Abstract
Human septins comprise a family of 13 genes that encode for >30 protein isoforms with ubiquitous and tissue-specific expressions. Septins are GTP-binding proteins that assemble into higher-order oligomers and filamentous polymers, which associate with cell membranes and the cytoskeleton. In the last decade, much progress has been made in understanding the biochemical properties and cell biological functions of septins. In parallel, a growing number of studies show that septins play important roles for the development and physiology of specific tissues and organs. Here, we review the expression and function of septins in the cardiovascular, immune, nervous, urinary, digestive, respiratory, endocrine, reproductive, and integumentary organ systems. Furthermore, we discuss how the tissue-specific functions of septins relate to the pathology of human diseases that arise from aberrations in septin expression.
Keywords: cardiovascular, endocrine, human disease, immune, nervous, pathology, reproductive, respiratory, septins
Introduction
The biological function of the septin G proteins lies in their ability to dimerize and assemble into filamentous polymers, which serve as scaffolds and diffusion barriers that control the localization of cellular proteins (Kinoshita, 2006; Caudron and Barral, 2009; Gasper et al., 2009; Spiliotis and Gladfelter, 2012). Evolved from a primordial eukaryotic ancestor through gene duplications and deletions, septin genes vanished from plants and proliferated in eukaryotic lineages as divergent as algae, ciliates, fungi, and metazoa (Pan et al., 2007; Nishihama et al., 2011). In vertebrates, the number of septin genes nearly doubled that of single-cell (e.g., yeast) and invertebrate organisms (e.g., insects, worms). Thus, septin paralogs evolved together with highly complex organ systems, adapting and diversifying their scaffolding and barrier functions to new physiological processes.
Septins are phylogenetically classified under the translation factor (TRAFAC) class of P-loop nucleotide-binding proteins, which includes the superfamilies of Ras-like GTPases and the kinesin and myosin cytoskel-etal motors (Leipe et al., 2002). The nucleotide-binding domain of these proteins consists of alternating α-helices and β-strands separated by flexible loops, which contain sequence motifs that interact with the phosphate groups of GTP or ATP (Leipe et al., 2002). In eukaryotic septins, the motifs G1 (Walker A motif GxxxxGK[ST]), G3 (Walker B motif DxxG), and G4 (GTP-specificity motif NKxD) are highly conserved and the GTP-binding domains are flanked by an N-terminal polybasic region (Figure 1A), which interacts with membrane phospholipids (Zhang et al., 1999; Casamayor and Snyder, 2003) and a C-terminal 56 amino acid sequence of unknown function termed the septin unique element (Versele and Thorner, 2005). Septins vary mainly in the length and amino acid composition of their N- and C-terminal tails, which contain pro-line-rich domains and α-helical coiled coils, respectively (Figure 1A). Through these protein-protein interaction domains, septin polymers can form paired filaments and bind a diversity of cellular proteins in a septin-specific manner (Nakahira et al., 2010; DeMay et al., 2011).
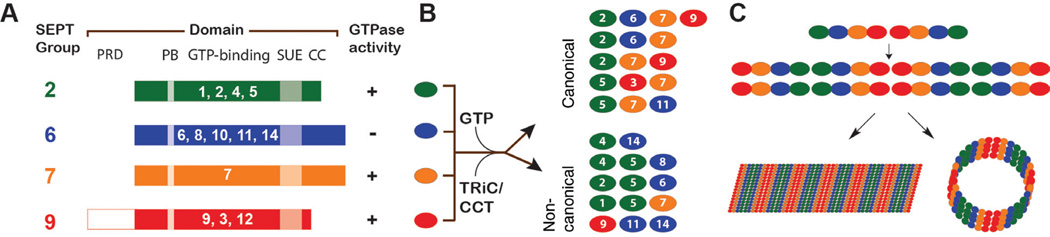
Figure 1. Septin classification, sequence domains, and assembly into oligomers and polymers.
(A) Classification, sequence domains, and GTPase activity of the mammalian septins. On the basis of sequence similarity, human septin proteins are classified into four groups: SEPT2 (SEPT1, SEPT2, SEPT4, and SEPT5), SEPT6 (SEPT6, SEPT8, SEPT10, SEPT11, and SEPT14), SEPT7, and SEPT9 (SEPT3, SEPT9, and SEPT12). Septins have a highly conserved GTP-binding domain, which is flanked by a conserved N-terminal polybasic (PB) sequence that interacts with phospholipids and a C-terminal 56 amino acid sequence of unknown function termed septin unique element (SUE). Septins vary mainly in the length and amino acid composition of their N- and C-terminal tails, which contain proline-rich domains (PRD) and α-helical coiled coil (CC) domains. Most septins have a slow intrinsic GTPase activity, and septins of the SEPT6 group are posited to lack catalytic activity. (B) Septin assembly into oligomeric complexes of variable size and composition. After GTP-binding and folding by the cytosolic TRiC/CCT chaperonin, septins dimerize combinatorially forming oligomeric complexes, which consist of equal number of subunits from each one of the four septin groups (e.g., SEPT2/6/7/9 or SEPT2/6/7; canonical complexes) or more than one subunit from the same septin group (e.g., SEPT4/5/8 or SEPT9/11/14; non-canonical complexes). (C) Polymerization of septin oligomers into higher-order filamentous structures. Septin hetero-oligomers assemble into non-polar filaments, which can pair with one another forming linear bundles and circular rings.
On the basis of sequence similarity, the products of 13 mammalian septin genes are classified into four groups (Kinoshita, 2003): SEPT2 (SEPT1, SEPT2, SEPT4, and SEPT5), SEPT6 (SEPT6, SEPT8, SEPT10, SEPT11, and SEPT14), SEPT7, and SEPT9 (SEPT3, SEPT9, and SEPT12); many of these septins exist in multiple isoforms (e.g., SEPT9_i1, SEPT9_i2). In contrast to the majority of septins, which hydrolyze GTP slowly, members of the SEPT6 group are posited to be catalytically inactive and always bound to GTP (Sirajuddin et al., 2009). Using the G-domain as a binding interface (Sirajuddin et al., 2007), a member of each septin group dimerizes in tandem with itself and/ or a septin from another group to form hetero-oligomeric complexes (Figure 1B). End-to-end binding of these oligomers leads to the assembly of non-polar polymers such as straight, curved, or circular filaments (Figure 1C), which can make lateral contacts with each other through their C-terminal coiled-coil domains that extend perpendicularly from their G-domain interfaces (Kinoshita, 2002; Bertin et al., 2008; de Almeida Marques et al., 2012). Septins follow a combinatorial mode of assembly that is dictated by preferential affinities for one another and availability of specific isoforms (Kinoshita, 2003; Nakahira et al., 2010). Members of the same septin group can substitute each other within a SEPT2/6/7/9 complex (Sandrock et al., 2011; Sellin et al., 2011b, 2012) and thus, SEPT7 is a ubiquitous subunit and the presence of all other septins depends on their expression in various cell types and developmental stages. In vivo, the precise composition and size of septin oligomers are unknown; however, complexes including SEPT2/6/7/9 (Sellin et al., 2011b), SEPT2/6/7 (Joberty et al., 2001; Kinoshita, 2002), SEPT7/9/11 (Nagata et al., 2004), SEPT3/5/7 (Fujishima et al., 2007), and SEPT5/7/11 (Xie et al., 2007) have been isolated from cultured cells. Interestingly, septin complexes such as SEPT2/5/6/7 (Hsu et al., 1998), SEPT4/5/8 (Blaser et al., 2002; Martinez et al., 2004), SEPT4/14 (Shinoda et al., 2010), SEPT2/5 (Beites et al., 1999), and SEPT1/5 (Mizutani et al., 2013) have also been reported. These non-canonical complexes include multiple subunits from the same septin group and do not appear to conform to the canonical SEPT2/6/7/9 arrangement (Figure 1B).
Assembly of septins into oligomers and polymers is a dynamic process regulated by many factors, including GTP-binding and hydrolysis, binding partners, and post-translational modifications. GTP binding triggers conformational changes within the G domains of septins, affecting the stability of their oligomerizing interfaces (Sirajuddin et al., 2009). Little is known about how GTP turnover and hydrolysis influence septin heteromerization, but subunits of the SEPT6 group, which are constitutively bound to GTP, are thought to stabilize septin oligomers. Septin assembly can be further modulated by a variety of intracellular factors such as the TRiC/CCT chaperone system (Dekker et al., 2008), which is implicated in the folding and possibly dimerization of septins, the Cdc42-binding protein Borg3, which in the absence of Cdc42 inhibits septin assembly (Joberty et al., 2001), and the Orc6 subunit of the origin recognition complex, which enhances septin GTPase activity and filament formation (Huijbregts et al., 2009). In addition, posttranslational modifications such as phosphorylation, sumoylation, acetylation, and ubiquitylation have all been reported to affect septin assembly (reviewed in Hernandez-Rodriguez and Momany, 2012). Association of septins with membrane lipids and cytoskeletal elements such as actin microfilaments or microtubules can further influence their polymerization into filaments. Reconstitution of septin assembly on membrane bilayers and liposomes indicate that septin binding to phosphatidylinositol 4,5-biphosphate (PIP2) enhances filament formation (Tanaka-Takiguchi et al., 2009; Bertin et al., 2010). Actin filaments have also been shown to act as templates for the assembly of septin complexes into linear filaments and microtubules support the disk-like organization of septins in non-adherent cells (Kinoshita et al., 2002; Sellin et al., 2011a). Thus, assembly of septins depends not only on their biochemical properties and posttranslational modifications but also on their interaction with binding partners and intra-cellular structures and organelles.
Septin expression and roles in embryogenesis
Insights into the tissue expression and functions of mammalian septins have emerged from DNA microarray analyses and septin knockout mice (Table 1). Serial analysis of gene expression in a variety of human tissues shows a nearly ubiquitous expression of SEPT2, SEPT7, and SEPT9 (Hall et al., 2005; Cao et al., 2007; Connolly et al., 2011a). While absent from some tissues, the septins SEPT4, SEPT8, SEPT10, and SEPT11 are expressed widely (Hall et al., 2005; Cao et al., 2007). In contrast, expression of SEPT1, SEPT3, SEPT12, and SEPT14 is limited to specific tissues. SEPT1 and SEPT3 are expressed in lymphoid and nervous tissues, respectively (Hall et al., 2005), and SEPT12 is a testis-specific septin (Xue et al., 2004; Peterson et al., 2007). SEPT14 was originally identified as a novel testis-specific protein, but is also expressed in the nervous system (Peterson et al., 2007; Shinoda et al., 2010). Little is known about how septin expression varies throughout development and adult life, but a recent study showed that the levels of 10 different septin proteins change dramatically during brain development (Tsang et al., 2011).
Table 1.
Septin expression, binding partners, and links to human diseases.
| Gene | Locus | Tissue expression | Mouse model | Binding partner | Disease association | Referencesa |
|---|---|---|---|---|---|---|
| SEPT1 | 16p11.1 | Lymphoid – skin | Not done | Aurora B kinase | Alzheimer’s; oral, skin, and colorectal cancers; leukemia |
(Qi et al., 2005) |
| SEPT2 | 2q.37.3 | Ubiquitous | Not done | GLAST, MAP4, anillin, myosin II, exo70, IQGAP, p85 |
Alzheimer’s, renal cell carcinoma, brain cancer, leukemia, systemic lupus erythematosus |
(Beites et al., 1999; Kinoshita et al., 2002, 2004; Vega and Hsu, 2003; Kremer et al., 2005; Garcia et al., 2006; Joo et al., 2007) |
| SEPT3 | 22q13.2 | Neuronal | Viable | cGMP-dependent protein kinase-1 (PKG-I) |
Alzheimer’s, Down syndrome, brain cancer | (Xue et al., 2004) |
| SEPT4 | 17q22 | Widely expressed | Viable; sterile | X-linked inhibitor of apoptosis (XIAP), α-synuclein, DYRK1A kinase, kaposin A |
Alzheimer’s, Parkinson’s, melanoma, leukemia, urothelial cancers, schizophrenia, male infertility |
(Ihara et al., 2003; Gottfried et al., 2004; Lin et al., 2007; Sitz et al., 2008) |
| SEPTS | 22qll.2 | Widely expressed | Viable; behavioral defects |
Syntaxin 1A, syntaxin 4, parkin, dynamin 1 |
Pancreatic cancer, leukemia, bipolar disorder, schizophrenia |
(Beites et al., 1999; Choi et al., 2003; Suzuki et al., 2009; Maimaitiyiming et al., 2013) |
| SEPT6 | Xq24 | Ubiquitous | Viable | SOCS7, binder of Rho GTPases (BORG), hnRNP A1, NS5b, myelin and lymphocyte protein (MAL) |
Melanoma, leukemia, bipolar disorder, Down syndrome |
(Joberty et al., 2001; Kim et al., 2007; Kremer et al., 2007; Buser et al., 2009) |
| SEPT7 | 7p14.2 | Ubiquitous | Embryonic lethal | CENP-E, nephrin, CD2AP, ERK3, binder of Rho GTPases (BORG) |
Alzheimer’s, Down syndrome, bipolar disorder, schizophrenia |
(Joberty et al., 2001; Zhu et al., 2008; Brand et al., 2012; Wasik et al., 2012) |
| SEPT8 | 5q31 | Widely expressed | Not done | VAMP2, mitogen-activated protein kinase- 5 (MK5), CDK14 (PFTAIRE1) |
(Yang et al., 2002; Ito et al., 2009; Shiryaev et al., 2012) | |
| SEPT9 | 17q25 | Ubiquitous | Embryonic lethal | Microtubules, rhotekin, septin- associated (SA)-RhoGEF, HIF1-α, internalin B |
Hereditary neuralgic amyotrophy; colorectal, breast, ovarian, liver, and head and neck cancers; leukemia; Down syndrome; bipolar disorder; schizophrenia |
(Pizarro-Cerda et al., 2002; Nagata et al., 2003; Ito et al., 2005; Nagata and Inagaki, 2005; Amir et al., 2006) |
| SEPT10 | 2q13 | Widely expressed | Not done | |||
| SEPT11 | 4q21.1 | Widely expressed | Embryonic lethal | Dynamin 1 | Renal cell carcinoma, leukemia, bipolar disorder, schizophrenia |
(Maimaitiyiming et al., 2013) |
| SEPT12 | 16p13.3 | Testicular | Not done | Male infertility | ||
| SEPT14 | 7p11.2 | Testicular –neuronal |
Not done | Testicular cancer |
References correspond to the binding partners column of the table. Disease associations are discussed and referenced throughout the main text of this review.
To date, knockout mice have been generated for 7 of the 13 septins. Genetic ablation of the ubiquitous Sept7, Sept9, and Sept11 resulted in embryonic lethality. Sept7−/−mice were never born, arresting early in development possibly due to mitotic failure (Kinoshita, 2008). Development of Sept11−/− mice ceased at day 11.5 in utero and the mice died by day 13.5 (Roseler et al., 2011). Despite the development of a healthy yolk sac, heartbeat, and blood vessels, Sept9−/− embryos died by day 10 of gestation, exhibiting mesenchymal tissue degeneration and extensive cell death (Fuchtbauer et al., 2011). Surprisingly, loss of the neuron-specific Sept3 did not have any discernible effects on the physiology and function of Sept3−/− mice (Tsang et al., 2008). Functional redundancy between Sept3 and Sept9 or compensation of Sept3 loss by Sept9 isoforms could account for the lack of phenotype. Similarly, Sept5−/− and Sept6−/− mice were reported to be normal; however, newer behavioral assays indicate changes in the social interaction, rewarded goal approach, and anxiety-related behavior of Sept5−/− mice (Ono et al., 2005a; Suzuki et al., 2009; Harper et al., 2012). Among all the septin knockout mice, Sept4−/− mice are the most well studied and characterized. Male Sept4−/− mice are sterile due to a morphologically altered and immotile sperm (Ihara et al., 2005; Kissel et al., 2005). Neurologically, the cerebellum of Sept4−/− mice is mildly maldeveloped and hypo-dopaminergic phenotypes such as an increase in the inhibition of the startling response by weaker prestimuli have been reported (Kinoshita, 2008). Loss of Sept4 from mice that express the α-synuclein mutant A53T, which is common among familial forms of Parkinson’s disease, increased amyloid deposition and neurodegenerative pathology, leading to shorter lifespans (Ihara et al., 2007). Moreover, Sept4−/− mice are more susceptible to tumor growth and liver fibrosis, and contain high levels of hematopoietic and hair follicle stem cells (HFSCs) (Iwaisako et al., 2008; Garcia-Fernandez et al., 2010; Fuchs et al., 2013).
The embryonic lethality phenotype of the Sept7−/−, Sept9−/−, and Sept11−/− mice suggests that septins play an essential role in embryogenesis. It is unknown how septins are involved in the embryonic development of mammals, but studies in model organisms such as the fruit fly Drosophila melanogaster, the roundworm Caenorhabditis elegans, and the frog Xenopus laevis have shed some light on the developmental functions of septins. In early Drosophila development, multiple rounds of mitotic nuclear divisions lead to the formation of a multinucleated single-cell embryo. The nuclei of this syncytial blastoderm are partitioned into individual cells by a process termed cellularization. In septin-deficient embryos, cellularization is incomplete, resulting in multinucleated cells, less imaginal discs (precursors of epithelial tissues), and larval death (Neufeld and Rubin, 1994; Adam et al., 2000). In C. elegans, septins localize similarly to the cytokinetic furrows, but are not essential for embryonic development (Nguyen et al., 2000). Septin-depleted embryos rarely fail to complete cytokinesis; however, the asymmetric geometry of furrow ingression is disrupted and the contractile apparatus is less robust and more prone to stochastic errors (Maddox et al., 2007). Postembryonic development of C. elegans tissues is more severely affected by septin mutations, which disrupt gonadogenesis and the formation of a functional sensory and motor nervous system (Nguyen et al., 2000; Finger et al., 2003). Recently, a study in Xenopus showed that septins are involved in the planar cell polarity (PCP) signaling pathway, which directs the collective cell movements of embryogenesis during gastrulation, axial elongation, and organogenesis. The PCP signaling protein Fritz, which interacts directly with Sept2, was shown to control septin localization to the cortical membrane of gastrulating cells (Kim et al., 2010). Moreover, Fritz and septins synergized toward the formation of cilia, which are critical for the transduction of the Sonic hedgehog signals that regulate organogenesis (Kim et al., 2010). Thus, septins are essential components of the molecular and cellular mechanisms that give rise to complex organ and tissue systems. Next, we focus on how septins function in the development, maintenance, and disease states of the cardiovascular, immune, nervous, reproductive, urinary, digestive, respiratory, endocrine, and integumentary systems (Figure 2).
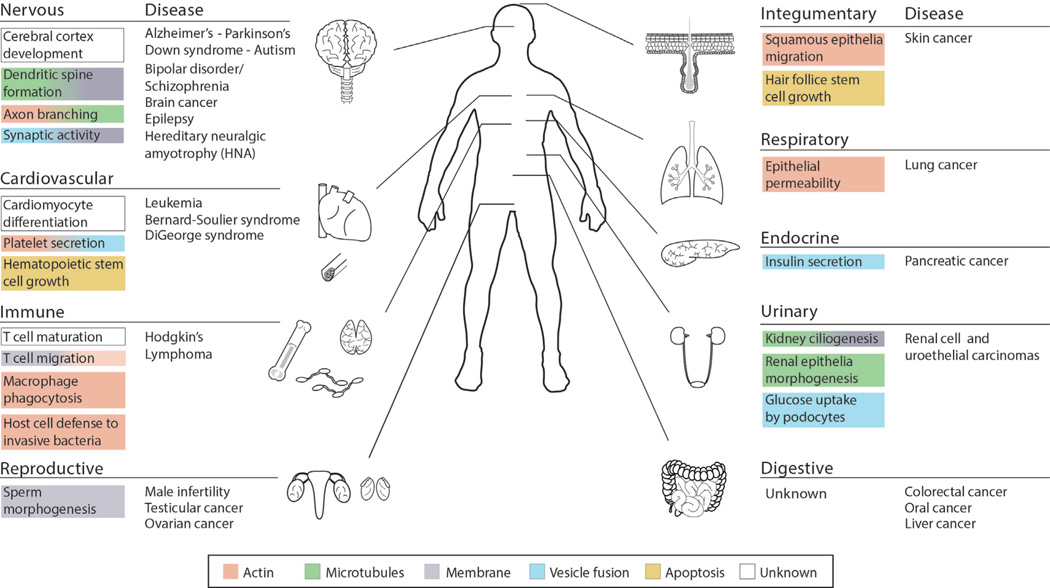
Figure 2. Septin functions in organ systems and their connection to human disease.
The schematic diagram depicts the main organ systems of the human body and outlines organ- and cell type-specific structures and processes that require septins. Color codes indicate the intracellular involvement of septins, which includes septin functions in the organization of actin (red), microtubules (green) and cell membranes (gray), vesicle fusion (blue), and apoptosis (yellow). Human diseases are listed based on genetic, histological, genomic, and proteomic evidence that implicates septins in the pathology of various organ disorders.
Cardiovascular system
The heart is the first organ to develop during embryogenesis. Terminal differentiation of cardiomyocytes occurs near birth and is characterized by the cessation of cell division and the development of contractile multinucleated cells. In mouse embryonic cardiac cells, septin 2, 6, 7, and 9 levels are the highest in early development and decrease at birth and adulthood (Ahuja et al., 2006). In embryonic cardiomyocytes, septins localize to the cytokinetic ring and midbody of dividing cells and are absent from sarcomeric actomyosin (Ahuja et al., 2006). Thus, septins could function in early cardiac development by interacting with key components of the cytokinetic machinery (e.g., myosin, anillin). Moreover, septin downregulation could trigger mitotic arrest and the formation of terminally differentiated multinucleated cells. Interestingly, SEPT5 is located within the 22q11.2 locus that is commonly deleted in the DiGeorge/ velo-cardial-facial syndrome (DGS), which is characterized by cardial malformations and lesions (McKie et al., 1997). Studies in animal models suggest that 22q11.2 hemizygosity, which occurs in 90% of DGS cases, results in developmental impairment of the right ventricle and outflow tract (Scambler, 2000). It is unknown if partial loss of SEPT5 contributes to the pathology of DGS, but decreased septin (SEPT8) expression is also implicated in the toxic effects that anti-inflammatory drugs have on the development of embryonic cardiomyocytes (Baek et al., 2010).
Septin expression and functions in the vascular network have been identified in platelets, which are specialized secretory cells that regulate hemostasis, thrombosis, and injury repair. SEPT5 (CDCrel-1) localizes around platelet α-granules, storage vesicles that contain growth factors and effector molecules involved in adhesion and clot formation (Dent et al., 2002). Additionally, SEPT5 coprecipitates with syntaxin 4, a component of the SNARE-SNAP complex that mediates vesicle fusion with the plasma membrane (Dent et al., 2002). Platelets isolated from Sept5 null mice secrete serotonin more readily upon stimulation, suggesting that Sept5 negatively regulates α-granule fusion with the plasma membrane (Dent et al., 2002).
Abnormal SEPT5 expression has been linked to the Bernard-Soulier syndrome (BSS), a rare autosomal recessive blood disorder characterized by excessive bleeding and abnormal platelet morphology, count, and secretion (Lopez et al., 1998). BSS patients harbor mutations in the quaternary platelet glycoprotein Ibβ-IX (GpIBB) receptor subunit, which regulates platelet adhesion, activation, and aggregation. Mice lacking the GpIBB gene exhibit symptoms that phenocopy BSS pathology and possess platelets with abnormally large α-granules and elevated levels of SEPT5 (Kato et al., 2004). More recently, genotypic analysis of a juvenile patient with a severe case of BBS showed homozygous deletions of the GpIBB and SEPT5 genes, which are positioned directly next to each other (Zieger et al., 1997; Bartsch et al., 2011).
Aberrant septin expression has been implicated in other blood disorders, including acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) (Cerveira et al., 2011). Mixed lineage leukemia (MLL) is a histone methyltransferase that regulates homeobox (HOX) gene expression during hematopoiesis (Muntean and Hess, 2012). Chromosomal translocations of the MLL gene account for ~10% of the de novo and therapy-induced leukemias (Muntean and Hess, 2012). Septins 2, 5, 6, 9, and 11 have all been identified as MLL fusion partners, accounting for the largest known protein family associated with MLL rearrangements (Cerveira et al., 2011). The MLL-septin chimera results from double-stranded intronic breaks in the MLL and septin genes, producing in-frame fusions of the MLL N-terminus with almost the entire open-reading frame of the septin (Cerveira et al., 2011). Ectopic expression of the MLL-SEPT6 chimera has been shown to immortalize hematopoietic progenitor cells, upregulating the expression of the Hox-7a and Hox-9a genes (Ono et al., 2005b). Although MLL-SEPT6 expression in mice was not sufficient to induce leukemogenesis (Ono et al., 2005a), mice transduced with an active FMS-like receptor tyrosine kinase 3, which is frequently overexpressed in AML, required MLL-SEPT6 to stimulate leukemogenesis (Ono et al., 2005b). MLL-SEPT6 expression also led to a lethal myeloproliferative disease with long latency (Ono et al., 2005b).
Recent studies have shown that hematopoietic stem cell maturation and malignancy are affected by the SEPT4 splice variant ARTS (apoptosis-related protein in the TGF-β signaling; SEPT4_v2) (Garcia-Fernandez et al., 2010). SEPT4/ARTS binds to inhibitors of apoptosis proteins, initiating caspase activation and cell death (Larisch et al., 2000; Gottfried et al., 2004). Sept4-deficient mice contain elevated levels of hematopoietic stem cells, including progenitor and immature B-cells that are resistant to pro-apoptotic stimuli (Garcia-Fernandez et al., 2010). Genetic crosses between Sept4−/− and the leukemogenic Eµ-Myc mice showed that Sept4 ablation enhances lymphomagenesis and reduces lifetime by 50% (Garcia-Fernandez et al., 2010). Significantly, ARTS expression is frequently altered in bone marrow lymphocytes derived from patients with childhood ALL, the most common malignancy found in children (Elhasid et al., 2004). Lymphocytes from ALL patients exhibit a significant reduction in SEPT4/ARTS mRNA levels, and SEPT4/ARTS-deficient lymphocytes are highly resistant to apoptosis when treated with the chemotherapeutic agent ara-C (Elhasid et al., 2004). DNA methylation inhibitors can rescue SEPT4/ARTS expression, suggesting that aberrant methylation downregulates its expression during tumorigenesis (Elhasid et al., 2004). Clinically, SEPT4/ARTS expression was rescued in patient lymphocytes following therapy, indicating that SEPT4/ ARTS expression suppresses growth and possibly malignant transformation of hematopoietic cells.
Immune system
The mammalian innate and adaptive immune systems encompass several tissues and specialized cell types that defend against infectious agents. Despite that the first mammalian septin (Sept1) was cloned from lymphocytes 25 years ago (Carol et al., 1990), it was not until recently that researchers began to investigate the immunological functions of septins.
Cells of the innate immunity (e.g., dendritic cells, macrophages) recognize and engulf foreign bodies, and elicit a larger response through antigen presentation. SEPT10 was initially identified in dendritic cells, where it is abundantly expressed (Sui et al., 2003). In contrast, SEPT10 is weakly expressed in the spleen, thymus, and peripheral blood leukocytes. In dendritic cells, SEPT10 localizes to the cytoplasm and the nucleus, and is transcriptionally upregulated upon stimulation with lipopolysaccharide (Sui et al., 2003). Septins 2, 6, 8, 10, and 11 are abundantly expressed in macrophages, and SEPT2 and SEPT11 localize to actin-based structures at the base and periphery of the phagocytic cup (Huang et al., 2008). Disrupting septin assembly by expressing the septin-binding domain of BORG3 or targeted knockdown of SEPT2 or SEPT11 reduced phagosome formation and uptake of IgG-coated beads (Huang et al., 2008). Further studies are necessary to understand the precise functions of septins in dendritic and macrophage cell biology.
T lymphocytes proliferate and mature in lymphoid tissues (e.g., thymus, spleen, lymph nodes) and migrate to peripheral tissues, searching for antigen-presenting cells. Septins are essential for both T-cell development and migration. During mouse T-cell development, the transition from a double-negative (CD4−CD8−) to a double-positive (CD4+CD8+) stage is accompanied by a 5- to 10-fold increase in Sept9 expression (Lassen et al., 2013). Sept9 deletion affects T-cell development in the thymus, leading to an increase in stage 3 double- negative cells (CD4−CD8−CD25+CD44−) at the expense of stage 4 double-negative cells (CD4−CD8−CD25−CD44−). Peripheral CD8+ and CD4+ T cells are reduced concomitantly, and in vitro proliferation of Sept9-deleted CD8+ T-cells is impaired; the effects of Sept9 deletion in vivo are more severe on CD8+ than CD4+ T cells (Lassen et al., 2013). These studies are the first to implicate septins in T-cell development, but more work is needed to determine the mechanism by which SEPT9 regulates T-cell maturation and selection.
Lymphocytes migrate by forming a leading pseudopod and a trailing uropod, both of which require polarized assembly of filamentous actin and contraction of the cortical membrane. In T cells, septins localize to the middle of the cell cortex and are distinctly absent from the protruding pseudopod and uropod (Tooley et al., 2009). SEPT7-depleted lymphocytes have elongated uropods and show poor persistent migration despite maintaining normal levels of actin, microtubules, and phosphorylated (active) myosin (Tooley et al., 2009). In addition, SEPT7 depletion results in excessive blebbing and reduces the rate of cortical retraction in a hydrostatic volume change assay, which measures the ability of cells to expand and retract their cell membrane (Gilden et al., 2012). In migrating lymphocytes, septins (SEPT6 and SEPT7) are absent from blebs and filopodia, but are recruited to plasma membrane sites adjacent to these protrusive structures (Sellin et al., 2011a; Gilden et al., 2012). Overall, the plasma membrane organization and dynamics of migrating T cells appear to depend on septins, which affect persistent migration.
In non-immune cells, septins are essential for host defense against infectious bacteria. The invasive bacterium Listeria monocytogenes enters mammalian cells through the interaction of its surface proteins internalin A and B (InIA, InIB) with E-cadherin and the Met receptor, respectively (Cossart, 2011). Targeted depletion of SEPT2 inhibits bacterial invasion and impairs the InIB/Met signaling pathway (Mostowy et al., 2009). Septins also thwart the intracellular mobility of Shigella flexneri, a pathogenic bacterium that propels itself through the cytoplasm by recruiting and activating the Arp2/3 complex, which induces the formation of an actin tail (Egile et al., 1999). Septin filaments are recruited to cytoplasmic Shigella and assemble cage-like structures that inhibit bacterial mobility and facilitate the formation of an autophagosome for bacterial degradation (Mostowy et al., 2010).
Nervous system
Septins are abundantly present in the nervous system, exhibiting cell type-specific and developmentally regulated patterns of expression.
Development of a functional nervous system involves the migration of embryonic stem cells and neural precursors, and their differentiation into neurons and glial cells. Neuronal morphogenesis requires the formation of axons, dendrites, and synapses. Recent studies have shown that septins play essential roles in neuronal migration, axonal and dendritic arborization, and synaptic activity. In mouse embryos, Sept4 and Sept14 are highly expressed in the cortical plate of the developing cortex, which is formed by postmitotic neurons that migrate tangentially from the subventricular zone to the outer layers of the neocortex (Shinoda et al., 2010). In vivo knockdown of Sept14 or Sept4 expression and inhibition of their interaction perturb neuronal migration to the cortical plate; neurons fail to extend leading processes and stall at the ventricular and intermediate zones without reaching the cortical plate (Shinoda et al., 2010). The sensory and motor neurons of the C. elegans septin mutants unc-59 and unc-61 exhibit similar migration defects during larval development (Finger et al., 2003).
In cultured hippocampal neurons, septins are required for the formation of dendritic spines (Tada et al., 2007; Xie et al., 2007; Cho et al., 2011). SEPT5/7/11 complexes localize to dendritic branch points and at the base of dendritic spines (Xie et al., 2007). Targeted depletion of SEPT7, SEPT11, or SEPT6 reduces dendritic arborization and alters the length, density, and head morphology of dendritic spines (Tada et al., 2007; Xie et al., 2007; Cho et al., 2011). In contrast, SEPT7 overexpression increases dendritic protrusions and branch points (Tada et al., 2007; Xie et al., 2007). Because dendritic spines are enriched with membrane proteins (e.g., neurotransmitter receptors) that localize specifically at the postsynaptic cleft, septins are posited to form a diffusion barrier at the base of dendritic spines blocking free diffusion of membrane proteins in and out of the dendritic shaft (Caudron and Barral, 2009). In addition, septins may also synergize with the actin and microtubule cytoskeleton for the formation of dendritic spines.
Septin-mediated reorganization of the actin and microtubule cytoskeleton is required for the collateral branching of axons. In sensory neurons isolated from chicken dorsal root ganglia, SEPT6 and SEPT7 localize at the base of axonal filopodia and axon branch points (Hu et al., 2012). This localization is reminiscent of septin complexes at the base of dendritic spines, but in axons septins control the initiation and maturation of collateral branches (Hu et al., 2012). SEPT6 enhances the transition of axonal patches of F-actin to filopodia by enhancing the recruitment of the actin-binding protein cortactin, an activator of Arp2/3-mediated actin polymerization (Hu et al., 2012). In contrast, SEPT7 is involved in the entry of axonal microtubules into nascent filopodia, enabling the formation of collateral branches (Hu et al., 2012). These regulatory roles of septins are likely to influence not only the outgrowth and differentiation of neurites into axons and dendrites, but also axon extension and growth cone motility. Indeed, septins have been reported to affect axon length (Vega and Hsu, 2003), but more studies are needed to understand their precise roles in axonal differentiation and growth.
Neurotransmission and organ innervation rely on the formation and function of chemical synapses, which release and receive neurotransmitter molecules through their presynaptic and postsynaptic clefts, respectively. Neurotransmitter secretion and uptake are coupled to ion channels that sense and generate membrane potential. Initial studies indicated that septins inhibit the release of synaptic vesicles (Beites et al., 2001), but new evidence suggests that septins could also regulate the localization of ion channels in membrane microdomains and their endocytosis from synaptic membranes. Septins 3, 5, 6, 7, and 11 localize to the presynaptic cleft and their filamentous organization coincides with the filament-like strands, which have been observed by electron microscopy to connect synaptic vesicles with each other and the plasma membrane (Hirokawa et al., 1989; Kinoshita et al., 2000; Tsang et al., 2011). SEPT5 and SEPT8 have been reported to interact with components of the molecular machinery of synaptic vesicle fusion (Beites et al., 1999, 2005; Ito et al., 2009). SEPT5 and SEPT7 are posited to inhibit vesicle fusion by inhibiting the formation of productive SNARE complexes (Beites et al., 2005; Wasik et al., 2012). Interestingly, however, synaptic transmission and morphology were not altered in Sept5−/− and Sept3−/− mice (Tsang et al., 2008). Further analysis of the developing calyx of Held, which is a large glutamatergic synapse of the auditory nervous system, showed that Sept5 filaments play a key role in the positioning of predocked synaptic vesicles relative to the voltage gated calcium channels (VGCCs) of the active zone (Yang et al., 2010). In Sept5−/− synapses, synaptic vesicles are more tightly docked to the VGCCs. In addition, synaptic vesicles are more readily and cooperatively released, requiring a lower number of calcium channels to trigger single fusion events (Yang et al., 2010). Thus, SEPT5 introduces a physical barrier between synaptic vesicles and VGCCs regulating the efficiency of synaptic transmission. A new study suggests that SEPT5 and SEPT4 might modulate synaptic activity by controlling the membrane organization and clustering of ion channels (Sharma et al., 2013). Although this hypothesis has yet to be tested in neurons, SEPT4 is required for the organization of phosphatidyl-inositol-(4,5)-disphosphate-rich domains in the plasma membrane of HeLa cells and the clustering of the calcium channel ORAI1, which triggers calcium entry and signaling when calcium concentration is low in the endoplasmic reticulum (Sharma et al., 2013). Alternatively, septins may affect the internalization of ion channels from the synaptic membrane. SEPT2 interacts with the astrocyte glutamate transporter GLAST, and SEPT2 mutations increase GLAST internalization, and consequently, result in a decrease of glutamate uptake (Kinoshita et al., 2004). Interaction of septins (SEPT5, SEPT11) with dynamin, which mediates scission of vesicles from the plasma membrane, raises the possibility that septins regulate endocytosis at synaptic terminals, affecting the recycling of synaptic vesicles and ion transporters (McNiven, 1998; Maimaitiyiming et al., 2013).
In summary, septins have fundamental roles in the development, activity, and connectivity of the nervous system. Thus, abnormalities in septin expression are likely to compromise the function of the nervous system, contributing to the pathology of neurodevelopmental and neurodegenerative disorders. Hereditary neuralgic amyotrophy, a disorder characterized by shoulder and arm pain and muscle atrophy, is genetically linked to missense mutations and duplications in the N-terminal sequence of SEPT9 (Chance and Windebank, 1996; Kuhlenbaumer et al., 2005). These abnormalities may cause the axonal degeneration and focal demyelination observed in the brachial plexus nerves of patients with hereditary neuralgic amyotrophy (Ueda et al., 2010). In the maldeveloped brain of fetuses with Down syndrome, the expression of SEPT6 and SEPT7 is markedly reduced and SEPT4 might be hyperphosphorylated due to the overexpression of the DYRK1A kinase (Sitz et al., 2008). Increases in septin 5, 6, and 11 expression were also observed in schizophrenia and bipolar disorders (Pennington et al., 2008), and SEPT9 was abnormally overexpressed in a mouse model of demyelinating neuropathy (Patzig et al., 2011). Histological analysis of brains from Alzheimer’s patients identified septins 1, 2, and 4 in neurofibrillary tangles (Kinoshita et al., 1998), which are intracellular aggregates of the hyperphosphorylated microtubule-associated protein tau. In addition, allelic variations in SEPT3 isoforms have been observed in Alzheimer’s tissue samples, indicating a potential role or risk factor in Alzheimer’s disease pathogenesis (Takehashi et al., 2004). In the brains of patients with Parkinson’s disease, SEPT4 is found in the Lewy body aggregates of α-synuclein and overexpression of SEPT4 in the mouse brain results in behavioral abnormalities (Ihara et al., 2003; Ageta-Ishihara et al., 2013). Interestingly, SEPT4 interacts with α-synuclein directly, and loss of SEPT4 expression in the A53T familial Parkinson’s mouse model enhances amyloid-like aggregation and neurodegeneration (Ihara et al., 2007).
Reproductive system
Septins have been genetically linked to male infertility and ovarian cancer. The importance of septins for sperm development was discovered by two independent studies of SEPT4 knockout mice. Male Sept4−/− mice are sterile and their sperm is immotile and morphologically bent with an abnormal L-shape (Ihara et al., 2005; Kissel et al., 2005). This phenotype correlates with Sept4 localization to the annulus, a cortical ring separating the middle and principal pieces of spermatozoa. In Sept4−/− sperm, the annular structure is abolished (Ihara et al., 2005; Kissel et al., 2005). Moreover, Sept4−/− sperm mitochondria have abnormal size, cristiae, and membrane morphology, and an excess of cytoplasmic droplets is observed in the head and neck regions of the sperm (Kissel et al., 2005). While these data suggest that Sept4 functions in mitochondrial division and caspase-mediated removal of the cytoplasm during spermiogenesis, follow-up studies explored the hypothesis that Sept4 maintains a cortical diffusion barrier at the annulus. Kwitny et al. (2010) tracked the localization of the diffusing membrane protein basigin, which localizes to the principal piece during spermato-genesis and shifts to the middle piece during epididymal maturation. In the sperm of Sept4−/− mice, basigin fails to localize to either the principal or middle pieces, indicating that Sept4 is critical for maintaining the proper localization of sperm proteins through the establishment of an annular diffusion barrier (Kwitny et al., 2010). Because a defective annulus is common among patients with asthenospermia, screening for Sept4 localization is now used as a diagnostic tool for sperm malformation (Sugino et al., 2008).
In addition to Sept4, septins 1, 6, 7, and 12 also localize to the annulus of the sperm (Toure et al., 2011). Notably, Sept12 is expressed only in the testis (Peterson et al., 2007) and similar to the Sept4−/− mice, Sept12+/− chimeric mice are sterile and their sperm is immotile with nuclear defects and distorted shape (Lin et al., 2009). In vitro fertilization of mouse oocytes with Sept12+/− sperm has low success rates, and fertilized oocytes do not progress past the morula stage (Lin et al., 2011). Interestingly, genetic analysis of infertile men revealed two missense SEPT12 mutations, T89M and D197N, which map to the switch I and GTP recognition motifs of Sept12, respectively (Kuo et al., 2012). Biochemical analysis showed that the SEPT12-T89M mutant had reduced rates of GTP hydrolysis and SEPT12-D197N did not bind GTP (Kuo et al., 2012). Both mutants failed to form filaments in tissue culture cells, and SEPT12 was absent from the sperm annulus of SEPT12-D197N patients (Kuo et al., 2012). Thus, mutations in the testis-specific SEPT12 are genetically linked to male infertility. In addition, septins are linked to testicular cancer, in which SEPT14 expression is lost, suggesting that SEPT14 could be a suppressor of testicular cancer (Peterson et al., 2007).
Septin expression in ovarian tissue was first identified in Drosophila where sep1 localizes to follicle cells during embryonic development (Fares et al., 1995). The precise function of septins in ovarian physiology is unknown. Septin 9 expression, however, is significantly altered in ovarian cancers. Initial studies in sporadic ovarian tumor samples showed that the chromosomal locus 17q25.3, which includes the SEPT9 gene, was partially deleted leading to allelic imbalances and loss of heterozygosity (Kalikin et al., 2000; Russell et al., 2000). Subsequent studies revealed that SEPT9 has a complex genomic organization encoding for up to 18 different mRNA transcripts and 15 protein isoforms (McIlhatton et al., 2001). Analysis of SEPT9 mRNA transcripts in benign, malignant and borderline, low-grade ovarian carcinomas showed that the SEPT9_v1 and SEPT9_v4* transcripts were overexpressed in serous and mucinous borderline tumors (Scott et al., 2006). The enhanced translational efficiency of SEPT9_v4* relative to SEPT9_v4 suggested that increased levels of SEPT9_i4 could promote the malignant and metastatic potential of ovarian carcinomas (McDade et al., 2007). Consistent with this possibility, overexpression of SEPT_i4 enhances cell motility and resistance to anticancer drugs that target microtubules (Chacko et al., 2012). Upregulation of SEPT9_i1 could also enhance tumor growth and angiogenesis by slowing down the degradation of the c-Jun-N-terminal kinase (JNK) and the hypoxia-inducible factor HIF1a (Amir et al., 2009; Gonzalez et al., 2009). While the cause of the amplification of certain SEPT9 mRNA transcripts is not understood, epigenetic changes, such as the hypermethylation of alternative intragenic promoters, could alter the relative expression of mRNA transcripts (Connolly et al., 2011b).
Urinary and digestive systems
The tissue and organ surfaces of the urinary and digestive systems are composed of epithelial cells. The epithelial plasma membrane is biochemically differentiated into the apical and basolateral membrane domains. The directional transport of water, ions, and solutes depends on the proper localization of specific channels (e.g., aquaporins, Na/K pump) and transporters (e.g., P-glycoprotein) to the epithelial membrane domains. Establishment and maintenance of the polarized distribution of these proteins is key for the development and function of organs such as the kidney, intestine, and lung.
In Madin-Darby canine kidney cells (MDCKs), a well-established model for studying renal epithelial morphogenesis in two- and three-dimensional cultures, SEPT2 is essential for the organization of the microtubule cytoskeleton and the transport of membrane vesicles from the Golgi to the plasma membrane (Spiliotis et al., 2008; Bowen et al., 2011). During the establishment of columnar epithelial morphology, SEPT2 guides microtubule growth and microtubule-microtubule interactions for the establishment of the subapical microtubule network. Moreover, SEPT2 localization to a subset of perinuclear, microtubule bundles enables the egress of Golgi-derived vesicles by hindering the binding of the microtubule-associated protein 4 (MAP4), which impedes microtubule motor-driven transport (Spiliotis et al., 2008). Without proper microtubule architecture and post-Golgi membrane traffic, SEPT2-depleted epithelia fail to assume a columnar shape and most apical and basolateral membrane proteins accumulate in the cytoplasm. New studies suggest that septins are also important for the two-dimensional expansion of the renal epithelium, which occurs by planar division and asymmetric ingression of the cleavage furrow from the basal to the apical membrane. In Drosophila epithelia, septins enable the closure of the cytokinetic furrow by strengthening the cytokinetic actomyosin ring, which must overcome the tensile forces exerted by the apical adherens junctions (Founounou et al., 2013; Guillot and Lecuit, 2013).
Renal epithelia not only maintain water and ion homeostasis but also monitor the flow of fluid in the lumen of the nephron, which in turn can affect cell morphology and proliferation. Fluid flow is sensed by the primary cilium, a microtubule-based antenna-like organelle that projects from the epithelial apical membrane into the lumen of the nephron (Pazour and Witman, 2003). Septins are integral and essential components of the primary cilium (Hu et al., 2010; Chih et al., 2012; Ghossoub et al., 2013). In the inner medullary collecting duct cells IMCD3, SEPT2 localizes to the base of the primary cilium and is required for the formation of functional cilia (Hu et al., 2010). SEPT2 depletion results in complete loss of primary cilia and the formation of shorter cilia, in which ciliary membrane proteins diffuse freely into the surrounding plasma membrane (Hu et al., 2010). Thus, SEPT2 is posited to be part of a membrane diffusion barrier, which maintains the localization of ciliary membrane proteins. Recent studies, however, indicate that SEPT2/7/9 complexes may also interact with axonemal microtubules controlling the length of cilia (Ghossoub et al., 2013).
Podocytes are specialized epithelial cells that wrap around the capillaries of the kidney’s glomeruli and respond to insulin. Podocytes prevent serum albumin and other macromolecules from leaving the blood, while allowing water, salts, and glucose to enter the kidney lumen. Loss of podocyte function is prevalent in diabetic nephropathy, the most common cause of renal failure (Pagtalunan et al., 1997). A new study shows that SEPT7 colocalizes with the glucose transporter isoform 4 (GLUT4) storage vesicles and interacts with nephrin, the CD2-associated protein CD2AP, and the vesicle SNARE protein VAMP2 (Wasik et al., 2012). SEPT7 knockdown increased the interaction of VAMP2 with nephrin and syntaxin, and glucose uptake (Wasik et al., 2012). Thus, SEPT7 could be involved in the regulation of glucose transport in the insulin-sensitive podocytes.
Similar to many tumors of epithelial origin, septins are overexpressed in renal cell carcinoma (RCC), which is an aggressive and highly metastatic cancer of the kidney with poor response rates to therapeutics (Craven et al., 2006b). SEPT2 and SEPT11 are upregulated in RCCs defective in the von Hippel-Lindau (VHL) tumor suppressor, a ubiquitin E3 ligase that targets the hypoxia-inducible factor 1 (HIF1) transcription factor for degradation (Craven et al., 2006a). It is unknown if SEPT2 and SEPT11 are transcriptionally regulated by HIF1 or directly degraded by VHL. Moreover, it is unclear if and how septin upregulation contributes to RCC growth and metastasis. An increase in the mRNA and protein levels of the Bradeion isoform of SEPT4 has also been detected in urine samples from patients with RCCs and transitional bladder cancers (Tanaka et al., 2003; Bongiovanni et al., 2012). Quantification of SEPT4 Bradeion expression by immunochromatography and RT-PCR of urine samples has been introduced for the diagnosis of urothelial cancers in Japan and Europe (Tanaka et al., 2003; Bongiovanni et al., 2012).
Abnormal expression of SEPT4 has also been observed in colorectal cancer (CRC). While absent from patient-matched healthy tissues, two SEPT4 isoforms (Bradeion α and β) were identified in colorectal tissue isolated from human patients with mucinous carcinoma and rectal malignant melanoma (Zieger et al., 2000; Tanaka et al., 2001). Ribozyme-mediated cleavage of either or both isoforms arrests CRC growth in the G2 phase of the cell cycle in vitro and prevents tumor growth in mice injected with CRC cells (Tanaka et al., 2002). The SEPT4 isoforms exhibit cytoplasmic and nuclear localization (Tanaka et al., 2001); however, further studies are necessary to elucidate their cellular functions and the molecular mechanisms that contribute to their abnormal expression in CRC.
SEPT9 is highly expressed in normal colonic glandular and surface epithelia, but is markedly reduced in adenomas and almost completely absent from tumor tissues, indicating that SEPT9 levels decrease progressively during tumorigenesis (Toth et al., 2011). Treatment of HT29 CRC cells with a demethylating agent results in increased levels of SEPT9, suggesting that SEPT9 expression is downregulated by gene methylation during CRC progression (Toth et al., 2011). Epigenetic modifications in stem cell DNA within the colonic crypt are known to change with age and have been identified in a subset of CRCs (Taylor et al., 2003; Humphries and Wright, 2008). More specifically, hypermethylation of CpG islands (GC-rich regulatory sequences found in the promoters of almost half of all human genes) are common in many cancers (Herman and Baylin, 2003). Plasma DNA from CRC patients contains abnormally high levels of methylated SEPT9 (Grutzmann et al., 2008). Significantly, SEPT9 is hypermethylated in 87% of early stage I and II colorectal tumors (Warren et al., 2011). These findings have led to the development of the septin 9 test, which is a commercial non-invasive, blood-based test that screens for the methylation of the SEPT9 gene as a diagnostic marker of early-stage colon cancer.
Respiratory system
Respiratory epithelia perform a number of key functions, including gas exchange and protection from particulate matter. In the lower respiratory tract, the multiciliated bronchial epithelia protect the underlying tissue from noxious agents and push the mucus toward the pharynx (Fahy and Dickey, 2010). Maintenance of the epithelial cell-cell junctions is critical for limiting paracellular permeability, subepithelial inflammation, and injury (Frank, 2012). Physiological stresses in bronchial epithelia (e.g., particulate matter) stimulate dynamic reorganization of the actin cytoskeleton at the cell cortex, strengthening cell-cell junctions and restricting paracellular permeability (Youakim and Ahdieh, 1999). In shear-stressed bronchial epithelia, SEPT2 is recruited to the apical plasma membrane and interacts with the F-actin network more strongly (Sidhaye et al., 2011). In SEPT2-depleted MDCK and bronchial cells challenged with shear stress or particulate matter, cortical F-actin is reduced and low-molecular-weight dextran enters into the basolateral space more readily (Sidhaye et al., 2011). Taken together, these data indicate that SEPT2 is critical for the maintenance of epithelial integrity during respiratory stress.
Endocrine system
Tissues and cells of the endocrine system secrete hormones that regulate the physiology and function of peripheral tissues and organs. In the pancreas, SEPT5 is highly expressed in the islet of Langerhans, while its expression is markedly reduced and absent from the acinar and ductal epithelia, respectively (Capurso et al., 2005). The islet of Langerhans consists of α- and β-cells that secrete glucagon and insulin, respectively, stimulating glucose release and uptake from the blood. The role of SEPT5 in exocytosis was tested in the HIT-T15 insulinoma cell line by assaying for the release of exogenous human growth hormone (hGH) in the presence of wild-type or dominant-negative SEPT5. Secretion of hGH was significantly reduced in cells expressing wild-type SEPT5, indicating that SEPT5 inhibits exocytosis (Beites et al., 1999). In pancreatic β-cells, SEPT2 interacts with EXO70, a component of the octameric exocyst complex that mediates vesicle tethering to the plasma membrane (Rittmeyer et al., 2008). The SEPT2-EXO70 complex localizes to the β-cell membrane and interacts directly with the Rho GTPase effector, IQGAP1 (Rittmeyer et al., 2008). Interestingly, overexpression of the SEPT2-EXO70 binding domain of IQGAP1 displaces SEPT2 from the plasma membrane, and the constitutively active form of CDC42 inhibits the interaction of IQGAP1 with the SEPT2-EXO70 complex and blocks insulin secretion (Rittmeyer et al., 2008). Although the precise role of septins in vesicle docking and fusion is unknown, regulation of septin localization and function by IQGAP and Rho signaling might be important for the secretion of insulin from pancreatic β-cells.
Integumentary system
The integumentary system consists of the skin, hair follicles, and sebaceous glands. Collectively, these structures make up the largest organ system of the body protecting all other organs from physical damage, infectious agents, and water loss. SEPT1 localizes diffusely throughout the cytoplasm of epidermal keratinocytes, and Western blotting of epidermal lysates shows expression of septins 1, 4, 8, 11, and 14 (Mizutani et al., 2013). In squamous cell carcinoma (SCC) and malignant melanoma tissue sections, SEPT1 is significantly upregulated (Mizutani et al., 2013). In SCC cells, SEPT1 and SEPT5 coimmunoprecipitate and colocalize with SEPT4 in the lamellipodia, a dendritic actin network at the leading edge of migrating cells (Mizutani et al., 2013). SEPT1-depleted DJM-1 cells exhibit defective cell spreading, a process that depends on actin polymerization and cell adhesion with the extracellular matrix. While absent from healthy epidermis, SEPT9 is highly expressed in cultured SCC cells and coprecipitates with SEPT7/10/11/14 (Mizutani et al., 2013). In contrast to SEPT1, septins 9, 11, and 14 colocalize with microtubules and are absent from actin-based structures. These data indicate that different septins and septin complexes interact with different cytoskeletal elements in SCC cells. However, the role of septins in the malignant transformation and metastasis of epidermal cells remains unknown.
Regeneration of the epidermis after injury relies on skin follicle stem cells and HFSCs, whose growth and differentiation are tightly regulated so that tissue structure is preserved without overgrowth. A recent study showed that the SEPT4 splice variant ARTS (SEPT4_v2) is specifically expressed in HFSCs (Fuchs et al., 2013). Hair follicle bulges in mice lacking Sept4/ARTS are morphologically normal, but contain twice as many CD34+ progenitor cells (Fuchs et al., 2013). Sept4/ARTS-deficient CD34+ cells grow faster in culture without feeder cells and are highly resistant to pro-apoptotic stimuli, suggesting that SEPT4/ ARTS restricts HFSC proliferation (Fuchs et al., 2013). Remarkably, wound recovery in Sept4/ARTS-deficient mice is extraordinarily fast. Sept4/ARTS has previously been shown to promote apoptosis by targeting XIAP, an inhibitor of apoptosis, for degradation (Larisch et al., 2000). HFSCs from mice deficient in both SEPT4/ARTS and XIAP showed reduced wound repair (Fuchs et al., 2013), supporting the hypothesis that SEPT4/ARTS-mediated apoptosis can limit stem cell growth.
Conclusion
Septins constitute a large family of GTP-binding proteins with ubiquitous and tissue-specific patterns of expression. Septins assemble into hetero-oligomers and polymers, whose subunit composition varies depending on developmental stage and cell type. By regulating the spatial organization of membrane and cytoplasmic proteins, septins are essential for the morphogenesis and physiological functions of many cell types of the human body. For several tissues and organs, the identity of septin complexes remains unknown, but a number of septin-specific interactions suggest that septins fulfill tissue-specific functions. In the future, the challenge lies in identifying the septin complexes of most tissues and organs, and determining how they function. This will be an important step toward understanding how alterations in septin expression contribute to the pathogenesis of specific diseases. Male infertility, neuromuscular, and bleeding disorders are genetically linked to septin mutations and deletions. Alterations in septin expression are common in many cancers and neurodevelopmental disorders (e.g., Down syndrome, schizophrenia), and septins have been found in the neurofibrillary tangles and Lewy bodies of Alzheimer’s and Parkinson’s brains. Despite this knowledge, the precise role of septins, if any, remains unknown for many of these diseases. Alterations in septin localization and expression have been used as infertility and cancer biomarkers; however, therapeutic treatments that target septins or use septin-derived peptides are yet to be developed. New exciting research shows that septins play key regulatory roles in the proliferation of stem cells. Thus, the study of septins can provide valuable insights into the emerging field of regenerative medicine while paving the way for new diagnostics and treatments of human disease.
Acknowledgments
Work in the Spiliotis laboratory was supported by grant GM097664 from the National Institutes of Health. Q.H. was supported by U.S. Department of Defense Breast Cancer Research Predoctoral Training Grant BC083077. We apologize to our colleagues whose work was omitted due to space and reference limitations.
Biographies

Lee Dolat received his Bachelor of Science in Molecular and Cell Biology from the University of Connecticut and worked as a research technician at 454 Life Sciences (Roche Diagnostics) and the Center for Computational and Integrative Biology at Massachusetts General Hospital (Harvard Medical School). Lee Dolat is a PhD graduate student in the laboratory of Elias Spiliotis in the Department of Biology at Drexel University. Lee Dolat is interested in understanding how septins function in cell motility and actin organization.

Qicong Hu graduated from the University of Sciences and Technology of China with a BS in Biological Sciences and joined the Biological Sciences PhD program at Stanford University. In the laboratory of James Nelson, Qicong Hu worked together with Elias Spiliotis on forchlorfenuron, a septin-targeting compound, and investigated the role of septins in the biogenesis and membrane organization of the primary cilium. Since 2012, Qicong Hu works as a consultant at the Boston Consultant Group.

Elias Spiliotis received his Bachelor of Science in Biology from Boston College with the distinctions of summa cum laude and Scholar of the College. Following his PhD studies with Michael Edidin at the Johns Hopkins University, Elias Spiliotis received a post-doctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research and joined the lab of cell biologist James Nelson at Stanford University, where he began investigating the role of septins in microtubule-dependent functions. Currently, Elias Spiliotis is an Assistant Professor in the Department of Biology and the Director of the Drexel Biology Graduate Program and Cell Imaging Center.
Contributor Information
Lee Dolat, Department of Biology, Drexel University, 3245 Chestnut Street, Philadelphia, PA 19104, USA.
Qicong Hu, Department of Biology, Stanford University, Stanford, CA 94305, USA.
Elias T. Spiliotis, Email: ets33@drexel.edu, Department of Biology, Drexel University, 3245 Chestnut Street, Philadelphia, PA 19104, USA.
References
- Adam JC, Pringle JR, Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol. Biol. Cell. 2000;11:3123–3135. doi: 10.1091/mbc.11.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ageta-Ishihara N, Yamakado H, Morita T, Hattori S, Takao K, Miyakawa T, Takahashi R, Kinoshita M. Chronic overload of SEPT4, a parkin substrate that aggregates in Parkinson’s disease, causes behavioral alterations but not neurodegeneration in mice. Mol. Brain. 2013;6:35. doi: 10.1186/1756-6606-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja P, Perriard E, Trimble W, Perriard J-C, Ehler E. Probing the role of septins in cardiomyocytes. Exp. Cell Res. 2006;312:1598–1609. doi: 10.1016/j.yexcr.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Amir S, Wang R, Matzkin H, Simons JW, Mabjeesh NJ. MSF-A interacts with hypoxia-inducible factor-1α and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66:856–866. doi: 10.1158/0008-5472.CAN-05-2738. [DOI] [PubMed] [Google Scholar]
- Amir S, Wang R, Simons JW, Mabjeesh NJ. SEPT9_ v1 up-regulates hypoxia-inducible factor 1 by preventing its RACK1-mediated degradation. J. Biol. Chem. 2009;284:11142–11151. doi: 10.1074/jbc.M808348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SM, Ahn JS, Noh HS, Park J, Kang SS, Kim DR. Proteomic analysis in NSAIDs-treated primary cardiomyocytes. J. Proteomics. 2010;73:721–732. doi: 10.1016/j.jprot.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Bartsch I, Sandrock K, Lanza F, Nurden P, Hainmann I, Pavlova A, Greinacher A, Tacke U, Barth M, Busse A, et al. Deletion of human GP1BB and SEPT5 is associated with Bernard-Soulier syndrome, platelet secretion defect, polymicrogyria, and developmental delay. Thromb. Haemost. 2011;106:475–483. doi: 10.1160/TH11-05-0305. [DOI] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Beites CL, Peng XR, Trimble WS. Expression and analysis of properties of septin CDCrel-1 in exocytosis. Methods Enzymol. 2001;329:499–510. doi: 10.1016/s0076-6879(01)29111-3. [DOI] [PubMed] [Google Scholar]
- Beites C, Campbell KA, Trimble WS. The septin Sept5/CDCrel-1 competes with α-SNAP for binding to the SNARE complex. Biochem. J. 2005;385:347–353. doi: 10.1042/BJ20041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G, 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. USA. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Thai L, Garcia G, 3rd, Votin V, Grob P, Allyn T, Thorner J, Nogales E. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J. Mol. Biol. 2010;404:711–731. doi: 10.1016/j.jmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser S, Jersch K, Hainmann I, Wunderle D, Zgaga-Griesz A, Busse A, Zieger B. Human septin-septin interaction: CDCrel-1 partners with KIAA0202. FEBS Lett. 2002;519:169–172. doi: 10.1016/s0014-5793(02)02749-7. [DOI] [PubMed] [Google Scholar]
- Bongiovanni L, Pirozzi F, Guidi F, Orsini M, Chiurazzi P, Bassi PF, Racioppi M. Bradeion (SEPT4) as a urinary marker of transitional cell bladder cancer: a real-time polymerase chain reaction study of gene expression. J. Urol. 2012;187:2223–2227. doi: 10.1016/j.juro.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Bowen JR, Hwang D, Bai X, Roy D, Spiliotis ET. Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J. Cell Biol. 2011;194:187–197. doi: 10.1083/jcb.201102076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand F, Schumacher S, Kant S, Menon MB, Simon R, Turgeon B, Britsch S, Meloche S, Gaestel M, Kotlyarov A. The extracellular signal-regulated kinase 3 (mitogen-activated protein kinase 6 [MAPK6])-MAPK-activated protein kinase 5 signaling complex regulates septin function and dendrite morphology. Mol. Cell. Biol. 2012;32:2467–2478. doi: 10.1128/MCB.06633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser AM, Erne B, Werner HB, Nave KA, Schaeren-Wiemers N. The septin cytoskeleton in myelinating glia. Mol. Cell. Neurosci. 2009;40:156–166. doi: 10.1016/j.mcn.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Cao L, Ding X, Yu W, Yang X, Shen S, Yu L. Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Lett. 2007;581:5526–5532. doi: 10.1016/j.febslet.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Capurso G, Crnogorac-Jurcevic T, Milione M, Panzuto F, Campanini N, Dowen SE, Di Florio A, Sette C, Bordi C, Lemoine NR, et al. Peanut-like 1 (septin 5) gene expression in normal and neoplastic human endocrine pancreas. Neuroendocrinology. 2005;81:311–321. doi: 10.1159/000088449. [DOI] [PubMed] [Google Scholar]
- Carol G, Michael W, St. John T. Lymphocyte HEV adhesion variants differ in the expression of multiple gene sequences. Gene. 1990;95:279–284. doi: 10.1016/0378-1119(90)90372-x. [DOI] [PubMed] [Google Scholar]
- Casamayor A, Snyder M. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol. Cell. Biol. 2003;23:2762–2777. doi: 10.1128/MCB.23.8.2762-2777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F, Barral Y. Septins and the lateral compart-mentalization of eukaryotic membranes. Dev. Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Cerveira N, Bizarro S, Teixeira MR. MLL-SEPTIN gene fusions in hematological malignancies. Biol. Chem. 2011;392:713–724. doi: 10.1515/BC.2011.072. [DOI] [PubMed] [Google Scholar]
- Chacko A, McDade S, Chanduloy S, Church S, Kennedy R, Price J, Hall P, Russell SEH. Expression of the SEPT9_i4 isoform confers resistance to microtubule-interacting drugs. Cell Oncol. 2012;35:85–93. doi: 10.1007/s13402-011-0066-0. [DOI] [PubMed] [Google Scholar]
- Chance PF, Windebank AJ. Hereditary neuralgic amyotrophy. Curr. Opin. Neurol. 1996;9:343–347. doi: 10.1097/00019052-199610000-00005. [DOI] [PubMed] [Google Scholar]
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 2012;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Lee H, Dutta S, Song J, Walikonis R, Moon IS. Septin 6 regulates the cytoarchitecture of neurons through localization at dendritic branch points and bases of protrusions. Mol. Cells. 2011;32:89–98. doi: 10.1007/s10059-011-1048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P, Snyder H, Petrucelli L, Theisler C, Chong M, Zhang Y, Lim K, Chung KK, Kehoe K, D’Adamio L, et al. SEPT5_v2 is a parkin-binding protein. Brain Res. Mol. Brain Res. 2003;117:179–189. doi: 10.1016/s0169-328x(03)00318-8. [DOI] [PubMed] [Google Scholar]
- Connolly D, Abdesselam I, Verdier-Pinard P, Montagna C. Septin roles in tumorigenesis. Biol. Chem. 2011a;392:725–738. doi: 10.1515/BC.2011.073. [DOI] [PubMed] [Google Scholar]
- Connolly D, Yang Z, Castaldi M, Simmons N, Oktay MH, Coniglio S, Fazzari MJ, Verdier-Pinard P, Montagna C. Septin 9 isoform expression, localization and epigenetic changes during human and mouse breast cancer progression. Breast Cancer Res. 2011b;13:R76. doi: 10.1186/bcr2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA. 2011;108:19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RA, Hanrahan S, Totty N, Harnden P, Stanley AJ, Maher ER, Harris AL, Trimble WS, Selby PJ, Banks RE. Proteomic identification of a role for the von Hippel Lindau tumour suppressor in changes in the expression of mitochondrial proteins and septin 2 in renal cell carcinoma. Proteomics. 2006a;6:3880–3893. doi: 10.1002/pmic.200500811. [DOI] [PubMed] [Google Scholar]
- Craven RA, Stanley AJ, Hanrahan S, Dods J, Unwin R, Totty N, Harnden P, Eardley I, Selby PJ, Banks RE. Proteomic analysis of primary cell lines identifies protein changes present in renal cell carcinoma. Proteomics. 2006b;6:2853–2864. doi: 10.1002/pmic.200500549. [DOI] [PubMed] [Google Scholar]
- de Almeida Marques I, Valadares NF, Garcia W, Damalio JC, Macedo JN, de Araujo AP, Botello CA, Andreu JM, Garratt RC. Septin C-terminal domain interactions: implications for filament stability and assembly. Cell Biochem. Biophys. 2012;62:317–328. doi: 10.1007/s12013-011-9307-0. [DOI] [PubMed] [Google Scholar]
- Dekker C, Stirling PC, McCormack EA, Filmore H, Paul A, Brost RL, Costanzo M, Boone C, Leroux MR, Willison KR. The interaction network of the chaperonin CCT. EMBO J. 2008;27:1827–1839. doi: 10.1038/emboj.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMay BS, Bai X, Howard L, Occhipinti P, Meseroll RA, Spiliotis ET, Oldenbourg R, Gladfelter AS. Septin filaments exhibit a dynamic, paired organization that is conserved from yeast to mammals. J. Cell Biol. 2011;193:1065–1081. doi: 10.1083/jcb.201012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J, Kato K, Peng XR, Martinez C, Cattaneo M, Poujol C, Nurden P, Nurden A, Trimble WS, Ware J. A prototypic platelet septin and its participation in secretion. Proc. Natl. Acad. Sci. USA. 2002;99:3064–3069. doi: 10.1073/pnas.052715199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhasid R, Sahar D, Merling A, Zivony Y, Rotem A, Ben-Arush M, Izraeli S, Bercovich D, Larisch S. Mitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patients. Oncogene. 2004;23:5468–5475. doi: 10.1038/sj.onc.1207725. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N. Engl. J. Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Peifer M, Pringle JR. Localization and possible functions of Drosophila septins. Mol. Biol. Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger FP, Kopish KR, White JG. A role for septins in cellular and axonal migration in C. elegans. Dev. Biol. 2003;261:220–234. doi: 10.1016/s0012-1606(03)00296-3. [DOI] [PubMed] [Google Scholar]
- Founounou N, Loyer N, Le Borgne R. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev. Cell. 2013;24:242–255. doi: 10.1016/j.devcel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Frank JA. Claudins and alveolar epithelial barrier function in the lung. Ann. N.Y. Acad. Sci. 2012;1257:175–183. doi: 10.1111/j.1749-6632.2012.06533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, Brown S, Gorenc T, Rodriguez J, Fuchs E, Steller H. Sept4/ARTS regulates stem cell apoptosis and skin regeneration. Science. 2013;341:286–289. doi: 10.1126/science.1233029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchtbauer A, Lassen LB, Jensen AB, Howard J, Quiroga Ade S, Warming S, Sorensen AB, Pedersen FS, Fuchtbauer EM. Septin9 is involved in septin filament formation and cellular stability. Biol. Chem. 2011;392:769–777. doi: 10.1515/BC.2011.088. [DOI] [PubMed] [Google Scholar]
- Fujishima K, Kiyonari H, Kurisu J, Hirano T, Kengaku M. Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons. J. Neurochem. 2007;102:77–92. doi: 10.1111/j.1471-4159.2007.04478.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez M, Kissel H, Brown S, Gorenc T, Schile AJ, Rafii S, Larisch S, Steller H. Sept4/ARTS is required for stem cell apoptosis and tumor suppression. Genes Dev. 2010;24:2282–2293. doi: 10.1101/gad.1970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Z, Silio V, Marques M, Cortes I, Kumar A, Hernandez C, Checa AI, Serrano A, Carrera AC. A PI3K activity-independent function of p85 regulatory subunit in control of mammalian cytokinesis. EMBO J. 2006;25:4740–4751. doi: 10.1038/sj.emboj.7601324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: regulation of G proteins by dimerization. Nat. Rev. Mol. Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- Ghossoub R, Hu Q, Failler M, Rouyez M-C, Spitzbarth B, Mostowy S, Wolfrum U, Saunier S, Cossart P, James Nelson W, et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J. Cell Sci. 2013;126:2583–2594. doi: 10.1242/jcs.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden JK, Peck S, Chen YC, Krummel MF. The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J. Cell Biol. 2012;196:103–114. doi: 10.1083/jcb.201105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez ME, Makarova O, Peterson EA, Privette LM, Petty EM. Up-regulation of SEPT9_v1 stabilizes c-Jun-N-terminal kinase and contributes to its pro-proliferative activity in mammary epithelial cells. Cell Signal. 2009;21:477–487. doi: 10.1016/j.cellsig.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–1635. doi: 10.1038/sj.emboj.7600155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzmann R, Molnar B, Pilarsky C, Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F, Roblick UJ, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS ONE. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot C, Lecuit T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev. Cell. 2013;24:227–241. doi: 10.1016/j.devcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J. Pathol. 2005;206:269–278. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- Harper KM, Hiramoto T, Tanigaki K, Kang G, Suzuki G, Trimble W, Hiroi N. Alterations of social interaction through genetic and environmental manipulation of the 22q11.2 gene Sept5 in the mouse brain. Hum. Mol. Genet. 2012;21:3489–3499. doi: 10.1093/hmg/dds180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rodriguez Y, Momany M. Posttranslational modifications and assembly of septin heteropolymers and higher-order structures. Curr. Opin. Microbiol. 2012;15:660–668. doi: 10.1016/j.mib.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J. Cell Biol. 1989;108:111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr. Biol. 2012;22:1109–1115. doi: 10.1016/j.cub.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YW, Yan M, Collins RF, Diciccio JE, Grinstein S, Trimble WS. Mammalian septins are required for phagosome formation. Mol. Biol. Cell. 2008;19:1717–1726. doi: 10.1091/mbc.E07-07-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts RP, Svitin A, Stinnett MW, Renfrow MB, Chesnokov I. Drosophila Orc6 facilitates GTPase activity and filament formation of the septin complex. Mol. Biol. Cell. 2009;20:270–281. doi: 10.1091/mbc.E08-07-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat. Rev. Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- Ihara M, Tomimoto H, Kitayama H, Morioka Y, Akiguchi I, Shibasaki H, Noda M, Kinoshita M. Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmic inclusions found in Parkinson’s disease and other synucleinopathies. J. Biol. Chem. 2003;278:24095–24102. doi: 10.1074/jbc.M301352200. [DOI] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ihara M, Yamasaki N, Hagiwara A, Tanigaki A, Kitano A, Hikawa R, Tomimoto H, Noda M, Takanashi M, Mori H, et al. Sept4, a component of presynaptic scaffold and Lewy bodies, is required for the suppression of α-synuclein neurotoxicity. Neuron. 2007;53:519–533. doi: 10.1016/j.neuron.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Ito H, Iwamoto I, Morishita R, Nozawa Y, Narumiya S, Asano T, Nagata K. Possible role of Rho/Rhotekin signaling in mammalian septin organization. Oncogene. 2005;24:7064–7072. doi: 10.1038/sj.onc.1208862. [DOI] [PubMed] [Google Scholar]
- Ito H, Atsuzawa K, Morishita R, Usuda N, Sudo K, Iwamoto I, Mizutani K, Katoh-Semba R, Nozawa Y, Asano T, et al. Sept8 controls the binding of vesicle-associated membrane protein 2 to synaptophysin. J. Neurochem. 2009;108:867–880. doi: 10.1111/j.1471-4159.2008.05849.x. [DOI] [PubMed] [Google Scholar]
- Iwaisako K, Hatano E, Taura K, Nakajima A, Tada M, Seo S, Tamaki N, Sato F, Ikai I, Uemoto S, et al. Loss of Sept4 exacerbates liver fibrosis through the dysregulation of hepatic stellate cells. J. Hepatol. 2008;49:768–778. doi: 10.1016/j.jhep.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Joberty G, Perlungher RR, Sheffield PJ, Kinoshita M, Noda M, Haystead T, Macara IG. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev. Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Kalikin LM, Sims HL, Petty EM. Genomic and expression analyses of alternatively spliced transcripts of the MLL septin-like fusion gene (MSF) that map to a 17q25 region of loss in breast and ovarian tumors. Genomics. 2000;63:165–172. doi: 10.1006/geno.1999.6077. [DOI] [PubMed] [Google Scholar]
- Kato K, Martinez C, Russell S, Nurden P, Nurden A, Fiering S, Ware J. Genetic deletion of mouse platelet glycoprotein Ibβ produces a Bernard-Soulier phenotype with increased α-granule size. Blood. 2004;104:2339–2344. doi: 10.1182/blood-2004-03-1127. [DOI] [PubMed] [Google Scholar]
- Kim CS, Seol SK, Song OK, Park JH, Jang SK. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J. Virol. 2007;81:3852–3865. doi: 10.1128/JVI.01311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M. Assembly of mammalian septins. J. Biochem. 2003;134:491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Diversity of septin scaffolds. Curr. Opin. Cell Biol. 2006;18:54–60. doi: 10.1016/j.ceb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Insight into septin functions from mouse models. In: Hall PA, Russell SEH, Pringle JR, editors. The Septins. West Sussex, UK: Wiley-Blackwell; 2008. [Google Scholar]
- Kinoshita A, Kinoshita M, Akiyama H, Tomimoto H, Akiguchi I, Kumar S, Noda M, Kimura J. Identification of septins in neurofibrillary tangles in Alzheimer’s disease. Am. J. Pathol. 1998;153:1551–1560. doi: 10.1016/S0002-9440(10)65743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Noda M, Kinoshita M. Differential localization of septins in the mouse brain. J. Comp. Neurol. 2000;428:223–239. doi: 10.1002/1096-9861(20001211)428:2<223::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of mammalian septins. Dev. Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Kimura K, Matsumoto N, Watanabe M, Fukaya M, Ide C. Mammalian septin Sept2 modulates the activity of GLAST, a glutamate transporter in astrocytes. Genes Cells. 2004;9:1–14. doi: 10.1111/j.1356-9597.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell. 2005;8:353–364. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol. Biol. Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlenbaumer G, Hannibal MC, Nelis E, Schirmacher A, Verpoorten N, Meuleman J, Watts GD, De Vriendt E, Young P, Stogbauer F, et al. Mutations in SEPT9 cause hereditary neuralgic amyotrophy. Nat. Genet. 2005;37:1044–1046. doi: 10.1038/ng1649. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin YH, Chen HI, Wang YY, Chiou YW, Lin HH, Pan HA, Wu CM, Su SM, Hsu CC, et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012;33:710–719. doi: 10.1002/humu.22028. [DOI] [PubMed] [Google Scholar]
- Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol. Reprod. 2010;82:669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D, et al. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat. Cell Biol. 2000;2:915–921. doi: 10.1038/35046566. [DOI] [PubMed] [Google Scholar]
- Lassen LB, Fuchtbauer A, Schmitz A, Sorensen AB, Pedersen FS, Fuchtbauer EM. Septin9 is involved in T-cell development and CD8(+) T-cell homeostasis. Cell Tissue Res. 2013;352:695–705. doi: 10.1007/s00441-013-1618-6. [DOI] [PubMed] [Google Scholar]
- Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- Lin C-W, Tu P-F, Hsiao N-W, Chang C-Y, Wan L, Lin Y-T, Chang H-W. Identification of a novel septin 4 protein binding to human herpesvirus 8 kaposin A protein using a phage display cDNA library. J. Virol. Methods. 2007;143:65–72. doi: 10.1016/j.jviromet.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Lin YH, Lin YM, Wang YY, Yu IS, Lin YW, Wang YH, Wu CM, Pan HA, Chao SC, Yen PH, et al. The expression level of septin12 is critical for spermiogenesis. Am. J. Pathol. 2009;174:1857–1868. doi: 10.2353/ajpath.2009.080955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Chou CK, Hung YC, Yu IS, Pan HA, Lin SW, Kuo PL. SEPT12 deficiency causes sperm nucleus damage and developmental arrest of preimplantation embryos. Fertil. Steril. 2011;95:363–365. doi: 10.1016/j.fertnstert.2010.07.1064. [DOI] [PubMed] [Google Scholar]
- Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell. 2007;12:827–835. doi: 10.1016/j.devcel.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Maimaitiyiming M, Kobayashi Y, Kumanogoh H, Nakamura S, Morita M, Maekawa S. Identification of dynamin as a septin-binding protein. Neurosci. Lett. 2013;534:322–326. doi: 10.1016/j.neulet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Martinez C, Sanjuan MA, Dent JA, Karlsson L, Ware J. Human septin-septin interactions as a prerequisite for targeting septin complexes in the cytosol. Biochem. J. 2004;382:783–791. doi: 10.1042/BJ20040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade SS, Hall PA, Russell SE. Translational control of SEPT9 isoforms is perturbed in disease. Hum. Mol. Genet. 2007;16:742–752. doi: 10.1093/hmg/ddm003. [DOI] [PubMed] [Google Scholar]
- McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Johnston PG, Russell SE. Genomic organization, complex splicing pattern and expression of a human septin gene on chromosome 17q25.3. Oncogene. 2001;20:5930–5939. doi: 10.1038/sj.onc.1204752. [DOI] [PubMed] [Google Scholar]
- McKie JM, Sutherland HF, Harvey E, Kim U-J, Scambler PJ. A human gene similar to Drosophila melanogaster peanut maps to the DiGeorge syndrome region of 22q11. Hum. Genet. 1997;101:6–12. doi: 10.1007/s004390050576. [DOI] [PubMed] [Google Scholar]
- McNiven MA. Dynamin: a molecular motor with pinchase action. Cell. 1998;94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Ito H, Iwamoto I, Morishita R, Kanoh H, Seishima M, Nagata K. Possible role of a septin, SEPT1, in spreading in squamous cell carcinoma DJM-1 cells. Biol. Chem. 2013;394:281–290. doi: 10.1515/hsz-2012-0258. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Danckaert A, Tham TN, Machu C, Guadagnini S, Pizarro-Cerda J, Cossart P. Septin 11 restricts InlB-mediated invasion by Listeria. J. Biol. Chem. 2009;284:11613–11621. doi: 10.1074/jbc.M900231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 2010;8:433–444. doi: 10.1016/j.chom.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Ann. Rev. Pathol. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Inagaki M. Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene. 2005;24:65–76. doi: 10.1038/sj.onc.1208101. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J. Biol. Chem. 2003;278:18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- Nagata K, Asano T, Nozawa Y, Inagaki M. Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J. Biol. Chem. 2004;279:55895–55904. doi: 10.1074/jbc.M406153200. [DOI] [PubMed] [Google Scholar]
- Nakahira M, Macedo JN, Seraphim TV, Cavalcante N, Souza TA, Damalio JC, Reyes LF, Assmann EM, Alborghetti MR, Garratt RC, et al. A draft of the human septin interactome. PLoS ONE. 2010;5:e13799. doi: 10.1371/journal.pone.0013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell Sci. 2000;113:3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Onishi M, Pringle JR. New insights into the phylogenetic distribution and evolutionary origins of the septins. Biol. Chem. 2011;392:681–687. doi: 10.1515/BC.2011.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono R, Ihara M, Nakajima H, Ozaki K, Kataoka-Fujiwara Y, Taki T, Nagata K, Inagaki M, Yoshida N, Kitamura T, et al. Disruption of Sept6, a fusion partner gene of MLL, does not affect ontogeny, leukemogenesis induced by MLL-SEPT6, or phenotype induced by the loss of Sept4. Mol. Cell. Biol. 2005a;25:10965–10978. doi: 10.1128/MCB.25.24.10965-10978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono R, Nakajima H, Ozaki K, Kumagai H, Kawashima T, Taki T, Kitamura T, Hayashi Y, Nosaka T. Dimerization of MLL fusion proteins and FLT3 activation synergize to induce multiple-lineage leukemogenesis. J. Clin. Invest. 2005b;115:919–929. doi: 10.1172/JCI22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J. Clin. Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. Evol. Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzig J, Jahn O, Tenzer S, Wichert SP, de Monasterio-Schrader P, Rosfa S, Kuharev J, Yan K, Bormuth I, Bremer J, et al. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J. Neurosci. 2011;31:16369–16386. doi: 10.1523/JNEUROSCI.4016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, Wait R, Dunn MJ, Cotter DR. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol. Psychiatr. 2008;13:1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- Peterson EA, Kalikin LM, Steels JD, Estey MP, Trimble WS, Petty EM. Characterization of a SEPT9 interacting protein, SEPT14, a novel testis-specific septin. Mamm. Genome. 2007;18:796–807. doi: 10.1007/s00335-007-9065-x. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Jonquieres R, Gouin E, Vandekerckhove J, Garin J, Cossart P. Distinct protein patterns associated with Listeria monocytogenes InlA- or InlB-phagosomes. Cell. Microbiol. 2002;4:101–115. doi: 10.1046/j.1462-5822.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- Qi M, Yu W, Liu S, Jia H, Tang L, Shen M, Yan X, Saiyin H, Lang Q, Wan B, et al. Septin1, a new interaction partner for human serine/threonine kinase aurora-B. Biochem. Biophys. Res. Commun. 2005;336:994–1000. doi: 10.1016/j.bbrc.2005.06.212. [DOI] [PubMed] [Google Scholar]
- Rittmeyer EN, Daniel S, Hsu S-C, Osman MA. A dual role for IQGAP1 in regulating exocytosis. J. Cell Sci. 2008;121:391–403. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- Roseler S, Sandrock K, Bartsch I, Busse A, Omran H, Loges NT, Zieger B. Lethal phenotype of mice carrying a Sept11 null mutation. Biol. Chem. 2011;392:779–781. doi: 10.1515/BC.2011.093. [DOI] [PubMed] [Google Scholar]
- Russell SE, McIlhatton MA, Burrows JF, Donaghy PG, Chanduloy S, Petty EM, Kalikin LM, Church SW, McIlroy S, Harkin DP, et al. Isolation and mapping of a human septin gene to a region on chromosome 17q, commonly deleted in sporadic epithelial ovarian tumors. Cancer Res. 2000;60:4729–4734. [PubMed] [Google Scholar]
- Sandrock K, Bartsch I, Blaser S, Busse A, Busse E, Zieger B. Characterization of human septin interactions. Biol. Chem. 2011;392:751–761. doi: 10.1515/BC.2011.081. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum. Mol. Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Scott M, McCluggage WG, Hillan KJ, Hall PA, Russell SEH. Altered patterns of transcription of the septin gene, SEPT9, in ovarian tumorigenesis. Int. J. Cancer. 2006;118:1325–1329. doi: 10.1002/ijc.21486. [DOI] [PubMed] [Google Scholar]
- Sellin ME, Holmfeldt P, Stenmark S, Gullberg M. Microtubules support a disk-like septin arrangement at the plasma membrane of mammalian cells. Mol. Biol. Cell. 2011a;22:4588–4601. doi: 10.1091/mbc.E11-09-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin ME, Sandblad L, Stenmark S, Gullberg M. Deciphering the rules governing assembly order of mammalian septin complexes. Mol. Biol. Cell. 2011b;22:3152–3164. doi: 10.1091/mbc.E11-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin ME, Stenmark S, Gullberg M. Mammalian SEPT9 isoforms direct microtubule-dependent arrangements of septin core heteromers. Mol. Biol. Cell. 2012;23:4242–4255. doi: 10.1091/mbc.E12-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda T, Ito H, Sudo K, Iwamoto I, Morishita R, Nagata K. Septin 14 is involved in cortical neuronal migration via interaction with Septin 4. Mol. Biol. Cell. 2010;21:1324–1334. doi: 10.1091/mbc.E09-10-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev A, Kostenko S, Dumitriu G, Moens U. Septin 8 is an interaction partner and in vitro substrate of MK5. World J. Biol. Chem. 2012;3:98–109. doi: 10.4331/wjbc.v3.i5.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhaye VK, Chau E, Breysse PN, King LS. Septin-2 mediates airway epithelial barrier function in physiologic and pathologic conditions. Am. J. Respir. Cell Mol. Biol. 2011;45:120–126. doi: 10.1165/rcmb.2010-0235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Zent E, Wittinghofer A. GTP-induced conformational changes in septins and implications for function. Proc. Natl. Acad. Sci. USA. 2009;106:16592–16597. doi: 10.1073/pnas.0902858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitz JH, Baumgartel K, Hammerle B, Papadopoulos C, Hekerman P, Tejedor FJ, Becker W, Lutz B. The Down syndrome candidate dual-specificity tyrosine phosphorylation-regulated kinase 1A phosphorylates the neurodegeneration-related septin 4. Neuroscience. 2008;157:596–605. doi: 10.1016/j.neuroscience.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Spiliotis ET, Gladfelter AS. Spatial guidance of cell asymmetry: septin GTPases show the way. Traffic. 2012;13:195–203. doi: 10.1111/j.1600-0854.2011.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J. Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino Y, Ichioka K, Soda T, Ihara M, Kinoshita M, Ogawa O, Nishiyama H. Septins as diagnostic markers for a subset of human asthenozoospermia. J. Urol. 2008;180:2706–2709. doi: 10.1016/j.juro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sui L, Zhang W, Liu Q, Chen T, Li N, Wan T, Yu M, Cao X. Cloning and functional characterization of human septin 10, a novel member of septin family cloned from dendritic cells. Biochem. Biophys. Res. Commun. 2003;304:393–398. doi: 10.1016/s0006-291x(03)00601-6. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Harper KM, Hiramoto T, Sawamura T, Lee M, Kang G, Tanigaki K, Buell M, Geyer MA, Trimble WS, et al. Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum. Mol. Genet. 2009;18:1652–1660. doi: 10.1093/hmg/ddp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehashi M, Alioto T, Stedeford T, Persad AS, Banasik M, Masliah E, Tanaka S, Ueda K. Septin 3 gene polymorphism in Alzheimer’s disease. Gene Expr. 2004;11:263–270. doi: 10.3727/000000003783992243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Takiguchi Y, Kinoshita M, Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Curr. Biol. 2009;19:140–145. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tanaka T, Kijima H, Itoh J, Matsuda T, Hori S, Yamamoto M. Characterization of tissue- and cell-type-specific expression of a novel human septin family gene, bradeion. Biochem. Biophys. Res. Commun. 2001;286:547–553. doi: 10.1006/bbrc.2001.5413. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kijima H, Itoh J, Matsuda T, Tanaka T. Impaired expression of a human septin family gene Bradeion inhibits the growth and tumorigenesis of colorectal cancer in vitro and in vivo. Cancer Gene Ther. 2002;9:483–488. doi: 10.1038/sj.cgt.7700460. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tanaka T, Matsuzaki S, Seto Y, Matsuda T, Komori K, Itoh J, Kijima H, Tamai K, Shibayama M, et al. Rapid and quantitative detection of human septin family Bradeion as a practical diagnostic method of colorectal and urologic cancers. Med. Sci. Monit. 2003;9:MT61–MT68. [PubMed] [Google Scholar]
- Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, Greaves LC, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley AJ, Gilden J, Jacobelli J, Beemiller P, Trimble WS, Kinoshita M, Krummel MF. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat. Cell Biol. 2009;11:17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Galamb O, Spisak S, Wichmann B, Sipos F, Valcz G, Leiszter K, Molnar B, Tulassay Z. The influence of methylated septin 9 gene on RNA and protein level in colorectal cancer. Pathol. Oncol. Res. 2011;17:503–509. doi: 10.1007/s12253-010-9338-7. [DOI] [PubMed] [Google Scholar]
- Toure A, Rode B, Hunnicutt GR, Escalier D, Gacon G. Septins at the annulus of mammalian sperm. Biol. Chem. 2011;392:799–803. doi: 10.1515/BC.2011.074. [DOI] [PubMed] [Google Scholar]
- Tsang CW, Fedchyshyn M, Harrison J, Xie H, Xue J, Robinson PJ, Wang LY, Trimble WS. Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission. Mol. Cell. Biol. 2008;28:7012–7029. doi: 10.1128/MCB.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CW, Estey MP, DiCiccio JE, Xie H, Patterson D, Trimble WS. Characterization of presynaptic septin complexes in mammalian hippocampal neurons. Biol. Chem. 2011;392:739–749. doi: 10.1515/BC.2011.077. [DOI] [PubMed] [Google Scholar]
- Ueda M, Kawamura N, Tateishi T, Sakae N, Motomura K, Ohyagi Y, Kira JI. Phenotypic spectrum of hereditary neuralgic amyotrophy caused by the SEPT9 R88W mutation. J. Neurol. Neurosurg. Psychiatr. 2010;81:94–96. doi: 10.1136/jnnp.2008.168260. [DOI] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. Neuroreport. 2003;14:31–37. doi: 10.1097/00001756-200301200-00006. [DOI] [PubMed] [Google Scholar]
- Versele M, Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. doi: 10.1186/1741-7015-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik AA, Polianskyte-Prause Z, Dong M-Q, Shaw AS, Yates JR, Farquhar MG, Lehtonen S. Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol. Biol. Cell. 2012;23:3370–3379. doi: 10.1091/mbc.E11-12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr. Biol. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Xue J, Tsang CW, Gai WP, Malladi CS, Trimble WS, Rostas JA, Robinson PJ. Septin 3 (G-septin) is a developmentally regulated phosphoprotein enriched in presynaptic nerve terminals. J. Neurochem. 2004;91:579–590. doi: 10.1111/j.1471-4159.2004.02755.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Gao YK, Chen JY. KIAA0202, a human septin family member, interacting with hPFTAIRE1. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao Acta Biochim. Biophys. Sin. 2002;34:520–525. [PubMed] [Google Scholar]
- Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS, Wang LY. Septins regulate developmental switching from microdomain to nanodomain coupling of Ca2+ influx to neurotransmitter release at a central synapse. Neuron. 2010;67:100–115. doi: 10.1016/j.neuron.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youakim A, Ahdieh M. Interferon-γ decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 1999;9:1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- Zhu M, Wang F, Yan F, Yao PY, Du J, Gao X, Wang X, Wu Q, Ward T, Li J, et al. Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization. J. Biol. Chem. 2008;283:18916–18925. doi: 10.1074/jbc.M710591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieger B, Hashimoto Y, Ware J. Alternative expression of platelet glycoprotein Ib(β) mRNA from an adjacent 5′ gene with an imperfect polyadenylation signal sequence. J. Clin. Invest. 1997;99:520–525. doi: 10.1172/JCI119188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieger B, Tran H, Hainmann I, Wunderle D, Zgaga-Griesz A, Blaser S, Ware J. Characterization and expression analysis of two human septin genes, PNUTL1 and PNUTL2. Gene. 2000;261:197–203. doi: 10.1016/s0378-1119(00)00527-8. [DOI] [PubMed] [Google Scholar]