To the Editor: Infections with typhus group rickettsiae (TGR), spotted fever group rickettsiae (SFGR), and scrub typhus group orientiae (STGO) have been reported among persons in South Korea in increasing numbers over the past decade (1,2). During 2001–2011 in South Korea, 51,825 orientiae group infections were reported (mean incidence 9.95 cases/100,000 residents/year) (2). TGR (Rickettsia typhi), SFGR (R. akari, R. japonica, R. monacensis, and R. felis), and STGO (Orientia tsutsugamusi) have been identified in their arthropod vectors and reservoirs in northern provinces and at US military training facilities in South Korea (3–5).
Currently, little data exist on the risk for rickettsioses and scrub typhus for US military deployed to South Korea. Thus, a retrospective serologic investigation to determine the level of exposure to rickettsiae among 9,303 military personnel deployed to South Korea was conducted. The study used de-identified predeployment and postdeployment serum samples made available from the Department of Defense Serum Repository (6). The study group consisted of men in combat-related jobs at US military training sites and military installations in South Korea during 1990–1995 while on active duty continuously for >1 year. This study protocol was reviewed and approved by the Naval Medical Research Command Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects.
Age range of the 9,303 soldiers in the study group was 17–52 (median 24) years. Most (99.6%) were stationed in Dongducheon, Yongtaeri, and Seoul, located in the Gyeonggi and Gangwon provinces in northern South Korea. Primary military occupation specialties were infantryman (58.8%), fighting vehicle infantryman (22.4%), indirect fire infantryman (12.3%), and heavy anti-armor weapons infantryman (6.5%).
The soldiers’ postdeployment serum samples (n = 9,303) were screened at a dilution of 1:100 for IgG against TGR, SFGR, and STGO by using group-specific ELISA whole-cell antigen preparations from R. typhi Wilmington, R. conorii Morrocan, and a mixture of O. tsutsugamushi Karp, Kato, and Gilliam, respectively (7,8). TGR, SFGR, and STGO IgG ELISA titers (range 100–>6,400) were determined for screen-positive (net absorbance >0.500) postdeployment serum samples, and results were compared with matched predeployment serum samples. Samples with a net total absorbance >1.000 for serum dilutions 1:100, 1:400, 1:1,600, and 1:6,400 were considered titer positive. The inverse of the highest dilution of titer positive serum that produced a net absorbance >0.200 was determined to be the titer. Serum samples from laboratory animals infected with R. felis reacted specifically in the SFGR ELISA but not in the TGR ELISA (K.R. Macaluso and A.L. Richards, unpub. data); thus, any soldier infected with R. felis would have reacted in the SFGR but not the TGR ELISA.
The postdeployment seropositivity in US military personnel for antibodies against TGR, SFGR, and STGO at a titer ≥100 were 1.3% (117/9,249), 9.0% (805/8,918), and 0.5% (44/9,135), respectively (Figure). Seropositivity occurred for 10 (0.1%), 181 (2.0%), and 15 (0.2%) men who showed evidence of infection (seroconversion or 4-fold rise in antibody titer) with TGR, SFGR, and STGO, respectively, during their deployment to South Korea (Figure). The chance of a soldier having an infection with SFGR was significantly higher than the chance of having an infection with TGR or STGO (χ2 test, p<0.05) (analysis performed in SAS version 9.4; SAS Institute Inc., Cary, NC, USA). For personnel who seroconverted or had a 4-fold rise in titer to TGR, SFGR, or STGO, the age range was 19–49 (median 25) years, and job specialties were infantrymen (63.5%), fighting vehicle infantrymen (16.4%), indirect fire infantrymen (14.2%), and heavy anti-armor weapons infantrymen (5.9%).
Figure.
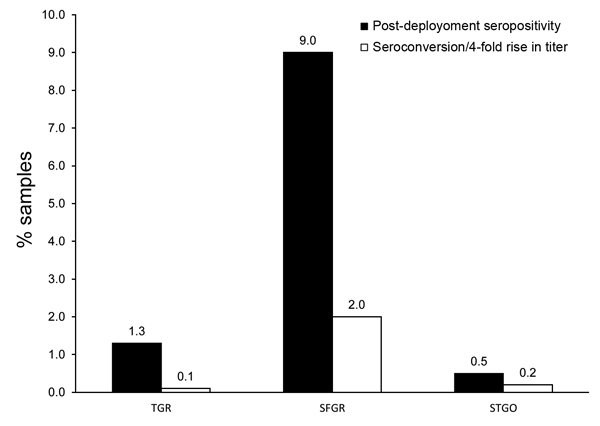
Evidence of rickettsiosis or scrub typhus among US military personnel deployed to South Korea. Black bars indicate postdeployment serum samples from US military personnel with a titer ≥1:100 (seropositive) to typhus group rickettsiae (TGR), spotted fever group rickettsiae (SFGR), or scrub typhus group orientia (STGO) IgG, as determined by ELISA. White bars indicate personnel determined by paired serum sample analyses to have seroconversion or 4-fold rise in titer between predeployment and postdeployment serum samples, indicating evidence of infection with the corresponding pathogen during deployment.
These results indicate that many US military personnel were exposed to rickettsiae and orientiae before their deployment to South Korea (Figure), perhaps because of previous deployments around the world or because of exposure to rickettsial agents at home (8–10). However, 206 (2.2%) of the men became infected with either a typhus group (n = 10) or spotted fever group (n = 181) rickettsia or a scrub typhus group orientia (n = 15) during their deployment to South Korea.
More SFGR infections occurred than TGR and STGO infections, although the pathogens for the latter infections (R. typhi and O. tsutsugamushi) are considered endemic to South Korea and are believed to affect the public and military health more than SFGR (3). The SFGR infections might correlate with recent observations of highly prevalent rickettsia-infected tick and R. felis–infected flea populations seen in South Korea (4,5). No evidence of co-infection was found in the men assessed during the deployment. These results suggest a risk for rickettsial disease, including scrub typhus and especially spotted fever, among US military personnel stationed in or visiting South Korea.
Acknowledgments
This study was supported by the Armed Forces Health Surveillance Center (Global Emerging Infections Surveillance and Response System) work unit #0000188M.0931.001.A0074.
The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government. Copyright protection for this work is not available under Title 17 U.S.C. §105 and Title 17 U.S.C. §101.
Footnotes
Suggested citation for this article: Jiang J, Myers TE, Rozmajzl PJ, Graf PCF, Chretien JP, Gaydos JC, et al. Seroconversions to rickettsiae in US military personnel in South Korea [letter]. Emerg Infect Dis. 2015 Jun [date cited]. http://dx.doi.org/10.3201/eid2106.141487
References
- 1.Choi YJ, Jang WJ, Kim JH, Ryu JS, Lee SH, Park KH, et al. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis. 2005;11:237–44. 10.3201/eid1102.040603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin HS, Chu C, Han DY. Spatial distribution analysis of scrub typhus in Korea. Osong Public Health Res Perspect. 2013;4:4–15. 10.1016/j.phrp.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Guinn ML, Klein TA, Lee JS, Richards AL, Kim HC, Ha SJ, et al. Serological surveillance of scrub typhus, murine typhus, and leptospirosis in small mammals captured at Firing Points 10 and 60, Gyeonggi Province, Republic of Korea, 2001–2005. Vector Borne Zoonotic Dis. 2010;10:125–33. 10.1089/vbz.2008.0123 [DOI] [PubMed] [Google Scholar]
- 4.Ko S, Kim HC, Yang YC, Chong ST, Richards AL, Sames WJ, et al. Detection of Rickettsia felis and Rickettsia typhi and seasonal prevalence of fleas collected from small mammals at Gyeonggi Province in the Republic of Korea. Vector Borne Zoonotic Dis. 2011;11:1243–51. 10.1089/vbz.2010.0261 [DOI] [PubMed] [Google Scholar]
- 5.Shin SH, Seo HJ, Choi YJ, Choi MK, Kim HC, Klein TA, et al. Detection of Rickettsia monacensis from Ixodes nipponensis collected from rodents in Gyeonggi and Gangwon Provinces, Republic of Korea. Exp Appl Acarol. 2013;61:337–47. 10.1007/s10493-013-9699-1 [DOI] [PubMed] [Google Scholar]
- 6.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900–4. 10.2105/AJPH.92.12.1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards AL, Soeatmandji DW, Widodo MA, Sardjono TW, Yanuwiadi B, Hernowati TE, et al. Seroepidemiological evidence for murine and scrub typhus in Malang, Indonesia. Am J Trop Med Hyg. 1997;57:91–5. [DOI] [PubMed] [Google Scholar]
- 8.Graf PC, Chretien JP, Ung L, Gaydos JC, Richards AL. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin Infect Dis. 2008;46:70–7. 10.1086/524018 [DOI] [PubMed] [Google Scholar]
- 9.Richards AL, Malone JD, Sheris S, Weddle JR, Rossi CA, Ksiazek TG, et al. Arboviral and rickettsial infections among combat troops during Operation Desert Shield/Storm. J Infect Dis. 1993;168:1080–1. 10.1093/infdis/168.4.1080 [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Marienau KJ, May LA, Beecham HJ, Wilkinson R, Ching W-M, et al. Laboratory diagnosis of two scrub typhus outbreaks at Camp Fuji, Japan in 2000 and 2001 by enzyme-linked immunosorbent assay, rapid flow assay, and Western blot assay using outer membrane 56 kDa recombinant proteins. Am J Trop Med Hyg. 2003;69:60–6. [PubMed] [Google Scholar]


