Abstract
Carriage of the New Delhi metallo-β-lactamase variant 1 (NDM-1) enables drug resistance to move between communities and hospitals. In Bangladesh, we found the blaNDM-1 gene in 62% of environmental waters and in fermentative and nonfermentative gram-negative bacteria. Escherichia coli sequence type (ST) 101 was most commonly found, reflecting a common global relationship between ST101 and NDM-1.
Keywords: New Delhi metallo-β-lactamase, NDM, multilocus sequence typing, sequence type, ST101, ST405, ST648, CTX-M-15, extensively drug resistant, bacteria, Bangladesh, environment, Escherichia coli, environmental water, sewage
Carbapenemases, bacterial enzymes that typically inactivate most of the β-lactam class of antimicrobial drugs, have emerged rapidly over the past decade (1). These resistance mechanisms are often accompanied by other resistance alleles, and together they can confer extensive drug resistance, leaving minimal treatment options (2). The New Delhi metallo-β-lactamase variant 1 (NDM-1), a chimera formed by the fusion of 2 resistance genes, is unique among the carbapenemases (3). Since its description in 2009, NDM-1 has spread rapidly to many countries worldwide and appears to be endemic in South Asia (1,4,5). A study of the environment in New Delhi, India, showed that ≈30% of surface waters and sewage was contaminated with NDM-1; the enzyme was also detected in drinking water (6). In addition, high rates of NDM-1 gut carriage have been found in the community and in hospitals in Pakistan (7). High rates of gut carriage can lead to contamination of drinking water and food through inadequate sewage treatment. Furthermore, gut carriage of NDM-1–encoding Escherichia coli can lead to common community-acquired infections (e.g., urinary tract infections), which often require hospitalization (8) and enable resistance mechanisms to move between community and hospital sectors. Indirect studies in 2009 and 2010 showed that NDM-1 was not present in the Bangladesh environment (9,10). To determine whether NDM-1 is now present in Bangladesh, we surveyed the environmental waters of Dhaka.
The Study
During October 19–27, 2012, we collected environmental water/sewage samples from 7 regions (58 sites) in Dhaka, Bangladesh (Figure 1). Control samples were from the United Kingdom. Each sample was investigated for bacterial growth on UTI brilliance agar plates (Thermo Fischer Scientific, Basingstoke, UK) containing vancomycin (30 mg/L) plus meropenem (0.5 mg/L). The species of individual colonies of different colors and morphologies were determined by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Bacteria were genetically characterized by blaNDM-specific PCR. Genetic location of the blaNDM-1 gene was determined by probing S1 nuclease pulsed-field gels. A subset of isolates of each species was further investigated for MICs of relevant antimicrobial drugs. All E. coli isolates were genotyped to determine multilocus sequence typing group; examples of each group were characterized for additional relevant resistance mechanisms. Details are provided in the Technical Appendix.
Figure 1.
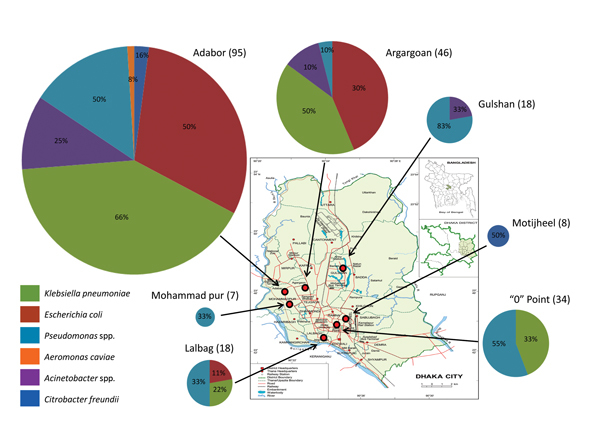
Diversity of New-Delhi metallo-β-lactamase variant 1–encoding species and the number found in 58 locations in 7 regions (red circles on map) of Dhaka, Bangladesh, October 2012. Individual sampling sites were within 2 km of each sampling region, and the number of sites varied from 6 to 12 per region. Pie charts indicate the proportions of different blaNDM-1–positive bacteria isolated in each region; colors indicate specific species. The diameter of each pie chart is directly proportional to the number of blaNDM-1–positive isolates collected in each region; actual numbers are shown in parentheses after the region name. Numbers within pie charts indicate the percentage of sites in each region in which the individual positive blaNDM-1–positive species were found. blaNDM-1 was detected in samples from all 7 regions and from 36 (62%) of the 58 sampling sites.
The carbapenemase and extended-spectrum β-lactamase genes blaNDM-1 and blaCTX-M-15 were detected by PCR in 36 (62%) and 41 (71%), respectively, of the 58 water samples. Both genes were found at all 7 sample region sites in Dhaka. Gene blaCTX-M-15, but not blaNDM-1, was detected in sewage samples from the United Kingdom; neither was detected in UK water samples from the River Thames.
We identified 226 gram-negative NDM-1–producing isolates to the species level (Figure 1; Technical Appendix Table 1); 15 isolates harboring blaNDM-1 could not be identified and were not investigated further. The most widely disseminated bacteria in samples from Dhaka were pseudomonads (6/7 regions) and Klebsiella pneumoniae (4/7 regions). Nine different species of Pseudomonas spp. and 5 Acinetobacter spp., mostly belonging to nonpathogenic strains, were among the nonfermentative bacteria (Technical Appendix Table 1). Carbapenem resistance in the Pseudomonas spp. isolates was unstable; all strains lost the blaNDM-1 gene after 2 days’ growth or when frozen for storage.
With the exception of 4 isolates, all bacterial isolates contained the original blaNDM-1 allele; 3 E. coli sequence type (ST) 101 isolates carried the blaNDM-3 variant, and 1 ST648 isolate carried the blaNDM-4 variant (Technical Appendix Table 2). S1 nuclease pulsed-field gel electrophoresis combined with blaNDM-1 probes detected blaNDM-1 on plasmids of limited size diversity in E. coli (ST101, 160 kb; ST405, 100 kb; ST648, 150 kb); however, other species included blaNDM-1–positive plasmids in a wide diversity of sizes (30 kb–450 kb); some of these species had multiple positive plasmids, and blaNDM-1 was also found on the chromosome (Technical Appendix Table 1 and Figure 1).
The E. coli isolates were further analyzed by PCR to identify additional resistance mechanisms often associated with blaNDM-1 (Table). blaCTX-M-15 and 16s ribosomal methylase genes (armA or rmtB) were associated with most E. coli strains, which explains the extensively drug-resistant phenotype of the E. coli isolates (Technical Appendix Table 3). Plasmids of plasmid incompatibility groups incFII (ST101, ST405, ST648) and incX (ST405, ST648) were also closely associated with E. coli strains (Table). E. coli harboring blaNDM-1 were isolated from 10 sampling sites (Figure 1; Technical Appendix Table 1). The E. coli isolates belonged to 3 different multilocus sequence typing groups: ST101 (phylogroup B1, 20/53 samples); ST405 (phylogroup D, 5/53 samples); and ST648 (phylogroup D, 28/53 samples) (Technical Appendix Table 2). ST101, which was found in samples from 6 (10.3%) of the 58 sites, was the most prevalent NDM-1–encoding E. coli genotype. ST648 represented an intermediate prevalence (5/58 [8.6%] sites), and ST405 was the least prevalent (1/58 [1.7%] sites) (Technical Appendix Tables 1, 2).
Table. Resistance genes and plasmid profiles for a subset of Escherichia coli strains in a study of extensively drug-resistant New Delhi metallo-β-lactamase–encoding bacteria in the environment, Dhaka, Bangladesh, October 2012*.
| E. coli strain, ST |
Resistance genes
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| bla CTX-M-15 | bla NDM | 16S methylase | bla ampC | incX | incFII | incL/M | incA/C | incN2 | |
| 18, ST101 | + | NDM-3 | rmtB | − | − | + | − | − | − |
| 24, ST101 | + | NDM-3 | rmtB | − | − | + | − | − | − |
| 25, ST101 | + | + | rmtB | − | − | + | − | − | − |
| 28, ST101 | + | NDM-3 | rmtB | − | − | + | − | − | − |
| 221, ST101 | + | NDM-1 | rmtB | − | − | + | − | − | − |
| 34, ST648 | + | + | armA | cmy, dha | + | + | − | − | − |
| 192, ST648 | + | NDM-4 | armA | cmy, dha | + | + | − | − | − |
| 346, ST648 | + | + | armA | cmy, dha | + | + | − | − | − |
| 43, ST405 | + | NDM-1 | armA | cmy, dha | + | + | − | − | − |
| 54, ST405 | + | NDM-1 | armA | cmy, dha | + | + | − | − | − |
*armA and rmtB, aminoglycoside methylase genes; CTX-M-15, cmy, and dha, β-lactamases; inc, plasmid incompatibility group; NDM, New-Delhi metallo-β-lactamase; ST, sequence type; −, negative; +, positive.
Conclusions
Our findings indicate that NDM-1 is widespread in the Dhaka environment. We detected 241 NDM-1–encoding bacterial isolates; they were found in all 7 sampled regions and at 36 (62%) of the 58 sampling sites. This high level of environmental blaNDM-1 contamination is of concern, especially because drinking water in Bangladesh usually carries high levels of sewage-derived bacteria (11). It is therefore likely that blaNDM-1 carriage rates will rise rapidly. Future environmental studies could provide indicators of epidemics of emerging resistant bacteria before they are realized in hospitals.
Despite the widespread presence of NDM-1 in Dhaka, it appears that this carbapenemase has recently emerged in the Bangladesh environment. Studies in northern Bangladesh did not find NDM-1 in wild ducks and poultry in 2009 (9) or in crow and gull feces in 2010 (10). Similarly, NDM-1 was not detected in drinking water in Dhaka during 2008–2009 (11) even though all samples had high levels of fecal and blaCTX-M-15 contamination. Furthermore, a study of 1,879 clinical E. coli and Shigella spp. isolates collected during 2009–2010 in Bangladesh did not detect blaNDM-1 (12). The first known clinical isolates date from 2008 (12), and the first evidence of human gut carriage of blaNDM-1 was found in samples collected in Dhaka (13) a month before our study.
Because E. coli is the leading cause of human urinary tract infections, bloodstream infections, and neonatal meningitis, the ability of NDM-1 to give this bacterium clinical resistance to carbapenems is of concern (14). E. coli is also universally carried in the human gut. Therefore, we focused on this species because it is likely to be the greatest threat to human health. E. coli encoding NDM-1 were found in 3 of the 7 sampled regions, and genotyping showed they belonged to only 3 STs: ST648, ST101, and ST405. These same 3 E. coli genotypes are responsible for 80% of clinical NDM-1–encoding E. coli isolates in the United Kingdom (15). Furthermore, ST101 is the most common E. coli genotype in the Bangladesh environment (10.3% prevalence) and in clinical isolates from the United Kingdom (50%). Results of a literature search for NDM-1–encoding E. coli belonging to ST101 showed that this genotype has been detected in 15 nations (Figure 2). Thus, E. coli ST101 appears to be a successful global genotype that is often associated with NDM-1. This association with a single global genotype is analogous to the association between E. coli ST131 and the cephalosporinase CTX-M-15. Because of the critical nature of extensively drug-resistant bacteria, we are investigating the underlying factors responsible for the success of these particular antimicrobial drug–resistant strains.
Figure 2.
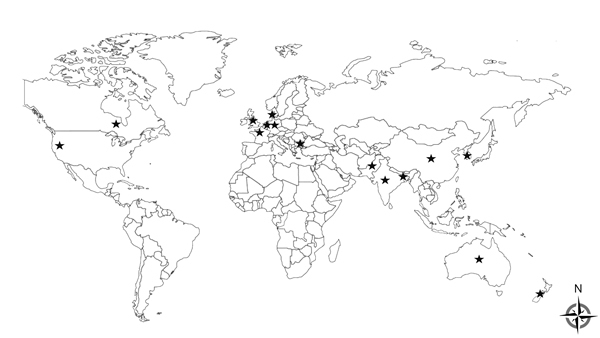
Sites where New-Delhi metallo-β-lactamase variant 1 (NDM-1)–encoding Escherichia coli sequence type (ST) 101 isolates have been detected worldwide. Stars indicate countries where NDM-encoding E. coli ST101 has been detected: Australia, Bangladesh, Belgium, Bulgaria, China, Canada, Denmark, France, Germany, India, Korea, New Zealand, Pakistan, the United Kingdom, and the United States.
Technical Appendix. Methods and Results for a study of extensively drug-resistant New Delhi metallo-β-lactamase–encoding bacteria in the environment, Dhaka, Bangladesh, October 2012; genomic location of blaNDM-1 in environmental bacteria from Dhaka; list of isolates; and phylogenetic analysis of NDM-positive Escherichia coli from the environment
Acknowledgments
This work was funded by grants from the National Institute for Social Care and Health Research (grant no. HF-11-24) and from the Medical Research Council (grant no. G1100135).
Biography
Dr. Toleman is a senior lecturer at Cardiff University. His recent work includes the discovery of the ISCR (insertion sequence common region) elements, NDM-1, and the formation of NDM-1 by an unusual genetic fusion event.
Footnotes
Suggested citation for this article: Toleman MA, Bugert JJ, Nizam SA. Extensively drug-resistant New Delhi metallo-β-lactamase–encoding bacteria in the environment, Dhaka, Bangladesh, 2012. Emerg Infect Dis. 2015 Jun [date cited]. http://dx.doi.org/10.3201/eid2106.141578
References
- 1.Patel G, Bonomo RA. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol. 2013;4:48.http:// [DOI] [PMC free article] [PubMed]
- 2.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 3.Toleman MA, Spencer J, Jones L, Walsh TR. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56:2773–6. 10.1128/AAC.06297-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62. 10.1016/S1473-3099(11)70059-7 [DOI] [PubMed] [Google Scholar]
- 7.Perry JD, Naqvi SH, Mirza IA, Alizai SA, Hussain A, Ghirardi S, et al. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J Antimicrob Chemother. 2011;66:2288–94. 10.1093/jac/dkr299 [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM. Has the era of untreatable infections arrived? J Antimicrob Chemother. 2009;64(Suppl 1):i29–36. 10.1093/jac/dkp255 [DOI] [PubMed] [Google Scholar]
- 9.Hasan B, Sandegren L, Melhus A, Drobni M, Hernandez J, et al. Antimicrobial drug-resistant Escherichia coli in wild birds and free-range poultry, Bangladesh. Emerg Infect Dis. 2012;18:2055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan B, Drobni P, Drobni M, Alam M, Olsen B. Dissemination of NDM-1. Lancet Infect Dis. 2012;12:99–100, author reply 101–2. 10.1016/S1473-3099(11)70333-4 [DOI] [PubMed] [Google Scholar]
- 11.Talukdar PK, Rahman M, Nabi A, Islam Z, Hoque MM, et al. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. PLoS ONE. 2013;8:e61090. 10.1371/journal.pone.0061090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam MA, Huq M, Nabi A, Talukdar PK, Ahmed D, et al. Occurrence and characterization of multidrug-resistant New Delhi metallo-β-lactamase-1–producing bacteria isolated between 2003 and 2010 in Bangladesh. J Med Microbiol. 2013;62:62–8. 10.1099/jmm.0.048066-0 [DOI] [PubMed] [Google Scholar]
- 13.Islam MA, Nabi A, Rahman M, Islam M, Ahmed D, et al. Prevalence of faecal carriage of NDM-1–producing bacteria among patients with diarrhoea in Bangladesh. J Med Microbiol. 2014;63:620–2. 10.1099/jmm.0.064527-0 [DOI] [PubMed] [Google Scholar]
- 14.Pitout JD. Extraintestinal pathogenic Escherichia coli: a combination of virulence with antibiotic resistance. Front Microbiol. 2012;3:9. [DOI] [PMC free article] [PubMed]
- 15.Mushtaq S, Irfan S, Sarma JB, Doumith M, Pike R, et al. Phylogenetic diversity of Escherichia coli strains producing NDM-type carbapenemases. J Antimicrob Chemother. 2011;66:2002–5. 10.1093/jac/dkr226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical Appendix. Methods and Results for a study of extensively drug-resistant New Delhi metallo-β-lactamase–encoding bacteria in the environment, Dhaka, Bangladesh, October 2012; genomic location of blaNDM-1 in environmental bacteria from Dhaka; list of isolates; and phylogenetic analysis of NDM-positive Escherichia coli from the environment


