SUMMARY
Background
Microparticles (MPs) are submicron size cell fragments that are released from cells.
Objectives
We hypothesise that MPs increase during red blood cell (RBC) storage and are part of the pro-inflammatory activity, which accumulates in the RBC supernatant.
Methods/Materials
RBC units were separated from whole blood of eight healthy donors: 5 U were split, with 50% undergoing leucoreduction (LR) and the remaining left as unmodified controls. The remaining 3 U were leucoreduced. Samples were obtained at days (D) 1 and 42 and cell-free supernatants separated and stored. The supernatants were centrifuged at 17 000 × g (60 min) or 100 000 × g (120 min) into microparticle-rich (MPR) and microparticle-poor (MPP) portions, resuspended in albumin, incubated with antibodies to CD235 (RBCs), CD45 [white blood cells (WBCs)] and CD41a [platelets (Plts)], and analysed by flow cytometry. Isolated neutrophils were incubated with these samples, and priming activity measured.
Results
Total MPs increased during storage; however, MPs that marked for precursor cell types did not. Significant priming accumulated in the MPP fraction during storage with some activity present in the MPR fraction from D1 and D42 LR-RBCs.
Conclusion
Most of the pro-inflammatory priming activity from stored RBCs resides in the MPP supernatant, although the MPR fraction from D42 LR-RBCs does contain some priming activity.
Keywords: leucoreduction, neutrophils, priming, supernatants, TRALI
Microparticles (MPs) are submicron size fragments which are released from leucocytes, platelets (Plts), red blood cells (RBCs) and endothelial cells (Dey-Hazra et al., 2010). Although their role in disease is still not completely known, they have been theorised to be involved in inflammation, atherosclerosis, coagulation and tumour metastasis (Piccin et al., 2007; Italiano, et al., 2010; Grimshaw et al., 2011). Recently, MPs have been postulated to have a cyto-protective/anti-inflammatory effect in the transfused host (Sadallah et al., 2008; Morel et al., 2011).
One of the difficulties of assessing MPs has been detection and isolation due to their small size. MPs have multiple names, typically relating to their relative size. The smallest MPs, which are membrane-derived, are called ectosomes with diameters <200 nm. Larger membrane derived MPs, with sizes in the 200–1200 nm range, are referred to as microvessicles. The collective group of MPs typically range anywhere from 100 to 1000 nm (Distler et al., 2006; Jy et al., 2010). Other particles similar to MPs include exosomes and apoptotic bodies, the former are by-products of exocytosis, and the later are released from apoptotic bodies, both typically in the same size range as ectosomes (Mathivanan et al., 2010). Careful analysis using flow cytometry can help identify MPs of varying sizes and the inclusion of sizing beads may help in identifying particles of the right size and determine the lower limit of detection for the flow cytometer (Lacroix et al., 2010).
One of the major interests in MPs relates to adverse events in the transfused host, namely transfusion-related reactions where MPs have been theorised to play a key role (Jy et al., 2011; Belizaire et al., 2012; Kriebardis et al., 2012; Almizraq et al., 2013). Because MPs have been shown to contain pro-inflammatory activity, particularly neutrophil (PMN) priming, which has been linked to transfusion-related acute lung injury (TRALI), there has been interest in classifying and understanding their biology (Cardo et al., 2008; Jy et al., 2011). In addition, MPs have been reported to increase during storage of RBC units and are considered part of the ‘storage lesion’ (Kim-Shapiro et al., 2011). Two recent studies have looked at indirect mediators of PMN priming, particularly via P-selectin: P-selectin glycoprotein ligand-1 (PSGL-1) interaction and elevation of C-receptors and immunoglobulins, as well as elevation of CD11b in supernatant from RBC’s (Cardo et al., 2008; Jy et al., 2011). Pre-storage leucoreduction (LR) has been reported to decrease the risk of TRALI (Blumberg et al., 2010), which has been postulated to be due to a reduction in MPs (Sugawara et al., 2010).
The objective of this article is to determine if the pro-inflammatory activity that accumulates during RBC storage resides in the MPs or in the microparticle-poor (MPP) supernatant. To this end, the cell-free supernatant of RBC units was separated into microparticle-rich (MPR) and the MPP-supernatant, and we hypothesise that MPs increase during routine RBC storage, contribute to the priming activity that accumulates in the supernatant of RBCs, and that pre-storage LR affects these processes.
MATERIALS AND METHODS
Materials
All chemicals unless otherwise specified were purchased from Sigma Chemical Corporation (St. Louis, MO, USA). Antibodies for flow cytometry were purchased from BD Biosciences (San Jose, CA, USA), including CD41a-PE, CD45-Per-CP and CD235-FITC. Counting beads (Flow-Count Fluorospheres™) were obtained from Beckman Coulter Inc. (Brea, CA, USA). Sizing beads were purchased from Spherotech Inc. (Lake Forest, IL, USA). Plasma, either fresh frozen plasma or FP24, was obtained from Bonfils Blood Center.
RBC collection and storage
Whole blood (500 mL) was collected from eight different healthy donors after informed consent was obtained in accordance with the Declaration of Helsinki under a protocol approved by the Colorado Multiple Internal Review Board at the University of Colorado, Denver. Whole blood was processed via industry standards which satisfy the AABB and the Food and Drug Administration (FDA) regulations and Circular of information in AS-5 (FDA, 2011). Five RBC units were divided by equal weight with 50% w/w undergoing LR via filtration (Pall BPF4 leucoreduction filter, Pall Corporation, Westbury, NY, USA), and the residual 50% w/w left unmodified. The three additional RBC units were also leucoreduced. All units were stored in AS-5 at 4 °C according to AABB standards. Sterile couplers were inserted for sampling on day 1 (D1) and day 42 (D42) (the last day a unit can be transfused). The supernatant, plasma fraction, was isolated via centrifugation at 5000 × g for 7 min at room temperature, and then 12 500 × g for 6 min at 4 °C, and the cell-free supernatant aliquoted and stored at −80 °C for further use (Bercovitz et al., 2012).
MP isolation
The cell-free supernatant was thawed and centrifuged at either 17 000 × g for 60 min or 100 000 × g for 120 min into MPR and MPP portions, and the MPR were resuspended in an equal volume of 1·25% fatty acid free, globulin free human serum albumin (HSA), which does not prime the PMN oxidase or cell-free plasma (FP).
Flow cytometry
MPs were incubated with CD235-fluorescein isothiocyanate (FITC) for RBCs, CD41a-PE for Plts and CD45-PerCP-Cy5·5 for leucocytes [white blood cells (WBCs)] for 30 min at 4 °C, fixed with 4% paraformaldyde, and then diluted to 1% with buffer. Samples were analysed on a FACS Canto II™ flow cytometer with bd facs diva™ software v. 6·1.1. (BD Biosciences, Franklin Lakes, NJ, USA). The flow cytometer was calibrated daily with BD FACS™ 7-Color Setup Beads (BD Biosciences) containing seven different fluorescent beads. Size events were defined using flow cytometry size beads of 0·22–1 μm (Spherotech). For the different windows used, the flow cytometer was set on a logarithmic scale. The majority of MPs were found to be in the 0·44–0·88 μm range, and the individual sizes of respective RBC, PLT and WBC MPs was not assessed, as all groups fell in the 0·44–0·88 μm. Samples were also analysed by flow cytometry utilising counting beads to determine relative quantities present. Means and the standard errors of the mean were calculated.
Priming activity
Isolated neutrophils (PMNs) collected from multiple different volunteers were incubated with the MPR fraction and MPP supernatant at (10%) FINAL for 5 min at 37 °C. Following incubation, the PMN NADPH oxidase was activated with formyl-methionyl-leucyl-phenylalanine (fMLF), and the maximal rate of O2− production was measured as the superoxide dismutase (SOD)-inhibitable reduction of cytochrome c at 550 nm (Silliman et al., 1994). Priming is the augmentation of the maximal rate of fMLF-activation of the NADPH oxidase in direct comparison to the vehicle control (buffer or albumin), as published previously (Silliman et al., 1994). For all data we report the maximal rate of O2− in response to fMLF as the gross numbers alone without data manipulation, and each assay had paired positive (Plt activating factor, a prototypical rapid priming agent) and negative (vehicle + albumin) controls (Silliman et al., 1994).
Lipid extractions and priming activity
Lipids were extracted from the MPP supernatants and the MPR fractions using a 1: 1: 1 mixture of chloroform: methanol: water with 0·2% acetic acid, as previously published (Silliman et al., 1994). The lipid fractions were resuspended in the original starting volume with 1·25% albumin, and these extractions were assayed their ability to prime the fMLF-activated respiratory burst.
Data analysis
The data are reported as the mean ± the standard error of the mean. The data were analysed for statistical differences via a paired analysis of variance (anova) with a post hoc Bonferroni or Newman Keuls test for multiple comparisons based upon the equality of variance employing GB Stat version 8.0.
RESULTS
Quantification of MPs
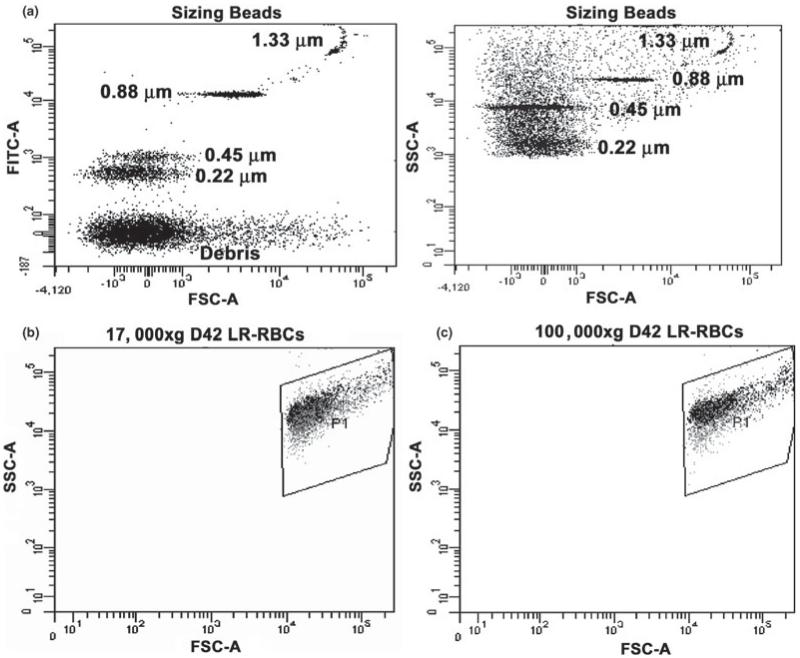
To determine any differences between centrifugation speeds and the isolation of MPs from RBCs, MPs isolated by centrifugation at 17 000 × g (60 min) and 100 000 × g (120 min) were compared via flow cytometry (Fig. 1b,c). Irrespective of the centrifugation speeds the MPs evidenced virtually identical gating characteristics, forward scatter and side scatter, demonstrating that both isolation techniques yielded similar MP profiles (Fig. 1b,c). To count the number of MPs, the mean florescence values were collected via flow cytometry and both these mean values, as well as calibrated counting beads, were employed to calculate the total number of MPs present in each of the samples and a representative scatter plot is shown to demonstrate the relative sizes of the MPs analysed (Fig. 1a). In addition, to determine that relative amount of MPs in the MPP- vs the MPR-fraction flow cytometry was performed on these paired fractions, and centrifugation was able to enrich the MPR fraction by 80 ± 5% with analogous depletion of the MPP-fraction. As each sample was collected, processed, stored and analysed in an identical fashion, the samples were comparable. Using these numbers, the total MPs increased during routine storage in all units irrespective of LR; however, this increase at D42 of storage was not significant when compared with D1 (Table 1). Unexpectedly, the MPs, which were specifically labeled for precursor cells, namely: RBCs (CD235), WBCs (CD45) or Plts (CD41a), decreased with storage time, although this observed decrease on D42 was not statistically different from D1 (Table 1). Importantly, this decrease was irrespective of LR. Of the MPs that specifically marked for precursor cells, RBC MPs were the most numerous with Plt MPs the least. In the LR samples, a non-significant increase in MP numbers was seen on D1 only (Table 1). However, when compared with the total number of MPs, the majority did not mark for the haematological cells tested.
Fig. 1.
Flow cytometric analyses of sizing beads and the MPs from the supernatants of stored D42 LR-RBCs. Panel a depicts scatter plots of the sizing beads with the fluorescence of the FITC channel as a function of forward scatter. As can be seen the specific sizing beads were present at 0·22, 0·45, 0·88 and 1·33 μm with a debris field at the bottom. The panel on the left shows the gating used for the MPs as defined by the sizing beads with the side scatter depicted as a function of the forward scatter of the sizing beads with the debris field not included in the MP gates. Panel b demonstrates the scatter plot of the MPs from the supernatant from D42 LR-RBCs isolated by centrifugation at 17 000 × g for 60 min. Panel c demonstrates the scatterplot of MPs isolated from the identical supernatants from D42 LR-RBCs isolated by centrifugation at 100 000 × g for 120 min. These data are virtually identical and represent a sample size of 6.
Table 1.
Microparticle counts in unmodified and LR-RBCs
| Flow cytometry: counts |
||||
|---|---|---|---|---|
| Total (unlabeled) | RBC (CD235)% Total MP | Platelet (CD41a)% Total MP | WBC (CD45)% Total MP | |
| NLR D1 | 146 336±15 560 | 41 293 (51·5%)±5121 | 11 443 (14·3%)±3249 | 27 431 (34·2%)±1527 |
| NLR D42 | 188 944±4284 | 25 836 (53·7%)±7095 | 7374 (15·3%)±1537 | 14 954 (31·0%)±5189 |
| LR D1 | 157 492±19 489 | 41 302 (47·8%)±3676 | 8866 (10·3%)±2719 | 36 173 (41·9%)±5612 |
| LR D42 | 173 421±19 892 | 33 957 (48·5%)±3920 | 6854 (10%)±683 | 29 063 (41·5%)±5797 |
LR, pre-storage leucoreduction by filtration (n=5); NLR, unmodified.
PMN priming activity
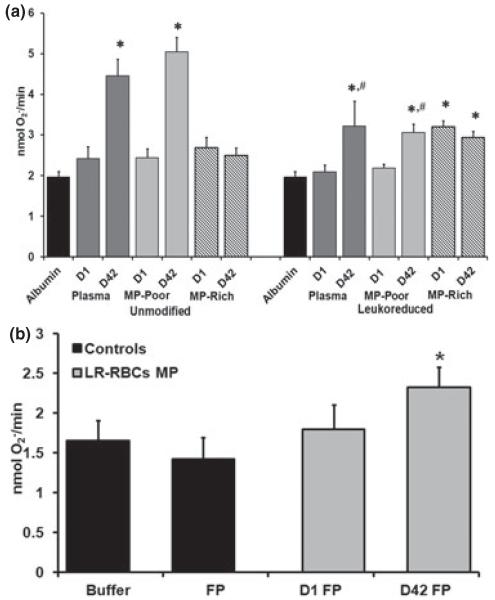
As PMNs are a key aspect of innate immunity and have been linked to TRALI, PMN priming activity was measured in the MPR- and MPP-fractions of the RBC supernatants, and the effects of storage time and pre-storage LR were assessed. There was a significant increase in priming found in the D42 supernatants in both unmodified and LR, with significantly greater priming activity present in the unmodified D42 RBCs (Fig. 2a). Importantly, these data are congruous with previous data, which demonstrated that pre-storage LR significantly decreased the accumulation of priming activity during routine storage (Silliman et al., 2011). When the supernatants were fractionated into MPR- and the MPP-supernatant, the priming activity was not present in the MPR-fraction; rather, it was present in the MPP supernatant (Fig. 2a). Surprisingly, PMN priming was found in the D1 MPR-fraction from LR-RBC that was not found in the D1 RBC supernatants. To determine if this priming activity was a possible experimental artifact due to resuspension in 1·25% HSA rather than in plasma, the MPR-fractions from D1 and D42 were resuspended in plasma, and the MPR priming activity was again evaluated. The MPR-fraction from D1 LR-RBCs suspended in plasma did not show significant priming activity compared with albumin-treated controls; however, the priming activity from the MPR-fraction from D42 LR-RBCs was significant from the buffer-treated, control PMNs but not the D1 LR-RBC MPR-fraction (Fig. 2b).
Fig. 2.
Priming activity of the supernatants of D1 vs D42 RBCs and LR-RBCs separated into MPR and MPP fractions and the role of plasma resuspension. The maximal rate of superoxide anion production in PMNs stimulated with 1 μM fMLF is shown as a function of treatment group in both panels. In panel a, significant PMN priming activity was present in the supernatants from D42 unmodified RBCs and the D42 RBC MPP-fraction; however, there was no priming activity in the MPR fraction of D42 RBCs. For LR-RBCs both the supernatants and the MPF-fractions from D42 had significant priming activity compared with the albumin-primed controls and the D1 LR-RBC supernatants. In contrast to RBCs, both the D1 and D42 MPR-fractions demonstrated significant priming activity vs the albumin-primed controls PMNs. *P < 0·05 as compared with the albumin-primed controls and the D1 RBC or D1 LR-RBC supernatants, n = 14 for each bar. In addition, both the D42 LR-RBC supernatants and the D42 LR RBC MPP-fractions were significantly decreased vs the supernatants from and the MPP-fractions of D42 RBCs; #P < 0·05 vs D42 supernatant and D42 MPP-fractions, respectively. In panel b, the MPR priming activity from D1 LR-RBCs was not present when the MPR-fraction was resuspended in plasma (v:v). In contrast, plasma resuspension did not alter the priming activity of the D42 LR-RBC MPR fractions vs the plasma primed control PMNs. The sample size for each bar in panel b was 6; *P < 0·05 as compared with plasma-primed PMNs activated with fMLF.
Lipid priming activity
Lipid extractions from D42 MPP-fraction primed the PMN oxidase while the MPR fractions did not: albumin/fMLF: 3·2 ± 0·3, D1 Supernatant-MPP: 3·0 ± 0·6, D1 MPR: 1·2 ± 1·1, D42 supernatant-MPP: 4·5 ± 0·7*, D42 MPR: 1·5 ± 0·7 (n = 4, *P < 0·05). These data indicate that the pro-inflammatory lipids reside in the MPF fraction most likely bound to albumin.
DISCUSSION
During routine storage of LR- and unmodified-RBCs, MPs increased in the supernatant; however, the numbers of MPs that can be associated with specific cell types, i.e. RBCs, WBCs or Plts, did not increase. In fact, the number of MPs that specifically marked for haematological cells actually decreased, although not significantly. While it is unclear as to the reason for this decrease, it may be due to: (i) ‘non-specific antigen loss’, (ii) proteolysis, as proteases are released and increase during RBC storage, or (iii) internalisation/capping of surface markers leading to MPs that do not have surface antigens for a specific cell type (de Angelis et al., 1990). The presence of MPs from Plts and WBCs In LR-RBC units are likely due to the filtration process, which is known to cause the release of WBC-derived proteins, which may be represented by the MPs. There remains debate as to whether LR leads to a decrease in MPs. Similar to the presented data, LR of apheresis Plt concentrates did not affect MP formation (Nollet et al., 2013). In contrast, the same research group demonstrated that whole blood LR did decrease MP formation (Sugawara et al., 2010). The discrepancy between these results may be related to different flow techniques because MPs are a very heterogeneous group of cells and different gating would lead to increased or decreased numbers of MPs; moreover, the inclusion of cellular debris could lead to a false increase in MPs, especially if sizing beads are not employed Second, whole blood LR, employed in the Sugawara article is not the same as LR following component separation (Sugawara et al., 2010). Importantly, in both D1 and D42 the MP fractions from LR-RBC supernatants did exhibit significant priming activity, likely a result of leukocyte activation caused by filtration. Upon resuspension in plasma, the pro-inflammatory activity from the MPs from D1 LR-RBC supernatants was inhibited back to the priming activity of the D1 LR-RBC supernatant. However, the pro-inflammatory activity of the MPR fraction from D42 LR-RBCs was unaffected. Lastly, this priming activity is not lipid in nature, and although significantly increased vs albumin controls, this activity did not differ from the priming activity of the D1 LR-RBC MPR fraction both resuspended in plasma.
MPs have been reported to cause the pro-inflammatory activity that accumulates during the routine storage of RBC units (Cardo et al., 2008; Grimshaw et al., 2011; Jy et al., 2011; Belizaire et al., 2012). In contrast, when the supernatant from unmodified RBCs was separated into MPR and MPP fractions, the PMN priming activity, was present in the MPP the supernatant, which would contain the lipids bound to albumin (Silliman et al., 1994; Silliman et al., 2011). In contrast, when the MPR- and MPF-fractions of the LR-RBC supernatants were assayed priming activity was present in both the MPR- and MPP-fractions of the supernatants on both D1 and D42. This activity in the MPs from D1 LR-RBC supernatants is likely due to filtration, although not lipid in nature.
The presence of lipid priming activity in the MPF fraction is not surprising as free fatty acids are known to avidly bind to albumin, a well-known lipid carrier in human plasma, and albumin and albumin-bound mediators would be expected to reside in the MPP fraction of the supernatant. (Shanbhag & Johansson, 1979; Silliman et al., 1994; Silliman et al., 2011). Moreover, the increased priming activity in the D42 supernatant from unmodified RBCs was not completely removed by pre-storage LR, although there was a significant reduction in priming activity most likely due to non-polar lipids and confirmed by the presence of AA and lipid priming activity as previously reported (Silliman et al., 2011).
The presented data agree that MPs may play a role in PMN priming leading to TRALI secondary to stored LR-RBC units (Jy et al., 2011); however, there is no PMN priming activity present in the MPR fraction from unmodified RBC units, and this activity is not lipid in nature. Pre-storage LR appears to impart a PMN priming activity that is present on D1 and persists to D42 which is not lipid in nature. In addition the priming activity from the D1 MPR fraction is inhibited by autologous plasma whereas the MPR-fraction from D42 RBCs was not. The lipid priming activity does not reside in the MPs and is present in the MPP supernatant. Thus, the pro-inflammatory activity in LR-RBCs consisted of activity in both MPR- and MPP-fractions of the supernatant and the persistence of this activity when the MPs were resuspended in plasma is indicative of possible in vivo activity. Previous data from this laboratory has demonstrated that non-polar lipids accumulate during the routine storage of LR-RBC units and cause pro-inflammatory activity, and these lipids require albumin binding for activity (Silliman et al., 2011). While it still appears that the majority of priming resided in the MPP fraction, there does appear to be some effect from the MPR fraction, both of which may represent a possible second event in the two-event pathogenesis of TRALI caused by stored LR-RBCs (Silliman et al., 1994; Silliman et al., 2003; Silliman et al., 2011).
ACKNOWLEDGMENTS
This work was supported by Bonfils Blood Center, and grant P50-GM049222 from NIGMS, NIH.
Footnotes
The manuscript has been seen and approved by all authors, it is not under active consideration for publication, has not been accepted for publication, nor has it been published in full or in part, except in abstract form.
M. K. performed most of the experiments and wrote the manuscript. M. R. K. helped to complete the experiments and aided in writing the manuscript. F. B. W. ensured that the drawing of whole blood, manufacture of components and LR was done properly via AABB guidelines and Bonfils Blood Center GMPs. C. C. S. designed the experiments, helped to complete the manuscript and analysed the data.
CONFLICT OF INTEREST
The authors have no competing interests.
REFERENCES
- Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–2267. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- de, Angelis V, de, Matteis MC, Orazi BM, Santarossa L, Della TL, Raineri A, Vettore L. Erythrocyte endogenous proteinase activity during blood bank storage. Vox Sanguinis. 1990;59:73–77. doi: 10.1111/j.1423-0410.1990.tb05012.x. [DOI] [PubMed] [Google Scholar]
- Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. Journal of the American College of Surgeons. 2012;214:648–655. doi: 10.1016/j.jamcollsurg.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitz RS, Kelher MR, Khan SY, Land KJ, Berry TH, Silliman CC. The pro-inflammatory effects of platelet contamination in plasma and mitigation strategies for avoidance. Vox Sanguinis. 2012;102:345–353. doi: 10.1111/j.1423-0410.2011.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, Kirkley SA, Fialkow LB. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50:2738–2744. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfusion and Apheresis Science. 2008;38:117–125. doi: 10.1016/j.transci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Dey-Hazra E, Hertel B, Kirsch T, Woywodt A, Lovric S, Haller H, Haubitz M, Erdbruegger U. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vascular Health and Risk Management. 2010;6:1125–1133. doi: 10.2147/VHRM.S13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–690. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- FDA [Accessed 5/12/13];Circular of Information: For the Use of Blood and Blood Products. 2011 [WWW document]. URL http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm364565.htm.
- Grimshaw K, Sahler J, Spinelli SL, Phipps RP, Blumberg N. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion. 2011;51:874–880. doi: 10.1111/j.1537-2995.2011.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano JE, Jr., Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Current Opinion in Hematology. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jy W, Horstman LL, Ahn YS. Microparticle size and its relation to composition, functional activity, and clinical significance. Seminars in Thrombosis and Hemostasis. 2010;36:876–880. doi: 10.1055/s-0030-1267041. [DOI] [PubMed] [Google Scholar]
- Jy W, Ricci M, Shariatmadar S, Gomez-Marin O, Horstman LH, Ahn YS. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion. 2011;51:886–893. doi: 10.1111/j.1537-2995.2011.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebardis A, Antonelou M, Stamoulis K, Papassideri I. Cell-derived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions? Blood Transfusion. 2012;10(Suppl. 2):s25–s38. doi: 10.2450/2012.006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Robert S, Poncelet P, Gnat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Seminars in Thrombosis and Hemostasis. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Seminars in Immunopathology. 2011;33:469–482. doi: 10.1007/s00281-010-0239-3. [DOI] [PubMed] [Google Scholar]
- Nollet KE, Saito S, Ono T, Ngoma A, Ohto H. Microparticle formation in apheresis platelets is not affected by three leukoreduction filters. Transfusion. 2013;53:2293–2298. doi: 10.1111/trf.12088. [DOI] [PubMed] [Google Scholar]
- Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Reviews. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. Journal of Leukocyte Biology. 2008;84:1316–1325. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- Shanbhag VP, Johansson G. Interaction of human serum albumin with fatty acids. Role of anionic group studied by affinity partition. European Journal of Biochemistry. 1979;93:363–367. doi: 10.1111/j.1432-1033.1979.tb12831.x. [DOI] [PubMed] [Google Scholar]
- Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. The Journal of Laboratory and Clinical Medicine. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G, Ambruso DR. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A, Nollet KE, Yajima K, Saito S, Ohto H. Preventing platelet-derived microparticle formation – and possible side effects-with prestorage leukofiltration of whole blood. Archives of Pathology and Laboratory Medicine. 2010;134:771–775. doi: 10.5858/134.5.771. [DOI] [PubMed] [Google Scholar]