Abstract
The brain is a target of HIV-1 and serves as an important viral reservoir. Astrocytes, the most abundant glial cell in the human brain, are involved in brain plasticity and neuroprotection. Several studies have reported HIV-1 infection of astrocytes in cell cultures and infected brain tissues. The prevailing concept is that HIV-1 infection of astrocytes leads to latent infection. Here, we provide our perspective on endocytosis-mediated HIV-1 entry and its fate in astrocytes. Natural entry of HIV-1 into astrocytes occurs via endocytosis. However, endocytosis of HIV-1 in astrocytes is a natural death trap where the majority of virus particles are degraded in endosomes and a few which escape intact lead to successful infection. Thus, regardless of artificial fine-tuning (treatment with cytokines or proinflammatory products) done to astrocytes, HIV-1 does not infect them efficiently unless the viral entry route or the endosomal enzymatic machinery has been manipulated.
Keywords: HIV-1 brain, lysosomotropic drugs, Chloroquine, HIV-1 reservoir, Rab, Rev, DDX3, TRBP, HIV-1 latency
Introduction
The central nervous system (CNS) is a target of HIV-1 infection (Epstein et al., 1984; Levy et al., 1985; Petito et al., 1985; Fox and Cottler-Fox, 1986; Gabuzda et al., 1986; Navia et al., 1986; Wiley et al., 1986) and serves as an important viral reservoir (Dahl et al., 2014). HIV-1 enters the CNS soon after its transmission (Resnick et al., 1988; Churchill et al., 2006; Ragin et al., 2012) and persists through the life of the infected individual. HIV-1 infection in significant number of patients produces HIV-associated neurocognitive disorders (HAND). Astrocytes are the predominant neuro-glial cells involved in brain plasticity and neuroprotection (Giuliani et al., 1993; Piani and Fontana, 1994). Astrocytes are also important in HIV-1-mediated neuropathology, serving as inflammatory cells in response to viral- and inflammatory -products (Chauhan et al., 2003, Bruce-Keller et al., 2003; Rostasy et al., 2003; Chauhan et al, 2007, Yadav and Collman, 2009; Xing et al., 2009; Muratori et al., 2010; Woods et al., 2010; Mehla et al., 2012). In brain, HIV-1 infection in microglia and macrophages is often productive (production of infectious virus particles) (Gabuzda et al., 1986; Gartner et al., 1986; Koenig et al., 1986; Wiley et al., 1986; Pumarola-Sune et al., 1987; Vazeux et al., 1987; Strizki et al., 1996; Fischer-Smith et al., 2001), while infection in astrocytes ranges from unproductive to productive (Stoler et al., 1986; Wiley et al., 1986; Dewhurst et al., 1987; Koyanagi et al., 1987; Ward et al., 1987; Cheng-Mayer, et al., 1987; Brack-Werner et al., 1992; Saito et al., 1994; Ranki et al., 1995; Takahashi et al., 1996; Wiley and Achim, 1997; Sabri et al., 1999; Gorry et al., 1998 and 1999; Boutet et al., 2001; Trillo-Pazos et al., 2003; Vijaykumar et al., 2008; Churchill et al., 2009; Chauhan et al., 2014). Recently, using immunohistochemistry on HIV-associated neurocognitive disorders brain sections, productive HIV-1 infection was undetectable (Tavazzi et al., 2014).
Although several studies showed astrocytes to be restricted to HIV-1 infection (Wiley, 1986; Brack-Werner et al., 1992; Blumberg et al., 1994, Neumann et al., 1995; Gorry et al., 2003; Petito 2004; Deiva et al., 2006), but few have reported non-permissiveness of astrocytes to the virus infection (Sharpless et al., 1992; Clark et al., 2006). Several others have, however, shown that HIV-1 infection in astrocytes is moderately productive (Nath et al., 1995; Brengel-Pesce et al., 1997; Wiley and Achim, 1997; McCarthy et al., 1998; Brack-Werner, 1999; Li et al; 2007; Vijaykumar et al., 2008; Chauhan et al., 2014; Chauhan 2014 in press). In infected brain tissues, 3–19% of astrocytes have been found to carry HIV-1 DNA as an unproductive infection (Dewhurst et al., 1987; An et al., 1999a; An et al., 1999b; Trillo-Pazos et al., 2003; Lambotte et al., 2003; Churchill et al., 2006; Churchill et al., 2009; Desplats et al., 2013; Smith et al., 2014). Recently, we have found that an extremely low rate (0.025%) of productive HIV-1 infection occurs in astrocytes (Chauhan et al., 2014). Based on the published evidences, the current status of HIV-1 infection in astrocytes is discussed.
HIV-1 infection in astrocytes
In several studies, evidence of HIV-1 infection in astrocytes was based on the detection of viral antigens by immunocytochemistry (Ward et al., 1987; Kure et al., 1990; Tornatore et al., 1994; Saito et al., 1994; Ranki et al., 1995; Bagasra et al., 1996; Trillo-Pazos et al., 2003; Thompson et al., 2011) and HIV-1 DNA (Dewhurst et al., 1987; An et al., 1999a; An et al., 1999b; Trillo-Pazos et al., 2003; Lambotte et al., 2003; Churchill et al., 2006; Desplats et al., 2013; Smith et al., 2014). In a study on HIV-1-infected brain tissues, infection was detected in perivascular macrophages and astrocytes without p24 expression and with no other histopathological changes (Thompson et al., 2006). Evidence of HIV-1 infection in these brain tissues was obtained by triple nested polymerase chain reaction (PCR) for viral DNA and the infection was assumed to be latent. Further, sequencing data on the gag gene from these HIV-1-infected brain tissue macrophages showed homology with astrocyte-derived HIV-1 DNA with substitution of one amino-acid. Identification of this small variation in the gag gene sequence could be possible by PCR. None the less, the significance of these observations is that the same type of HIV might be infecting macrophages and astrocytes.
In another study, 19% of astrocytes were found to contain viral DNA when double immunohistochemistry was coupled with laser capture microdissection on HIV-demented patents’ brain-tissue sections (Churchill et al., 2009). As in an earlier study, these astrocytes were in close proximity to perivascular macrophages and HIV-1 DNA was detected by triple-nested PCR. However, in another study using in-situ DNA PCR on HIV-demented brain tissue sections showed the viral DNA signal in only 3% of astrocytes. Several other studies, using in-situ PCR or PCR on DNA extracted from astrocytes have demonstrated viral DNA (Nuovo et al., 1994; Bagasra et al., 1996; An et al., 1999a,b; Trillo-Pazos et al., 2003). Detection of HIV-1 DNA in astrocytes by PCR in the above studies is a significant revelation; nevertheless, it is not obligatory that viral DNA is in the integrated state – a condition critical to HIV-1 replication. Seldom studies have reported on the integrated viral DNA in HIV-infected brain tissues. Overall, the investigations on HIV-infected brain tissues have inferred latent or unproductive infection in astrocytes.
In vitro, restricted HIV-1 infection was shown in astrocytes because of the absence of CD4-receptor (Sabri et al., 1996; Boutet et al., 2001; Canki et al., 2001; Schweighardt and Atwood, 2001; Schweighardt et al., 2001; Willey et al, 2003; Deiva et al., 2006; Li et al., 2007; Chauhan et al., 2014). In addition, a few studies in astrocytes have reported abundant ‘adsorption’ of HIV-1 particles (Clark et al., 2006; Li et al., 2007; Chauhan et al., 2014), although one study noted adsorption but no viral reverse transcription product (Clark et al., 2006). However, despite the absence of reverse transcription product, early viral transcripts were detected in astrocytes by RT-PCR. Although several in-vitro studies have been done on astrocytes, the majority of studies have been done on transformed astrocytic cells and primary human fetal astrocytes, but only a few on adult astrocytes (Sharpless et al., 1992; Willey et al., 2003; Neil et al., 2005). The studies on primary adult human astrocytes, which have used several primary HIV-1 isolates for infection (Wiley et al., 2003; Neil et al., 2005), have found only one or two HIV-1 strains to be infectious, suggesting the existence of an astrocyte-tropic strain. Given the scenario in brain, it seems unconceivable that astrocyte-tropic strains have been able to evolve when virus per se cannot even efficiently enter these cells. Another question that remains unanswered is why virus should adapt to astrocytes, when other permissive cells such as microglia and macrophages are present in the brain. However, limited evidence from a study using M- and T-tropic HIV-1 strains, including brain-derived HIV-1 strains, suggests limitedly productive infection in astrocytes (Chauhan et al., 2014). This indicated that astrocytes equally restrict both M- and T-tropic HIV-1, and was because of viral entry restriction.
The insufficiency in data on HIV-1 infection in astrocytes is presumably a consequence of the complexity of the mechanism of infection and failure to detect authentic viral infection. However, development of recombinant HIV-1-expressing fluorescent reporter and use of fluorescent reporter astrocytes has unambiguously revealed authentic, but low level of productive infection in astrocytes. Our use of recombinant fluorescent HIV-1 demonstrated an extremely low level of productive infection in astrocytes, far below the limit of the p24 detection assay (ELISA) (Chauhan et al., 2014). Fluorescent reporter HIV-1 infection in astrocytes was first observed on Day 4 and peaked on Day 12 post-infection, which did not correlate with extracellular p24 levels for the initial 10 days. Similar results, obtained by infecting ectopically-CD4-expressing HFA or astrocytic reporter cells, suggested an incubation period of 4–5 days (Chauhan et al., 2014). Further, positive signal for Alu-HIV LTR PCR in HIV-1-infected astrocytes corroborated infection as a post viral-DNA integration event. Authentic viral infection (productive) in astrocytes was further demonstrated by abrogating the import of nascent HIV cDNA into the nucleus by depleting TNPO3 (Zhang et al., 2010; Chauhan et al., 2014). Thus, we have provided precise evidence for the peculiar profile of extracellular p24 levels between Days 1 and 10 after HIV-1 infection of astrocytes is, and report that it is, in fact, a result of the adsorbed viral inoculum and not the true replicated virus (Chauhan et al., 2014). This was in corroboration with earlier study (Clark et al., 2006), which except for early viral transcripts could not detect authentic viral infection (productive) in astrocytes. It seems that the residual viral activity from Day 1 to 10 is more prominent in astrocytes than in other cells (Clark et al., 2006; Li et al., 2007; Chauhan et al, 2014). In general, contrary to the evidence on human brain tissues suggesting presence of viral DNA in 3–19% astrocytes as unproductive infection, in vitro data inferred the occurrence of infinitesimal productive HIV-1 infection due to restriction at the viral entry step. Recently, SHIV infection in rhesus monkeys showed virus-infected astrocytes in brain (Zhuang et al., 2014) thus, corroborating in-vitro HIV-1 infection results.
HIV-1 endocytosis, a kiss of death in astrocytes
As reported earlier, HIV-1 infection in astrocytes is impaired at the viral entry level (Boutet et al., 2001; Canki et al., 2001; Schweighardt and Atwood, 2001; Vijaykumar et al., 2008; Chauhan et al., 2014). Moreover, intracellular restrictions for HIV-1 infection in astrocytes have also been reported (Neumann et al., 1995; McCarthy et al., 1998; Gorry et al., 2003). In all these studies, HIV-1 infection in astrocytes was detected by conventional p24 assay. However, the most convincing findings on HIV-1 infection in astrocytes have emerged from studies where recombinant HIV-1-expressing fluorescent reporter is coupled with a traditional viral detection assay (Li et al., 2007; Vijaykumar et al., 2008; Chauhan et al., 2014; Chauhan 2014, in press). Using fluorescent labeled HIV-1 particles, virus infection was found to be merely 1% and the majority of virus particles were adsorbed onto astrocytes (Li et al., 2007).
In our extensive studies of HIV-1 infection in primary astrocytes using recombinant viruses expressing yellow fluorescent protein (YFP) reporter, we found that the virus enters these cells by endocytosis, resulting in infinitesimal productive infection (Fig. 1) (Vijayakumar et al, 2008; Chauhan et al., 2014; Chauhan, 2014, in press). Endocytosis is a complex process wherein virus particles move along the intracellular endocytic pathway, involving early, late, and recycling endosomes, whose functioning involves Rabs and associated proteins (Martinez and Goud, 1998). Rabs are gaunosine triphosphatases (GTPases) of the Ras superfamily. Rab (Ras-related proteins in brain) proteins are important factors in discriminating between pathways leading to different intracellular locations (Mainou and Dermody, 2012; Macovei et al., 2013). They regulate specific steps of endocytosed vesicles from the plasma membrane to early endosomes (Rabs 4 and 5) (Gorvel et al., 1991), late endosomes, lysosomes (Rab7) (Bucci et al., 2000), and vesicle-recycling endosomes (Rabs 4 and 11) (Urbe et al., 1993; Ullrich et al., 1996).
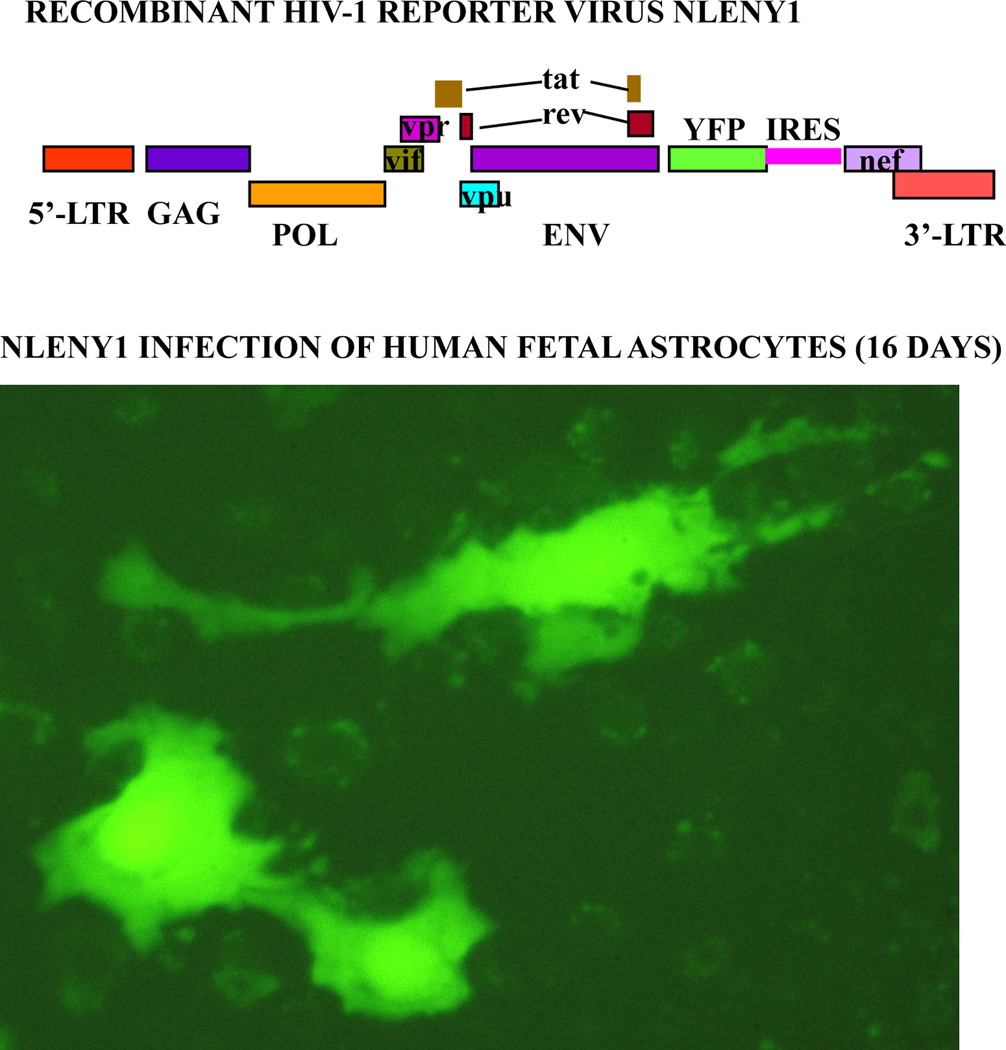
Figure 1. Natural HIV-1 infection in primary human fetal astrocytes.
(Upper panel): Diagrammatic view of recombinant HIV-1 infectious molecular clone, NLENY1 showing yellow fluorescent protein (YFP) gene insertion. (Lower panel): Human fetal astrocytes after culture for a month were infected with NLENY1 (1.0 µg/mL p24 concentration). Infection was detected by green fluorescence in HIV-1 infected astrocytes on day 16 post-infection.
In natural infection of lymphocytes, HIV-1 enters via a pH-independent pathway using a classical receptor-coreceptor mechanism. However, in natural infection of astrocytes, HIV-1 enters via a pH-dependent endocytic route in which endosomal internal machinery degrades virus particles (Vijaykumar et al., 2008; Chauhan et al., 2014). The latter has been amply evidenced by in our studies on treatment with lysosomotropic agents (Chloroquine and bafilomycin), which led to the increase in HIV-1 infection, in our studies (Vijaykumar et al., 2008; Chauhan et al., 2014). Corroborating above studies, similar observations in non-astrocytes were reported by others (Vidricaire and Tremblay, 2005; Khatua et al., 2010; Gobeil et al., 2013). Further evidence on the endocytic entry of HIV-1 in astrocytes was shown in our study, by abrogation of productive viral infection after depleting Rabs 5, 7 or 11, thereby indicating the involvement of early and late endosomes (Chauhan et al., 2014). Overall, endocytosis of HIV-1 in astrocytes is not an efficient process, given that endosomal and proteasomal inhibitors do not increase susceptibility to infection beyond 0.5% (Chauhan et al., 2014). Thus, vesicular internalization of HIV-1 particles, independent of viral receptor in CD4-negative astrocytes, provides resistance to productive infection. Although most HIV-1 particles internalized by the vesicular pathway appear to be degraded in endolysosomes, a few escape the acidic environment in endosome vesicles without damage and enter the cytoplasm. Thus, HIV-1 endocytosis in astrocytes is a kiss of death; only few virus particles escape the degradation and establish productive infection. When endosomal pH was increased with Chloroquine or bafilomycin A, infection with both M- and T-tropic HIV-1 were equally increased several fold, ruling out any viral tropism bias in astrocytes (Chauhan et al., 2014).
Evidence of endocytic entry of HIV-1 was also supported by the finding that depletion of leukocyte-specific protein 1 (LSP1) substantially decreased the rate of endocytosis. LSP1, an F-actin binding protein, has been shown to direct HIV-1 particles to endosomes and proteasomes in non-astrocytes and hence facilitates viral degradation (Smith et al., 2007). Its depletion with RNAi decreased HIV-1 infection in astrocytes, supporting the conclusion that whatever little productive viral infection occurred in astrocytes, it was a result of endocytosis (Chauhan et al., 2014). Impairing the endocytic pathway disrupts HIV-1 trafficking, but crippling endosomal enzymatic machinery facilitates viral survival. Vesicular stomatitis virus (VSV) enters target cells via endocytosis and produces robust productive infection. Enhancing HIV-1 endocytosis by using VSV envelope to pseudotype HIV-1, leads to extremely productive viral infection in astrocytes (Fig. 2) (Canki et al., 2001; Li et al., 2007; Vijaykumar et al, 2008; Chauhan et al., 2014). Mechanistically, the VSV envelope is internalized by endocytosis and, being pH-dependent, it needs an acidified environment to reach the cytosol. Therefore, natural endocytosis of HIV-1 in astrocytes is an inefficient route for productive infection because the viral envelope is sensitive to acidic pH of the endolysosomes.
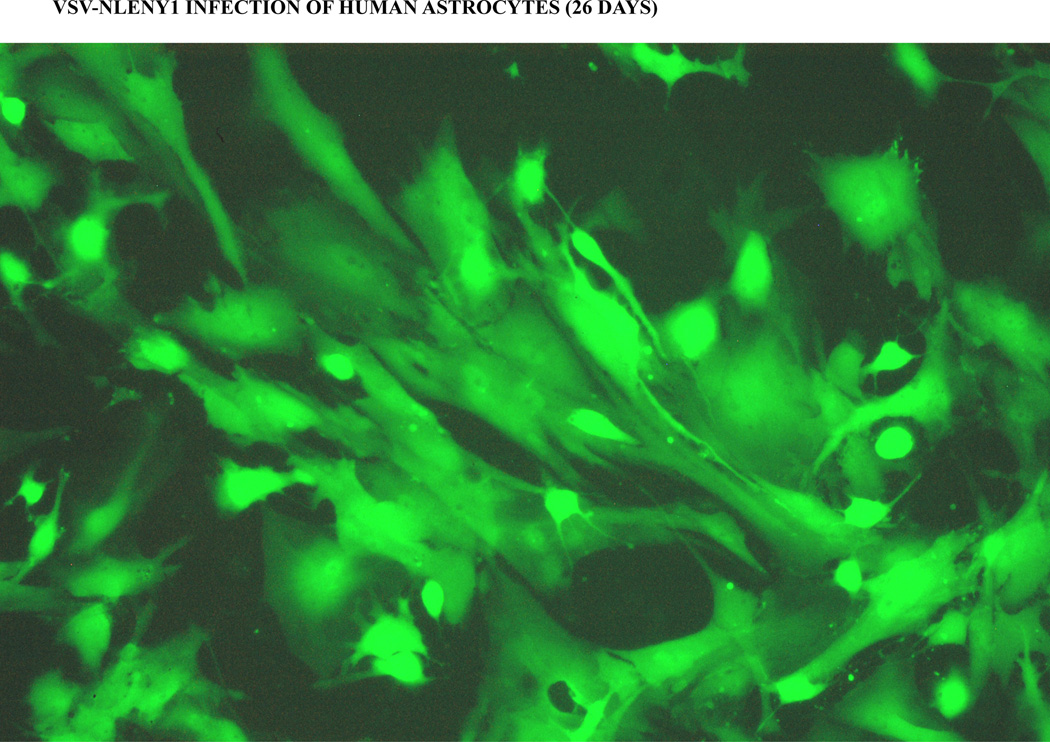
Figure 2. Viral entry bypass produces robust HIV-1 infection in astrocytes.
Primary human fetal astrocytes were infected with VSV-pseudotyped NLENY1 virus (200 ng/mL p24 concentration) and infection was monitored for green fluorescence under fluorescent microscope. Green fluorescent astrocytes were showing HIV-1 infection (live) on day 26 post-infection.
Does HIV-1 replication in astrocytes face intracellular restrictions
Several factors in astrocytes have been implicated in restricting HIV-1 infection. As noted previously, the natural endocytic route offers intracellular resistance to HIV-1 in astrocytes. What other intracellular restrictions for HIV-1 replication in astrocytes may possibly exist? The significant among these is the malfunction of the HIV-1 Rev protein (Fisher et al., 1994; Neumann et al., 1995; Noguchi et al., 2012), which is involved in transporting unspliced and partially spliced viral mRNA transcripts from the nucleus to the cytoplasm (Fischer et al., 1994; Noguchi et al., 2012). Rev-interacting human protein fragment (16.4.1) (Risp) was shown to be present at high levels in astrocytes. The Risp impedes Rev activity and cripples HIV-1 replication in astrocytes, whilst its ablation leads to efficient HIV-1 replication (Vincendeau et al., 2010). Ludwig et al. (1999) found that in astrocytes Rev accumulates in the nucleus, resulting in impairment of its function. The same group has reported that Rev is restricted to the cytoplasm and may not function efficiently in exporting HIV-1 RNA from the nucleus to the cytoplasm (Neumann et al., 1995 and 2001). Currently, there is concrete evidence that not only Rev function is intact after HIV-1 bypasses viral entry by adopting endocytic route, but also the viral replication machinery works optimally, in astrocytes (Fig. 2) (Canki et al., 2001; Li et al., 2007; Vijaykumar et al., 2008; Chauhan et al, 2014; Chauhan 2014, in press).
Dead box RNA helicases, DDX1 and DDX3, as well as an RNA helicase A (RHA), have been implicated in HIV-1 replication by imparting normal functioning of Rev (Yedavalli et al., 2004; Fang et al., 2005; Ishaq et al., 2008). DDX3, an ATP-dependent RNA helicase, functions as a cellular co-factor for CRM1-dependent nuclear export of HIV-1 RNA ( Yedavalli et al., 2004; Nashchekin et al., 2006). Similarly, another double-stranded RNA binding protein TRBP, a TAR-binding protein involved in inhibiting PKR activation and a component of miRNA (micro RNA) processing machinery, is under-expressed in astrocytes (Gatignol et al., 2005; Daher et al., 2009; Daniels et al., 2009; Sanghvi et al., 2011) . It was found that primary fetal astrocytes express barely detectable levels of TRBP (Ong et al., 2005; Chauhan et al., 2014; Chauhan, 2014, in press) and DDX3, compared to astrocytic cells (Chauhan 2014, in press). DDX3 is essential for HIV-1 replication and its barely detectable natural levels were adequate to support viral replication (Chauhan, 2014, in press).
Natural under-expression of TRBP in astrocytes has been implicated in restricted HIV-1 replication (Gatignol et al., 2005; Ong et al., 2005). In these studies, ectopic TRBP supplementation in astrocytes resulted in normalization of HIV-1 replication which occurred through inhibition of PKR activation (Ong et al., 2005) . Apart from its direct activation effect, TRBP has also been found to reverse PKR-induced suppression of HIV-1 LTR promoter activity (Park et al., 1994; Daher et al., 2009; Sanghvi and Steele 2011). In contrast, we have found that supplementation with DDX3 or TRBP or ablation of PKR yielded no advantage to HIV-1. However, unnatural strategies such as the use of VSV-pseudotyped virus or ectopic introduction of HIV-1 infectious molecular clone in astrocytes to bypass natural viral entry led to robust viral replication (Fig. 2) and extracellular release of infectious virus particles (Tornatore et al., 1991; Bencheikh et al., 1999; Canki et al., 2001; Li et al., 2007; Vijaykumar et al., 2008; Chauhan et al., 2014). These investigations indicate that the intracellular environment in astrocytes is conducive to HIV-1 replication. Above all, in 110-day to 160-day follow-up of VSV-HIV-1-infected primary astrocytes, robust viral replication observed by fluorescence (Fig. 3) and extracellular viral release again suggested that astrocytes do not lack the factors required for normal viral replication (Chauhan et al., 2014; Chauhan, 2014 in press). Also, ectopic CD4-expression in primary astrocytes has led to efficient HIV-1 infection (Schweighardt and Atwood, 2001; Chauhan et al., 2014). Although some regulatory factors in astrocytes are barely expressed and many have not yet been investigated, HIV-1 entering astrocytes by other than the natural entry route do not face major intracellular resistance.
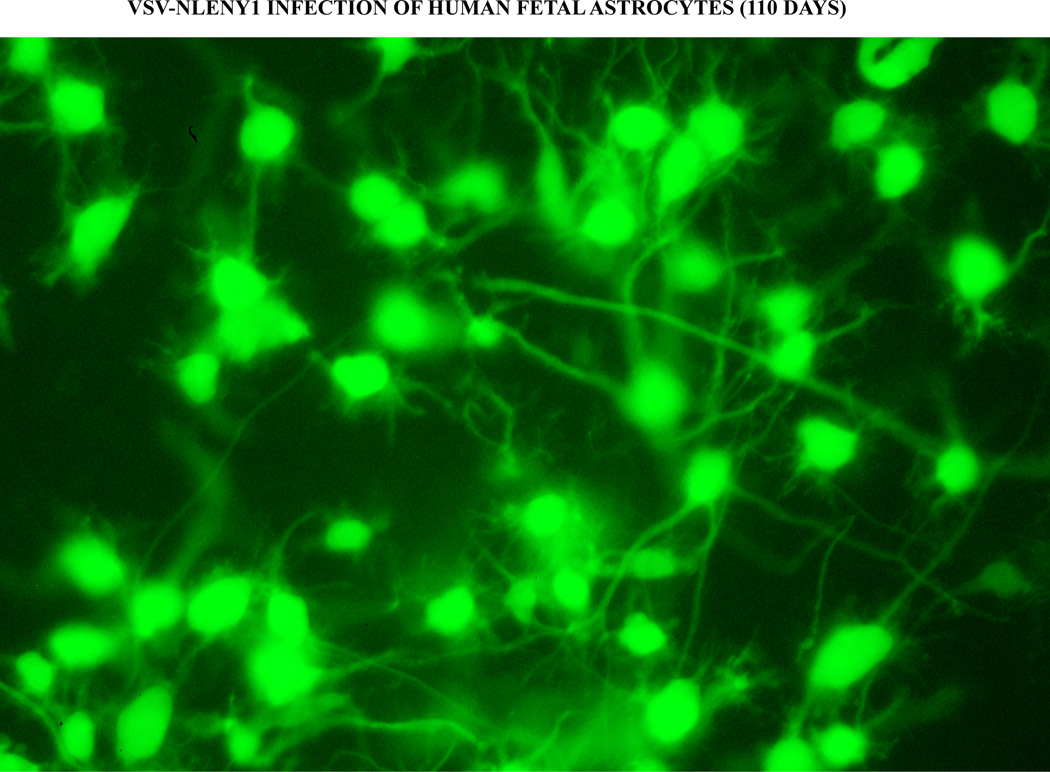
Figure 3. Persistent long term HIV-1 infection in astrocytes.
Primary human fetal astrocytes were infected with VSV-pseudotyped NLENY1 virus (200 ng/mL p24 concentration) and monitored for green fluorescence using fluorescent microscope. VSV-NLENY1 infected astrocytes were showing green fluorescence at day 110 post-infection.
Does HIV-1 infection persist in astrocytes
Even though long-term studies on HIV-1-infected astrocytes are lacking, HIV-1 infection has been shown to enter into a latent state that is amenable to reactivation (Chiodi et al., 1987; Tornatore et al., 1991; Atwood et al., 1994; Lawrence et al., 2004; Narasipura et al., 2014). In one of the studies (Tornatore et al., 1991), HIV-1 infection in astrocytes was inducible, although active virus was recovered only after co-culture with lymphocytes. It was concluded that reactivation of latent infection in astrocytes was too meager to be detectable by p24 assay, so that co-culture was needed to detect new virus particles. Given the limited infection in astrocytes, this could be possible.
Given the nature of productive HIV-1 life cycle to revert to a latent state in permissive cells (lymphocytes), it is possible that astrocytes may show this quiescent state of the virus. Also, limited division of adult astrocytes in the brain is another reason to believe that HIV-1 infection may undergo a latent state in this cell type. Several factors, such as underexpression of DDX3 and TRBP and high basal levels of antiviral PKR in astrocytes (Chauhan, 2014, in press), could contribute to this latent state. p-TEF-b is another factor that regulates HIV latency in permissive cells (Choudhary et al., 2008; Hoque et al., 2011; Bartholomeeusen et al., 2013; Xing and Siliciano, 2013; Budhiraja et al., 2013; Mbonye and Karn, 2014; Ramakrishnan et al., 2014), but has never been investigated in astrocytes. In our study, low levels of DDX3 and TRBP did not affect HIV-1 replication in astrocytes, except upon complete depletion of DDX3. Ectopic overexpression of DDX3 and TRBP, and ablation of PKR by RNAi did not provide any advantage to HIV-1 replication in astrocytes. Intriguingly, we found persistently productive HIV-1 infection in primary astrocytes (Fig. 3) throughout the 160-day duration of follow-up with no effect of latent viral reactivators (Chauhan et al., 2014; Chauhan 2014, in press). Similarly, other studies have found persistent productive infection in human astrocytes using wild type HIV-1 (Rothenaigner et al., 2007) or VSV-pseudotyped HIV-1 viruses (Bencheikh et al., 1999; Canki et al., 2001). Since the introduction of fluorescent reporter HIV-1, the detection of authentic viral infection in astrocytes has resolved ambiguity, from viral entry to latent infection. Indeed, more studies are needed to substantiate the claim in adult astrocytes.
A recent study has shown latent viral infection in the brain tissue of HIV-1-infected patients. Immunocytochemical examination of these tissues showed elevated levels of Bcl IIB, a factor described as regulating HIV-1 silencing, in microglia and astrocytes. In this study, latent infection was identified on the criteria of the presence of viral DNA but the absence of viral RNA and p24 protein (Desplats et al., 2013). Although, microglia and astrocytes were indicated to have latent HIV-1 infection, lack of precise evidence gives rise to a need for further investigations. In an in-vitro study, Bcl-IIB protein was identified as a factor in silencing HIV-1 in microglia and was reported to regulate HIV-1 latency in U1 and microglia (Marban et al. (2007). However, this study lacked data on true viral latency. Thus, even if Bcl IIB regulates HIV-1 silencing in microglia (Marban et al., 2006), there still is no information on its role in HIV-1 latent infection of astrocytes. The pertinent question that still remains unanswered is whether HIV-1 latency in astrocytes is a consequence of a suspended state (unintegrated) or integrated viral DNA. Overall, studies of the occurrence of a latent HIV-1 state in adult astrocytes need use of fluorescent reporter HIV-1.
Perspective and conclusion
Given the current scenario, our perspective is that HIV-1 entry into astrocytes leads to barely detectable productive infection because most of the endocytosed virus particles perish in endosomes or proteasomes (Vijaykumar et al., 2008; Chauhan et al., 2014). In earlier studies, ectopic CD4 expression in primary fetal astrocytes led to robust wild-type HIV-1 infection (Reeves et al., 1999; Schweighardt and Atwood 2001; Chauhan et al., 2014), exquisitely demonstrating that the intracellular environment in astrocytes is conducive to viral replication. However, the natural HIV-1 endocytic entry route is least productive as compared to by-passed entry by VSV-pseudotyped HIV-1. Inhibition of endosomal acidification in astrocytes bolstered the natural HIV-1 infectivity (Vijaykumar et al., 2008; Chauhan et al., 2014). This inferred that in-vitro natural HIV-1 infection in astrocytes is an uncommon phenomenon (Chauhan et al., 2014; Chauhan, 2014, in press). Even using co-culture of astrocytes with HIV-1-infected lymphocytes led to only little infection. Thus, it is conclusively established that no matter what artificial fine-tuning (treatment with cytokines or proinflammatory products) is done to astrocytes, HIV-1 will not efficiently infect them unless the viral entry route or, to certain extent, the endosomal enzymatic machinery has been manipulated. Also, in an earlier study (Mehla et al., 2012) authors were unsuccessful to provoke astrocytes into increasing their permissiveness to HIV-1, using cytokines or proinflammatory molecules (IFN-γ, TNF-α, IL-6, and IL-8) that were present at elevated levels in the CSF of HIV-1-infected demented patients. However, lysosomotropic agents significantly augmented HIV-1 infection in astrocytes, generating caution against using such drugs on HIV-infected patients. Lysosomotropic drugs such as Chloroquine will help to expand an unnatural HIV-1 reservoir in nonpermissive cells not only in the brain, but also elsewhere in the body. HIV-1 indeed can productively infect astrocytes, but minimally by endocytosis irrespective of viral tropism, and productive infection could persist for long duration as shown in our follow up (Chauhan et al., 2014; Chauhan 2014, in press). Moreover, astrocytes take up, transiently retain, and extracellularly release HIV-1 over several days (7–10 days), indicating the existence of transient survival mechanism under unfavorable conditions. Finally, the limited HIV-1 infection in primary astrocytes may not contribute to the overall viral load in brain. However, astrocytes may serve as elusive long-term viral reservoirs.
Acknowledgments
The work was supported by NIH (NINDS) grant RO1 NS0064 (AC) and internal funding from the University of South Carolina School of Medicine. Arwa Mujahid Abdullah was supported by a fellowship from Ministry of Education, Govt. of Iraq, and Jankiben Patel was supported by a Magellan Scholar grant and the Science Undergraduate Research Fellowships Grant from the University of South Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An SF, Groves M, Giometto B, Beckett AA, Scaravilli F. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. 1999a;98(5):481–487. doi: 10.1007/s004010051113. [DOI] [PubMed] [Google Scholar]
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999b;58(11):1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Atwood WJ, Tornatore CS, Traub R, Conant K, Drew PD, Major EO. Stimulation of HIV type 1 gene expression and induction of NF-kappa B (p50/p65)-binding activity in tumor necrosis factor alpha-treated human fetal glial cells. AIDS Res Hum Retroviruses. 1994;10(10):1207–1211. doi: 10.1089/aid.1994.10.1207. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10(6):573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Bartholomeeusen K, Fujinaga K, Xiang Y, Peterlin BM. Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem. 2013;288(20):14400–14407. doi: 10.1074/jbc.M113.464834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]
- Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32(2):253–267. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Boutet A, Salim H, Taoufik Y, Lledo PM, Vincent JD, Delfraissy JF, Tardieu M. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001;34:165–177. [PubMed] [Google Scholar]
- Brack-Werner R, Kleinschmidt A, Ludvigsen A, Mellert W, Neumann M, Herrmann R, Khim MC, Burny A, Müller-Lantzsch N, Stavrou D, et al. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS. 1992;6(3):273–285. [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neuroscience. 2003;23(23):8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brengel-Pesce K, Innocenti-Francillard P, Morand P, Chanzy B, Seigneurin JM. Transient infection of astrocytes with HIV-1 primary isolates derived from patients with and without AIDS dementia complex. J Neurovirol. 1997;3(6):449–454. doi: 10.3109/13550289709031191. [DOI] [PubMed] [Google Scholar]
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja S, Famiglietti M, Bosque A, Planelles V, Rice AP. Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J Virol. 2013;87(2):1211–1220. doi: 10.1128/JVI.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A. Unperturbed posttranscriptional regulatory Rev protein function and HIV-1 replication in astrocytes. PLOS ONE. 2014 doi: 10.1371/journal.pone.0106910. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278(15):13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Hahn S, Gartner S, Pardo CA, Netesan SK, et al. Molecular programming of endothelin-1 in HIV-infected brain: role of Tat in up-regulation of ET-1 and its inhibition by statins. FASEB J. 2007;21:777–789. doi: 10.1096/fj.06-7054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Mehla R, Theophilus-Sunder VK, Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the HIV-1 in astrocytes. Virology. 2014;456–457C:1–19. doi: 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C, Rutka JT, Rosenblum ML, McHugh T, Stites DP, Levy JA. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi F, Fuerstenberg S, Gidlund M, Asjo B, Fenyo EM. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987;61:1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary SK, Archin NM, Margolis DM. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4(+) T cells. J Infect Dis. 2008;197(8):1162–1170. doi: 10.1086/529525. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Clarke JN, Lake JA, Burrell CJ, Wesselingh SL, Gorry PR, Li P. Novel pathway of human immunodeficiency virus type 1 uptake and release in astrocytes. Virology. 2006;348:141–155. doi: 10.1016/j.virol.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Daher A, Laraki G, Singh M, Melendez-Pena CE, Bannwarth S, et al. TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Molecular and cellular biology. 2009;29:254–265. doi: 10.1128/MCB.01030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl V, Gisslen M, Hagberg L, Peterson J, Shao W, Spudich S, Price RW, Palmer S. An Example of Genetically Distinct HIV Type 1 Variants in Cerebrospinal Fluid and Plasma During Suppressive Therapy. J Infect Dis [Epub ahead of print] 2014 doi: 10.1093/infdis/jit805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SM, Melendez-Pena CE, Scarborough RJ, Daher A, Christensen HS, et al. Characterization of the TRBP domain required for dicer interaction and function in RNA interference. BMC molecular biology. 2009;10:38. doi: 10.1186/1471-2199-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiva K, Khiati A, Hery C, Salim H, Leclerc P, Horellou P, Tardieu M. CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes. AIDS Res Hum Retroviruses. 2006;22:1152–1161. doi: 10.1089/aid.2006.22.1152. [DOI] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology. 2013;80(15):1415–1423. doi: 10.1212/WNL.0b013e31828c2e9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges MJ, Volsky DJ. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987;61:3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Cho ES, Myenhofer M, Navia B, Price RW. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1:447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, et al. The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology. 2005;336:299–307. doi: 10.1016/j.virol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Fischer U, Meyer S, Teufel M, Heckel C, Luhrmann R, et al. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. The EMBO journal. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Régulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Fox CH, Cottler-Fox M. AIDS in the human brain. Nature. 1986;319:8. doi: 10.1038/319008a0. [DOI] [PubMed] [Google Scholar]
- Gabuzda DH, Ho DD, de la Monte SM, Hirsch MS, Rota TR, Sobel RA. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Betts RF, Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986;256:2365–2371. [PubMed] [Google Scholar]
- Gatignol A, Laine S, Clerzius G. Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology. 2005;2:65. doi: 10.1186/1742-4690-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36:681–693. doi: 10.1002/jnr.490360609. [DOI] [PubMed] [Google Scholar]
- Gobeil LA, Lodge R, Tremblay MJ. Macropinocytosis-like HIV-1 internalization in macrophages is CCR5 dependent and leads to efficient but delayed degradation in endosomal compartments. J Virol. 2013;87(2):735–745. doi: 10.1128/JVI.01802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Howard JL, Churchill MJ, Anderson JL, Cunningham A, Adrian D, McPhee DA, Purcell DF. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1(4):463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Hoque M, Shamanna RA, Guan D, Pe'ery T, Mathews MB. HIV-1 replication and latency are regulated by translational control of cyclin T1. J Mol Biol. 2011;410(5):917–932. doi: 10.1016/j.jmb.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq M, Hu J, Wu X, Fu Q, Yang Y, et al. Knockdown of cellular RNA helicase DDX3 by short hairpin RNAs suppresses HIV-1 viral replication without inducing apoptosis. Molecular biotechnology. 2008;39:231–238. doi: 10.1007/s12033-008-9040-0. [DOI] [PubMed] [Google Scholar]
- Khatua AK, Taylor HE, Hildreth JE, Popik W. Non-productive HIV-1 infection of human glomerular and urinary podocytes. Virology. 2010;408(1):119–127. doi: 10.1016/j.virol.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kure K, Lyman WD, Weidenheim KM, Dickson DW. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990;136(5):1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathol. 2013;13(1):95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78(14):7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Shimabukuro J, Hollander H, Mills J, Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985;2:586–588. [PubMed] [Google Scholar]
- Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007;12(8):31. doi: 10.1186/1471-2202-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig E, Silberstein FC, van Empel J, Erfle V, Neumann M, Brack-Werner R. Diminished rev-mediated stimulation of human immunodeficiency virus type 1 protein synthesis is a hallmark of human astrocytes. J Virol. 1999;73(10):8279–8289. doi: 10.1128/jvi.73.10.8279-8289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macovei A, Petrareanu C, Lazar C, Florian P, Branza-Nichita N. Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J Virol. 2013;87:6415–6427. doi: 10.1128/JVI.00393-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainou BA, Dermody TS. Transport to late endosomes is required for efficient reovirus infection. J Virol. 2012;86:8346–8358. doi: 10.1128/JVI.00100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, Aunis D, Rohr O. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26(2):412–423. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O, Goud B. Rab proteins. Biochim Biophys Acta. 1998;1404:101–112. doi: 10.1016/s0167-4889(98)00050-0. [DOI] [PubMed] [Google Scholar]
- Mbonye U, Karn J. Transcriptional control of HIV latency: cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology. 2014;454–455:328–339. doi: 10.1016/j.virol.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M, He J, Wood C. HIV-1 strain-associated variability in infection of primary neuroglia. J Neurovirol. 1998;4(1):80–89. doi: 10.3109/13550289809113484. [DOI] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation. 2012;9:239. doi: 10.1186/1742-2094-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergener K, Facke M, Welker R, Brinkmann V, Gelderblom HR, Krausslich HG. Analysis of HIV particle formation using transient expression of subviral constructs in mammalian cells. Virology. 1992;186:25–39. doi: 10.1016/0042-6822(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Muratori C, Bona R, Ruggiero E, D’Ettorre G, Vullo V, Andreotti M, Federico M. DC contact with HIV-1-infected cells leads to high levels of Env-mediated virion endocytosis coupled with enhanced HIV-1 Ag presentation. Eur J Immunol. 2009;39:404–416. doi: 10.1002/eji.200838751. [DOI] [PubMed] [Google Scholar]
- Nashchekin D, Zhao J, Visa N, Daneholt B. A novel Ded1-like RNA helicase interacts with the Y-box protein ctYB-1 in nuclear mRNP particles and in polysomes. The J Biol Chem. 2006;281:14263–14272. doi: 10.1074/jbc.M600262200. [DOI] [PubMed] [Google Scholar]
- Narasipura SD, Kim S, Al-Harthi L. Epigenetic regulation of HIV-1 latency in astrocytes. J Virol. 2014;88(5):3031–3038. doi: 10.1128/JVI.03333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Hartloper V, Furer M, Fowke KR. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. J Neuropathol Exp Neurol. 1995;54:320–330. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Aasa-Chapman MM, Clapham PR, Nibbs RJ, McKnight A, Weiss RA. The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. J Virol. 2005;79(15):9618–9624. doi: 10.1128/JVI.79.15.9618-9624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Felber BK, Kleinschmidt A, Froese B, Erfle V, Pavlakis GN, Brack-Werner R. Restriction of human immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Afonina E, Ceccherini-Silberstein F, Schlicht S, Erfle V, Pavlakis GN, Brack-Werner R. Nucleocytoplasmic transport in human astrocytes: decreased nuclear uptake of the HIV Rev shuttle protein. J Cell Sci. 2001;114(Pt 9):1717–1729. doi: 10.1242/jcs.114.9.1717. [DOI] [PubMed] [Google Scholar]
- Nguyen DG, Hildreth JE. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol. 2003;33:483–493. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishibashi K, Miyokawa K, Hokari M, Kanno T, Hirano T, Yamamoto N, Takaku H. HIV-1 suppressive sequences are modulated by Rev transport of unspliced RNA and are required for efficient HIV-1 production. PLoS One. 2012;7:e51393. doi: 10.1371/journal.pone.0051393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CL, Thorpe JC, Gorry PR, Bannwarth S, Jaworowski A, Howard JL, Chung S, Campbell S, Christensen HS, Clerzius G, Mouland AJ, Gatignol A, Purcell DF. Low TRBP levels support an innate human immunodeficiency virus type 1 resistance in astrocytes by enhancing the PKR antiviral response. J Virol. 2005;79(20):12763–12772. doi: 10.1128/JVI.79.20.12763-12772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo GJ, Gallery F, MacConnell P, Braun A. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am J Pathol. 1994;144(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Paquette JS, Fortin JF, Blanchard L, Tremblay MJ. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J Virol. 1998;72:9329–9336. doi: 10.1128/jvi.72.11.9329-9336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Davies MV, Langland JO, Chang HW, Nam YS, et al. TAR RNA binding protein is an inhibitor of the interferon-induced protein kinase PKR. PNAS. 1994;91:4713–4717. doi: 10.1073/pnas.91.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Navia BA, Cho ES, Jordan BD, George DC, Price RW. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;312:874–879. doi: 10.1056/NEJM198504043121402. [DOI] [PubMed] [Google Scholar]
- Petito CK. Human immunodeficiency virus type 1 compartmentalization in the central nervous system. J Neurovirol. 2004;10(Suppl 1):21–24. doi: 10.1080/753312748. [DOI] [PubMed] [Google Scholar]
- Piani D, Fontana A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol. 1994;152:3578–3585. [PubMed] [Google Scholar]
- Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, Epstein LG. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79:2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Liu H, Rice AP. SAHA (Vorinostat) Induces CDK9 Thr-186 (T-Loop) Phosphorylation in Resting CD4+ T Cells: Implications for Reactivation of Latent HIV. AIDS Res Hum Retroviruses. [Epub ahead of print] PMID. 2014 doi: 10.1089/aid.2013.0288. 24528253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 1995. 1995;9(9):1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Reeves JD, Hibbitts S, Simmons G, McKnight A, Azevedo-Pereira JM, Moniz-Pereira J, Clapham PR. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J Virol. 1999;73(9):7795–7804. doi: 10.1128/jvi.73.9.7795-7804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA. SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol. 2003;62(6):617–626. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- Rothenaigner I, Kramer S, Ziegler M, Wolff H, Kleinschmidt A, Brack-Werner R. Long-term HIV-1 infection of neural progenitor populations. AIDS. 2007;21(17):2271–2281. doi: 10.1097/QAD.0b013e3282f12f27. [DOI] [PubMed] [Google Scholar]
- Sabri F, Chiodi F, Fenyo EM. Lack of correlation between V3 amino acid sequence and syncytium-inducing capacity of some HIV type 1 isolates. AIDS Res Hum Retroviruses. 1996;12:855–858. doi: 10.1089/aid.1996.12.855. [DOI] [PubMed] [Google Scholar]
- Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999;264(2):370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44(3 Pt 1):474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sanghvi VR, Steel LF. The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. J Virol. 2011;85:12614–12621. doi: 10.1128/JVI.05240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E, Soros VB, Greene WC. Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes. J Virol. 2004;78:1375–1383. doi: 10.1128/JVI.78.3.1375-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighardt B, Atwood WJ. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retroviruses. 2001;17:1133–1142. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- Schweighardt B, Shieh JT, Atwood WJ. CD4/CXCR4-independent infection of human astrocytes by a T-tropic strain of HIV-1. J Neurovirol. 2001;7:155–162. doi: 10.1080/13550280152058816. [DOI] [PubMed] [Google Scholar]
- Sharpless N, Gilbert D, Vandercam B, Zhou JM, Verdin E, Ronnett G, Friedman E, Dubois-Dalcq M. The restricted nature of HIV-1 tropism for cultured neural cells. Virology. 1992;191(2):813–825. doi: 10.1016/0042-6822(92)90257-p. [DOI] [PubMed] [Google Scholar]
- Smith AL, Ganesh L, Leung K, Jongstra-Bilen J, Jongstra J, Nabel GJ. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med. 2007;204:421–430. doi: 10.1084/jem.20061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014;20(1):28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler MH, Eskin TA, Benn S, Angerer RC, Angerer LM. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986;256(17):2360–2364. [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39(6):705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain Inflammation is a Common Feature of HIV-Infected Patients Without HIV Encephalitis or Productive Brain Infection. Curr HIV Res. 2014 May 26; doi: 10.2174/1570162x12666140526114956. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, McLean CA. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179(4):1623–1629. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44(3 Pt 1):481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, et al. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987;126:403–410. [PMC free article] [PubMed] [Google Scholar]
- Vidricaire G, Tremblay MJ. Rab5 and Rab7, but not ARF6, govern the early events of HIV-1 infection in polarized human placental cells. J Immunol. 2005;175:6517–6530. doi: 10.4049/jimmunol.175.10.6517. [DOI] [PubMed] [Google Scholar]
- Vijaykumar TS, Nath A, Chauhan A. Chloroquine mediated molecular tuning of astrocytes for enhanced permissiveness to HIV infection. Virology. 2008;381:1–5. doi: 10.1016/j.virol.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincendeau M, Kramer S, Hadian K, Rothenaigner I, Bell J, Hack SM, Bickel C, Nagel D, Kremmer E, Werner T, Leib-Mösch C, Brack-Werner R. Control of HIV replication in astrocytes by a family of highly conserved host proteins with a common Rev-interacting domain (Risp) AIDS. 2010;24(16):2433–2442. doi: 10.1097/QAD.0b013e32833e8758. [DOI] [PubMed] [Google Scholar]
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- Ward JM, O’Leary TJ, Baskin GB, Benveniste R, Harris CA, Nara PL, Rhodes RH. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987;127(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Achim CL. No evidence of significant abortive HIV infection of the brain. AIDS. 1997;11:252. [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. PNAS. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey SJ, Reeves JD, Hudson R, Miyake K, Dejucq N, Schols D, De Clercq E, Bell J, McKnight A, Clapham PR. Identification of a subset of human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus strains able to exploit an alternative coreceptor on untransformed human brain and lymphoid cells. J Virol. 2003;77(11):6138–6152. doi: 10.1128/JVI.77.11.6138-6152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Dawson MS, Weber E, Grant I, Letendre SL. HIV-associated deficits in action (verb) generation may reflect astrocytosis. J Clin Exp Neuropsychol. 2010;32(5):522–527. doi: 10.1080/13803390903264130. HIV Neurobehavioral Research Center (HNRC) Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HQ, Hayakawa H, Izumo K, Kubota R, Gelpi E, Budka H, Izumo S. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology. 2009;29(4):433–442. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- Xing S, Siliciano RF. Targeting HIV latency: pharmacologic strategies toward eradication. Drug Discov Today. 2013;18(11–12):541–551. doi: 10.1016/j.drudis.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4(4):430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Zhang R, Mehla R, Chauhan A. Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus −1 preintegration complex (DNA) PLoS One. 2010;5:e15620. doi: 10.1371/journal.pone.0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang K, Leda AR, Tsai L, Knight H, Harbison C, Gettie A, Blanchard J, Westmoreland S, Cheng-Mayer C. Emergence of CD4 independence envelopes and astrocyte infection in r5 simian-human immunodeficiency virus model of encephalitis. J Virol. 2014;88(15):8407–8420. doi: 10.1128/JVI.01237-14. [DOI] [PMC free article] [PubMed] [Google Scholar]