Abstract
The purpose of this article was to review follow up studies of children with prenatal drug exposure from preschool through adolescence. Specifically, the authors focus on the effects of prenatal exposure to cocaine, methamphetamine, and opiates on behavior and development. The largest number of studies have examined cocaine-exposed children. The authors identified 42 studies that suggest that there are unique effects of prenatal cocaine exposure on 4- to 13-year-old children, particularly in the areas of behavior problems, attention, language, and cognition. In addition, studies make reasonable attempts to control for possible confounding factors. Systematic research on the long-term effects of prenatal methamphetamine exposure is just beginning but seems to be showing similar effects to that of cocaine. The literature on the on the long-term effects of children with prenatal opiate exposure is more substantial than the methamphetamine literature but it is still relatively sparse and surprising in that there is little recent work. Thus, there are no studies on the current concerns with opiates used for prescription mediation. There is a growing literature using neuroimaging techniques to study the effects of prenatal drug exposure that holds promise for understanding brain/behavior relationships. In addition to pharmacological and teratogenic effects, drugs can also be viewed from a prenatal stressor model. The author discuss this “fetal origins” approach that involves fetal programming and the neuroendocrine system and the potential implications for adolescent brain and behavioral development.
Keywords: Prenatal drug exposure, follow-up, fetal origins
INTRODUCTION
In the previous article, Bandstra et al. reviewed the effects of prenatal drug exposure through infancy and early childhood. Our review will consider prenatal drug exposure effects from preschool through adolescence, specifically the outcomes of exposure to cocaine, methamphetamine, and opiates related to behavior and development. We first provide a review of the published findings of exposure to each drug. We then describe how the effects of these drugs can be viewed from a prenatal stressor model involving fetal programming and the neuroendocrine system and the implications for adolescent brain development.
PRENATAL COCAINE EXPOSURE
Unlike the rather sparse prenatal methamphetamine and opiate literatures, our knowledge about the long-term effects of prenatal cocaine exposure is based on a reasonably large and growing corpus of studies. We were able to identify 42 published, empirical studies of the behavior and development of children with prenatal cocaine exposure between the ages of 4 and 13. These studies were published between 1996 and 2008, most (n = 34) between 2004 and 2008, many of which were longitudinal follow-up reports of the cohorts in the previous Bandstra review. We grouped the studies into categories based on the following outcomes: behavior problems, psychopathology, substance use, IQ, language, cognition (including attention), motor function, school and achievement, quality of relationships, and psychophysiology. In addition, there are 10 neuroimaging studies in this population. We will summarize the findings by category with effects for cocaine considered positive when adjusted for covariates (if applied) and then review covariate and related methodological issues.
Behavior Problems
Behavior problems formed the largest category and included 18 studies,1-18 10 of which showed negative effects of prenatal cocaine exposure.2-4,6-8,10,13,16-18 Of the 18 studies,8 used the parent report Child Behavior Checklist,1,2,5,11,12,16-18 4 used the teacher version (Teacher Report Form),9,13-15 4 used other tests (1 child report [Youth Risk Behavior Survey]4 and 3 teacher report [PROBES-14]6-8), and 2 used behavioral observations (frustration; aggression elicitation).3,10 For the 12 studies that used the comparable measures (Child Behavior Checklist or Teacher Report Form), only four showed adverse cocaine effects on major subscales (e.g., externalizing) or minor subscales,2,13,16 with two studies reporting interaction of cocaine exposure, gender, and alcohol exposure (girls exposed to cocaine but not alcohol were more aggressive, whereas boys exposed to both cocaine and alcohol were more delinquent).13,16
Psychopathology
Only two studies have examined psychopathology in these children,12,19 probably because the age of most of the cohorts limited these examinations. One study showed an association between cocaine exposure and ADHD and oppositional defiance disorder12 and the other studied showed no effects on suicide ideation.19
Substance Use
Only 1 study included substance use as an outcome in children. In this study of preadolescents at 10.5 years of age, cocaine-exposed children were more likely to have smoked cigarettes.4
IQ
IQ was reported in 14 studies5,11,14,15,20-29 and, perhaps surprisingly, negative effects of prenatal cocaine exposure were only observed in five of those.5,21,22,24,28 Of the five studies, gender effects were observed in two studies with lower IQ in boys.5,22 Among the seven studies reporting analysis of summary IQs and subtests,5,14,21-23,27,28 six reported significant cocaine effects on Perceptual Reasoning,28 Verbal Comprehension,21 Verbal Reasoning (boys only),5 Short Term Memory,5 Picture Completion,23 Object Assembly,23 Abstract/Visual Reasoning (boys only),22 Visual-Spatial Skills,27 General Knowledge,27 and Arithmetic Skills.27 In only two cases, summary and subtests scores were reduced by prenatal cocaine exposure but the full IQ score was not.23,27
Language
There were eight studies of language in cocaine exposed children.5,11,24,26,30-34 Only one study did not find negative exposure effects,11 and one study reported adverse effects for boys only.5 In a study that examined the trajectory of overall language function from 3 to 7 years, language deficits associated with cocaine exposure were stable across all age points with no increase or decrease over time.31
Cognition
Cognition is a somewhat heterogeneous category. The largest single category among cocaine exposure studies is sustained attention, which was examined in seven studies,14,15,26,35-38 with all but one study14 reporting adverse exposure effects. Six studies used a continuous performance or vigilance task14,15,26,35,36,38 that yields scores for omission (poor sustained attention) and commission errors (impulsivity); of these 6 studies,2 reported exposure effects on omission errors,35,36 2 reported both omission and commission errors,15,26 and 1 reported the effects on both outcomes.38 Other attention tests (contrary tapping/delay task efficiency) also reported poor attention in exposed children.37
Nine studies examined the relationship of cocaine exposure to other cognitive processes, including visual motor integration,20,26 task persistence observation during a mastery paradigm,36 central processing (PROBES) for all subjects.6 or for boys only,7 visual-spatial short term memory (Gordon Maze),39,40 maintaining set with interference (Stroop Task),41 visual attention span and sequencing (Knox Cube),26 and set switching (Trail Making).15 Of the nine studies, all but two studies reported harmful exposure effects, both on visual motor integration.15,20 The diversity of findings is similar to the subscales on IQ tests in that no specific ability emerges across the relevant studies that relate to cocaine exposure. However, in this case, the study-specific selection of paradigms makes replication less likely.
Motor
Of three studies assessing motor abilities, one showed more motor problems in cocaine exposed boys7 and two showed no cocaine effects on fine motor behavior.20,26 Unlike the infancy studies described by Bandstra et al. in the previous article in this supplement, relatively few studies examined motor function in older exposed children.
School Performance and Academic Achievement
Of the seven studies of school performance and academic achievement,14,17,18,24,25,28,42 three showed negative effects of prenatal cocaine exposure.24,25,42 Two effects were on achievement tests…;24,25 the other showed an increase in referrals for special education and academic support services.42 In addition, the one study that anticipated school success by examining school readiness reported negative prenatal cocaine exposure effects.26
Relationships
No effects of prenatal cocaine exposure were found in the study of the mother-child relationship using observation of play.11 No other relationships, such as relationships to peers or teachers, were examined in any study.
Psychophysiology
Only two physiological studies have been reported, and both showed cocaine effects. One showed changes on event-related potentials during the Stroop executive function test of working memory.41 In the other study, skin conductance, which measures electrodermal activity and reflects autonomic arousal, was altered during stress in children with prenatal cocaine exposure.18
Covariates
As has been well documented, early studies of the effects of prenatal cocaine exposure were difficult to interpret because of methodological problems, including small sample size and poor control for confounding factors (e.g., prenatal exposure to other substances and measures of the postnatal caregiving environment) that could also explain what has been ascribed to cocaine exposure. The 42 studies reported above were based on reasonably large sample sizes, with 20 studies based on samples of over 300 participants with the maximum over 1,000 participants. Of course, some studies are based on the same cohorts. There are 14 different cohorts for a total of 4,419 children. Large population studies also allow statistical control for many prenatal and postnatal confounding variables. Studies vary in the criteria used to determine what potentially confounding variables are, such as variables on which the exposed group and the comparison group show statistical differences or variables that have a predetermined relationship (e.g., P < .10) with both the predictor variable (prenatal cocaine exposure) and the outcome variable (e.g., IQ). Thus, in the description below, the fact that a variable was not controlled could simply mean that it did not need to be controlled. For example, a study of full-term infants would not control for gestational age. Lack of control could also mean that a factor was not measured.
Prenatal Confounding Variables
Use of legal or illegal substances other than cocaine during pregnancy (tobacco, alcohol, marijuana, or opiates) was controlled in 33 of the 42 studies. Measures of fetal growth, including birthweight, prematurity, head circumference, birth length, and neonatal medical problems, were controlled in 20 studies, and 12 studies controlled for prenatal care.
Postnatal Confounding Variables
Poverty or socioeconomic status was controlled in 27 of the 42 studies. A variety of measures have been used to control for specific aspects of the home environment. The Home Observation Measure of the Environment (Home scale) was used as a covariate in 15.43 Placement in foster care was used in 23 studies. Related measures of the household included the number of children in the home, the number of regular caregivers, chaos, social support, and early case management. Measures of the caretaker included age, education, marital status, parity, employment, IQ, and self-esteem. Parental psychological problems or symptoms were controlled in 15 studies, and maternal IQ was controlled in 16 studies. Parent’s postnatal use of drugs was controlled in 22 studies. Measures of the child included daycare, head start, enrichment programs, services, and blood lead level. Child measures that could be outcomes were also included as covariates, including IQ, physical growth, and depressive symptoms. Two studies controlled for child abuse, one for domestic violence, and six for community violence. Race was used as a covariate in 10 studies and gender was used in 28 studies.
Dose-Response Relationships
One question that is often asked is whether the effects of prenatal cocaine exposure are related to the level of cocaine use. There are no studies in this group in which results of toxicology analysis (e.g., urine or meconium) were reported in relation to prenatal cocaine exposure. However, studies have used maternal interviews to derive measures of cocaine use patterns, such as the frequency of use per week. Of the 42 studies,10 found effects of heavy cocaine use related to worse outcome: six on behavior problems, two on language, and two on IQ.
Summary
The review of these 42 studies suggests that there are unique effects of prenatal cocaine exposure on 4- to 13-year-old children across a range of domains, including behavior problems, attention, language, and cognition. The evidence is weaker that IQ, school performance, and achievement are affected. In the domains of psychopathology, physiology, and motor performance, only a few studies have been reported. It is important to note that, in general, the effects that have been observed have been conducted on reasonably large samples with adjustment for confounding variables, suggesting that these are “true” cocaine effects. There is also evidence for dose response relationships, especially with behavior problems.
It is also interesting to note dimensions that have not been well studied. Substance use by the children and more studies of psychopathology will undoubtedly be undertaken as the age of children in longitudinal cohorts increases. It is somewhat surprising that there is only one study of parent-child relationships because this was an important focus in the infancy research, and that there are only two psychophysiological studies. There are also few studies using direct observation of behavior. Maternal report, paper and pencil forms, or computerized tests were used in 39 of the 42 studies.
In addition to determining the unique effects of cocaine, it is also important to determine how cocaine interacts with other factors in predicting child outcome. There are no studies on the interaction between genetic markers and prenatal cocaine exposure. The field of genetics provides an interesting metaphor in that we now know that most diseases do not obey the laws of classical Mendelian genetics and this is likely even more the case for behavioral phenotypes. For most outcomes, the genetic “main effect” is relatively small, allowing the study of gene-environment interactions to take center stage. This approach is starting to appear in the prenatal cocaine exposure literature. For example, studies have examined mediators or moderators of prenatal cocaine exposure effects, including gender, alcohol, head circumference, and foster care. The impact of puberty on this complex matrix has yet to be studied. More sophisticated statistical modeling is starting to be used to study more complex relationships between prenatal cocaine exposure and child outcome, including structural equation modeling, generalized estimated equations, and hierarchical linear modeling.
Neuroimaging
Quantitative neuroimaging studies represent another way in which the field of prenatal cocaine exposure has matured. We chose to review these 10 studies separately because this is a new area for this field and many are preliminary with relatively small sample sizes and were not able to control for confounding variables as in the 42 studies reviewed above. Quantitative neuroimaging studies in children with prenatal cocaineinclude anatomical magnetic resonance imaging (MRI), diffusion tensor imaging, functional MRI, and magnetic resonance spectroscopy (MRS).
In anatomicor structural MRI, the volumes of cortical gray matter, white matter, subcortical structures, corpus callosum, and cerebellum have been studied. In a study of 41 11-year-old children, Behnke et al. reported a decrease in the volume of the right anterior cerebellum in cocaine-exposed children.44 Singer et al. reported a decrease in gray matter volume in the right parietal and left occipital lobes, as well as a decrease in corpus callosum volume in 35 cocaine-exposed children aged 7 to 8.44 They also found a decrease in the corpus callosum, which contains the largest fiber tract in the brain. A mean decrease in the caudate volumes in 49 cocaine-exposed children at 13 to 15 years of age has also been reported.45 We46 reported a volumetric decrease in cortical gray matter, thalamus, and putamen in a sample of 22 cocaine-exposed and comparison children between 9 and 11 years of age. We also found an inverse “dose response” relationship between prenatal exposure level and these volumes. The caudate and putamen findings in these last two studies45,46 are noteworthy because these are major dopaminergic areas. Finally, although lower mean cortical gray matter and total parenchymal volumes were reported by Rivkinin in a study of 35 children aged 10 to 14, cocaine effects did not remain47 when adjusted for prenatal exposure to other drugs.
In a study using diffusion tensor imaging, cocaine-exposed children (age: 10.6 years, N = 53) had increased average diffusion in left frontal callosal and right frontal projection fibers.48 They found no differences in fractional anisotropic values. A brain MRS study of children exposed to cocaine in utero showed increased Creatine in the frontal white matter, with normal N-acetyl aspartate in the absence of any visible volumetric brain abnormalities on MRI.49 It was suggested that the increased Creatine could be due to glial proliferation or an abnormal energy metabolism. Using perfusion fMRI, Rao et al. reported a 10% decrease in global cerebral blood flow in 49 adolescents with prenatal cocaine exposure.50 They found an increase in cerebral blood flow in the anterior and superior brain regions and suggested that this may be due to development of compensatory mechanisms for reduced global cerebral blood flow during neural ontogeny. In an fMRI study of 34 adolescents, both fMRI activation patterns during task and performance on a separate executive function test were similar between cocaine exposed and comparison groups.51 We52 measured fMRI activation during a response inhibition task in 24 children aged 8 and 9 years. Groups did not differ in task performance by design. Cocaine-exposed children showed greater activation in the right inferior frontal cortex and the caudate during response inhibition, suggesting that prenatal cocaine may affect the development of brain systems involved in the regulation of attention and response inhibition.
Recent advances in MRI quality have greatly contributed to our understanding of prenatal cocaine exposure effects on the developing human brain. Longitudinal studies with careful control of other factors (exposure to other drugs and postnatal environmental factors) will add to the excitement of these findings. It will be important to determine whether these imaging findings are related to the child’s behavior and performance on psychological tests outside of the scanner. It is also important to be mindful of the fact that imaging findings do not denote causal mechanisms, but rather associations between a condition and brain involvement, not that the condition was caused by said brain findings. For example, we might speculate that the increases in fMRI,52 Creatine,53 diffusion,48 and cerebral blood flow50 could represent compensatory mechanisms related to other (as yet unknown) areas of brain involvement. Nor do we know the developmental course of these findings, their presence or absence at birth, the effects of prenatal cocaine exposure on normative processes of brain development and maturation, or the role of the postnatal environment in shaping brain architecture, structure, and function.
PRENATAL METHAMPHETAMINE EXPOSURE
Systematic research on the long-term effects of prenatal methamphetamine exposure is limited.54 As mentioned in the previous Bandstra review in this supplement, the only published findings from the NIH longitudinal Infant Development Environment and Lifestyle (IDEAL) study are from the infancy period. IDEAL is a multisite (Iowa, Oklahoma, California, and Hawaii) longitudinal study of children with prenatal exposure to methamphetamines. Preliminary findings from approximately half of that sample at age 3 show (Figure 1) no differences between methamphetamine-exposed and comparison children on the Bayley Scales (MDI shown here) at ages 1, 2, or 3 years of age.
FIGURE 1.
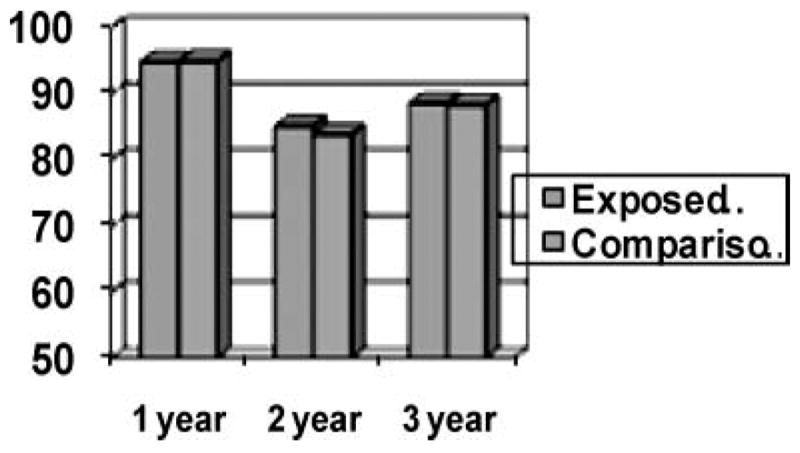
Mean Bayley MDI scores in methamphetamine exposed and comparison children at ages 1, 2, 3.
On the Child Behavior Checklist at age 3, the number of children with total problem behavior scores above the clinical cutoff was similar for the methamphetamine exposed (28 of 133; 21%) and comparison group (25 of 138; 18%). This finding should be considered in light of our study of cocaine exposed children. Differences in IQ between cocaine exposed and controls did not emerge until 4.5 years of age and increased from ages 4.5 to 7.55 Adverse cocaine effects on the Child Behavior Checklist were not observed until age 5 but remained stable from ages 5 to 7.2 It is possible that stimulant drugs, such as cocaine and methamphetamine, affect areas of the brain whose functions become more easily tested as children are forced to deal with settings that place more demands on executive function abilities and require higher cortical functioning, such as school. Continued follow-up is critical to determine whether the methamphetamine effects continue to parallel the cocaine effects.
The only published work on prenatal methamphetamine exposure in school-age children draws from a series of studies from Sweden examining 66 amphetamine-exposed children who have been studied to the age of 14.56 At age 4,57 there were no differences in physical growth or health. Mean IQ was lower than a separately selected control group from the population. The amphetamine group had more disturbed or problem children if the mother was still addicted. Also at age 4,58 child adjustment correlated with maternal alcohol and drug use, maternal stress, and paternal criminal convictions. At age 8,59 the extent of prenatal exposure could be related to psychometric outcome, aggression, peer problems, adjustment, and general assessment, as well as alcohol exposure and pregnancy attitudes. Maternal psychiatric treatment, alcohol abuse, and the number of custodians correlated with aggressive behavior and general assessment. The children had problems with advancement in school due to delays in math and language, and at age 14 they had difficulties with physical fitness activities.56 The limitations of this work include lack of a control group, small sample size, and prenatal polydrug use (30% of the mothers used heroin,81% used alcohol with 1/3 meeting criteria for alcohol abuse, and 80% smoked more than 10 cigarettes a day). The study is also based on self-report and the route of administration of amphetamine was injection, which is less common today in the United States.
Neuroimaging
Two MRI studies of children exposed prenatally to methamphetamine have been reported.53,60 Smith et al.53 used proton magnetic resonance spectroscopy (1H MRS) to evaluate neurochemical alterations in 12 children between 7 and 8 years old who were exposed prenatally to methamphematine and other substances (alcohol and tobacco) and 14 controls. Increases were found in total creatine in the basal ganglia of the methamphetamine exposed group, suggesting possible alterations in cellular energy metabolism; however, no differences were found in N-acetyl-containing compounds that would indicate neuronal damage or loss. In the second study,60 volumetric MRI differences in brain regions were found between 13 methamphetamine-exposed (and other drug) children and a group of 15 non-drug exposed controls at 7 and 8 years of age. Methamphetamine was associated with reduced volumes of the globus pallidus, and hippocampus. However, the reductions in the methamphetamine-exposed group were not related to performance on attention and verbal memory tests.
PRENATAL EXPOSURE TO OPIATES
The literature on the long-term effects of children with prenatal opiate exposure is more substantial than the methamphetamine literature but it is still relatively sparse61 and surprising in that little work has been done since the thorough reviews published ten years ago.61,62 The opiates studied include heroin and methadone. Currently, there is concern about the abuse of opiate-based prescription medication (e.g., Vicodin or hydrocodone and OxyContin or oxycodone) by pregnant women, as well as the recently approved drug, buprenorphine. No studies have yet been published on the effects of these drugs on the post-infancy child. Methodological similarities and differences exist between the literature studying prenatal cocaine (and to some extent methamphetamine) and opiate exposure. Both literatures face the problem of how to control for virtually the same set of confounding factors when trying to isolate the effects of the target drug. Most pregnant women who use cocaine or opiates are polydrug users and most of their children are raised in high-risk social environments. One major difference between the prenatal cocaine/methamphetamine and opiate studies is that the opiates studies tend to have small sample sizes (20 subjects per group is typical), which precludes statistical adjustment for confounding variables, whereas cocaine studies deal with larger population samples, allowing more valid adjustment for confounding variables.
Some studies were cross-sectional rather than longitudinal. In one such study,63 3 to 6 year old children exposed to heroin performed more poorly than the comparison groups on the general cognitive index and on the perceptual, quantitative, and memory subscales of the McCarthy Scales. There were three comparison groups—drug-nave subjects in drug-using households, a group with multiple high risk factors, and a socioeconomic comparison group—but there were only 15 to 22 children in each group, including the heroin exposed group. No effects of heroin exposure on measures of speech and language, activity level, mental maturity, or developmental status were found. In a study of 126 offspring of heroin addicts between 8 and 17 years old,64 school absence, school failure, and behavior problems in school were more common than in comparison group from the same neighborhoods. Cubas and Field65 compared 20 children aged 6 to 13 with prenatal exposure to methadone and a control group of 20 non-exposed children. No differences were found on cognitive tests, although methadone exposure was associated with lower IQ scores. Methadone-exposed children exhibited greater anxiety, aggression, and rejection than did those in the control group, and their mothers reported more behavior problems.
looseness-1In three longitudinal studies, no differences were found on the McCarthy scales related to prenatal opiate exposure. These include: a study comparing methadone-exposed (N = 26), heroin-exposed (N = 25), and a drug-free comparison group (N = 41) in 3 to 6 year old children;66 a study of 27 methadone-exposed and 17 non-drug exposed children at age 4 years;67 and a study of methadone-exposed (N = 33) and non-drug exposed comparison children (N = 30) at age 5 years.68 In the latter study, during the testing session, the drug-exposed children were more active, energetic, immature, and showed more task irrelevant activity during the testing session.68 In a longitudinal follow-up of the previously reported sample,63 32 prenatally exposed 6- to 11-year-old children were compared with 12 controls.69 Behavior problems in school and psychiatric referrals were related to maternal opiate use, although the number of cases was small (7 psychiatric referrals in the exposed group and 1 in the comparison group). In a 10-year follow-up of 36 methadone-exposed children and a similar group of comparison children, Hans62 found that drug-exposed children were somewhat more likely to receive ADHD and disruptive behavior diagnoses and made more errors on an attentional (continuous performance) task.
As mentioned above, it is known that many of these children grow up in high-risk environments.62 Similar learning and behavioral problems have been reported in school-age children either exposed to drugs in utero70 or living with drug-dependent parents who were not exposured in utero.71,72 Some studies have attempted to isolate the effects of in utero opiate exposure by using different types of comparison groups. An early study compared 6- to 15-year-old, opiate-exposed children to a narcotic-environment group (no in utero exposure but caregivers used opiates) using measures of neurological and behavioral functioning.73 Opiate-exposed children had impairments in perceptual, motor, and attentional functioning, whereas the narcotic environmental group did not. In more recent work, lower scores were found on the McCarthy scales at age 4.5 in a Norwegian study of 64 exposed and 52 comparison children under conditions of minimal postnatal social risk.74 Social risk was “controlled” by studying children of heroin users (and other drugs) placed in the care of foster or adoptive parents who were specially selected to provide care for infants at risk. In an Israeli study attempting to control the postnatal environment, the development of children aged 5 to 12 years born to mothers with heroin dependency who were raised at home or adopted was studied in comparison with children with environmental deprivation alone (i.e., low parental socioeconomic status and evidence of neglect), children born to fathers with heroin dependency,75 and control individuals of average socioeconomic status.76 Children born to parents with heroin dependency who were raised at home showed impaired verbal, performance, and reading and arithmetic skills. Children born to mothers with heroin dependency but who were adopted at a young age had normal intellectual and learning abilities, but some had reduced function on performance IQ. Children born to parents with heroin dependency had a high rate of ADHD, including those who were adopted; the highest rate of ADHD was seen in children born to mothers with heroin dependency who were raised at home.
One physiological study attempted to control for opiate-related environmental factors, comparing a group of children born to mothers who began drug use after the child was born to a group of children with intrauterine opiate exposure for environmental influences associated with parental drug use.77 Heart rate variability (vagal tone) was measured during an attention task. When distracters were added to the attention task, opiate-exposed boys failed to suppress vagal tone compared to both control groups. However, both the opiate-exposed boys and the environmental controls made fewer correct responses than non-drug-exposed controls on this task. Thus, physiological responses to increased attention demands may be affected by prenatal opiate exposure as well as environmental influences.
In a neuroimaging study of this population,78 morphometric cerebral characteristics were studied in 9- to 11-year-old children with prenatal exposure to opiates and other drugs (n = 14) compared to unexposed controls (n = 14). Compared to controls, the drug-exposed children had smaller intracranial and brain volumes, including smaller cerebral cortex, amygdala, accumbens area, putamen, pallidum, brainstem, cerebellar cortex, cerebellar white matter, and inferior lateral ventricles, as well as a thinner cortex of the right anterior cingulate and lateral orbitofrontal cortex. There was some association between volumes of the right anterior cingulate, the right lateral orbitofrontal cortex, and the accumbens area and cognitive ability and behavior problems.
UNDERSTANDING THE EFFECTS OF PRENATAL DRUG EXPOSURE
One determinant of the long-term effects of prenatal drug exposure is the pathophysiology of the drug itself. Typically, we think of drugs as teratogens that cause malformations or defects depending on the drugs’ mechanisms of action. For example, the effects of cocaine on blocking the reuptake of neurotransmitters, especially dopamine, have been well documented. However, in addition to teratogenic effects, there may also be non-teratogenic effects of drugs. We79 have described a model in which cocaine, as well as other substances, acts as an intrauterine stressor that disrupts the neuroendocrine environment and the genetic programming of fetal-placental development.
Increasing evidence from preclinical, prospective clinical and epidemiological studies suggest that, at least in the case of disease, early development does echo throughout life.80-82 The literature on the influence of prenatal stress on the offspring suggests that many biological factors acting during prenatal life are associated not only with the development of common adult cardiovascular and metabolic disorders but also with neurobehavioral abnormalities83-93 and behavioral disorders.94-97 These findings have given rise to the concept of “fetal origins of adult disease” or the “developmental origins of health and disease.” Although original studies related low birthweight to adult disease, it is generally accepted that low birth weight per se is not at the heart of these disorders, but that there are common factors that influence intrauterine growth, as well as adult physiological systems.82 The “fetal origins” observations are due, in part, to environmental factors acting early in life that “program” developing systems, altering structure and function and probably behavioral expression. Prenatal drug exposure may be one of those factors that alter the set-points or “hard-wire” physiological systems. Stress hormones such as catecholamines and glucocorticoids can alter regulation of the neuroendocrine environment by acting on the hypothalamic-pituitary-adrenal (HPA) axis, which results in an altered set point for physiologic, metabolic, and behavioral outcomes.98 Because they are an important feature of the stress response, glucocorticoids have become prominent candidates as mediators of the effects of “programming.”
Fetal Programming and Brain
The brain is particularly sensitive to prenatal programming including effects on the HPA axis. Pregnant dams exposed to stress show increased adrenocorticotrophic hormone (ACTH) and corticosterone (CORT).82,99, 100 Prenatal stress also increases CORT and ACTH levels in adult offspring.80-82, 96,98 The effect of prenatal stress on adult hippocampal corticosteroid receptor density95,101-104 may have implications for emotional reactivity. Prenatally, stressed rats have a high degree of “emotionality,”105 indicated by decreased locomotion and increased defecation.105-108 They also show less play,109 more defensive freezing,110 less movement in an activity wheel,111 and increased vocalizations.100 Prenatal stress affects cognitive abilities including operant discrimination,112 reversal task in a water maze,113,114 and memory.115 In other animal studies, maternal stress during pregnancy results in offspring that are more irritable, anxious, and difficult to control.82,116-119
Brain neurotransmitter systems and glucocorticoids interact to modulate both behavior and HPA activity.120 Disturbances in HPA regulation and brain monoamine levels have been associated with affective and anxiety disorders in humans.121-124 Also, in human studies, poor health outcomes, such as low birthweight, preterm birth, and intrauterine growth retardation, have been related to prenatal stress.125,126 Moderate to severe stressful life events during midgestation was related to birth weight and small head circumference, suggesting effects on the brain.127 Maternal first-trimester exposure to the stress of war has also been associated with an increased risk of the offspring developing schizophrenia in adult life.128 Similar to the “emotionality” reported in animal studies, human infants exposed to stress in utero show high reactivity, activity, and irritability.129-131 Psychological and behavioral abnormalities, including learning and behavior problems, have also been reported in children exposed to prenatal stress.132-135 The effects of prolonged exposure to chronic stress, called allostatic load,136 and, concomitantly, prolonged activation of the neuroendocrine stress axes has also been related to physical disease and behavioral disorders.137
Drugs and Gene Expression
Our model79 argues for the effects of an adverse intrauterine environment, created by maternal use of cocaine and other substances, on catecholamines and glucocorticoids. As a stressor, a drug such as cocaine programs the HPA axis as well as behavior, due in part to plasticity of brain monoamine systems. Specifically, a drug as a stressor alters the expression of key candidate genes and gene networks important to placental function in late gestation. We focus on the norepinephrine transporter (NET) and a steroid metabolic enzyme, 11β-HSD-2 and placental gene networks.
Placental NET and 11ß-HSD-2 are pivotal placental genes that program the intrauterine neuroendocrine environment during development by protecting the fetus from excess catecholamines and glucocorticoids, which have harmful effects on the fetus.138 Lower placental 11ß-HSD-2 activity is associated with smaller fetuses in rats139 and humans.140-143 Mutations of 11ß-HSD-2 are also associated with low birth weight in human infants144 and increased fetal cortisol levels are associated with IUGR145 11ß-HSD-2 modulates the programming effects of prenatal glucocorticoid exposure.146,147 The HPA axis is highly sensitive to the effects of glucocorticoids on perinatal programming.82,148-150 High levels of maternal glucocorticoids have been shown to disrupt intrauterine growth, postnatal HPA axis function, and neurobehavioral outcome in offspring. Placental expression of this key enzyme is potently downregulated at the RNA, protein, and functional level by norepinephrine, which is in turn regulated by NET. Downregualtion of NET is associated with adverse intrauterine events, including maternal/placental disorders such as preeclampsia and exposure to drugs such as cocaine and nicotine.151,152 Reduced placental NET expression from drug exposure may lead to increased circulating catecholamines, downregulation of 11β-HSD-2 and chronic fetal hyper-cortisolism, leading to altered neuroendocrine (HPA axis) activity and dysregulated neurobehavioral functioning. Because catecholamines are released during stress, this may be a mechanism linking prenatal stress with altered fetal development mediated by effects on 11β-HSD-2.
The altered expression of these two key candidate genes is likely associated with changes in networks of genes involved in critical placental functions that maintain physiological homeostasis in utero and otherwise promote intrauterine growth, development, and preparation for postnatal life. Epigenetic mechanisms (i.e., environmentally induced changes in gene expression without altering DNA sequences) are thought to be involved. Our preliminary findings79 showed that these changes in placental gene expression are associated with altered methylation of placental genomic DNA, particularly in promoter regions. We found that dysregulation of 11β HSD2 related to cocaine or nicotine use during pregnancy was associated with hyper-methylation, suggesting DNA silencing. Significant interest exists in the concept that modifications of DNA mechanistically affect “adaptation to the environment.” Complex processes such as the development of memory are now being attributed to chromatin remodeling, epigenetic modifications of DNA, and lasting alterations in gene expression.153 Drugs of abuse induce “adaptations” in brain regions. This may occur through alterations in gene expression, altered chromatin remodeling, and alterations in the number and projections of neurons in specific regions. Recently, cocaine was shown to induce neuroadaptations through altered gene expression.154 It is becoming widely accepted that chromatin remodeling is an important regulatory mechanism underlying gene environment interactions, learning, and drug-induced neural and behavioral plasticity. Similar alterations in DNA methylation and histone acetylation are seen following intrauterine growth restriction.155 It is thus likely that altered expression of these key candidate genes and associated changes in networks of genes involved in placental function follows intrauterine exposure to stress, cocaine, and other substances. Some of these changes may lead to permanent “epigenetic” alterations in placental gene expression through DNA methylation and chromatin remodeling. Because of the unique nature of intrauterine development, these observations suggest mechanisms whereby an altered placental environment can have effects on neurodevelopmental outcome.
Behavioral Dysregulation
In our model,79 these disruptions in the placental environment alter HPA set points, resulting in neuroendocrine and behavioral dysregulation in the newborn that can lead to the type of childhood behavioral, emotional, and neurocognitive deficits related to prenatal drug exposure described earlier. These deficits may, in turn, have implications for adolescent psychopathology, including early onset of substance use in adolescence. Behavioral dysregulation can begin in utero and is proposed to be a dynamic developmental process as alterations in the quality of the environment (prenatal and postnatal) modify behavioral expression. Behavioral dysregulation is evidenced during infancy as neurobehavioral and neuroendocrine disorganization. During childhood, indicators of behavioral dysregulation reflect a deficient capacity to control behavior and regulate emotion. These phenotypes are important because they appear to be prognostic indicators for substance use.156 The earlier a drug is used, the greater the likelihood of its abuse during adolescence and adulthood.157,158 These phenotypes reflect major domains of psychological function, cognition, affect, and behavior and include impulsivity, reactive aggression, sensation seeking, excessive risk taking,159,160 irritability, negative affect, difficult temperament,161-163 conduct disorder, attention deficit hyperactivity disorder, oppositional defiant disorder, anxiety, depression,164and impaired executive function.165-167 This collection of disturbances in emotion regulation and behavior control is included in the construct of neurobehavioral disinhibition and is thought to reflect disturbances in the prefrontal cortex.168 Some of these disturbances have already been reported in the prenatal cocaine exposure studies reported above and may become more visible as these youths enter adolescence. Disruption of neuroendocrine homeostasis in utero by prenatal drug exposure can be observed at birth and may lead to lasting behavioral dysregulation that increases vulnerability to substance use, resulting in early onset substance use in adolescents.
Adolescent Brain Development
The understanding that addiction is a developmental disease means that it is critical to consider the influence of specific developmental periods.169 In addition to fetal development, we know that brain development continues well beyond childhood and adolescence.170,171 The “immaturity” or abnormal development of the adolescent brain may be related to risk taking behavior, including substance use.
Extensive maturational changes that occur in brain development in adolescence may confer vulnerability to substance use through disruption of the HPA system.172-174 Maturation of high-order association cortices, including changes in prefrontal cortex, occurs later than low-order sensory cortices during late adolescence.75 Neurophysiologic,175,176 and fMRI177 studies have shown disruption of the frontal cortex in youths at high risk for substance use onset. These youths show deficits related to substance use onset, including poor affect modulation, behavior control, and executive function processes thought to reflect a neuromaturational disturbance subserved by neural systems in the anterior cerebral cortex.160,178-180 Stress reactivity is related to pubertal development and brain regions, such as the hippocampus, pre-frontal cortex, and amygdale, and anatomic areas that are highly sensitive to stress hormones and regulate emotionality and continue to mature during the peripubertal period.181 Glucocorticoid receptors are present in both subcortical (paraventricular nucleus and other hypothalamic nuclei, the hippocampus and parahippocampal gyrus) and cortical structures, with a preferential distribution in the prefrontal cortex.120,182-184 The distribution of glucocorticoid receptors in the primate brain are more closely related to the human brain in terms of neocortex development.185,186 Non-human primate studies suggest that the behavioral effects of prenatal cocaine exposure may not be manifested until later in development.187,188 Chronic exposure to stress or allostatic load leads to atrophy and impaired neuronal function in the hippocampus and medial prefrontal cortex,189-191 regions that are responsible for executive function and adaptation to stress. Cocaine and other substance related to “fetal programming” could have long-term effects on HPA axis activity, resulting in a physiological endophenotype of cortisol reactivity that could be a harbinger of substance use. Although there are studies of cortisol reactivity related to prenatal drug exposure in infancy, as described in the previous article by Bandstra et al., we did not find any studies relating prenatal cocaine, methamphetamine, or opiate exposure to cortisol in older children.
The advent of the “Genome Era” has dissolved disciplinary boundaries and transformed the research landscape. The sequencing of the human genome has lead to advances in our understanding of the cellular, molecular, and biochemical mechanisms that regulate development, learning, behavior, and the molecular pathways that could be altered due to prenatal substance exposure. These are exciting times as advances in neurobiology, including genetics, epigenetics, fetal programming, and neural plasticity, will provide a better understanding of the pathophysiology and long-term implications of prenatal drug exposure that should have implications for public policy, preventive intervention and treatment.
References
- 1.Accornero VH, Morrow CE, Banstra ES, Johnson AL, Anthony JC. Behavioral outcome of preschoolers exposed to cocaine: role of maternal behavioral health. J Ped Psych. 2002;27:259–69. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–59. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 3.Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–72. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional–behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psych. 2002;38:648–58. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covington C, Nordstrom–Klee B, Delaney–Black V, Templin T, Ager J, Sokol RJ. Development of an instrument to assess problem behavior in first grade students prenatally exposed to cocaine. Part II: validation. Subst Abus. 2001;22:217–33. doi: 10.1080/08897070109511464. [DOI] [PubMed] [Google Scholar]
- 7.Delaney–Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, Chiodo L, Sokol RJ. Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–63. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Delaney–Black V, Covington C, Templin T, Ager J, Martier S, Sokol R. Prenatal cocaine exposure and child behavior. Pediatrics. 1998;102:945–50. doi: 10.1542/peds.102.4.945. [DOI] [PubMed] [Google Scholar]
- 9.Delaney–Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, Leddick L, Czerwinski RH, Sokol RJ. Teacher–assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:783–91. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- 10.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–97. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilbride HW, Castor CA, Fuger KL. School–age outcome of children with prenatal cocaine exposure following early case management. J Dev Behav Pediatr. 2006;27:181–7. doi: 10.1097/00004703-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S. Mental health outcomes of cocaine–exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordstrom Bailey B, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, et al. Gender and alcohol moderate prenatal cocaine effects on teacher–report of child behavior. Neurotoxicol Teratol. 2005;27:181–9. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school–age children. Neurotoxicol Teratol. 1996;18:627–34. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- 15.Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine–exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–7. [PubMed] [Google Scholar]
- 16.Sood BG, Nordstrom Bailey B, Covington C, Sokol RJ, Ager J, Janisse J, Hannigan JH, Delaney-Black V. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol. 2005;27:191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hurt H, Brodsky NL, Roth H, Malmud E, Giannetta JM. School performance of children with gestational cocaine exposure. Neurotoxicol Teratol. 2005;27:203–11. doi: 10.1016/j.ntt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Kable JA, Coles CD, Lynch ME, Platzman K. Physiological responses to social and cognitive challenges in 8–year olds with a history of prenatal cocaine exposure. Dev Psychobiol. 2008;50:251–65. doi: 10.1002/dev.20285. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary CC, Frank DA, Grant–Knight W, Beeghly M, Augustyn M, Rose–Jacobs R, Cabral HJ, Gannon K. Suicidal ideation among urban nine and ten year olds. J Dev Behav Pediatr. 2006;27:33–9. doi: 10.1097/00004703-200602000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, et al. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Ped. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asanbe CB, Lockert E. Cognitive abilities of African American children with prenatal cocaine/polydrug exposure. J Health Care Poor Underserved. 2006;17:400–12. doi: 10.1353/hpu.2006.0054. [DOI] [PubMed] [Google Scholar]
- 22.Bennett DS, Bendersky M, Lewis M. Children’s cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44:919–28. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank DA, Rose–Jacobs R, Beeghly M, Wilbur M, Bellinger D, Cabral H. Level of prenatal cocaine exposure and 48–month IQ: importance of preschool enrichment. Neurotoxicol Teratol. 2005;27:15–28. doi: 10.1016/j.ntt.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Marques PR, Pokorni JL, Long T, Teti LO. Maternal depression and cognitive features of 9–year–old children prenatally–exposed to cocaine. Am J Drug Alcohol Abuse. 2007;33:45–61. doi: 10.1080/00952990601082647. [DOI] [PubMed] [Google Scholar]
- 25.Morrow CE, Culbertson JL, Accornero VH, Xue L, Anthony JC, Bandstra ES. Learning disabilities and intellectual functioning in school–aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30:905–31. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulsifer MB, Radonovich K, Belcher HME, Butz AM. Intelligence and school readiness in preschool children with prenatal drug exposure. Child Neuropsychology. 2004;10:89–101. doi: 10.1080/09297040490911104. [DOI] [PubMed] [Google Scholar]
- 27.Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kirchner HL. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA. 2004;291:2448–56. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–11. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasserman GA, Kline JK, Bateman DA, Chiriboga C, Lumey LH, Friedlander H, Melton L, Heagarty MC. Prenatal cocaine exposure and school–age intelligence. Drug Alcohol Depend. 1998;50:203–10. doi: 10.1016/s0376-8716(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 30.Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir AY, Dausa AT, Xue L, Anthony JC. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotox Teratol. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 31.Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Substance Use and Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeghly M, Martin B, Rose–Jacobs R, Cabral H, Augustyn M, Bellinger D, Frank DA. Prenatal cocaine exposure and children’s language functioning at 6 and 9.5 years: moderating effects of child age, birthweight, and gender. J Pediatr Psychol. 2006;31:98–115. doi: 10.1093/jpepsy/jsj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis BA, Kirchner HL, Short EJ, Minnes S, Weishampel P, Satayathum S, Singer LT. Prenatal cocaine and tobacco effects on children’s language trajectories. Pediatrics. 2007;120:e78–85. doi: 10.1542/peds.2006-2563. [DOI] [PubMed] [Google Scholar]
- 34.Lewis BA, Singer Lt SEJ, Minnes S, Arendt R, Weshampel P, Klein N, Min MO. Four year language outcomes of children exposed to cocaine in utero. Neurotoxicol Teratol. 2004;26:617–27. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–59. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 37.Bendersky M, Gambini LS, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Ped. 2003;24:345–51. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–38. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Mayes L, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school–aged children exposed in utero to cocaine. Child Neuropsychol. 2007;13:205–18. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- 40.Schroder MD, Snyder PJ, Sielski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain Cog. 2004;55:409–12. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- 41.Mayes LC, Molfese DL, Key AP, Hunter NC. Event–related potentials in cocaine–exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Levine TP, Liu J, Das A, Lester B, Lagasse L, Shankaran S, Bada HS, Bauer CR, Higgins R. Effects of prenatal cocaine exposure on special education in school–aged children. Pediatrics. 2008;122:e83–91. doi: 10.1542/peds.2007-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell B, Bradley R. HOME inventory and administration manual. Littlerock: University of Arkansas for Medical Sciences and University of Arkansas at Littlerock; 2001. [Google Scholar]
- 44.Dow–Edwards DL, Benveniste H, Behnke M, Bandstra ES, Singer LT, Hurd YL, et al. Neuroimaging of prenatal drug exposure. Neurotoxicol Teratol. 2006;28:386–402. doi: 10.1016/j.ntt.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, et al. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37:275–9. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Neyzi N, Quinn B, Kekatpure D, Kennedy S, Sheinkopf B, Lester B, et al. Automated segmentation of brain structures in a pediatric population with prenatal cocaine exposure. 2007 meeting of the Society for Neuroscience; San Diego, CA. 2007. [Google Scholar]
- 47.Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, Robson CD, Rose-Jacobs R, Frank DA. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–50. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine–exposed children. Pediatrics. 2006;118:2014–24. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–31. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao H, Wang J, Giannetta J, Korczykowski M, Shera D, Avants BB, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–54. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- 51.Hurt H, Giannetta JM, Korczykowski M, Hoang A, Tang KZ, Betancourt L, Brodsky NL, Shera DM, Farah MJ, Detre JA. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. J Pediatr. 2008;152:371–7. doi: 10.1016/j.jpeds.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Dev Neurosci. 2009;31(1–2):159–66. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–60. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 54.Wouldes T, LaGasse L, Sheridan J, Lester B. Maternal methamphetamine use during pregnancy and child outcome: what do we know? NZ Med J. 2004;117(1206):1–10. [PubMed] [Google Scholar]
- 55.Lester B, Das A, LaGasse L, Seifer R, Bauer C, Shankaran S, Bada H, Messinger D, Wright L, Smeriglio V, Poole K. Prenatal cocaine exposure and 7–year outcome: IQ and special education. Ped Res. 2004;53:534A. [Google Scholar]
- 56.Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14–year follow–up of growth and school performance. Acta Paediatr. 1996;85:204–8. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 57.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Pre–school children of amphetamine–addicted mothers. I. Somatic and psychomotor development. Acta Paediatr Scand. 1985;74:179–84. doi: 10.1111/j.1651-2227.1985.tb10946.x. [DOI] [PubMed] [Google Scholar]
- 58.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4–year–old children whose mothers used amphetamine during pregnancy. Child Abuse Negl. 1988;12:503–7. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- 59.Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. The influence of environmental factors on behavioral problems in 8–year–old children exposed to amphetamine during fetal life. Child Abuse Negl. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 60.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ertist T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Kaltenbach KA. Exposure to opiates: behavioral outcomes in preschool and school– age children. NIDA Res Monogr. 1996;164:230–41. [PubMed] [Google Scholar]
- 62.Hans SL. Prenatal drug exposure: behavioral functioning in late childhood and adolescence. NIDA Res Monogr. 1996;164:261–76. [PubMed] [Google Scholar]
- 63.Wilson GS, McCreary R, Kean J, Baxter JC. The development of preschool children of heroin–addicted mothers: a controlled study. Pediatrics. 1979;63:135–41. [PubMed] [Google Scholar]
- 64.Sowder B, Burt M. Children of Heroin addicts: an assessment of health, learning, behavioral, and adjustment problems. New York, NY: Praeger; 1980. [Google Scholar]
- 65.de Cubas MM, Field T. Children of methadone–dependent women: developmental outcomes. Am J Orthopsychiatry. 1993;63:266–76. doi: 10.1037/h0079429. [DOI] [PubMed] [Google Scholar]
- 66.Lifschitz MH, Wilson GS, Smith EO, Desmond MM. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics. 1985;75:269–74. [PubMed] [Google Scholar]
- 67.Kaltenbach K, Finnegan L. Children exposed to methadone in–utero: Assessment of developmental and cognitive ability. Ann N Y Acad Sci. 1989;562:360–362. [Google Scholar]
- 68.Strauss ME, Lessen–Firestone JK, Chavez CJ, Stryker JC. Children of methadone–treated women at five years of age. Pharmacol Biochem Behav. 1979;11(Suppl):3–6. [PubMed] [Google Scholar]
- 69.Wilson GS. Clinical studies of infants and children exposed prenatally to heroin. Ann N Y Acad Sci. 1989;562:183–94. doi: 10.1111/j.1749-6632.1989.tb21017.x. [DOI] [PubMed] [Google Scholar]
- 70.Olofsson M, Buckley W, Andersen GE, Friis–Hansen B. Investigation of 89 children born by drug–dependent mothers. II. Follow–up 1–10 years after birth. Acta Paediatr Scand. 1983;72:407–10. doi: 10.1111/j.1651-2227.1983.tb09737.x. [DOI] [PubMed] [Google Scholar]
- 71.Hejanic B, Barredo V, Hejanic M, Tomelleri C. Children of heroin addicts. Inter J Addict. 1979;14:919–31. doi: 10.3109/10826087909073936. [DOI] [PubMed] [Google Scholar]
- 72.Nichtem S. The children of drug users. J Amer Acad Child Adolesc Psych. 1973;12:24–31. doi: 10.1097/00004583-197301000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Davis DD, Templer DI. Neurobehavioral functioning in children exposed to narcotics in utero. Addict Behav. 1988;13:275–83. doi: 10.1016/0306-4603(88)90054-8. [DOI] [PubMed] [Google Scholar]
- 74.Moe V. Foster–placed and adopted children exposed in utero to opiates and other substances: prediction and outcome at four and a half years. J Dev Behav Pediatr. 2002;23:330–9. doi: 10.1097/00004703-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ornoy A, Segal J, Bar–Hamburger R, Greenbaum C. Developmental outcome of school–age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol. 2001;43:668–75. doi: 10.1017/s0012162201001219. [DOI] [PubMed] [Google Scholar]
- 77.Hickey JE, Suess PE, Newlin DB, Spurgeon L, Porges SW. Vagal tone regulation during sustained attention in boys exposed to opiates in utero. Addict Behav. 1995;20:43–59. doi: 10.1016/0306-4603(94)00044-y. [DOI] [PubMed] [Google Scholar]
- 78.Walhovd KB, Moe V, Slinning K, Due-Tonnessen P, Bjornerud A, Dale AM, van der Kouwe A, Quinn BT, Kosofsky B, Greve D, Fischl B. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 2007;36:1331–44. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lester B, Padbury J. The Third Pathophysiology of Prenatal Cocaine Exposure. Dev Neurosci. 2009;31(1–2):23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 80.Barker DJ, Osmond C, Rodin I, Fall CH, Winter PD. Low weight gain in infancy and suicide in adult life. BMJ. 1995;311:1203. doi: 10.1136/bmj.311.7014.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 82.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–28. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 83.Barker D. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–8. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 84.Barker DJ. The fetal origins of adult hypertension. J Hypertens Suppl. 1992;10:S39–44. [PubMed] [Google Scholar]
- 85.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falkner B. Birth weight as a predictor of future hypertension. Am J Hypertens. 2002;15(2 Pt 2):43S–5S. doi: 10.1016/s0895-7061(01)02297-x. [DOI] [PubMed] [Google Scholar]
- 87.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 89.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. Eur J Endocrinol. 2004;151(Suppl 3):U131–9. doi: 10.1530/eje.0.151u131. [DOI] [PubMed] [Google Scholar]
- 90.Phillips D, Barker D, Hales C, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–4. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 91.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Speizer FE, Manson JE. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130(4 Pt 1):278–84. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 92.Sallout B, Walker M. The fetal origin of adult diseases. J Obstet Gynaecol. 2003;23:555–60. doi: 10.1080/0144361031000156483. [DOI] [PubMed] [Google Scholar]
- 93.Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996;348:1269–73. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- 94.Allin M, Rooney M, Cuddy M, Wyatt J, Walshe M, Rifkin L, Murray R. Personality in young adults who are born preterm. Pediatrics. 2006;117:309–16. doi: 10.1542/peds.2005-0539. [DOI] [PubMed] [Google Scholar]
- 95.Gale CR, Martyn CN. Birth weight and later risk of depression in a national birth cohort. Br J Psychiatry. 2004;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- 96.Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–5. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 97.Wals M, Reichart CG, Hillegers MH, Van Os J, Verhulst FC, Nolen WA, Ormel J. Impact of birth weight and genetic liability on psychopathology in children of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2003;42:1116–21. doi: 10.1097/01.CHI.0000070242.24125.78. [DOI] [PubMed] [Google Scholar]
- 98.Matthews SG. Antenatal glucocorticoids and the developing brain: mechanisms of action. Semin Neonatol. 2001;6:309–17. doi: 10.1053/siny.2001.0066. [DOI] [PubMed] [Google Scholar]
- 99.Slotkin TA, Orband–Miller L, Queen KL, Whitmore WL, Seidler FJ. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J Pharmacol Exp Ther. 1987;240:602–11. [PubMed] [Google Scholar]
- 100.Williams MT, Hennessy MB, Davis HN. Stress during pregnancy alters rat offspring morphology and ultrasonic vocalizations. Physiol Behav. 1998;63:337–43. doi: 10.1016/s0031-9384(97)00428-9. [DOI] [PubMed] [Google Scholar]
- 101.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long–term effects of prenatal stress. J Neurosci. 1996;16:3943–9. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo–pituitary–adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–5. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 103.Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long–term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15(1 Pt 1):110–6. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress–induced corticosterone secretion. J Neurosci. 1997;17:2626–36. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fride E, Dan Y, Feldon J, Halevy G, Weinstock M. Effects of prenatal stress on vulnerability to stress in pre-pubertal and adult rats. Physiol Behav. 1986;37:681–7. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- 106.Pfister HP, Muir JL. Prenatal exposure to predictable and unpredictable novelty stress and oxytocin treatment affects offspring development and behavior in rats. Int J Neurosci. 1992;62(3–4):227–41. doi: 10.3109/00207459108999774. [DOI] [PubMed] [Google Scholar]
- 107.Poltyrev T, Keshet GI, Kay G, Weinstock M. Role of experimental conditions in determining differences in exploratory behavior of prenatally stressed rats. Dev Psychobiol. 1996;29:453–62. doi: 10.1002/(SICI)1098-2302(199607)29:5<453::AID-DEV4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 108.Wakshlak A, Weinstock M. Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiol Behav. 1990;48:289–92. doi: 10.1016/0031-9384(90)90315-u. [DOI] [PubMed] [Google Scholar]
- 109.Takahashi LK, Haglin C, Kalin NH. Prenatal stress potentiates stress–induced behavior and reduces the propensity to play in juvenile rats. Physiol Behav. 1992;51:319–23. doi: 10.1016/0031-9384(92)90147-t. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress–induced behavior in adult rats. Brain Res. 1992;574:131–7. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- 111.Lambert KG, Kinsley CH, Jones HE, Klein SL, Peretti SN, Stewart KM. Prenatal stress attenuates ulceration in the activity stress paradigm. Physiol Behav. 1995;57:989–94. doi: 10.1016/0031-9384(94)00340-b. [DOI] [PubMed] [Google Scholar]
- 112.Weller A, Glaubman H, Yehuda S, Caspy T, Ben–Uria Y. Acute and repeated gestational stress affect offspring learning and activity in rats. Physiol Behav. 1988;43:139–43. doi: 10.1016/0031-9384(88)90229-6. [DOI] [PubMed] [Google Scholar]
- 113.Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–16. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 114.Szuran T, Zimmermann E, Welzl H. Water maze performance and hippocampal weight of prenatally stressed rats. Behav Brain Res. 1994;65:153–5. doi: 10.1016/0166-4328(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 115.Vallee M, MacCari S, Dellu F, Simon H, Le Moal M, Mayo W. Long–term effects of prenatal stress and postnatal handling on age–related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur J Neurosci. 1999;11:2906–16. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 116.Meaney M, Seckl J. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 117.Roughton EC, Schneider ML, Bromley LJ, Coe CL. Maternal endocrine activation during pregnancy alters neurobehavioral state in primate infants. Am J Occup Ther. 1998;52:90–8. doi: 10.5014/ajot.52.2.90. [DOI] [PubMed] [Google Scholar]
- 118.Schneider M, Moore C, Kraemer G. Moderate level alcohol during pregnancy, prenatal stress, or both and limbic–hypothalamic–pituitary–adrenocortical axis response to stress in rhesus monkeys. Child Dev. 2004;75:96–109. doi: 10.1111/j.1467-8624.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 119.Schneider ML. Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Dev Psychobiol. 1992;25:529–40. doi: 10.1002/dev.420250706. [DOI] [PubMed] [Google Scholar]
- 120.McEwen BS. Glucocorticoid–biogenic amine interactions in relation to mood and behavior. Biochem Pharmacol. 1987;36:1755–63. doi: 10.1016/0006-2952(87)90234-6. [DOI] [PubMed] [Google Scholar]
- 121.Maes M, Meltzer HY, D’Hondt P, Cosyns P, Blockx P. Effects of serotonin precursors on the negative feedback effects of glucocorticoids on hypothalamic–pituitary–adrenal axis function in depression. Psychoneuroendocrinology. 1995;20:149–67. doi: 10.1016/0306-4530(94)00049-g. [DOI] [PubMed] [Google Scholar]
- 122.Meador–Woodruff JH, Greden JF, Grunhaus L, Haskett RF. Severity of depression and hypothalamic–pituitary–adrenal axis dysregulation: identification of contributing factors. Acta Psychiatr Scand. 1990;81:364–71. doi: 10.1111/j.1600-0447.1990.tb05465.x. [DOI] [PubMed] [Google Scholar]
- 123.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin–releasing factor–like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 124.van Praag HM. Depression. Lancet. 1982;2:1259–64. doi: 10.1016/s0140-6736(82)90115-5. [DOI] [PubMed] [Google Scholar]
- 125.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin–releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–9. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 126.Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131–42. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- 127.Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J, Hemmingsen R. Prenatal stressors of human life affect fetal brain development. Dev Med Child Neurol. 1994;36:826–32. doi: 10.1111/j.1469-8749.1994.tb08192.x. [DOI] [PubMed] [Google Scholar]
- 128.van Os J, Selten J. Prenatal exposure to maternal stress and subsequent schizophrenia. Br J Psychiatry. 1998;172:324–6. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 129.Field T. Stress and coping from pregnancy through the postnatal period. In: Cummings EG, Greene AL, editors. Life–Span developmental psychology: perspectives on stress and coping. Vol. 199. Hillsdale, NJ: Lawrence Erlbaum Associates; pp. 45–59. [Google Scholar]
- 130.Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–8. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- 131.Levy–Shiff R, Dimitrovsky L, Shulman S, et al. Cognitive appraisals, coping strategies, and support resources as correlates of parenting and infant development. Dev Psychobiol. 1998;34:1417–27. doi: 10.1037//0012-1649.34.6.1417. [DOI] [PubMed] [Google Scholar]
- 132.Meijer A. Child psychiatric sequelae of maternal war stress. Acta Psychiatr Scand. 1985;72:505–11. doi: 10.1111/j.1600-0447.1985.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 133.Stott DH. Follow–up study from birth of the effects of prenatal stresses. Dev Med Child Neurol. 1973;15:770–87. doi: 10.1111/j.1469-8749.1973.tb04912.x. [DOI] [PubMed] [Google Scholar]
- 134.Ward AJ. Prenatal stress and childhood psychopathology. Child Psychiatry Hum Dev. 1991;22:97–110. doi: 10.1007/BF00707788. [DOI] [PubMed] [Google Scholar]
- 135.DePietro J. The role of prenatal maternal stress in child development. Current Directions in Psychological Science. 2004;13:71–4. [Google Scholar]
- 136.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 137.McEwen BS. Early life influences on life–long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–54. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 138.Meyer JS. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985;65:946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- 139.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–41. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 140.McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM. Reduced placental 11beta–hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86:4979–83. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- 141.Murphy VE, Zakar T, Smith R, Giles WB, Gibson PG, Clifton VL. Reduced 11beta–hydroxysteroid dehydrogenase type 2 activity is associated with decreased birth weight centile in pregnancies complicated by asthma. J Clin Endocrinol Metab. 2002;87:1660–8. doi: 10.1210/jcem.87.4.8377. [DOI] [PubMed] [Google Scholar]
- 142.Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, et al. 11Beta–hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod. 1998;13:799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- 143.Stewart P, Roberson F, Mason J. Type 2 11–hydroxysteroid dehydrogenase messenger RNA and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal steroidogenesis. J Clin Endocrinol Metab. 1995;80:885–90. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- 144.Dave–Sharma S, Wilson R, Harbison M. Extensive personal experience: examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endocrinol Metab. 1998;83:2244–54. doi: 10.1210/jcem.83.7.4986. [DOI] [PubMed] [Google Scholar]
- 145.Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta–hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 2000;57:1412–7. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 146.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–7. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 147.Seckl JR. Glucocorticoids, feto–placental 11 beta–hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 1997;62:89–94. doi: 10.1016/s0039-128x(96)00165-1. [DOI] [PubMed] [Google Scholar]
- 148.Bohn MC. Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience. 1980;5:2003–12. doi: 10.1016/0306-4522(80)90045-7. [DOI] [PubMed] [Google Scholar]
- 149.Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–93. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- 150.Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–85. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- 151.Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslen B, Marsal K, Hansson SR. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre–eclamptic and normotensive pregnancies. Placenta. 2004;25:518–29. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 152.Bzoskie L, Yen J, Tseng YT, Blount L, Kashiwai K, Padbury JF. Human placental norepinephrine transporter mRNA: expression and correlation with fetal condition at birth. Placenta. 1997;18:205–10. doi: 10.1016/s0143-4004(97)90094-1. [DOI] [PubMed] [Google Scholar]
- 153.Jacob S, Moley KH. Gametes and embryo epigenetic reprogramming affect developmental outcome: implication for assisted reproductive technologies. Pediatr Res. 2005;58:437–46. doi: 10.1203/01.PDR.0000179401.17161.D3. [DOI] [PubMed] [Google Scholar]
- 154.Colvis CM, Pollock JD, Goodman RH, Impey S, Dunn J, Mandel G, Champagne PA, Mayford M, Korzus E, Kumar A, Renthal W, Theobald DE, Nestler EJ. Epigenetic mechanisms and gene networks in the nervous system. J Neurosci. 2005;25:10379–89. doi: 10.1523/JNEUROSCI.4119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine–induced plasticity in striatum. Neuron. 2005;48:303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 156.Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry. 2000;39:1468–84. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 157.Ellickson PL, Tucker JS, Klein DJ. High–risk behaviors associated with early smoking: results from a 5–year follow–up. J Adolesc Health. 2001;28:465–73. doi: 10.1016/s1054-139x(00)00202-0. [DOI] [PubMed] [Google Scholar]
- 158.Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–90. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brook JS, Whiteman MM, Finch S. Childhood aggression, adolescent delinquency, and drug use: a longitudinal study. J Genet Psychol. 1992;153:369–83. doi: 10.1080/00221325.1992.10753733. [DOI] [PubMed] [Google Scholar]
- 160.Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: a maturational perspective. Dev Psychopathol. 1999;11:657–83. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- 161.Blackson TC, Butler T, Belsky J, Ammerman RT, Shaw DS, Tarter RE. Individual traits and family contexts predict sons’ externalizing behavior and preliminary relative risk ratios for conduct disorder and substance use disorder outcomes. Drug Alcohol Depend. 1999;56:115–31. doi: 10.1016/s0376-8716(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 162.Chassin L, Barrera MJ. Substance use escalation and substance use restraint among adolescent children of alcoholics. Psychol Addict Bahav. 1993;7:3–20. [Google Scholar]
- 163.Tarter RE, Blackson T, Brigham J, Moss H, Caprara GV. The association between childhood irritability and liability to substance use in early adolescence: a 2–year follow–up study of boys at risk for substance abuse. Drug Alcohol Depend. 1995;39:253–61. doi: 10.1016/0376-8716(95)01175-6. [DOI] [PubMed] [Google Scholar]
- 164.Clark DB, Parker AM, Lynch KG. Psychopathology and substance–related problems during early adolescence: a survival analysis. J Clin Child Psychol. 1999;28:333–41. doi: 10.1207/S15374424jccp280305. [DOI] [PubMed] [Google Scholar]
- 165.Aytaclar S, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. J Am Acad Child Adolesc Psychiatry. 1999;38:172–8. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 166.Giancola PR, Martin CS, Tarter RE, Pelham WE, Moss HB. Executive cognitive functioning and aggressive behavior in preadolescent boys at high risk for substance abuse. J Stud Alcohol. 1996;57:352–9. doi: 10.15288/jsa.1996.57.352. [DOI] [PubMed] [Google Scholar]
- 167.Shoal G, Giancola P. Executive cognitive functioning, negative affectivity, and drug use in adolescent boys with and without a family history of a substance use disorder. J Child Adolesc Subst Abuse. 2001;10:111–21. [Google Scholar]
- 168.Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–85. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 169.Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 170.Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002;17:1807–19. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- 171.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–92. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 172.Barnea–Goraly N, Eliez S, Menon V, Bammer R, Reiss AL. Arithmetic ability and parietal alterations: a diffusion tensor imaging study in velocardiofacial syndrome. Brain Res Cogn Brain Res. 2005;25:735–40. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 173.Giedd J. Brain development, IX: human brain growth. Am J Psychiatry. 1999;156:4. doi: 10.1176/ajp.156.1.4. [DOI] [PubMed] [Google Scholar]
- 174.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–11. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 175.Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–72. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- 176.Giancola P, Tarter R. Executive cognitive functioning and risk for substance abuse. Psychol Sci. 1999;10:203–5. [Google Scholar]
- 177.Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–4. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- 178.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- 180.Spear L. Neurobehavioral changes in adolescence. Curr Dir Psycholog Sci. 2000;9:111–4. [Google Scholar]
- 181.Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–14. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- 182.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic–pituitary–adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–88. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 184.Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–80. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- 185.Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–92. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 186.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–68. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chelonis JJ, Gillam MP, Paule MG. The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicol Teratol. 2003;25:437–46. doi: 10.1016/s0892-0362(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 188.Paule MG, Gillam MP, Allen RR, Chelonis JJ. Effects of chronic in utero exposure to cocaine on behavioral adaptability in rhesus monkey offspring when examined in adulthood. Ann N Y Acad Sci. 2000;914:412–7. doi: 10.1111/j.1749-6632.2000.tb05215.x. [DOI] [PubMed] [Google Scholar]
- 189.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal–juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 190.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–3. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 191.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]


