Abstract
Brain-derived neurotrophic factor (BDNF) decreases food intake and body weight, but few central sites of action have been identified for its effect on energy expenditure. The hypothalamic ventromedial nucleus (VMH) is important in regulating energy metabolism. Our previous work indicated that BDNF in the VMH reduced food intake. The purposes of the study were to determine: 1) if BDNF in the VMH increases energy expenditure (EE); 2) if BDNF-enhanced thermogenesis results from increased spontaneous physical activity (SPA) and resting metabolic rate (RMR); and 3) if VMH BDNF thermogenic effects are mediated by uncoupling protein 1 (UCP1) in brown adipose tissue (BAT). BDNF (0.5 μg) was injected into the VMH of male Sprague Dawley rats and oxygen consumption, carbon dioxide production, food intake and SPA were measured for 24 h in an indirect calorimeter. Animals were sacrificed 4 h after BDNF injection, and BAT UCP1 gene expression was measured with quantitative real-time polymerase chain reaction. BDNF significantly decreased food and water intake, and body weight gain. Heat production and RMR were significantly elevated for 9 h immediately after BDNF injection. BDNF increased SPA and aEE within 9 h after injection although BDNF had no effect on 0–24h SPA and EE during SPA (aEE). BDNF did not induce a significant increase in BAT UCP1 expression. In conclusion, VMH BDNF reduces body weight by decreasing food intake and increasing EE consequent to increased SPA and RMR, suggesting that the VMH is an important site of BDNF action to influence energy balance.
Keywords: thermogenesis, resting metabolic rate, spontaneous physical activity, energy homeostasis, food intake regulation
Introduction
The neurotrophin BDNF is not only important to the processing, differentiation, survival, and plasticity of the central nervous system (CNS), but it also plays important roles in the central regulation of energy metabolism as suggested by recent studies. Intracerebroventricular (ICV) administration of BDNF decreases food intake (Ono et al., 2000; Pelleymounter et al., 1995; Sauer et al., 1993) and body weight gain (Pelleymounter et al., 1995; Sauer et al., 1993). In animals with conditional BDNF deletion (Rios et al., 2001), mutation (Sha et al., 2007), or in BDNF (+/−) heterozygous mice (Kernie et al., 2000; Lyons et al., 1999), hyperphagia and obesity are accompanied by significantly reduced BDNF gene expression in the hypothalamus, including the ventromedial nuclei (VMH) (Kernie et al., 2000; Rios et al., 2001). Further, exogenous BDNF reversed the phenotype of these animals (Kernie et al., 2000), suggesting that endogenous BDNF reduces feeding and body weight gain.
Several studies have reported thermogenic effects of BDNF. Long-term peripherally administered BDNF increased body temperature and oxygen (O2) consumption (Nakagawa et al., 2000), increased uncoupling protein 1 (UCP1, an indicator of thermogenesis) mRNA and protein in brown adipose tissue (Tsuchida et al., 2001), and enhanced norepinephrine turnover (Tsuchida et al., 2001). ICV BDNF also reversed cold-induced reductions in body temperature in db/db mice (Tsuchida et al., 2001). In a previous study (Wang et al., 2007b), we showed that BDNF in the paraventricular nucleus of the hypothalamus (PVN) increased energy expenditure (EE) via elevating resting metabolic rate (RMR).
The VMH is important to the regulation of energy metabolism. Electrical stimulation of the VMH suppresses feeding and induces lipolysis (Ruffin and Nicolaidis, 1999), whereas lesions of the VMH result in hyperphagia and obesity (Inoue et al., 1977; King, 2006; Sakaguchi et al., 1988). Obesity in VMH-lesioned animals persists even when caloric intake is restricted to control levels, suggesting that VMH lesions reduce energy expenditure (Cox and Powley, 1981). Conversely, electrical stimulation of the VMH enhances basal metabolic rate, and this precedes and parallels inhibition of feeding behavior (Ruffin and Nicolaidis, 1999). Studies using indirect calorimetry show that VMH lesions produce a 15.5% reduction in both total EE and resting post-absorptive EE (Hustvedt et al., 1984), and many studies suggest that the VMH may positively regulate sympathetic nervous system activity (Minokoshi et al., 1986; Saito et al., 1989; Vander Tuig et al., 1982; Niijima et al., 1984). Both mRNA and protein for BDNF and its receptor TrkB have been identified in the VMH (Conner et al., 1997; Kernie et al., 2000; Wetmore et al., 1990; Yan et al., 1997). Recent studies have indicated that lack of the orphan nuclear receptor steroidogenic factor-1 (SF-1) results in VMH structural abnormalities, impaired VMH development, and reduced expression of BDNF and other genes in the area, leading to hyperphagia and obesity (Davis et al., 2004; Majdic et al., 2002; Tran et al., 2003; Tran et al., 2006; Zhao et al., 2004). Furthermore, selective deletion of BDNF in the VMH results in hyperphagia and obesity (Unger et al., 2007).
The VMH also appears to be important to the regulation of physical activity. Suppression of GABA synthesis in the VMH decreased food intake and enhanced locomotor activity in rats (Bannai et al., 1998). Narita et al found that VMH-injected kainate (a type of glutaminergic agonist) or bicuculline methiodide (a GABAA receptor antagonist) resulted in an increase in running activity, plasma glucose, norepinephrine, epinephrine and corticosterone in conscious rats (Narita et al., 1994). In “VMH-specific” SF-1 knock out (KO) rodents, locomotor activity was significantly decreased (Zhao et al., 2004).
Several peptides have been reported to control expression of BDNF in the VMH. Coexpression of SF-1 and BDNF in VMH neurons, activation of BDNF promoters by SF-1, and association between reduced hypothalamic BDNF expression and reduced SF-1 gene expression has been observed in SF-1 heterozygous (+/−) mice (Tran et al., 2006). This suggests that BDNF expression in the VMH may be a downstream effector of SF-1 for normal VMH functioning. Deficiency of SF-1 directly results in low BDNF levels in the VMH (Tran et al., 2006) and obesity (Majdic et al., 2002). BDNF also has been proposed as a downstream effector of melanocortins as its expression in the VMH is affected by melanocortins (Xu et al., 2003). Leptin has been reported to induce BDNF mRNA and protein expression in the dorsomedial part of the VMH (Komori et al., 2006).
In a previous study, we demonstrated that BDNF in the VMH significantly reduced food intake and reduced body weight at doses not causing taste aversion (Wang et al., 2007c). In the current study, we injected BDNF into the VMH and measured energy expenditure (EE) by indirect calorimetry and spontaneous physical activity (SPA) with an activity monitoring system. We tested the following hypotheses: 1) injection of BDNF into the VMH increases EE, resulting in a decrease in body weight gain; 2) changes in EE induced by BDNF result from heat produced by increased SPA and RMR; and 3) VMH BDNF thermogenic effects are mediated by UCP1 in brown adipose tissue BAT. Our findings indicate that a single injection of BDNF in the VMH reduces feeding and body weight gain, and increases EE by elevating both resting metabolic rate and physical activity, especially immediate post-injection.
Results
Study I: Food intake, energy expenditure and spontaneous physical activity in an indirect calorimetry chamber
1) Food intake and body weight
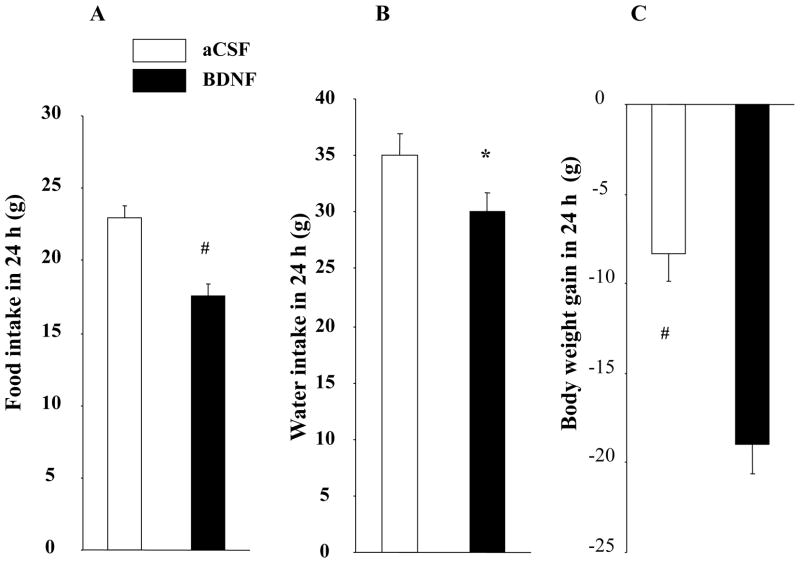
BDNF in the VMH significantly decreased feeding by 23.9% compared with rats treated with aCSF (17.5 ± 0.85 g for BDNF vs. 23.0 ± 0.8 g for aCSF, F1, 16 = 56.287, P < 0.0001, Cohen’s d = −1.564, Fig. 1A), and significantly decreased body weight gain (−19.0 ± 1.7 g for BDNF vs. −8.4 ± 1.5 g for aCSF, F1,16 = 25.023, P = 0.0001, Cohen’s d = −1.638, Fig. 1C).
Fig. 1.
Effect of BDNF in the VMH on food intake (A), water intake (B) and body weight gain (C) during the 24 h post-injection period. * P < 0.05; # P < 0.01; n = 17.
BDNF-treated rats also had significantly lower water intake (30.1 ± 1.5 g for BDNF vs. 35.1 ± 1.8 g for aCSF, F1, 16 = 4.904, P = 0.0417, Cohen’s d = −0.729, Fig. 1B). Using a regression analysis, we found a positive association between food and water intake in both treatments, with coefficient of 0.66 for the aCSF treatment (P = 0.004, data not shown) and 0.507 for the BDNF treatment (P = 0.0376, data not shown).
2) Total energy expenditure
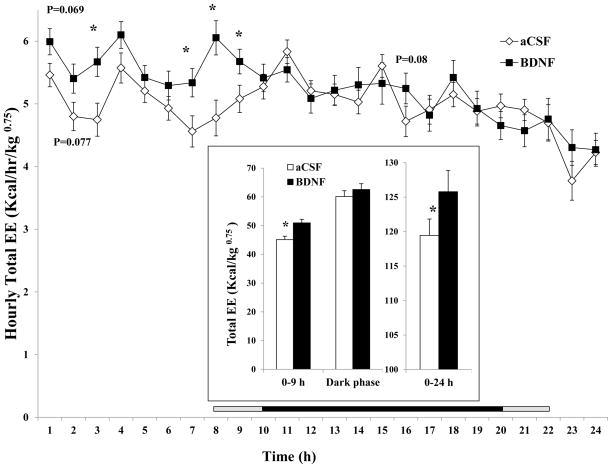
BDNF significantly increased 24-h EE by 5.3% (125. ± 3.1 kcal/kg0.75 for BDNF vs. 119.5 ± 2.4 kcal/kg0.75 for aCSF, F1, 16 = 7.006, P = 0.0176, Cohen’s d = 0.534, inset of Fig. 2). The time course analysis indicates the dramatic increases in total EE occurred within the first 9 h after BDNF injection (Fig. 2), with differences being significant at 3, 7, 8, and 9 h after injection. Cumulatively, BDNF significantly increased total EE during 0–9 h (50.9 ± 1.3 kcal/kg0.75 for BDNF vs. 45.1 ± 1.2 kcal/kg0.75 for aCSF, F1, 16 = 20.735, P = 0.000325, Cohen’s d = 1.15). There were no significant differences between treatment groups beyond 9 h post-injection.
Fig. 2.
Time course of total energy expenditure (EE) calorimetric index after BDNF injection into the VMH. Total EE in 0–9 h, dark phase and 0–24 h post-injection period is shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively. * P < 0.05; n = 17.
3) Spontaneous physical activity
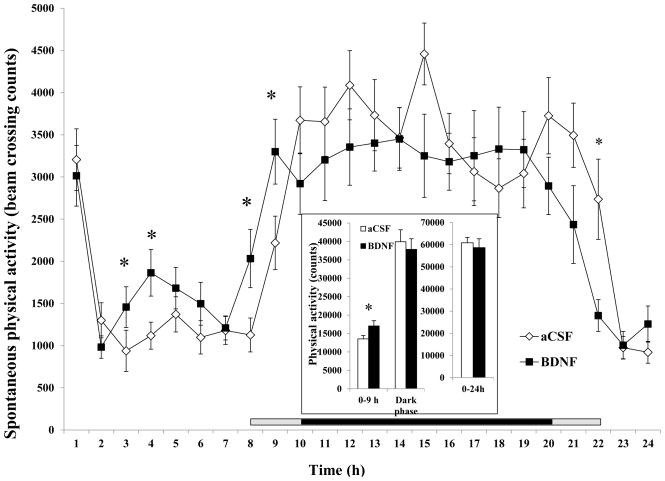
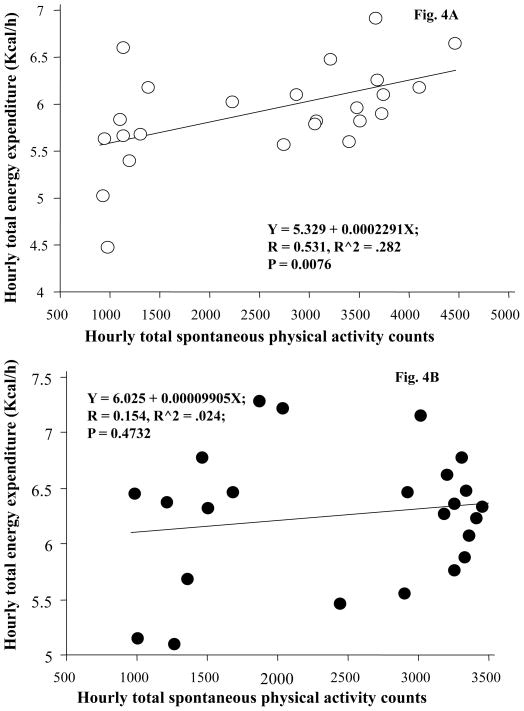
SPA (total number of beam breaks caused by natural motion in the cage) was significantly increased at 3, 4, 8, and 9 h post-BDNF injection, leading to a significant increase in 0–9 h (17,039 ± 1,452 for BDNF vs. 13,557 ± 892 for aCSF, F1, 16 = 9.599, P = 0.0069, Cohen’s d = 0.701, inset of Fig. 3). At 22 h post-injection, animals treated with BDNF had significantly lower activity compared to aCSF treatment (Fig 3). BDNF did not increase 24-h SPA (as measured by beam breaks in the horizontal and vertical planes) or ambulatory activity counts. Total SPA (sum of the horizontal and vertical beam breaks) was not significantly affected by BDNF (inset of Fig. 3) in the 12-h dark phase or the 24-h period. A regression analysis was performed to examine the relationship between hourly total SPA and hourly total EE. There was a significant correlation between hourly total SPA and hourly total EE when animals were given aCSF (r = 0.531, P < 0.0076, Fig. 4a). When the same animals were treated with BDNF, this significant association no longer existed (r = 0.154, P = 0.4732, Fig. 4b).
Fig. 3.
Time course of total SPA after administration of BDNF in the VMH. The SPA (total number of beam breaks caused by natural motion in the cage) in the 0 – 9 h, dark phase, and 0–24 h post-injection intervals are shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively.* P < 0.05;; n = 17.
Fig. 4.
Association between hourly total energy expenditure (EE) and hourly SPA after administration of aCSF (A) or BDNF (B). Each dot represents the average value during each one hour time period. n = 17.
4) Time spent and active
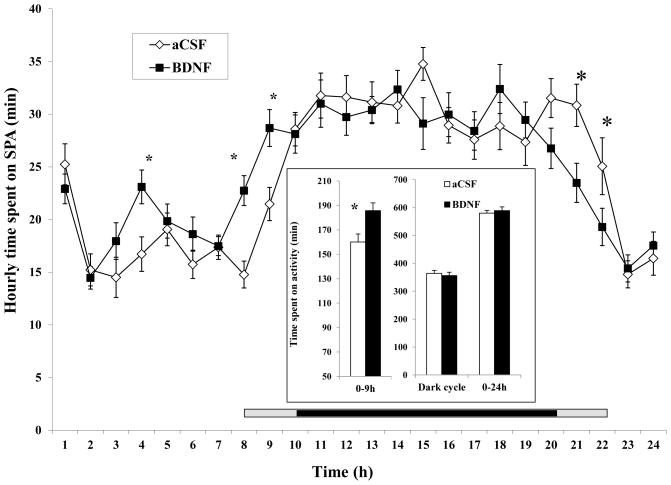
Similar to SPA in Fig. 3, the time course analysis shows that time spent active was significantly increased at 4, 8, and 9 h post BDNF injection, resulting in a significant increase during the 0–9 h period (185.79 ± 6.27 min for BDNF vs. 160.11 ± 6.48 min for aCSF, F1, 16 = 24.038, P = 0.000159, Cohen’s d = 0.977). Rats treated with BDNF spent significantly less time active at 21 and 22 h (Fig. 5). There were no significant differences between groups in the time spent active in the dark phase and 24-h period (inset of Fig. 5).
Fig. 5.
Time course of time spent on SPA after administration of BDNF in the VMH. The time spent on SPA in the 0 – 9 h, dark phase, and 0–24 h post-injection intervals are shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively.* P < 0.05; n = 17.
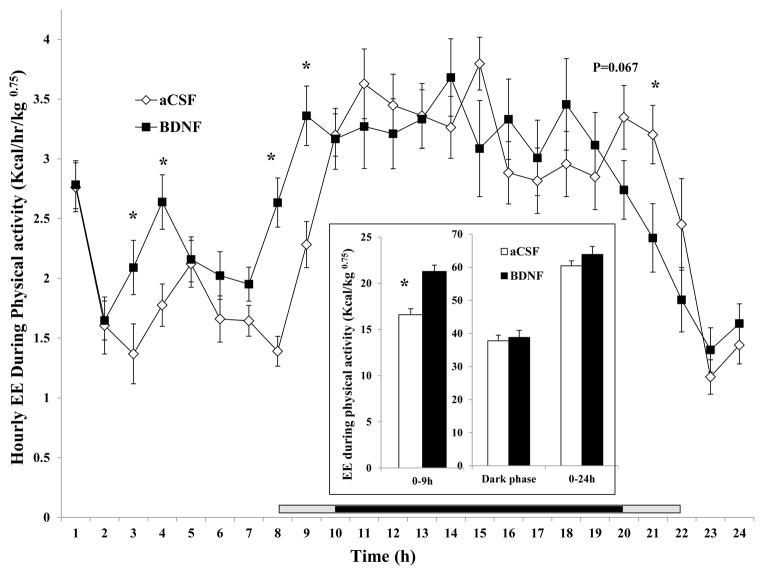
5) Energy expenditure during spontaneous physical activity (aEE)
Time course analysis indicates a significant increase in aEE at 3, 4, 8, and 9 h, and a significant decrease at 21 h post-injection (Fig. 6). Although BDNF increased aEE in the 0–24 h interval, the difference did not reach significance (inset of Fig. 6) and there was no difference in aEE during the dark phase. Consistent with these findings, BDNF significantly increased aEE in the 0–9 h interval (21.29 ± 0.68 kcal/kg0.75 for BDNF vs. 16.61 ± 0.64 kcal/kg0.75 for aCSF, F1, 16 = 30.186, P = 0.00005, Cohen’s d = 1.713, inset of Fig. 6).
Fig. 6.
Time course of hourly energy expenditure during physical activity (aEE) after administration of BDNF in the VMH. The aEE in the 0 – 9 h, dark phase, and 0–24 h post-injection intervals are shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively.* P < 0.05; n = 17.
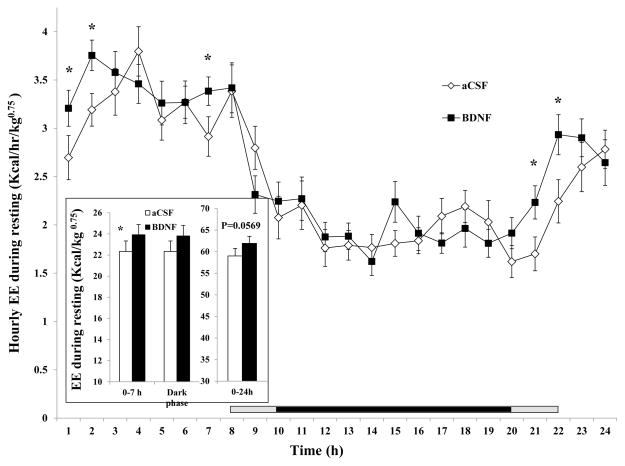
6) Resting energy expenditure (rEE) and metabolic rate
Time course analysis indicates that BDNF significantly increased rEE at 1, 2, 7, 21, and 22 hr after injection (Fig. 7). As a result, BDNF significantly increased rEE in the 0–7 hr post-injection interval (23.92 ± 0.96 kcal/kg0.75 for BDNF vs. 22.34 ± 0.95 kcal/kg0.75 for aCSF, F1, 16 = 5.710, P = 0.02953, Cohen’s d = 0.401, inset of Fig. 7). There was no difference during the 12-h dark cycle (inset of Fig. 7), and although BDNF increased rEE in the 0–24 h interval by 4.6%, it did not reach significance (P= 0.0596, inset of Fig. 7). There was no difference in the percent of total EE due to resting metabolism over the 24-h measurement period (data not shown).
Fig. 7.
Time course of hourly energy expenditure during resting (rEE) after administration of BDNF in the VMH. rEE in the 0 – 7 h, dark phase, and 0–24 h post-injection intervals are shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively.* P < 0.05; n = 17.
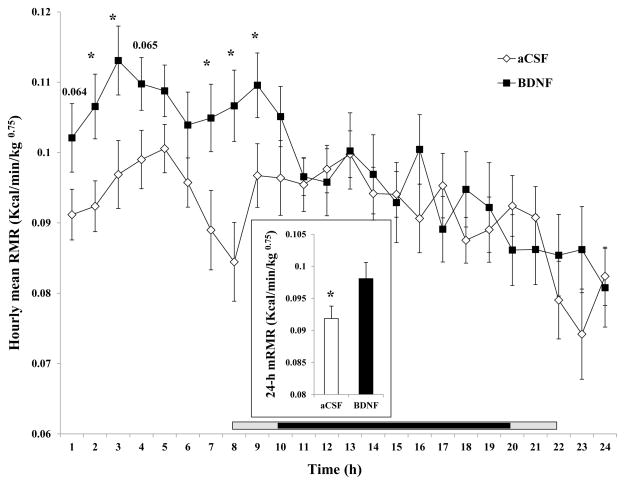
Mean resting metabolic rate (mRMR) and hourly average resting metabolic rate (hRMR) are shown in Fig. 8. BDNF significantly increased mRMR by 6.8% (0.0981 ± 0.0025 kcal/min/kg0.75 for BDNF vs. 0.0919 ± 0.0019 kcal/min/kg0.75 for aCSF, F1, 16 = 11.544, P = 0.0059, Cohen’s d = 0.672), as shown in the inset of the Fig. 8. A 24-h time course analysis indicates that BDNF significantly increased hRMR at 2, 3, 7, 8, and 9 h after injection, with no significant effect thereafter (Fig. 8).
Fig. 8.
Time course of the hourly mean resting metabolic rate (hRMR) after administration of BDNF in the VMH. The mean 24-h resting metabolic rate (mRMR) is shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively.* P < 0.05; n = 17.
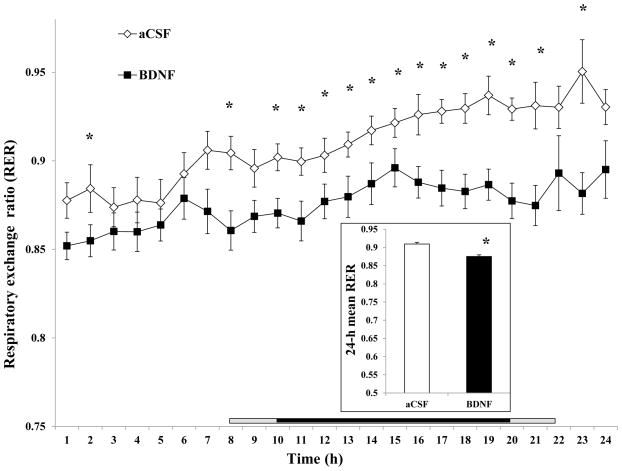
7) Respiratory exchange ratio (RER)
The time course showed increasing RER during the 0–24 h interval for both treatments. However, the RER for BDNF treatment was always lower than that for aCSF, with significantly decreases at 1, 8, 10 ~ 21 and 23 h after injection (Fig. 9). The 24-h mean RER for BDNF (0.875395 ± 0.006196) was significantly lower than that for aCSF (0.909346 ± 0.004383, F1, 16 = 26.268, P = 0.000102, Cohen’s d = −1.534).
Fig. 9.
Time course of the hourly mean respiratory exchange ratio (RER) after administration of BDNF in the VMH. The mean 24-h RER is shown in the inset. Since the measurement among rats started between 10:00 AM and 12:00 PM, the period lengths between injection and the beginning of the dark phase (7:00 PM) were variable. The horizontal bar above the x-axis represents the time spent in the dark phase after injection. The central dark bar plus the right or left short gray bar indicates 12 h spent in the dark phase and represents animals injected between 10 AM (the right gray bar plus the dark bar) and 12 PM (the left gray bar plus the dark bar), respectively.* P < 0.05; n = 17.
Study 2: The effect of BDNF on gene expression of uncoupling protein 1 in brown adipose tissue
There was no significant difference in iBAT or pBAT UCP expression between aCSF and BDNF treated rats (data not shown).
Discussion
Our studies demonstrate that bilateral administration of BDNF in the VMH significantly reduces body weight, which is due to reduced food and water intake, and increased EE. The enhanced EE in BDNF-treated animals results from an elevated RMR and SPA induced EE, especially during the first several hours post-injection. This study is the first to demonstrate VMH-injected BDNF effects on components of energy expenditure.
In this study, verification of cannulation placement was performed by means of a biological/behavioral assay: feeding response to NPY injection in VMH, which has been validated previously in many studies (Bouali et al., 1995; Currie and Coscina, 1997; Heinrichs et al., 1993; Jolicoeur et al., 1995; Lopez-Valpuesta et al., 1996; Myers et al., 1995; Stanley et al., 1985). The dose of 0.5 μg BDNF is based on a dose-response feeding and conditioned taste aversion study (Wang et al., 2007c) and represents a dose at which BDNF decreases feeding and body weight gain without producing taste aversion.
Based on principle of the energy homeostasis, changes in energy intake or/and energy expenditure contribute to body weight change. In this study BDNF significantly reduced food intake by 24% compared to control treatment (Fig. 1). BDNF has been reported to inhibit feeding when it is acutely or chronically administered in the periphery (Nakagawa et al., 2003), ventricle (Pelleymounter et al., 1995), dorsal vagal complex (DVC) (Bariohay et al., 2005) and PVN (Wang et al., 2007a). The current observation is consistent with our previous findings in which BDNF in the VMH reduced body weight by decreasing regular feeding and deprivation- and NPY-induced feeding (Wang et al., 2007c). The potency of feeding inhibition is different among these studies, most likely due to factors including dose range, site of administration, experimental protocol and species. It is reported that in mature BDNF mutant animals under balanced diet, their hyperphagia is mediated by increased meal number with normal meal size, meal duration, and satiety ratio (Fox and Byerly, 2004). Conversely this suggests that the reduced feeding in the current study might be due to inhibited meal frequency and meal initiation by BDNF. Since BDNF reduces food intake, the feeding related physical activity could be decreased. Thus we speculate that BDNF-increased SPA (see Results and below) is not related to feeding.
In the current study water intake was also significantly lower after BDNF treatment. Currently there are no data supporting direct effect of BDNF in the VMH on water intake. Xu et al showed lower food intake and water intake in wild type mice compared to that in hypothalamic TrkB mutant mice (Xu et al., 2003). Other studies suggested that water intake was proportional to food intake. Intracerebroventricular infusion of NPY increased both food intake and water intake; and infusion of melanocortin agonist MT-II decreased NPY-induced increases in feeding and water intake (Raposinho et al., 2003). Because water intake in the current study was significantly correlated with food intake, this suggests that the decreased water intake might be secondary to BDNF-induced reduction in dried pellet intake.
The current study shows that BDNF significantly increased total EE (Fig. 2), suggesting that the elevation of EE also contributed to BDNF-induced weight loss. This finding was inconsistent with a report showing that VMH-selective genetic deletion of BDNF does not affect EE (Unger et al., 2007), as indicated by unaffected body temperature or body weight after 5 weeks. One possible explanation is that increased tissue BDNF concentration in VMH in the current study triggers activation from baselines which cannot be stimulated by BDNF removal from VMH. Another possible explanation is that in Unger’s study food restriction might have resulted in compensatory responses in other brain areas.
Several studies have indicated that the VMH is important to physical activity behavior (Bannai et al., 1998; Narita et al., 1994; Narita et al., 2002) but the data are inconsistent. “VMH-specific” SF-1 knock-out rodents with diminished VMH BDNF expression and impaired VMH function had significantly decreased locomotor activity (Tran et al., 2003; Tran et al., 2006; Zhao et al., 2004), and increased locomotor activity was observed in animals with continuous lateral ventricle infusion of BDNF (Naert et al., 2006) or high VMH BDNF expression after BDNF gene delivery (Cao et al., 2009). Unger et al found unchanged physical activity during the light and dark phases in mice with selective deletion of BDNF in the VMH (Unger et al., 2007). BDNF heterozygous mutant mice with low BDNF expression in the whole brain have elevated locomotor activity (Kernie et al., 2000; Rios et al., 2001), suggesting that BDNF reduces physical activity behavior possibly resulting from different actions in multiple brain structures. The difference in sites of action, measurement intervals, and animal models might account above different observations. In the present study, VMH BDNF significantly increased SPA and time spent active during the first 9 h post injection but did not affect dark phase and 24-h SPA. This suggested that VMH BDNF stimulatory effects on physical activity were rapid and transient. This is in contrast to BDNF in the PVN, after which SPA was unaffected (Wang et al., 2007b). Together, these data suggest that VMH BDNF immediately increases physical activity responses, which results in elevated EE (inset of Fig. 6).
BDNF increased hRMR during the first 9 hours and 24-h mRMR (Fig. 8). In parallel, BDNF increased rEE in these approximate intervals (inset of Fig. 7), suggesting an immediate thermogenic response. A similar thermogenic response was also noticed with PVN BDNF (Wang et al., 2007b). In the VMH, other signals have been reported to elicit thermogenic responses. Electrical stimulation in the VMH for 15 min instantly increased “background metabolism” for about 25 min (Ruffin and Nicolaidis, 1999). ICV orexin A (0.5 nmol) immediately increased BAT and colonic temperature for almost 2 hours, while VMH lesions significantly reduced orexin-induced thermogenic responses (Monda et al., 2005). By comparison, BDNF in the VMH therefore exerts relatively long-term effects on thermogenesis.
Regression analysis indicated a significant correlation between hourly SPA and hourly EE in aCSF-treated animals, suggesting that EE is closely related to physical activity (Fig. 4). However, when the same animals were treated with BDNF, such a correlation was no longer significant, suggesting that BDNF-induced immediate increases in hRMR and rEE may interfere with the normal association between physical activity and total EE.
BDNF was given at the time animals would normally rest and sleep, and as BDNF induced aEE and rEE during the light cycle, this suggests that BDNF has an immediate effect on EE. Since aEE and rEE was not affected by BDNF during dark cycle, one question is whether the duration effect (increased aEE and rEE only seen in the light cycle but not in dark cycle) was due to a half-life of the BDNF or to circadian changes of sensitivity to BDNF. Our previous studies indicated that BDNF in the VMH reduced feeding only at 4–24 h (covering late light cycle and dark cycle) and 24–48 h post injection (Wang et al., 2007c). This delayed effect of VMH-BDNF on feeding rules out the above assumptions of BDNF inactivation or circadian efficacy changes. Therefore different mechanisms must be involved in the delayed BDNF effect on feeding and immediate BDNF effect on EE in the VMH..
In the current study BDNF did not elicit significantly increased UCP1expression in interscapular and perirenal BAT, which is consistent with a recent report that BDNF over expression in the VMH and arcuate nucleus significantly increased EE without concurrently increasing BAT UCP1(Cao et al., 2009). The lack of effect on BAT UCP1 may be explained by the extremely limited sympathetic projection from the VMH to interscapular BAT (only 1.6% neurons in the VMH were infected after injection of pseudorabies virus as a transneuronal viral tract tracer into the interscapular BAT (Bamshad et al., 1999)).
Tissues other than brown fat may contribute to the increased EE induced by VMH BDNF treatment. VMH lesions triggers reduction of sympathetic nervous system (SNS) activity in several organs including heart, pancreas, white adipose tissue (Vander Tuig et al., 1982), leading to decreased catecholamine turnover (Yoshida and Bray, 1984) and reaction to cold temperature (Niijima et al., 1984). Electrical stimulation of the VMH enhances both fat utilization and metabolic rate (Ruffin and Nicolaidis, 1999), and elicits a 3–8-fold increase in norepinephrine turnover rate in heart, liver, pancreas, spleen, submandibular gland and other peripheral tissues (Saito et al., 1989). VMH-injected compounds increase the firing rate of SNS (Sakaguchi and Bray, 1987), O2 consumption, heart rate, and colonic temperature (Kobayashi et al., 1999), plasma norepinephrine and epinephrine (Aizawa-Abe et al., 2000) and renal sympathetic activity (Tanida et al., 2003). These data suggest that SNS activation in the VMH may increase EE through pathways other than iBAT. BDNF-induced expression of CRH (Cao et al., 2009) and TRH (Guerra-Crespo et al., 2001; Ubieta et al., 2007) in the hypothalamus, of UCP2 in the liver and UCP3 in white adipose tissue (Cao et al., 2009) has also been reported. Thus it is possible that VMH-injected BDNF may induce sympathetic activation in other tissues and pathways, leading to increased heat production.
In conclusion, our studies demonstrate that BDNF in the VMH reduces body weight gain via rapid and transient inhibition of food intake and enhanced EE due to increased RMR and SPA.. Together these data suggest that the VMH may be an important site of BDNF action in the regulation of energy balance.
Experimental Procedures
Animals
Male Sprague-Dawley rats (Harlan, Madison, WI) weighing 280–320 g were housed individually in cages with a 12 h light/12 h dark photo-cycle (lights on at 07:00) in a room at 21–22 °C. Teklad lab Chow and water were allowed ad libitum, except where noted. The protocol was approved by the VAMC Institutional Animal Care and Use Committee.
Cannulation and Verification of Placement
Rats were anesthetized with intramuscular Xylazine (Butler, Dublin, OH, 3.5 mg/kg) and Ketamine (Ketaset, Fort Dodge, IA, 20 mg/kg) and were fitted with a 28-gauge stainless steel guide cannula placed just above the VMH bilaterally. Stereotaxic coordinates were determined from the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1998) and are as follows: 0.6 mm lateral and 2.5 mm posterior to bregma, and 8.5 mm below the skull surface. The injector extended 1 mm further than the end of the guide cannula. The animals were given at least one week to recover following surgery before experimental trials. To verify correct cannulae placement in the VMH, we used feeding response to injected NPY as behavioral assay {Jolicoeur, 1995 #5945}. This test was carried out before the EE study. We deemed that injection sites were correctly targeted when a rat had a 2.0 gram increase in food intake within 2h after injecting 100 pmol of NPY. In this test 17 of 25 animals responded to NPY and were included in the statistical analysis.
Drugs
BDNF was kindly provided by Regeneron Pharmaceuticals Inc (Tarrytown, NY), and stored at −70 C at 10 mg/ml of 150 mM NaCl, 10 mM NaHPO3 buffer and 0.004% Tween-20 until use. BDNF was diluted with artificial cerebrospinal fluid (aCSF) just before use.
Injections
A volume of 0.5 μl was injected slowly over 30 sec, with the injector left in place an additional 10 sec to ensure extrusion from the tip and to minimize distribution of drug upwards on the cannula tract. In total, animals received about 10 injections on each side. In previous studies, we demonstrated a lack of extensive tissue damage after 10 repeated injections as measured by gliosis around the injection site using light microscopy at 10x (Wang et al., 2007a; Wang et al., 2007b).
Specific experimental designs:
Study 1: Food intake, energy expenditure and spontaneous physical activity in an indirect calorimeter chamber
This experiment was designed to measure food intake, EE, and SPA simultaneously. Twenty-four-h EE was measured using a customized, high-precision, single-chamber indirect calorimeter (Columbus Instruments; Columbus, OH). Before each experiment, the calorimeter was calibrated using a primary gas standard, and the rat was placed inside the test chamber with food and water. The chamber was sealed and room air was pumped through the chamber at 3.0 – 4.7 L/min depending upon animal body weight, and verified by an optimal difference in O2 inflow and outflow (0.2 – 0.25) from the chamber during resting.
During EE measures, the levels of SPA were also recorded using Opto-M Verimex Minor activity monitors (Columbus Instruments; Columbus, OH) placed around the indirect calorimeter. This device contains 45 collimated infrared activity sensors that detect horizontal and vertical movement, as well as ambulatory movements (which exclude repetitive signals from a single beam). The total SPA counts are total number of beam breaks caused by horizontal and vertical movements.
This system allows us to measure continuous metabolic indices (O2 consumption and CO2 production) and physical activity at a user-defined interval – in this case every 10 s. As such, we are able to identify periods when the animals are resting or active and thereby quantify not only resting metabolism but also the EE of SPA.
Each rat was allowed to acclimate in a 14 L, 30 cm diameter, 20 cm high cylindrical chamber in the testing area for up to 24 h prior to testing, during which time most animals maintained their body weight. Between 10:00 and 12:00 the next morning, the animals were injected with 0.5 μg BDNF or aCSF and then monitored in the same chamber for an additional 24 h. Each animal received both treatments with at least 72 h between treatments. The timing of the gas measurements was as follows: reference air was allowed to “settle” for 120 sec, and then measured for 60 sec. This was then followed by a period in which cage air was allowed to settle for 120 sec. Finally the sample air was measured for 10 sec in each epoch for 20 min (120 epochs). The complete cycle (from reference air settling to completion of the sample measurement in the last epoch) was approximately 25 min. Thus this measurement protocol was repeated every 25 min.
The endpoints include food and water intake, body weight change, spontaneous physical activity, O2 consumption, CO2 production, respiratory exchange ratio (RER) and heat production. Heat production was calculated based on the equation for calculation of EE = (3.815 + 1.232 x RER) x VO2). Oxygen consumption and heat production were normalized by 0.75 power of mean individual body weight, using the average of body weights at the beginning and the end of the experiment. Individual activity counts for horizontal, vertical (standing and rearing), and ambulatory movement were collected, and total SPA counts (the sum of all beam break counts caused by horizontal and vertical movements) were calculated.
If there were zero SPA counts during a 10 sec epoch, the status in this period was defined as resting; otherwise, the status was defined as SPA. EE during resting (rEE) within each hour and 24-h period as well as the duration of resting (min) in each hour and 24-h were calculated. Hourly average resting metabolic rate (hRMR) was calculated by dividing hourly rEE by the resting time (min) in the same period. The 24-h mean RMR (mRMR) was defined as 24-h total rEE divided by total resting time (min). Using the same methods, EE during SPA (aEE) within each hour and 24-h period as well as the duration of SPA (min) in each hour and 24-h were also calculated. The above metabolic measurements were normalized for 0.75 power of body weight, and are expressed as kcal/kg0.75 for total EE, rEE and aEE, and kcal/min/kg0.75 for hRMR and mRMR.
Study 2: The effect of BDNF on gene expression of uncoupling protein 1 in brown adipose tissue
In this study, rat uncoupling protein 1 (UCP 1, NM012682) gene expression was measured at 4 h after administration of 0.5 μg BDNF. Food was removed after injection. The animals were sacrificed 4 h after injection by rapid decapitation. Interscapular and perirenal brown adipose tissues were taken for measurement of UCP1 mRNA. Total RNA from all tissues was extracted by the rapid guanidine thiocyanate-phenol-chloroform method (Chomczynski and Sacchi, 1987). Real time RT-PCR was used to measure relative UCP1 gene expression.
Real time, one step RT-PCR
The primers for UCP1 and the housekeeping gene, ribosomal protein L32 (Rpl32), were created using MacVector 7.2 (Accerlys, San Diego, CA). The primers for UCP1 were: 5′-TGG CAT CCA GAG GCA AAT CAG - 3′ (forward) and 5′-AGC ATT GTA GGT CCC AGT GTA GCG - 3′ (reverse). The primers for Rpl32 were 5′-CGG AAG TTT CTG GTC CAC AAT GTC - 3′ (forward) and 5′-GCT CTT TCT ACG ATG GCT TTT CGG - 3′ (reverse). One-step real time RT-PCR was performed using 100 ng of total RNA, and the reagents provided in the Roche RNA Amplification Kit SYBR Green I, and a Roche LightCycler (Roche Applied Science, Indianapolis, IN.). RT-PCR was performed as follows: reverse transcription for 30 min at 42°C, denaturation for 30 sec at 95°C, followed by 35 cycles of cDNA amplification consisting of a 15 sec denaturation at 95°C, primer annealing for 20 sec at 61°C (UCP1), and 59°C (Rpl32), and product elongation for 20 sec at 72°C. Each primer set yielded a single product that corresponds to the appropriate nucleotide lengths. Amplification products from PCR were purified (QIAquick PCR Purification Kit, Valencia, CA), determined by electrophoresis in a 4% Nuseive gel, and then verified by capillary electrophoresis. The 2−ΔΔCT method was used to calculate relative UCP1 and RPL32 mRNA, and fold changes in mRNA levels (Livak and Schmittgen, 2001). Fold change in UCP1 mRNA compared to RPL32 mRNA was expressed as the ratio of the mean relative UCP1 mRNA and mean relative RPL32 mRNA.
Statistics
In this study 17 rats were included in the statistical analysis. Data were analyzed using StatView 5.0 (Cary, NC) and are expressed as mean ± S.E.M. Repeated measures ANOVA was used to analyze group differences in food and water intake, body weight change, O2 consumption, total EE, rEE, aEE, percent total EE for resting, RMR, duration of resting, SPA, and UCP1/Rpl32. The effect size was calculated with the method proposed by Cohen (Cohen, 1988) and modified by Rosnow and Rosenthal(Rosnow, 1996), and represented by Cohen’s d.
Acknowledgments
We thank Regeneron Pharmaceuticals Inc (Tarrytown, NY) for providing BDNF and Mark R. Margosian for technical help in indirect calorimetry.
Grants:
This work was supported by the Department of Veterans Affairs, by Award R01DK080782 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and by the Minnesota Obesity Center (pilot & feasibility program #14).
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- VMH
hypothalamic ventromedial nucleus
- EE
energy expenditure
- UCP1
uncoupling protein 1
- BAT
brown adipose tissue
- SPA
spontaneous physical activity
- RMR
resting metabolic rate
- aEE
EE during SPA
- rEE
EE during resting
- mRMR
mean resting metabolic rate
References
- Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–52. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–78. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Bannai M, Ichikawa M, Nishihara M, Takahashi M. Effect of injection of antisense oligodeoxynucleotides of GAD isozymes into rat ventromedial hypothalamus on food intake and locomotor activity. Brain Res. 1998;784:305–15. doi: 10.1016/s0006-8993(97)01349-8. [DOI] [PubMed] [Google Scholar]
- Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–20. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- Bouali SM, Fournier A, St-Pierre S, Jolicoeur FB. Effects of NPY and NPY2-36 on body temperature and food intake following administration into hypothalamic nuclei. Brain Res Bull. 1995;36:131–5. doi: 10.1016/0361-9230(94)00177-3. [DOI] [PubMed] [Google Scholar]
- Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15:447–54. doi: 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JE, Powley TL. Intragastric pair feeding fails to prevent VMH obesity or hyperinsulinemia. Am J Physiol. 1981;240:E566–72. doi: 10.1152/ajpendo.1981.240.5.E566. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. Stimulation of 5-HT(2A/2C) receptors within specific hypothalamic nuclei differentially antagonizes NPY-induced feeding. Neuroreport. 1997;8:3759–62. doi: 10.1097/00001756-199712010-00020. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA. Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol. 2004;60:424–36. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R994–1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- Guerra-Crespo M, Ubieta R, Joseph-Bravo P, Charli JL, Perez-Martinez L. BDNF increases the early expression of TRH mRNA in fetal TrkB+ hypothalamic neurons in primary culture. Eur J Neurosci. 2001;14:483–94. doi: 10.1046/j.0953-816x.2001.01657.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Pich EM, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- Hustvedt BE, Jeszka J, Christophersen A, Lovo A. Energy metabolism in rats with ventromedial hypothalamic lesions. Am J Physiol. 1984;246:E319–26. doi: 10.1152/ajpendo.1984.246.4.E319. [DOI] [PubMed] [Google Scholar]
- Inoue S, Campfield LA, Bray GA. Comparison of metabolic alterations in hypothalamic and high fat diet-induced obesity. Am J Physiol. 1977;233:R162–8. doi: 10.1152/ajpregu.1977.233.3.R162. [DOI] [PubMed] [Google Scholar]
- Jolicoeur FB, Bouali SM, Fournier A, St-Pierre S. Mapping of hypothalamic sites involved in the effects of NPY on body temperature and food intake. Brain Research Bulletin. 1995;36:125–9. doi: 10.1016/0361-9230(94)00176-2. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19:1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–44. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T, Namba Y, Inoue S, Kimura S. Involvement of sympathetic activation and brown adipose tissue in calcitonin gene-related peptide-induced heat production in the rat. Brain Res. 1999;849:196–202. doi: 10.1016/s0006-8993(99)02154-x. [DOI] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Nanjo K, Senba E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–15. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Valpuesta FJ, Nyce JW, Griffin-Biggs TA, Ice JC, Myers RD. Antisense to NPY-Y1 demonstrates that Y1 receptors in the hypothalamus underlie NPY hypothermia and feeding in rats. Proc Biol Sci. 1996;263:881–6. doi: 10.1098/rspb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–44. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–14. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Saito M, Shimazu T. Sympathetic denervation impairs responses of brown adipose tissue to VMH stimulation. Am J Physiol. 1986;251:R1005–8. doi: 10.1152/ajpregu.1986.251.5.R1005. [DOI] [PubMed] [Google Scholar]
- Monda M, Viggiano AN, Viggiano A, Viggiano E, Lanza A, De Luca V. Hyperthermic reactions induced by orexin A: role of the ventromedial hypothalamus. Eur J Neurosci. 2005;22:1169–75. doi: 10.1111/j.1460-9568.2005.04309.x. [DOI] [PubMed] [Google Scholar]
- Myers RD, Wooten MH, Ames CD, Nyce JW. Anorexic action of a new potential neuropeptide Y antagonist [D-Tyr27,36, D-Thr32]-NPY (27–36) infused into the hypothalamus of the rat. Brain Research Bulletin. 1995;37:237–45. doi: 10.1016/0361-9230(94)00282-6. [DOI] [PubMed] [Google Scholar]
- Naert G, Ixart G, Tapia-Arancibia L, Givalois L. Continuous i.c.v. infusion of brain-derived neurotrophic factor modifies hypothalamic-pituitary-adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience. 2006;139:779–89. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–44. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ogawa Y, Ebihara K, Yamanaka M, Tsuchida A, Taiji M, Noguchi H, Nakao K. Anti-obesity and anti-diabetic effects of brain-derived neurotrophic factor in rodent models of leptin resistance. Int J Obes Relat Metab Disord. 2003;27:557–65. doi: 10.1038/sj.ijo.0802265. [DOI] [PubMed] [Google Scholar]
- Narita K, Nishihara M, Takahashi M. Concomitant regulation of running activity and metabolic change by the ventromedial nucleus of the hypothalamus. Brain Res. 1994;642:290–6. doi: 10.1016/0006-8993(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Narita K, Murata T, Honda K, Nishihara M, Takahashi M, Higuchi T. Subthalamic locomotor region is involved in running activity originating in the rat ventromedial hypothalamus. Behav Brain Res. 2002;134:275–81. doi: 10.1016/s0166-4328(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Niijima A, Rohner-Jeanrenaud F, Jeanrenaud B. Role of ventromedial hypothalamus on sympathetic efferents of brown adipose tissue. Am J Physiol. 1984;247:R650–4. doi: 10.1152/ajpregu.1984.247.4.R650. [DOI] [PubMed] [Google Scholar]
- Ono M, Itakura Y, Nonomura T, Nakagawa T, Nakayama C, Taiji M, Noguchi H. Intermittent administration of brain-derived neurotrophic factor ameliorates glucose metabolism in obese diabetic mice. Metabolism. 2000;49:129–33. doi: 10.1016/s0026-0495(00)90988-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–38. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, White RB, Aubert ML. The melanocortin agonist Melanotan-II reduces the orexigenic and adipogenic effects of neuropeptide Y (NPY) but does not affect the NPY-driven suppressive effects on the gonadotropic and somatotropic axes in the male rat. J Neuroendocrinol. 2003;15:173–81. doi: 10.1046/j.1365-2826.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–57. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people’s published data: General procedures for research consumers. Pyschological Methods. 1996;1:12. [Google Scholar]
- Ruffin M, Nicolaidis S. Electrical stimulation of the ventromedial hypothalamus enhances both fat utilization and metabolic rate that precede and parallel the inhibition of feeding behavior. Brain Res. 1999;846:23–9. doi: 10.1016/s0006-8993(99)01922-8. [DOI] [PubMed] [Google Scholar]
- Saito M, Minokoshi Y, Shimazu T. Accelerated norepinephrine turnover in peripheral tissues after ventromedial hypothalamic stimulation in rats. Brain Res. 1989;481:298–303. doi: 10.1016/0006-8993(89)90806-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Bray G. The effect of intrahypothalamic injections of glucose on sympathetic efferent firing rate. Brain Res Bull. 1987;18(5):591–595. doi: 10.1016/0361-9230(87)90128-6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Arase K, Bray GA. Sympathetic activity and food intake of rats with ventromedial hypothalamic lesions. Int J Obes. 1988;12:285–91. [PubMed] [Google Scholar]
- Sauer H, Fischer W, Nikkhah G, Wiegand SJ, Brundin P, Lindsay RM, Bjorklund A. Brain-derived neurotrophic factor enhances function rather than survival of intrastriatal dopamine cell-rich grafts. Brain Res. 1993;626:37–44. doi: 10.1016/0006-8993(93)90560-a. [DOI] [PubMed] [Google Scholar]
- Sha H, Xu J, Tang J, Ding J, Gong J, Ge X, Kong D, Gao X. Disruption of a novel regulatory locus results in decreased Bdnf expression, obesity, and type 2 diabetes in mice. Physiol Genomics. 2007;31:252–63. doi: 10.1152/physiolgenomics.00093.2007. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–4. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Tanida M, Nagai K, Kaneko H. Activation of the renal sympathetic nerve by leptin microinjection into the ventromedial hypothalamus in rats. In Vivo. 2003;17:213–7. [PubMed] [Google Scholar]
- Tran PV, Lee MB, Marin O, Xu B, Jones KR, Reichardt LF, Rubenstein JR, Ingraham HA. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci. 2003;22:441–53. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006;498:637–48. doi: 10.1002/cne.21070. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Nonomura T, Ono-Kishino M, Nakagawa T, Taiji M, Noguchi H. Acute effects of brain-derived neurotrophic factor on energy expenditure in obese diabetic mice. Int J Obes Relat Metab Disord. 2001;25:1286–93. doi: 10.1038/sj.ijo.0801678. [DOI] [PubMed] [Google Scholar]
- Ubieta R, Uribe RM, Gonzalez JA, Garcia-Vazquez A, Perez-Monter C, Perez-Martinez L, Joseph-Bravo P, Charli JL. BDNF up-regulates pre-pro-TRH mRNA expression in the fetal/neonatal paraventricular nucleus of the hypothalamus. Properties of the transduction pathway. Brain Res. 2007;1174:28–38. doi: 10.1016/j.brainres.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–74. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Tuig JG, Knehans AW, Romsos DR. Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sci. 1982;30:913–20. doi: 10.1016/0024-3205(82)90619-1. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R1003–12. doi: 10.1152/ajpregu.00011.2007. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R992–1002. doi: 10.1152/ajpregu.00516.2006. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007c;293:R1037–45. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990;109:141–52. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–42. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–57. [PubMed] [Google Scholar]
- Yoshida T, Bray GA. Catecholamine turnover in rats with ventromedial hypothalamic lesions. American Journal of Physiology. 1984;246:R558–65. doi: 10.1152/ajpregu.1984.246.4.R558. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Hanley NA, Majdic G, Stallings NR, Jeyasuria P, Parker KL. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol. 2004;215:89–94. doi: 10.1016/j.mce.2003.11.009. [DOI] [PubMed] [Google Scholar]