Abstract
It has been postulated that pubertal hormones may drive some neuroanatomical changes during adolescence, and may do so differently in girls and boys. Here, we use growth curve modeling to directly assess how sex hormones [testosterone (T) and estradiol (E2)] relate to changes in subcortical brain volumes utilizing a longitudinal design. 126 adolescents (63 girls), ages 10 to 14, were imaged and restudied ∼2 years later. We show, for the first time, that best‐fit growth models are distinctly different when using hormones as compared to a physical proxy of pubertal maturation (Tanner Stage) or age, to predict brain development. Like Tanner Stage, T and E2 predicted white matter and right amygdala growth across adolescence in both sexes, independent of age. Tanner Stage also explained decreases in both gray matter and caudate volumes, whereas E2 explained only gray matter decreases and T explained only caudate volume decreases. No pubertal measures were related to hippocampus development. Although specificity was seen, sex hormones had strikingly similar relationships with white matter, gray matter, right amygdala, and bilateral caudate volumes, with larger changes in brain volume seen at early pubertal maturation (as indexed by lower hormone levels), followed by less robust, or even reversals in growth, by late puberty. These novel longitudinal findings on the relationship between hormones and brain volume change represent crucial first steps toward understanding which aspects of puberty influence neurodevelopment. Hum Brain Mapp 35:5633–5645, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: development, puberty, magnetic resonance imaging, hormones, longitudinal studies
INTRODUCTION
Neuroimaging studies have shown that developmental trajectories across adolescence vary greatly by brain region, age, and sex [Dennison et al., 2013; Giedd et al., 1999; Gogtay et al., 2006; Koolschijn and Crone, 2013; Lenroot et al., 2007; Sowell et al., 1999; Sowell et al., 2002; Tamnes et al., 2010; Toga et al., 2006]. Pubertal maturation marks the onset of adolescence, with physical changes starting earlier on average in girls (∼age 10) compared to boys (age ∼11.5)[Herman‐Giddens et al., 2012; Marshall and Tanner, 1969; Marshall and Tanner, 1970; Sun et al., 2002]. In addition, dramatic increases are seen in the primary acting sex hormones: estradiol (E2) in girls and testosterone (T) in boys [McAnarney et al., 1992; Nottelmann et al., 1987]. Adolescence also marks the peak age of onset for many types of psychopathology, and does so in a sex‐specific fashion, with disproportionate increases in the prevalence of anxiety and depression in girls [Angold et al., 1998; Angold et al., 1999] and substance abuse and externalizing disorders in boys [American's Children: Key National Indicators of Well‐Being, 2009]. Because adolescence begins with puberty, it has been postulated that pubertal development may contribute to sexual dimorphisms in neurodevelopmental trajectories and vulnerabilities to pathological outcomes during adolescence [Blakemore et al., 2010; Giedd et al., 2006; Peper et al., 2011]. To date, support for this hypothesis largely comes from cross‐sectional analyses in humans [Angold et al., 1998; Angold et al., 1999; Bramen et al., 2011; Bramen et al., 2012; Graber et al., 1997; Paus et al., 2010; Peper et al., 2008; Peper et al., 2009; Peper et al., 2011] and castration paradigms in animals [Juraska and Markham, 2004; Sisk and Zehr, 2005], while other animal research suggest that effects may be region specific or that the two processes may occur in parallel [Anderson et al., 1995; Andersen et al., 2002b]. However, because these studies tend to be cross‐sectional, dichotomize groups into pubertal stages, or implement castration at a set point in the experimental design, they have failed to capture individual differences in pubertal and brain maturation. That is, key aspects of pubertal maturation are markedly different not only between males and females, but also between individuals of the same sex. In humans, these different pubertal trajectories include sex differences in age of onset, specific patterns and levels of hormone increases, and the rate of maturational changes leading to reproductive maturity [Herman‐Giddens et al., 2012; Marshall and Tanner, 1969; Marshall and Tanner, 1970; Sun et al., 2002; van den Berg et al., 2006].
Longitudinal designs and multiple measures of pubertal maturation are required to capture and accurately characterize how individual differences in pubertal onset and progression relate to subcortical and brain volume maturation. Yet, to date, only four longitudinal studies have included pubertal markers or sex hormones in examining brain development, and only one has focused on subcortical brain volumes [Goddings et al., 2013; Nguyen et al., 2013a, 2013b; Raznahan et al., 2010]. Raznahan et al. [2010] examined the degree to which brain maturation between the ages of 9 and 22 was affected by variation in signaling efficacy of the androgen receptor (AR), as determined by AR‐CAG length genotyping. Results showed that greater AR signaling attenuated age‐related decreases in superior parietal and portions of temporal lobe in boys, and accelerated age‐related decreases in the left inferior frontal gyrus in girls. Similarly, Nguyen et al. [2013a, 2013b] recently examined sex differences in the association between androgen levels and cortical thickness in prepubertal and postpubertal boys and girls, ages 4 to 22 years. Using T and dehydroepiandrosterone (DHEA), both age and sex‐specific relationships were seen. Higher DHEA in both sexes and T in girls predicted increases in cortical thickness at younger, prepubertal ages, whereas higher testosterone predicted decreases in cortical thickness in postpubertal males and females. However, by splitting the groups into pre and postpubertal, they did not ascertain the trajectory of pubertal change in relationship to neurodevelopment between boys and girls. In this regard, pubertal development, as assessed by self‐reported Tanner Stage, was recently shown to predict changes in subcortical volume, including the hippocampus, amygdala, and caudate, between the ages of 7 and 22 years [Goddings et al., 2013]. While providing great initial insight into pubertal changes in subcortical developmental trajectories in boys and girls, Tanner Staging by itself does not allow us to make inferences as to which aspect of puberty (hormones, etc.) contribute to these findings. In addition, self‐report Tanner Staging is not as valid as practitioner ratings [Coleman and Coleman, 2002], and, although hormones advance primary and secondary sexual characteristics, physical measurements are not a perfect match, but rather only a proxy for hormonal levels [Shirtcliff et al., 2009]. Furthermore, although Goddings et al. [2013] included both boys and girls, differences in subcortical developmental trajectories between the sexes and their possible interactions with age and puberty were not directly examined. That is, direct statistical comparisons, rather than qualitative description of differences, are necessary to determine where the sexes significantly differ from one another to confirm true sexually dimorphic patterns of pubertal‐specific neuromaturation. Despite recent advances in understanding the role of puberty in brain development, the associations between pubertal hormones and subcortical growth trajectories remain to be elucidated, and to our knowledge, no previous study has examined the relationships between within‐subject changes in E2 and subcortical development in girls.
In this study, we used growth curve modeling and utilized both physical exam (practitioner ratings of Tanner Stage) and hormonal markers (T and E2) of puberty, to more fully elucidate the relationships between age, sex, and puberty on cortical and subcortical brain volume development in a longitudinal sample of adolescents. The samples were recruited to be within a relatively narrow age range near the onset of puberty at time one assessment: 10–12 years in girls (mean = 11.83 ± 0.73) and 12–14 years in boys (mean = 12.89 ± 0.66). The asymmetry in ages was chosen because girls typically begin displaying physical pubertal characteristics earlier than boys [Herman‐Giddens et al., 2012; Marshall and Tanner, 1969; Marshall and Tanner, 1970; Sun et al., 2002]. Neuroimaging data and pubertal maturation indices were collected again approximately 2 years later. This longitudinal study design, with a relatively restricted age range, allows for sexes to be matched on pubertal status at the first time point. This provides the ability to statistically disentangle how age and puberty relate to neurodevelopment over time, because there is between‐subject variance in the amount of pubertal maturation occurring, but no between‐subject variance in change in age (all participants were sampled 2 years apart).
In preliminary cross‐sectional analyses using the first time point of this dataset, sex‐by‐puberty interactions were found for hippocampus and amygdala volumes when comparing late pubertal boys and girls (Tanner Stages 3–5) with their early pubertal peers (Tanner Stages 1–2) [Bramen et al., 2011]. Sex‐by‐puberty interactions were not seen for the caudate or thalamus. These findings led us to hypothesize that puberty‐driven brain maturation may contribute to region specific sex differences in trajectories of volume change only in the amygdala and hippocampus. Specifically, we expand on these findings by examining both between‐ and within‐subject change over time in pubertal markers and subcortical brain volumes. By utilizing the full longitudinal dataset, we present for the first time an assessment of how individual variability in puberty (Tanner Staging and T in both sexes, E2 in girls) relates to subcortical developmental trajectories across adolescence.
We further hypothesized that pubertal measures would predict the trajectory of brain volume change over time. Specifically, we predicted that greater pubertal maturation would be linked to a more developed brain phenotype, as indexed by lower gray matter and higher white matter volumes. Moreover, we hypothesized that greater changes in pubertal maturation would be associated with the slope of both cortical and subcortical brain volumes, above and beyond age. Based on our previous cross‐sectional findings [Bramen et al., 2011], we also hypothesized that boys and girls would have similar volumes in the amygdala and hippocampus at the beginning of adolescence, but sex differences would emerge as a function of pubertal development. We expected these associations to be localized, with subcortical regions dense in hormone receptors (amygdala and hippocampus) [Giedd et al., 1996; Sisk and Zehr, 2005] showing larger relationships than other subcortical regions (thalamus). Lastly, we predicted that the hormone levels (T and E2) would be better predictors of these hypothesized changes than outward physical change (Tanner Staging).
MATERIAL AND METHODS
Subjects
One hundred and twenty six (63 girls) typically developing adolescents, ages 10 to 14, were recruited through advertisements, flyers, and demographically targeted phone lists. Exclusionary criteria for all participants included a lifetime diagnosis of psychiatric disorders, braces, history of head injury, serious medical illness, or psychotropic medication. All participants and their legal guardians provided informed assent/consent according to the guidelines of the University of Pittsburgh Institutional Review Board, and research was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Portions of this longitudinal dataset, including fMRI data, have been previously reported [Spielberg et al., 2014a; Spielberg et al., 2014b].
For this study, a breakdown of the data for each time point by sex can be found in Table 1. For boys at Time 1, three participants were excluded due to unusable magnetic resonance imaging (MRI) data, eight were missing Tanner data, and three T values were not obtained. For boys at Time 2, 49 returned for follow‐up, but seven were excluded due to unusable MRI data, one was missing Tanner data, and nine T values were not obtained. For girls at Time 1, three were excluded due to unusable MRI data, 14 were missing Tanner data, two T values were not obtained, and four E2 values were excluded (two missing, two invalid). For girls at Time 2, 57 returned for follow‐up, but eight were excluded due to unusable MRI data, 10 were missing Tanner data, eight T values were not obtained, and four E2 values were not obtained.
Table 1.
Longitudinal study sample demographics
| Boys | Girls | |||
|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | |
| Age | 12.89 ±0.66 (n = 60) | 14.91 ± 0.69 (n = 42) | 11.83 ± 0.73 (n = 60) | 14.04 ± 0.85 (n = 38) |
| Tanner Stage | 2.90 ± 0.96 (n = 52) | 4.36 ± 0.93 (n = 40) | 2.65 ± 0.87 (n = 47) | 4.63 ± 0.68 (n = 40) |
| Testosterone (T) (ng/mL) | 199.81 ± 151.32 (n = 57) | 390.14 ± 152.56 (n = 33) | 45.89 ± 11.89 (n = 59) | 59.14 ± 15.00 (n = 40) |
| Estradiol (E2) (pg/mL) | — | — | 35.29 ± 17.31 (n = 57) | 87.46 ± 59.26 (n = 44) |
Number of subjects with usable imaging data are reported for each time point for each sex. Average and standard deviation are reported for age, as well as pubertal maturation indices (tanner stage, testosterone, and estradiol).
Pubertal Maturation
Stage of physical maturation on a scale of 1 to 5 was determined by a trained research nurse practitioner using Tanner Staging criterion [Marshall and Tanner, 1968]. A composite Tanner Stage score was calculated as the mean of individual scores for pubic hair and breast development in girls and pubic hair and penile/testicular development in boys. At Time 1, a large portion of both girls and boys had Tanner Stage values between 1 and 4, whereas at Time 2 the majority of each sex was at mid‐to‐late puberty (Tanner Stages 3–5) as by study design (Supporting Information Fig. 1). Morning blood samples were collected between 8:20 and 8:35 AM using a finger‐stick procedure developed by Worthman and Stallings [1997]. This technique allows for free index values of sex‐steroids (T in boys; T and E2 in girls) to be assessed via modification of commercially available serum/plasma radioimmunoassay kits (T: DSL/Beckman Coulter; E2: Siemens, Los Angeles, CA) [For full details see Worthman and Stallings, 1997]. The minimum sensitivity of T was 14.2 ng/dL for males and 14.0 ng/dL for females and interassay coefficients of variation (CV) for low, medium, and high BioRad external controls for T were 7.2% (low), 11.4% (medium), and 4.3% (high). None of the subjects were below the minimum sensitivity thresholds, indicating the assays were sensitive to T in prepubertal females and males. E2 sensitivity and interassay CV were also acceptable, with the Siemens BS‐serum regression curve of y = 2.7056 (pg/mL). As expected, values increased between Time 1 and Time 2 for Tanner Staging (Boys: β = 1.6, SE = 0.12, P < 0.0001; Girls: β = 1.9, SE = 0.14, P < 0.0001), T (Boys: β = 212.9, SE = 16.2, P < 0.0001; Girls: β = 13.4, SE = 1.8, P < 0.0001, and E2 (Girls: β = 51.2, SE = 8.6, P < 0.0001) (see Table 1 and Supporting Information Fig. 1).
Figure 1.
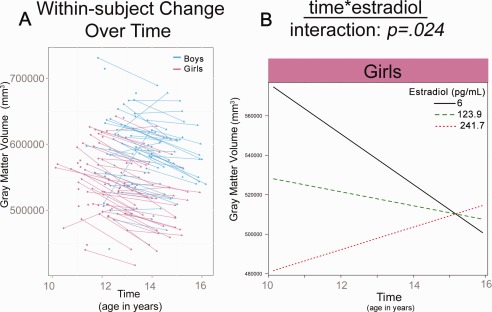
Significant interactions in total gray matter volume development (A) Plot shows raw total gray matter volumes for girls and boys. (B) Plot shows linear multilevel mixed effect fit lines for the effects of time *E2 on gray matter volume.
Structural Imaging Acquisition
Whole‐brain T1‐weighted magnetization‐prepared rapid gradient‐echo (MPRAGE) images were acquired for each participant at Time 1 and Time 2 using a 3‐Tesla Siemens Allegra scanner. Scan parameters for the MPRAGE were as follows: repetition time (TR) = 1,540 ms; echo time (TE) = 3.04 ms; flip angle = 8; field of view = 256 × 256; voxel size = 1 mm3.
Image Processing
Preprocessing and automatic segmentation of cortical and subcortical volumes were conducted using FreeSurfer's (v 5.1) longitudinal stream [Reuter et al., 2012], and manually checked by M.M.H. who was blind to participant demographics (sex, age, pubertal status) and scan session. This technique was chosen to determine reliable volume estimates that are unbiased with respect to any time point. The longitudinal preprocessing stream includes: (1) processing of all time points separately using the cross‐sectional pipeline (e.g., removal of nonbrain tissue, image registration to Talairach space, segmentation of intracranial volumes (ICV) into gray, white, and CSF tissue, subcortical parcellation, and intensity normalization) [Fischl et al., 2002; Fischl et al., 2004] (2) the creation of a probabilistic template for each participant that is unbiased to either time point, (3) using the cross‐sectional stream to process each participant's unbiased template, and (4) then reprocessing of each time point using the unbiased template. This last step allows for the unbiased template to be utilized in several processing steps including skull stripping, Talairach transformations, atlas registration, and ultimately, significant increases in reliability and statistical power when estimating volume segmentations [Reuter et al., 2012].
Statistical Analyses
Hierarchical linear modeling (HLM) (see below) statistical analyses were carried out in R [Pinheiro et al., 2013]. One of the many advantages of these statistical models is the ability to handle missing data using maximum likelihood (ML)‐based estimation [Singer and Willet, 2003]. Thus, individuals with missing data were not removed from the analysis; rather, all available (i.e., usable) data were incorporated in the estimation of model parameters for each HLM analysis.
Mixed‐level model analyses showed that age significantly predicted Tanner Stage (Age: β = 0.65, SE = 0.14, t(65) = 4.81, P < 0.0001), but this did not vary as a function of sex (Age‐by‐Sex Intx: β = 0.07, SE = 0.08, P = 0.413). Age was also found to significantly predict T in boys (Age: β = 99.6, SE = 7.6, P < 0.0001), as well as T (Age: β = 5.5, SE = 0.7, P < 0.0001) and E2 (Age: β = 19.8, SE = 3.0, P < 0.0001) in girls (Table 1). As expected, T levels varied depending on sex, with boys having higher values and larger increases in level with age (Age‐by‐Sex Intx: β = −94.16, SE = 6.7, P < 0.0001). Thus, to remove sex‐related differences in the mean and variance of testosterone scores, T values were converted to z‐scores (across time) separately within each sex.
Volumes for cortical gray matter, white matter, hippocampus, amygdala, caudate, and thalamus were obtained. One subject's (boy) right hippocampus and amygdala volumes were outliers (>3 standard deviations from the mean) and were excluded from further analyses. Consistent with the literature, boys showed significantly larger ICVs compared to girls (β = −144,143.4, SE = 24,337.75, P < 0.0001) and ICV was included as a covariate in further analyses to control for individual and sex variability in brain size. This method was deemed appropriate as, for each region of interest (ROI), ICV showed a linear trend with volume (P values < 0.09), and this relationship was not significantly different between the sexes (P values > 0.29). When homogeneous slopes between‐group (sexes) and linearity assumptions are met (as seen in this sample), this commonly used method has a number of statistical advantages and is a robust generalized modeling strategy over other adjustment techniques available for brain size in volumetric MR imaging (O'Brien et al., 2011). For each ROI, linear growth trajectories were examined. Given the small number of a priori ROIs, α < 0.05 was deemed significant. Trends toward significance (P < 0.06) are also reported.
HLM was used to determine the ML estimates for the contribution of pubertal maturation and sex in characterizing brain volume during adolescents, as well as how they relate to the rate of change (slope) in brain growth over a 2 year period. HLM, also known as “growth curve,” “random effects,” “mixed‐effects,” and “multilevel” modeling, expands multiple regression for repeated measures and allows statistical models to capture intra and interindividual differences in change over time [Singer and Willet, 2003]. This allows for characterization of general patterns of developmental change (such as those that occur with time/age), and determination of factors that may explain individual differences in these patterns of change by testing for significant population predictors, like sex and pubertal status, on the intercept and slope of each individual's growth trajectory [Singer and Willet, 2003].
HLM requires a series of model estimations and comparisons between the estimated models to determine the best fitting predictors for the dependent variable of interest. For each a priori ROI, the following model‐building procedure was used to predict brain volume. This procedure was implemented as outlined in Singer and Willet [2003]: first, the unconditional growth model was assessed to determine initial brain volume (intercept), and if brain volume changed over time (slope). For all models, the time variable was indexed by age in years, and the age term was centered at age 10. By centering at 10, the youngest age in the current adolescent sample, the initial status can be interpreted as the estimated dependent variable at the age of 10. In this model, the fixed effects were time (indexed by age in years) and ICV, with subject as the random variable. Second, model‐building procedures were used to determine whether adding the time‐invariant variable of sex, the time‐varying pubertal markers (Tanner Stage, T, E2), or both predicted individual differences in initial brain volume at age 10 (intercept), and how individuals changed over time (slope) across adolescence. Specifically, the HLM model for sex included ICV, time, sex, and time‐by‐sex interaction as the fixed effects and subject as the random variable. The model for E2 in girls included the fixed effects of ICV, time, E2, and a time‐by‐E2 interaction, with subject as the random variable. To determine if the effects of Tanner Stage and T on brain volume were different between boys and girls, these HLM models included fixed effects of ICV, time, pubertal marker (Tanner Stage or T), sex, and all interaction terms, as well as subject as the random variable. For models including the time‐invariant predictor sex, the main effect of sex reflects an effect of the variable on the intercept (brain volume at age 10), whereas the interaction terms with time‐varying predictors (time*sex, sex*Tanner Stage, sex*T, time*sex*Tanner Stage or time*sex*T) reflect the effect of sex on individuals' trajectories of change, or conditional rate of change, respectively (i.e., slopes). For the time‐varying pubertal markers, the intercept refers to the value of brain volume when all time‐varying predictors are zero and, the main effect of the puberty variable (T, Tanner Stage, E2) reflects the conditional rate of change in brain volume per unit change in the puberty variable, while controlling for the effect of other time‐varying predictors (i.e., time, as indexed by age), whereas the interaction term of the added variable with time (time*Tanner Stage, time*T, time*E2) reflects the effect of the pubertal variable on individuals' trajectories of change over time (i.e., slope for time, as indexed by age). Finally, model reduction and full information maximum criteria were used to produce a “best‐fit” model for each brain region. To do this, a best‐fit model was determined when the full information ML fit indices [log‐likelihood and Akaike Information Criteria (AIC)] reflected a significantly better model fit than the unconditional model and the individual predictor of interest (e.g., sex, Tanner Stage, T, E2) was P < 0.10. Specifically, both log‐likelihood and AIC estimates are standard model fit metrics that allow comparisons between nested and nonnested models, respectively. A lower absolute value of AIC values reflects a better model fit to the data. Thus, for each brain region, the log‐likelihood and AIC values are reported for the unconditional growth model, as well as the final models for each HLM.
RESULTS
Details for the best‐fit HLM models for each ROI can be found in Supporting Information Table 1 through 6. Highlighted below are the significant and trend‐level predictors (P < 0.06) from these models.
Total Gray Matter Volumes
Total gray matter volume was significantly predicted by sex and E2 (Table 2). The model for sex included a significant effect of time and sex (Table 2), with gray matter volume increasing over time and boys having larger gray matter volumes compared to girls. The best‐fit model for E2 in girls included a significant main effect of E2 and a significant time*E2 interaction (Fig. 1b). That is, girls with lower E2 levels showed more robust decreases or pruning in gray matter volume across adolescence when compared to their peers with higher E2 levels.
Table 2.
Regions of interest predicted by the fixed‐effects of sex, tanner stage, T and E2, controlling for ICV
| Time and Sex | ||||
|---|---|---|---|---|
| Predictor: | β | t | P | |
| Time and Sex | ||||
| Sex | ||||
| Gray Matter | sex | −26176 | −3.14 | 0.0021 |
| Left Amygdala | sex | −105 | −2.58 | 0.01 |
| Left Thalamus | sex | −296 | −1.97 | 0.05 |
| Time*Sex | ||||
| White Matter | time*sex | −2757 | −4.6 | <0.001 |
| Left Caudate | time*sex | −51 | −2.99 | 0.004 |
| Right Caudate | time*sex | −27 | −2.08 | 0.04 |
| Tanner Stage | ||||
| Time*Tanner Stage | ||||
| White Matter | time*tanner stage | −805 | −2.62 | 0.01 |
| Sex*Tanner Stage | ||||
| Left Caudate | sex* tanner stage | −30 | −3.53 | 0.0008 |
| Right Amygdala | sex*tanner stage | −75 | −2.01 | 0.048 |
| Testosterone | ||||
| Time*Sex*T | ||||
| Right Amygdala | time*sex*T | 41 | 2.31 | 0.024 |
| Time*T | ||||
| Left Caudate | time*T | −19 | −2.19 | 0.032 |
| Right Caudate | time*T | −14 | −1.88 | 0.06 |
| White Matter | time*T | −645 | −1.89 | 0.06 |
| Estradiol | ||||
| Time*E2 | ||||
| Gray Matter | time*E2 | 78.4 | 2.34 | 0.024 |
| White Matter | time*E2 | −25 | −2.23 | 0.03 |
| Right Amygdala | time*E2 | 0.52 | 1.95 | 0.058 |
For each model the regression coefficients for the fixed‐effect terms (and their associated t and P‐values) are presented. Bold reflects P < 0.05.
Total White Matter Volumes
Total white matter volume was predicted by sex, Tanner Stage, E2, and (at a trend‐level) T (Table 2). Although adding sex as a predictor did not significantly improve the best‐fit model for sex, the main effect of sex and the time*sex interaction reached significance (Fig. 2b), with boys showing a larger increase in volume over time compared to girls. A time*Tanner Stage interaction was also seen for total white matter volume; individuals with Tanner Stage 1 showed higher rates of total white matter growth over time compared to their more pubertally mature peers (Tanner Stage 5) (Fig. 2c). Similarly, the best‐fit model for T included a time*T interaction (Fig. 2d), although the significance was trend‐level. Lastly, the best‐fit model for E2 in girls included a significant time*E2 interaction (Fig. 2e) where individuals with lower E2 levels displayed more robust increases in white matter growth across adolescence compared to those with higher E2 levels.
Figure 2.
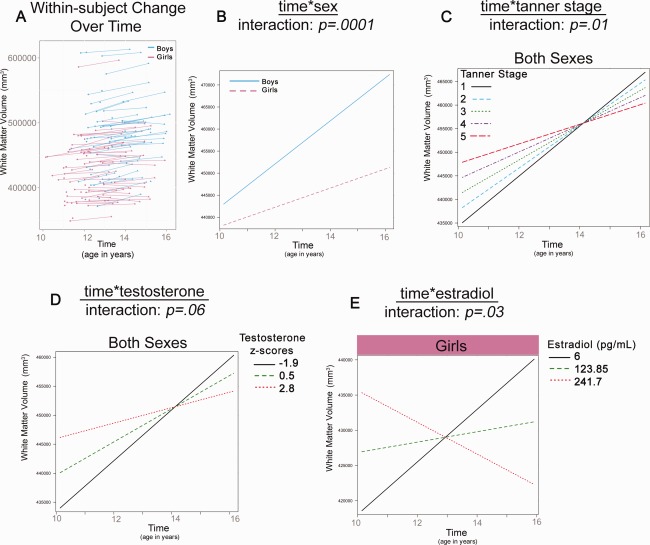
Significant interactions in total white matter volume development (A) Plots show raw total white matter volumes for girls and boys. The remaining plots show the linear multilevel mixed effects fit lines for the effects of time*sex (B), time*Tanner Stage (C), time*T (D) and time*E2 (E) on white matter volume.
Hippocampus Volumes
For the left hippocampus, the unconditional growth model showed an increase in volume over time; however, this did not remain significant after adding Tanner Stage, T, or E2. No change in volume was detected for the right hippocampus.
Amygdala Volumes
A significant main effect of sex was seen for left (but not right) amygdala, with boys having larger volumes compared to girls throughout adolescence (Table 2). Tanner Stage and T both significantly predicted right amygdala volume (Table 2). A sex*Tanner Stage interaction was found for the right amygdala, with boys showing increases in volumes, but girls showing decreases in volume with higher Tanner Stage (Fig. 3b). For T, a 3‐way time*sex*T interaction was found with the effect of T levels on right amygdala volume varying based on time (indexed by age) and sex (Fig. 3c). Specifically, boys with lower T levels showed increases in right amygdala volumes across adolescence, whereas those with high levels of T displayed decreases. Conversely, girls with low T levels showed robust decreases across adolescence, whereas no change was seen for those with high T levels (Fig. 3c). Trend‐level significance was also seen for E2, along with a significant main effect and time* E2 interaction (Table 2). Specifically, girls with lower E2 levels showed decreases, whereas girls with high levels of E2 displayed increases in right amygdala volumes across adolescence (Fig. 3d).
Figure 3.
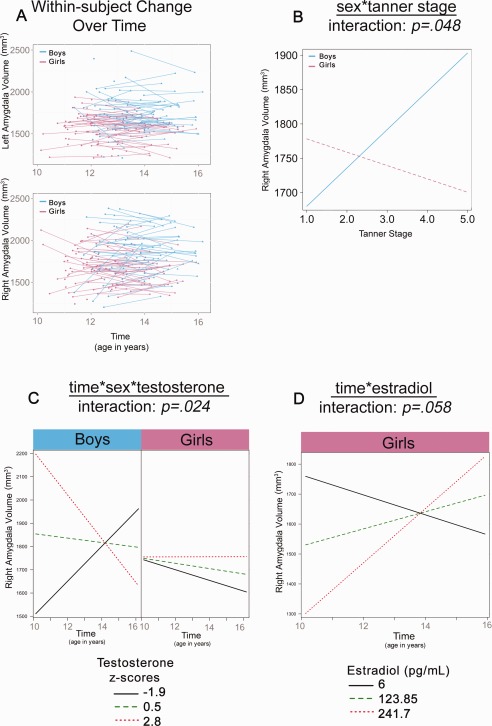
Significant interactions in amygdala volume development (A) Plots show raw left and right amygdala volumes for girls and boys. The remaining plots show the linear multilevel mixed effects fit lines for the effects of sex*Tanner Stage (B), time*sex*T (C) and time*E2 (D) on right amygdala volumes.
Caudate
Sex and T predicted bilateral caudate volumes, and Tanner Stage predicted the left caudate (Table 2). Bilateral time*sex interactions showed that girls had larger volumes compared to boys at age 10, but showed decreases in caudate volumes with age, while boys showed increases (left) or no change (right) across adolescence (Fig. 4b). A time*Tanner Stage interaction was also found for the left caudate, with increases seen for Tanner Stage 1 in girls and boys, but less change, and even decreases in volume seen for higher Tanner Staged individuals across adolescence (Fig. 4c). A time*T interaction (trend) for both the left and right caudate showed that both sexes showed increases in caudate volumes at low T levels, but decreases in caudate volume at higher T levels across adolescence (Fig. 4d).
Figure 4.
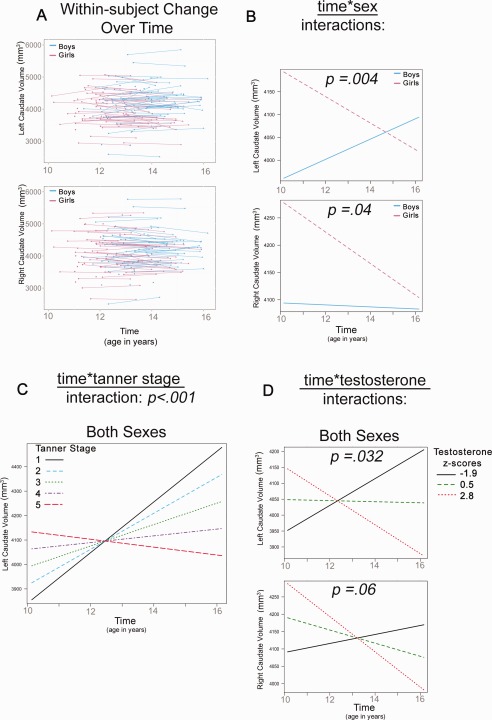
Significant interactions in caudate volume development (A) Plots show raw left and right caudate volumes for girls and boys. The remaining plots show the linear multilevel mixed effects fit lines for the effects of time*sex (bilaterally) (B), time*Tanner Stage (left only) (C) and time*T (bilaterally) (D) on caudate volumes.
Thalamus
A main effect of sex was found for the left thalamus (Table 2), suggesting boys had larger left thalamus volumes compared to girls. No change in volume was detected for the right thalamus.
DISCUSSION
Hormonal changes of puberty have been hypothesized to drive neuroanatomical sex differences across adolescence [Blakemore et al., 2010; Giedd et al., 2006; Peper et al., 2011]. For the first time, we empirically show that pubertal hormones, independent from age, interact with sex to drive individual differences in gray matter, white matter, amygdale, and caudate volumes across adolescence. We also show that using primary sex hormones T and E2 produce differing HLM results when compared to using Tanner Stage as a proxy of pubertal maturation. All three measures of puberty (Tanner Stage, T, and E2) were found to relate to white matter and right amygdala growth across adolescence. Tanner stage and T were also found to predict caudate volumes, whereas E2 also significantly predicted gray matter volumes in girls. Interestingly, pubertal measures were not found to be associated with within‐subject changes in hippocampal volumes. Despite the differences in model‐fits between the physical and hormonal measures, Tanner Stage, T, and E2 showed a strikingly similar pattern of how puberty relates to brain volume in these discrete brain regions across adolescence. Larger changes in cortical and subcortical volume were seen during early puberty, followed by less change during late puberty, regardless of brain region and the pubertal marker included in the model.
Given that Tanner Stage is a rough proxy for pubertal development, previously observed relationships between Tanner Stage and neuroanatomy have been posited to reflect hormonal effects on synaptic, dendritic, and axonal developmental processes [Blakemore et al., 2010; Giedd et al., 2006; Goddings et al., 2013]. Although MRI does not allow for direct assessment of cellular changes, by studying within‐subject changes in hormone levels and brain volume, here we provide further insight into the mechanism(s) by which puberty may contribute to neurodevelopment during adolescence. Changes in T were shown to relate to the development of total white matter, right amygdala, and bilateral caudate volumes, whereas E2 was associated with the development of total white matter, right amygdala, and total gray matter volume in girls. Interestingly, the relationship between testosterone and right amygdala development was different between the sexes, with decreases in right amygdala volume for boys, but increases for girls. These findings parallel those in animal models, which have shown an increase in new cell growth in the amygdala in male rats compared to females during puberty [Ahmed et al., 2008]. Moreover, individuals with higher E2 levels displayed increases in amygdala volumes across adolescence, which parallel previous animal research, as estrogen has been found to increase dendritic spine density in the medial amygdala in female rats [de Castilhos et al., 2008]. Together, these results suggest that estradiol and testosterone may contribute to sexually dimorphic changes in amygdala volumes across adolescence and into adulthood in both human and animal models. Beyond the amygdala, animal research has also shown estradiol to decrease the rate of myelination in female rats during puberty [Juraska and Markham, 2004], which is consistent with our findings where higher E2 levels predicted decreases in white matter volumes across adolescence in girls. Lastly, it seems that T may be driving the inverted‐U shaped patterns previously reported in caudate volumes seen to occur with age [Lenroot et al., 2007], as increases were seen at low T levels followed by steep decreases in volume at the highest T levels. However, less is known about T and its receptors in the caudate, making it is less clear what potential mechanism(s) may underlie how T influences bilateral caudate development. Further research is necessary to assess in more detail the actions of sex hormones on microstructural development during adolescence.
Given that high estradiol and AR densities are found in the hippocampus and amygdala [Sisk and Zehr, 2005], we expected hormonal associations with volume to be localized to these regions and to be larger in comparison to other subcortical regions, such as the thalamus. However, little change was seen in hippocampus volumes across this age range and neither T nor E2 levels predicted hippocampus volumes. Although in contrast to crosssectional studies [Bramen et al., 2011; Neufang et al., 2009], these results are consistent with the only other longitudinal study of hippocampus development, which also reported no changes in hippocampus volumes with age in either sex [Gogtay et al., 2006]. Hence, although somewhat unexpected, the hippocampus results highlight that some brain changes are likely to occur in parallel with hormonal levels without being directly influenced by the hormones themselves. This is consistent with research in animals where pyramidal neuronal development in layer III of the monkey medial prefrontal cortex and striatal dopamine receptor overexpression have been shown to occur independent of gonadal hormones across adolescence [Anderson et al., 1995; Andersen et al., 2002a].
As primary and secondary morphological maturation occur as a result of a milieu of hormonal processes [Shirtcliff et al., 2009], it is not surprising that HLM results from T and E2 were not identical when using Tanner Stage as a proxy of pubertal maturation. Although the coefficient estimates for Tanner Stage and age were not reported for HLM models by Goddings et al. [2013], our results are in agreement with their findings showing that a combination of both Tanner Stage and age is the best predictor of amygdala and caudate development. However, Goddings and colleagues found pubertal related amygdala growth for both sexes and decreases in the caudate volume in only girls, whereas the current Tanner Stage results were limited to the right amygdala in boys, but left caudate volumes in both sexes. Furthermore, Goddings et al. found Tanner Stage to predict increases in hippocampus volumes in a sex‐specific manner, whereas neither Tanner Stage (nor any other marker of puberty) was shown to relate to hippocampus development in the current study. Methodological differences between the two studies may potentially contribute to these discrepancies. Differences in the Tanner Stage procedures (self‐report versus practitioner ratings) may particularly help to explain the discrepancies seen in sex differences between the two studies, as accuracy of Tanner Stage self‐reporting varies between sexes, and does so as a function of pubertal stage and gonads/breast and pubic hair measurements [Dorn et al., 1990]. Because these two studies are the first of their kind, more research is needed to help disentangle the cause of these dissimilarities.
These interim and dynamic changes in brain volumes due to sex hormones may have important implications for understanding existing sex differences in behavior and mental health. For example, it is possible that structural changes seen across puberty in cortical, white matter, and amygdala volumes may contribute to affective processing differently in girls and boys. The amygdala interacts with cortical regions to regulate emotion and has been implicated in the pathophysiology of depression [Hulvershorn et al., 2011; Phelps and LeDoux, 2005]. It has been suggested that disparities in timing of cortical compared to limbic development, may lead to vulnerabilities to environmental stimuli in the adolescent brain and contribute to increased risk‐taking and poor emotional regulation [Somerville and Casey, 2010]. As only boys show increases in amygdala with puberty, our results support this view that amygdala connectivity and emotional regulation may develop differently between the sexes. Along these lines, affective reactivity in adolescents (i.e., threat) has recently been linked to testosterone changes in amygdala activation [Spielberg et al., 2014a] and amygdala and orbitofrontal connectivity during emotional processing [Spielberg et al., 2014b], while girls but not boys show more frontal relative to amygdala functional activity in response to fearful facial stimuli with age [Killgore et al., 2001]. These sex differences in pubertal‐related neurodevelopment may also help to explain previous findings that have shown puberty to be a better predictor than age for onset of depression during adolescence in girls but not boys [Angold et al., 1999]. In this regard, future research should explore relationships between sex, puberty, and internalizing and emotional behaviors.
By recruiting boys and girls matched for physical sexual maturity across a narrow age range, we were able to better disentangle hormonal associations from those of age and determine how they relate to within‐individual changes in brain volume over time. However, when using ML HLM with flexible and time‐unstructured data [Singer and Willet, 2003], caution must be used for estimates that extend beyond the range of the measured data. Since boys were recruited at a slightly older age in order to capture similar aspects of pubertal onset and progression between the sexes (Supporting Information Fig. 1), results for boys at ages 10 and 11 and girls at ages 16 are based on estimations alone. Future research encompassing a larger age range in boys is necessary to fully disentangle the potential confounding variables of age and sex at tail ends of the current sample. In addition, hormone levels in the current study were derived from plasma and E2 was not measured in this sample for boys. It is unclear if E2 also relates to brain volume in boys, or if E2 results shown here are sex‐specific. Furthermore, given that brain aromatization occurs to convert testosterone to estradiol [for review, see Roselli et al., 2009], the plasma levels measured may not directly reflect actual hormone concentration at the level of the brain. Thus, future research is warranted to determine how E2 may contribute to the patterns of brain development in boys, and to determine the degree of similarity between plasma and brain sex hormone levels in adolescent samples. Moreover, prior to and during the study, a great deal of effort was made to capture and control for hormone variations in girls across the cycle. However, it turned out that menarche did not occur in most girls until approximately 6 months prior to Time 2 (range 0 to 11 months), and their cycles were often quite irregular, making it uncertain precisely where in their cycle they were. This is in line with menstrual diary studies showing that population variability in menstrual cycle length are greatest 1–2 years following menarche [Treloar et al., 1967]. In addition, because 80% of the cycles are often anovulatory in the first year after menarche [Apter, 1980] and cycle phases and hormonal patterns of testosterone, progesterone, and estradiol vary as girls develop and maintain menstrual cycle function [Apter et al., 1978], menstrual cycle‐related influences are an inherent limitation to the current type of research. Moving forward, menstrual diaries as well as measuring hormone levels at multiple timepoints within the month of imaging postmenarcheal girls may help to reduce the possibility of this potential confound. Lastly, the current study aimed to understand the relationship between sex hormones and total gray and white matter as well as subcortical volumes, however, previous studies have shown sex steroids relate to other brain MRI metrics (e.g., thickness), highlighting that future studies are warranted to more fully elucidate how sex hormones relate to different aspects of within‐subject cortical maturation (e.g., thickness, surface area, gyrification).
CONCLUSIONS
This study quantifies the relationships between hormonal aspects of puberty and cortical and subcortical brain volume changes in girls and boys. The findings of the present longitudinal study support the idea that, independent of age, sex hormones uniquely relate to cortical and subcortical brain volumes in boys and girls across adolescence, but do so in a region‐specific manner. Regardless of the sex hormone studied (T, E2), the relationship between brain volume and puberty was consistent across cortical volumes and a number of subcortical regions. Large changes in brain volumes were seen during early puberty (as indexed by lower hormone levels), followed by less robust, or even a shift in growth, by late puberty. Future research expanding on how these relationships relate to behavior are likely to help us in understanding sex differences, and ultimately may provide us with better insight in clinical prevention and treatment for psychiatric disorders that emerge during the adolescent years.
Supporting information
Supplementary Information
Supplementary Information
Conflict of interest: Nothing to report.
REFERENCES
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL (2008): Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci 11:995–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- America's Children: Key National Indicators of Well‐Being (2009): Federal Interagency Forum on Child and Family Statistics. Washington, DC: U.S. Government Printing Office. http://www.childstats.gov/pdf/ac2009/ac_09.pdf
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA (1995): Synchronous development of pyramidal neuron dendritic spines and parvalbumin‐immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience 67(1):7–22. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH (2002a): Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 27:683–691. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH (2002b): Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 27:683–691. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM (1998): Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychol Med 28:51–61. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM (1999): Pubertal changes in hormone levels and depression in girls. Psychol Med 29:1043–1053. [DOI] [PubMed] [Google Scholar]
- Apter D (1980): Serum steroids and pituitary hormones in female puberty: A partly longitudinal study. Clin Endocrinol (Oxf) 12:107–120. [DOI] [PubMed] [Google Scholar]
- Apter D, Viinikka L, Vihko R (1978): Hormonal pattern of adolescent menstrual cycles. J Clin Endocrinol Metab 47(5):944–954. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE (2010): The role of puberty in the developing adolescent brain. Hum Brain Mapp 31:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE., Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER (2011): Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex 21:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER (2012): Sex matters during adolescence: Testosterone‐related cortical thickness maturation differs between boys and girls. PloS one 7(3):e33850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L, Coleman J (2002): The measurement of puberty: A review. J Adolesc 25:535–550. [DOI] [PubMed] [Google Scholar]
- de Castilhos J, Forti CD, Achaval M, Rasia‐Filho AA (2008): Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: A Golgi study. Brain Res. 1240:73–81. [DOI] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yucel M, Vijayakumar N, Kline A, Simmons J, Allen NB (2013): Mapping subcortical brain maturation during adolescence: Evidence of hemisphere‐ and sex‐specific longitudinal changes. Dev Sci 16:772–791. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff‐Germain G, Chrousos GP (1990): Perceptions of puberty: Adolescent, parent, and health care personnel. Dev Psychol 26:322–329. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL (1996): Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. J Comp Neurol 366:223–230. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango‐Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP (2006): Puberty‐related influences on brain development. Mol Cell Endocrinol 254–255 154–162. [DOI] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ (2014): The influence of puberty on subcortical brain development. Neuroimage 88:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, III , Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM (2006) Dynamic mapping of normal human hippocampal development. Hippocampus. 16:664–672. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks‐Gunn J (1997): Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry 36:1768–1776. [DOI] [PubMed] [Google Scholar]
- Herman‐Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, Wasserman R, Serwint JR, Smitherman L, Reiter EO (2012): Secondary sexual characteristics in boys: Data from the pediatric research in office settings network. Pediatrics 130:e1058–1068. [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, Anand A (2011): Toward dysfunctional connectivity: A review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav 5:307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Markham JA (2004): The cellular basis for volume changes in the rat cortex during puberty: White and gray matter. Ann N Y Acad Sci 1021:431–435. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun‐Todd DA (2001): Sex‐specific developmental changes in amygdala responses to affective faces. Neuroreport 12:427–433. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, Crone EA (2013): Sex differences and structural brain maturation from childhood to early adulthood. Dev Cognit Neurosci 5:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN (2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM (1968): Growth and physiological development during adolescence. Annu Rev Med 19:283–300. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM (1969): Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM (1970): Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnarney ER, Kreipe RE, Orr DP, Comerci GD (1992): Textbook of Adolescent Medicine. Philadelphia: W.B. Saunders Company. [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz‐Dahlmann B, Fink GR, Konrad K (2009) Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex 19:464–473. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S (2013a): Testosterone‐related cortical maturation across childhood and adolescence. Cereb Cortex 23:1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S (2013b) Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci Off J Soc Neurosci 33:10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottelmann ED, Susman EJ, Dorn LD, Inoff‐Germain G., Loriaux DL, Cutler GB, Jr. , Chrousos GP (1987): Developmental processes in early adolescence. Relations among chronologic age, pubertal stage, height, weight, and serum levels of gonadotropins, sex steroids, and adrenal androgens. J Adolesc Health Care 8:246–260. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ (2011): Statistical adjustments for brain size in volumetric neuroimaging studies: Some practical implications in methods. Psychiatry Res 193:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Nawaz‐Khan I, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Susman E, Veillette S, Pausova Z (2010): Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav 57:63–75. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre‐Van de Waal HA, Janke AL, Collins DL, Evans AC, Boomsma DI, Kahn RS, Hulshoff Pol HE (2008) Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology 33:909–915. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal, GC , van Leeuwen, M , van den Berg, SM , Delemarre‐Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE (2009): Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 34:332–342. [DOI] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, van Honk J (2011): Sex steroids and brain structure in pubertal boys and girls: A mini‐review of neuroimaging studies. Neuroscience 191:28–37. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48(2):175–187. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RDC (2013): nlme: Linear and nonlinear mixed effects models, R package version 3.1–113, http://CRAN.R-project.org/package=nlme.
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN (2010): Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci USA 107:16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012): Within‐subject template estimation for unbiased longitudinal image analysis. Neuroimage 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD (2009): Brain aromatization: Classic roles and new perspectives. Sem Reprod Med 27:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD (2009): Pubertal development: Correspondence between hormonal and physical development. Child Dev 80(2):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willet JB (2003): Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press. [Google Scholar]
- Sisk CL, Zehr JL (2005): Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26(3–4):163–174. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ (2010): Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol 20:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (1999): In vivo evidence for post‐adolescent brain maturation in frontal and striatal regions. Nat Neurosci 2:859–861. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL (2002): Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol 44:4–16. [DOI] [PubMed] [Google Scholar]
- Spielberg, J. M. , Olino TM, Forbes E. E. and Dahl R. E. (2014. a): Exciting fear in adolescence: Does pubertal development alter threat processing? Dev Cognit Neurosci 8:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Forbes EE, Ladouceur CD, Worthman CM, Olino TM, Ryan ND, Dahl RE (2014. b): Pubertal testosterone influences threat‐related amygdala‐orbitofrontal coupling. Soc Cognit Affect Neurosci. [Epub ahead of print] DOI: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS (2002): National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 110:911–919. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due‐Tonnessen P, Walhovd KB (2010): Brain maturation in adolescence and young adulthood: Regional age‐related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 20:534–548. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER (2006) Mapping brain maturation. Trends Neurosci 29:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW (1967): Variation of the human menstrual cycle through reproductive life. Int J Fertil 12:77–126. [PubMed] [Google Scholar]
- van den Berg SM, Setiawan A, Bartels M, Polderman TJ, van der Vaart AW, Boomsma DI (2006): Individual differences in puberty onset in girls: Bayesian estimation of heritabilities and genetic correlations. Behav Genet 36:261–270. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF (1997): Hormone measures in finger‐prick blood spot samples: New field methods for reproductive endocrinology. Am J Phys Anthropol 104:1–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information


