBCs) with untreated RBCs (x-axis) and the proportion of qualitative tests (fluorescent spot test [FST], solid line and filled circles or CareStart G6PD [CSG], dashed line and × marks) positive for G6PD deficiency (right y-axis). As in heterozygous states of G6PD deficiency, only CuCl-treated RBCs experience the effects of CuCl inhibition of G6PD. Hollow circles indicate quantitative G6PD measures defining a mean (solid lateral line) within boxes of 95% confidence intervals in the observed range of values (T lines at either end of the boxes).
Findings in the experiments modeling the heterozygous state model suggest that both the FST and CSG will perform inconsistently between the range of 40% and 70% of RBCs being G6PD deficient (at the approximately 10% of normal activity with 1.0-mM CuCl treatment). The odds of being classified as deficient increased in proportion to the diminishing net G6PD activity within that range.
Discussion
The laboratory findings reported here demonstrate noninferiority of a point-of-care screening device for G6PD deficiency (CSG) compared with a screening kit routinely used in the laboratory (FST). CSG has the enormous advantage over FST of appearing suitable for use in the impoverished rural tropics. The successful distribution and use of such a device may finally provide access to antirelapse therapy with primaquine to millions of patients otherwise suffering repeated attacks of acute vivax malaria. Definitive validation of that suitability and adequate diagnostic performance must await large scale, real world assessments in patients with G6PD deficiency and vivax malaria. The current laboratory findings lend to making the substantial investments required to do so.
Inhibiting G6PD activity in intact RBCs with CuCl allowed us to simulate the full range of G6PD activity phenotypes among hemizygous males and heterozygous females. Capturing such representation among naturally occurring phenotypes would be highly impractical, requiring a sample of many tens of thousands among dozens of distinct human populations. The CuCl model for G6PD deficiency is relatively simple and inexpensive, requiring no specialty chemicals or reagents and using only standard laboratory equipment. We have demonstrated the utility of this model in assessing diagnostic performance of 2 qualitative screening tests across the full range of possible G6PD phenotypes. The relative consistency of evenly decreasing G6PD activity across proportions of RBCs treated with 1.0 mM CuCl (Fig 5) suggests that this approach may be superior to variable CuCl concentration treatments (Fig 4) with respect to evaluating G6PD diagnostics performance in general. It was also less laborious.
We considered the diagnosis of G6PD deficiency and a diagnostic test guiding a decision to administer primaquine therapy as 2 distinct clinical objectives. This distinction is important because the FST and other qualitative tests are well known to be unreliable in the diagnosis of G6PD deficiency at residual activities between 30% and 70% of normal.24, 25 The findings in this study corroborated that trend and range. However, the most threatening acute hemolytic anemia caused by primaquine occurs among those having the very lowest levels or G6PD activity, for example, <10%, whereas otherwise healthy men having about 30% of normal G6PD activity have typically exhibited a mild and self-limited hemolysis.26, 27, 28, 29, 30 The scarcity of such evidence across the broad heterogeneity of G6PD activity phenotypes was the basis of applying the noninferiority analysis in this study—there is no definitive level of residual G6PD activity dividing safety vs harm with primaquine therapy. Noninferiority statistical testing across the tiers of impaired enzyme levels provided a more thorough assessment of diagnostic performance. We nonetheless also analyzed diagnostic performance using the conventional statistics, choosing 40% of normal G6PD activity as a reasoned margin of patient safety with primaquine therapy.
Subjective reading of color intensity imposes pitfalls in the FST and CSG. Although wholly normal and conspicuously deficient G6PD phenotypes may be distinguished with relative ease (see Fig 1), the intermediate phenotypes impose real difficulty. We dealt with this uncertainty by training readers to consider any test having diminished color development to be positive, that is, G6PD deficient and ineligible for primaquine therapy. Health care workers in the endemic tropics, we reasoned, would not be trained to make a classification of intermediate for the simple reason that such ambiguity defeats the aim of the test—a “go” vs “no go” decision on primaquine therapy. A classification of intermediate leaves that decision uninformed, so it was abandoned in this assessment. We further reasoned that favoring the classification of positive represented the appropriately conservative approach to the decision on primaquine therapy with regard to patient safety.
At intermediate G6PD activities in our experiments, the subjectivity of reading was most apparent between 2.75 and 3.5 U/gHb (37%–51% of normal; see Fig 3, Fig 4, Fig 5) with both positive and negative readings being relatively frequent. Reading with the CSG as negative in this range proved less likely than with FST (odds ratio = 0.44; 95% confidence interval = 0.20–0.95; P = 0.04). We viewed erring in favor patient safety in this range to be a likely advantage of CSG over FST, but acknowledge denying primaquine therapy to any patient who may safely receive it would also be a poor outcome. Improving specificity with the CSG could perhaps be achieved by the availability of a dummy cassette permanently exhibiting a color representing that occurring at an intermediate G6PD range, where less intense color should be considered positive and more intense color negative for deficiency. Such a simple device would help guide this difficult subjective decision by the reader.
Female heterozygotes impose uncertainty with G6PD diagnostics and primaquine safety. G6PD activity in any given blood sample represents the consensus activity of the many individual RBCs present in the sample. The mosaicism of female heterozygotes for G6PD activity phenotype among RBCs complicates that representation and has implications for the primaquine go vs no go output of a G6DPD diagnostic device. A hypothetical example illustrates this problem: a female presents a consensus G6PD activity of 50% of normal, and thus, she may often test negative for G6PD deficiency despite up to one half of her RBCs perhaps being fully vulnerable to primaquine-induced hemolysis.31 The data illustrated in Fig 5 affirm this problem. Both the FST and CSG performed erratically with >30% and <80% of RBCs being G6PD inhibited (by 1.0 mM CuCl). The proportion classified as negative at 50% of normal activity was approximately 50%. If the G6PD-deficient RBCs in such patients were indeed fully susceptible, neither the CSG nor FST would consistently prevent harmful exposure to primaquine. This problem will require clinical studies that would carefully assess the dangers imposed by this vulnerability.
The most severe G6PD deficiency variants appear to be most common where P. vivax occurs in greatest abundance, in South and Southeast Asia.32 Some evidence suggests that P. vivax drives selection for G6PD deficiency,33 which would require affecting the reticulocytes strictly preferred by this species—natural G6PD activity decays from the highest level in reticulocytes as RBCs age. Populations most likely to benefit from primaquine therapy against relapse may also be at greatest risk of suffering serious harm caused by it.
Primaquine in G6PD normal patients is remarkably safe and well tolerated and still exerts superb efficacy against relapse despite 60 years of continuous use.9, 34, 35, 36 The drug provides the enormous clinical benefit of preventing multiple pernicious and threatening attacks of acute malaria. Denying people access to this therapy undoubtedly imposes substantial and preventable burdens of morbidity and mortality, but providing it imposes risk of the serious harm. Practical and robust G6PD diagnostics at the point of care where most patients with malaria live would greatly mitigate this dilemma. The findings reported here suggest that the CSG may be suitable for this diagnostic task. We detailed a robust means of assessing G6PD diagnostic devices in the laboratory with relative ease, simplicity, and low cost.
The availability of G6PD screening in endemic zones would likely add to the already substantial number of patients who cannot receive primaquine therapy—pregnant or lactating women and infants,37 among the most vulnerable to serious illness with acute vivax malaria.38, 39 Further, some patients with relatively common mutations to 2D6 cytochrome P-450 may remain partially or fully susceptible to relapse despite primaquine therapy.40 These patients, including those screened out as G6PD deficient, will require alternative chemotherapeutic or chemopreventive strategies against relapse. Conceiving, optimizing, and validating such approaches should be a very high clinical research priority.
Acknowledgments
Conflicts of Interest: All authors have read the Journal's policy on disclosure of potential conflicts of interest and have none to declare.
An award from the Li Ka Shing Foundation to J.K.B. supported this work. J.K.B. was also supported by Wellcome Trust grant no. B9RJIXO. AccessBio provided the CareStart G6PD cassettes used in these experiments free of charge. They did so with no written agreements or expectations beyond informal promise of access to the data generated. The authors hold no financial stake or interest in either of the commercially available diagnostic kits evaluated in this study, or any other.
The authors are indebted to Arkasha Sadhewa, Rosalie Elvira, Ungke Antonjaya, Saraswati Soebianto, Jeny, Lia Waslia, Damian Oyong, Bimandra Djaafara, Ynigo Cristo, and Lenny Ekawati of the Eijkman Institute for Molecular Biology and the Eijkman-Oxford Clinical Research Unit within that Institute for the bulk of the considerable laboratory work represented in this report.
All authors have read and approved the journal's authorship agreement. All authors have read the manuscript and approved of its submission for publication.
References
- 1.Gething P.W., Elyazar I.R.F., Moyes C.L., et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price R.N., Tjitra E., Guerra C.A., Yeung S., White N.J., Anstey N.M. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(6 Suppl):79–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Baird J.K. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013;26:36–57. doi: 10.1128/CMR.00074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstey N.M., Douglas N.M., Poespoprodjo J.R., Price R.N. Plasmodium vivax: risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201. doi: 10.1016/B978-0-12-397900-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 5.von Seidlein L., Auburn S., Espino F., et al. Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J. 2013;12:112. doi: 10.1186/1475-2875-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White N.J. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyles D.E., Young M.D. The parasitological pattern of relapsing Plasmodium vivax in military patients. J Natl Malar Soc. 1948;7:23–37. [PubMed] [Google Scholar]
- 8.Pukrittayakamee S., Chantra A., Simpson J.A., et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44:1680–1685. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutanto I., Tjahjono B., Basri H., et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother. 2013;57:1128–1135. doi: 10.1128/AAC.01879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas N.M., Nosten F., Ashley E.A., et al. Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kinetics. Clin Infect Dis. 2011;52:612–620. doi: 10.1093/cid/ciq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird J.K., Owusu Agyei S., Utz G.C., et al. Seasonal malaria attack rates in infants and young children in northern Ghana. Am J Trop Med Hyg. 2002;66:280–286. doi: 10.4269/ajtmh.2002.66.280. [DOI] [PubMed] [Google Scholar]
- 12.Baird J.K., Maguire J.D., Price R.N. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol. 2012;80:203–270. doi: 10.1016/B978-0-12-397900-1.00004-9. [DOI] [PubMed] [Google Scholar]
- 13.Beutler E., Dern R.J., Alving A.S. The hemolytic effect of primaquine. III. A study of primaquine-sensitive erythrocytes. J Lab Clin Med. 1954;44:177–184. [PubMed] [Google Scholar]
- 14.Beutler E., Duparc S., G6PD Deficiency Working Group Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77:779–789. [PubMed] [Google Scholar]
- 15.Clark T.G., Fry A.E., Auburn S., et al. Allelic heterogeneity of G6PD deficiency in West Africa and severe malaria susceptibility. Eur J Hum Genet. 2009;17:1080–1085. doi: 10.1038/ejhg.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howes R., Piel F.B., Patil A.P., et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012;9:e1001339. doi: 10.1371/journal.pmed.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beutler E. Glucose-6-phosphate dehydrogenase: a historical perspective. Blood. 2008;111:16–24. doi: 10.1182/blood-2007-04-077412. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E. A series of new screening procedures for pyruvate kinase deficiency, glucose-6-phosphate dehydrogenase deficiency, and glutathione reductase deficiency. Blood. 1966;28:553–562. [PubMed] [Google Scholar]
- 19.Boulard M., Blume K.G., Beutler E. The effect of copper ion on red cell enzyme activities. J Clin Invest. 1972;51:459–461. doi: 10.1172/JCI106833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 21.Filosa S., Giacometti N., Wangwei C., et al. Somatic cell selection is a major determinant of the blood cell phenotype in heterozygotes for glucose-6-phosphate dehydrogenase mutations causing severe enzyme deficiency. Am J Hum Genet. 1996;59:887–895. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S., Nguon C., Guillard B., et al. Performance of the CareStart™ G6PD deficiency screening test, a point-of-care diagnostic for primaquine therapy screening. PLoS One. 2011;6:e28357. doi: 10.1371/journal.pone.0028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva G.T., Logan B.R., Klein J.P. Methods for equivalence and noninferiority testing. Biol Blood Marrow Transplant. 2008;15(1 Suppl):120–127. doi: 10.1016/j.bbmt.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf B.H., Weening R.S., Schutgens R.B., van Noorden C.J., Vogels I.M., Nagalkerke N.J. Detection of glucose-6-phosphate dehydrogenase deficiency in erythrocytes: a spectrophotometric assay and fluorescent spot test compared with a cytochemical method. Clin Chim Acta. 1987;168:129–136. doi: 10.1016/0009-8981(87)90281-6. [DOI] [PubMed] [Google Scholar]
- 25.Wong F.L., Boon N.Y., Ainoon O., Wang M.K. Comparison of detection of glucose-6-phosphate dehydrogenase deficiency using fluorescent spot test, enzyme assay, and molecular method for prediction of severe neonatal hyperbilirubinemia. Singapore Med J. 2009;50:62–67. [PubMed] [Google Scholar]
- 26.Clyde D.F. Clinical problems associated with the use of primaquine as a tissue schizontocidal and gametocytocidal drug. Bull World Health Organ. 1981;59:391–395. [PMC free article] [PubMed] [Google Scholar]
- 27.Salvidio E., Panncciulli I., Tizianello A., Ajmar F. Nature of hemolytic crises and the fate of G6PD deficient, drug-damaged erythrocytes in Sardinians. N Engl J Med. 1967;276:1339–1344. doi: 10.1056/NEJM196706152762402. [DOI] [PubMed] [Google Scholar]
- 28.Pannacciulli I., Tizianello A., Ajmar F., Salvidio E. The course of experimentally induced hemolytic anemia in a primaquine-sensitive Caucasian: a case study. Blood. 1965;25:92–95. [PubMed] [Google Scholar]
- 29.George J.N., Sears D.A., McCurdy M.E. Primaquine sensitivity in Caucasians: hemolytic reactions induced by primaquine in G6PD deficient subjects. J Lab Clin Med. 1967;70:80–93. [PubMed] [Google Scholar]
- 30.Alving A.S., Johnson C.F., Tarlov A.R., Brewer G.J., Kellermeyer R.W., Carson P.E. Mitigation of the haemolytic effect of primaquine and enhancement of its against the exoerythrocytic forms of the Chesson strain of Plasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bull World Health Organ. 1960;22:621–631. [PMC free article] [PubMed] [Google Scholar]
- 31.Peters A.L., van Noorden C.J.F. Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem. 2009;57:1003–1011. doi: 10.1369/jhc.2009.953828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howes R.E., Dewi M., Piel F.B., et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J. 2013;12:418. doi: 10.1186/1475-2875-12-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loucharoen C., Patin E., Paul R., et al. Positively selected G6PD-Mahidol mutation reduces Plasmodium vivax density in Southeast Asians. Science. 2009;326:1546–1549. doi: 10.1126/science.1178849. [DOI] [PubMed] [Google Scholar]
- 34.Baird J.K., Hoffman S.L. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 35.John G.K., Douglas N.M., von Seidlein L., et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11:280. doi: 10.1186/1475-2875-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durand S., Cabezas C., Lescano A.G., et al. Efficacy of three different regimens of primaquine for the prevention of relapses of Plasmodium vivax in the Amazon Basin of Peru. Am J Trop Med Hyg. 2014;91:18–26. doi: 10.4269/ajtmh.13-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . 2nd ed. WHO; Geneva: 2010. Guidelines for the treatment of malaria; p. 194. [Google Scholar]
- 38.Rijken M.J., McGready R., Boel M.E., et al. Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis. 2012;12:75–88. doi: 10.1016/S1473-3099(11)70315-2. [DOI] [PubMed] [Google Scholar]
- 39.McGready R., Lee S.J., Wiladphaingern J., et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment regimens in early pregnancy: a population-based study. Lancet Infect Dis. 2012;12:388–396. doi: 10.1016/S1473-3099(11)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett J.W., Pybus B.S., Yadava A., et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]