Abstract
Measures of immune outcomes in youth who initiate combination antiretroviral therapy (cART) early in HIV infection are limited.
Design
Adolescent Trials Network 061 examined changes over 48 weeks of cART in T cell subsets and markers of T cell and macrophage activation in subjects with pre-therapy CD4>350. All subjects had optimal viral suppression from weeks 24 through 48.
Methods
Subjects (n=48) initiated cART with tenofovir/emtricitabine plus ritonavir-boosted atazanavir. Data were collected at baseline and weeks 12, 24, and 48. Trends were compared to uninfected controls.
Results
Significant increases over 48 weeks were noted in all CD4 populations including total, naïve, central memory (CM), and effector memory RO (EM RO) and effector memory RA (EM RA) while numbers of CM and EMRO CD8 cells declined significantly. By week 48, CD4 naïve cells were similar to controls while CM CD4 cells remained significantly lower and EM RO and EM RA subsets were significantly higher. CD38 and HLA DR expression, both individually and when co-expressed, decreased over 48 weeks of cART on CD8 cells but remained significantly higher than controls at week 48. In contrast, markers of macrophage activation measured by sCD14 and sCD163 in plasma did not change with cART and were significantly higher than controls.
Conclusion
In youth initiating early cART, CD4 cell reconstitution is robust with decreases in CD8 cells. However CD8 T cell and macrophage activation persists at higher levels than uninfected controls.
Introduction
Infection with HIV-1 continues to impact youth in the US.1,2 While treatment with antiretroviral therapy is now recommended for most infected individuals,3 data are limited on the outcomes of antiretroviral treatment in infected youth who are unique for several reasons. For example, compared to adults following continuous antiretroviral therapy (cART), immune reconstitution in younger individuals shows higher proportions of naïve T cells.4–8 The timing of sexual debut is often close to HIV-1 acquisition among behaviorally infected adolescents indicating a relatively short duration of infection prior to initiating therapy. In older adults longer duration of infection and lower nadir CD4+ T cell (CD4) counts can diminish the depth and breadth of immune reconstitution.9–12 Younger age predicts less inflammation-mediated morbidity and presents opportunities to reduce the inflammatory consequences of HIV-1 infection such as cardiovascular disease.
Even with optimal viral suppression and immune reconstitution, high levels of immune activation following cART persist. While lack of adherence is a major cause of loss of viral suppression among youth on cART,13–17 immune activation and chronic inflammation may also contribute to viral breakthrough.18 Initiation of therapy before immune decline could reduce immune activation to levels similar to a uninfected individuals. In this study we examined changes in the distribution of naïve, memory, and effector memory T cell populations and extent of T cell activation following 48 weeks of cART in a population of youth with pre-therapy CD4 counts above 350 cells/mm3. We also determined if early cART resulted in decline in macrophage activation biomarkers associated with inflammation including soluble CD14 (sCD14) and soluble CD163 (sCD163).
Materials & Methods
Population
One hundred subjects from 23 clinical sites were enrolled between 2007 and 2010 into the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) and the International Maternal, Pediatric and Adolescent AIDS Clinical Trials Group (IMPAACT) study ATN 061: Preservation and Expansion of T-cell Subsets Following HAART De-intensification to Atazanavir/ritonavir. Of one hundred subjects enrolled, 75 were randomized to the early treatment arm with pre-entry CD4>350 cells/mm3 and begun on cART with tenofovir/emtricitabine plus ritonavir-boosted atazanavir after resistance testing. Forty-eight subjects achieved viral suppression, defined as HIV-1 RNA plasma viral load (VL) below 100 copies by week 24 and maintained through week 48, and are included in these analyses.
Fifty-one HIV-1 uninfected participants (HIV-) based on single time-point laboratory studies, similar to study cohort for age, gender, and African American ethnicity, were enrolled from one site (University of South Florida) as controls. Inclusion criteria for control subjects included no chronic illnesses or conditions, no infections or recent immunizations prior to blood studies, and documented HIV-1 sero-negative status.
The study was approved by Institutional Review Boards at each participating site. A Data Safety and Monitoring Board appointed by the Eunice Kennedy Shriver National Institute of Child Health and Human Development reviewed the data from the study semiannually.
Procedures
Analyses for T- cell subsets, plasma VL, extended flow cytometry, and plasma soluble factors were performed at entry and weeks 12, 24, and 48. Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque density centrifugation and cryopreserved at −160°C.19 Plasma samples were stored at −80°C.
Flow cytometry analysis of T cells subsets
Cryopreserved PBMCs were thawed, re-suspended in phosphate buffered saline containing 2% fetal bovine serum (PBS/2% FBS),20 divided into two tubes for incubation with anti-CD197 (CCR7, BD Bioscience, San Jose, CA) at 37°C, 5 % CO2 for 30 minutes, followed by incubation at 4°C for 30 minutes in a mixture of anti-CD3, anti-CD45RA, anti-HLA-DR, anti-CD57 (all BD Bioscience, San Jose, CA), anti-CD38 (Life Technologies, Carlsbad, CA) and either anti-CD4 or anti-CD8 (BD Bioscience, San Jose, CA). Cells were washed and re-suspended in PBS/2% FBS.
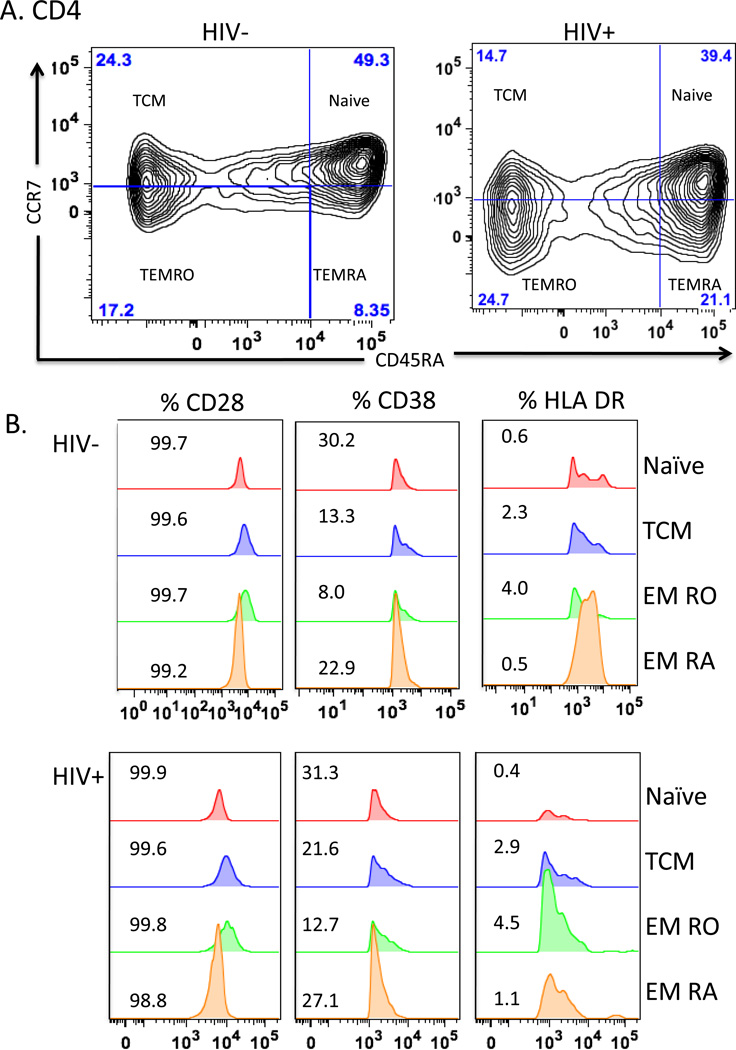
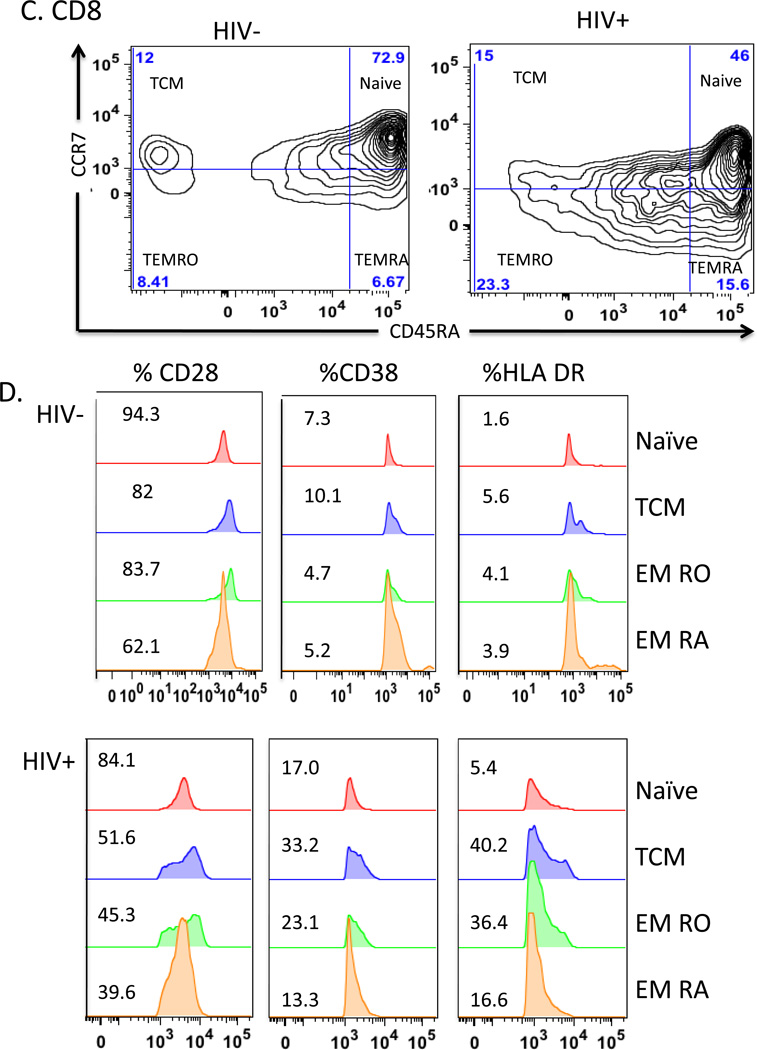
Markers of T cell differentiation/activation were analyzed with a multi-parameter LSR II flow cytometer (BD Bioscience, San Jose, CA). Lymphocytes were defined based on the forward scatter (FSC) and side scatter (SSC) from 100,000 events per sample. Lymphocyte subpopulations were subsequently acquired by gating on CD3+CD4+ or CD3+CD8+ cells identifying T helper and cytotoxic T cells, respectively. Naive CD4 and CD8 T cells were enumerated by gating CCR7+ and CD45RA+ cells, T central memory (TCM) by gating CD45RA− and CCR7+ T cells, T effector memory (EMRA) by gating CD45RA+ and CCR7−, and T effector RO cells (EMRO) by gating CD45RA− and CCR7− (Figure 1). Each subpopulation was individually analyzed for single expression and mean fluorescence intensity (MFI) of CD28, CD38, or HLA DR (Figure 1),21 as well as dual expression of CD38 and HLA DR. Data were acquired using BD FACSDiva acquisition software (BD Bioscience, San Jose CA) and analyzed with FlowJo v9.4.11 (Treestar, Inc, San Carlos, CA). Flow cytometry gates were established based on the cut-off determined by FMO controls.22
Figure 1. T cell subsets and expression of activation markers in PBMC.
Flow cytometry of T cells gated on CD3+CD4+ T cells (panels A and B) or CD3+CD8+ T cells (panels C and D) from representative uninfected (HIV−) and HIV-infected (HIV+) subjects. Panels A and C show density contour plots of CCR7 and CD45RA expression in CD4+ T cells (A & B) and CD8+ T cells (C & D). T cell subsets are defined as Naïve (CD3+ CD4/8+ CD45RA+ CCR7+); TCM (CD3+ CD4/8+ CD45RA− CCR7+); TEMRO (CD3+ CD4/8+ CD45RA−CCR7−); and TEMRA (CD3+ CD4/8+ CD45RA+ CCR7−). Percentage of cells based on FMO controls are shown for each quadrant. Activation markers CD28, CD38, and HLA DR are shown (B & D) as overlaid histogram plots for each subset.
Measurement of soluble markers of immune activation in plasma
Commercially available ELISA kits were used to detect sCD14 (R & D Systems, Minneapolis, MN), sCD27 (eBioscience, San Diego CA), and sCD163 (R & D Systems, Minneapolis, MN) within previously frozen plasma stored at −80°C. sCD14 was measured at a dilution factor 1:200 with a detection range of 250 – 8000 pg/ml; sCD27 at a dilution factor of 1:50, detection range 0.31 – 20 U/ml; and sCD163 at a dilution factor of 1:20, detection range of 1.6 – 100 ng/ml. The ELISA plates were read on Biotek® EL800 automated microplate reader (Winooski, VT) and results were analyzed using KCjunior™ microplate data analysis software, version 1.41.5 (Biotek®, Winooski, VT).
Statistical methods
Expression of immunological markers and soluble factors was analyzed using slopes and two-sample comparisons with healthy controls. Slopes of expression intensities over 48 weeks were estimated for each subject by linear regression and then analyzed by t-test to assess whether mean slope equaled zero. Baseline and week 48 values for subjects were compared with healthy controls using the Wilcoxon Rank-Sum test. Baseline and 48 week comparisons were conducted with the overall data, as well as data stratified by baseline CD4 counts (≤500 cells/mm3 versus >500 cells/mm3). All p-values were two-sided with statistical significance set at <0.05. Analyses were performed using SAS version 9.2.23
Results
Study Population
Forty-eight of the 75 subjects (64%) randomized to the treatment arm fully suppressed VL to <100 copies/ml between 24 and 48 weeks with the combination of ritonavir-boosted atazanavir, tenofovir, and emcitribine. Only two subjects had viral loads over 100 after week 24 (261 and 219 copies at week 36). Both subjects were below the level of detection with repeat testing and were continued on study. Baseline demographics of subjects who suppressed VL compared with the control group are shown in Table 1. The majority of subjects were male (87.5%), identified as African American (67%), and had CD4 counts above 500 cells/mm3 (58.33%). Substance use, including alcohol, tobacco, and marijuana, was similar between subjects and controls, as reported previously.24 Other psychosocial factors were not assessed as part of this study.
Table 1.
Baseline and Demographic Data
| Variable | HIV infected (N=48) |
HIV uninfected (N=52) |
p-value |
|---|---|---|---|
| Age at Entry (Years) - Continuous | |||
| Mean (SD) | 20.90 (1.55) | 21.62 (2.11) | 0.0533 |
| Range | 18 – 24 | 18 – 25 | |
| Age at Entry (Years) - Categorical | 0.2160 | ||
| 18 – 20 | 21 (43.75) | 16 (30.77) | |
| Over 21 | 27 (56.75) | 36 (69.23) | |
| Gender | 0.1317 | ||
| Male | 42 (87.50) | 39 (75.00) | |
| Female | 6 (12.50) | 13 (25.00) | |
| Race | 1.0000 | ||
| African American | 32 (66.67) | 35 (67.31) | |
| Other | 16 (33.33) | 17 (32.69) | |
| Baseline CD4+ Count | 0.0028 | ||
| < 350 | 0 (0) | 4 (7.69) | |
| 350 – 500 | 20 (41.67) | 8 (15.38) | |
| > 500 | 28 (58.33) | 40 (76.92) | |
| Baseline CD4+ Percent | <0.0001 | ||
| < 15% | 2 (4.17) | 1 (1.92) | |
| 15-25% | 19 (39.58)) | 1 (1.92) | |
| > 25% | 27 (56.25) | 50 (96.15) | |
| Baseline CD4+ Count | |||
| Mean (SD) | 553 (158) | 730 (284) | 0.0002 |
| Range | 355 – 1107 | 192 – 1416 | |
| Baseline CD4+ Percent (%) | |||
| Mean (SD) | 27.07 (8.60) | 41.94 (9.70) | <0.0001 |
| Range | 0.19 – 55.00 | 12.00 – 60.00 | |
| Baseline viral load | |||
| Mean (SD) | 20916 (21574) | NA | |
| Range | 1152 – 88900 |
CD4 count, CD4 percentage and Viral Load from the Pre-Entry visits were used as Baseline. P-values were calculated using Fishers exact test for categorical variables and t-test for continuous variables.
Expansion of CD4 and contraction of CD8 cells over 48 weeks on cART
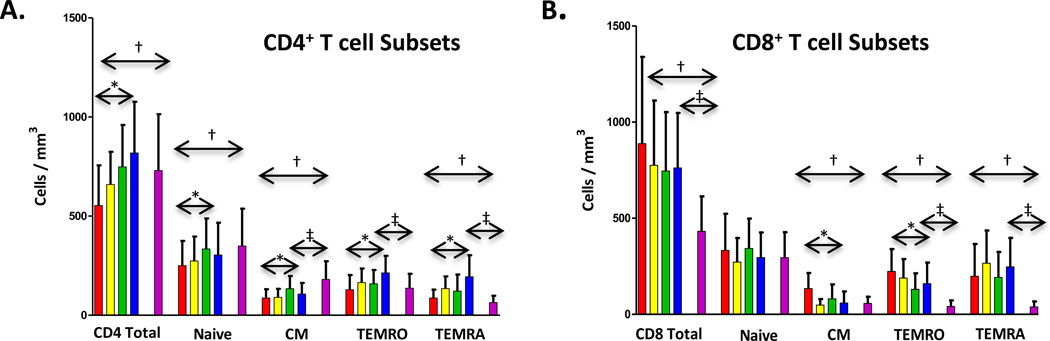
When compared to control youth, HIV-1 infected youth had significantly lower baseline CD4 counts for total (p=0.0019), naïve (p=0.0055), and CM (p<0.0001) subsets (Figure 2A). EM RA were higher (p=0.0019), but EM RO cells were not significantly different (p=0.4573). Following cART, significant increases developed in all CD4 populations including total (<0.0001), naïve (p=0.0029), CM (p=0.0001), and EM RO (p<0.0001) and EM RA (p<0.0001) subsets (Figure 2A). When T cell subsets were compared after week 48 of cART, mean total CD4 counts were higher and not different from control individuals (p=0.0543) (Figure 2A).
Figure 2. Trends in mean CD8+ and CD4+ T cells following antiretroviral therapy.
The y axis shows change in total cells/mm3 for CD4+ T-cell subsets (panel A), change in CD4+ T-cell in HIV+ subjects with baseline CD4+ T-cells >500 cells/mm3 (panel B), and with baseline CD4+ T-cells ≤ 500 cells/mm3 (panel C). Change in CD8+ T-cell subsets (panel D), CD8+ T-cell subsets in HIV+ subjects with baseline CD4+ T-cells > 500 cells/mm3 (panel E), and with baseline CD4+ T-cells ≤ 500 cells/mm3 (panel F) are also shown. Bars represent baseline ( ), week 12 (
), week 12 ( ), week 24 (
), week 24 ( ), week 48 (
), week 48 ( ), and HIV − controls (
), and HIV − controls ( ) and are shown for each subset. Significant changes over 48 weeks are shown with arrows. Changes from entry to 48 weeks among HIV+ subjects are represented with (*) (p<0.05, Wilcoxon Rank-Sum test). Significant differences between HIV+ subjects at baseline to HIV− controls and HIV+ subjects at 48 weeks to HIV− controls are shown with (†) and (‡), respectively (p<0.05, Wilcoxon Rank-Sum test). Error bar indicates 1 standard deviation.
) and are shown for each subset. Significant changes over 48 weeks are shown with arrows. Changes from entry to 48 weeks among HIV+ subjects are represented with (*) (p<0.05, Wilcoxon Rank-Sum test). Significant differences between HIV+ subjects at baseline to HIV− controls and HIV+ subjects at 48 weeks to HIV− controls are shown with (†) and (‡), respectively (p<0.05, Wilcoxon Rank-Sum test). Error bar indicates 1 standard deviation.
Total CD8 T cells and CM, EM RA, and EM RO subsets were significantly higher between subjects and controls (p<0.0001 for each), with no differences in CD8 naïve cells (p=0.5275). (Figure 2B). Total CD8 T cells decreased after therapy initiation, although the overall trend was not significant (p=0.0674). While no significant trends appeared for CD8 naïve or EM RA subpopulations, other populations, including CM (p<0.0001) and EM RO (p=0.0006) subsets, decreased (Figure 2B).
Changes among CD4 and CD8 T cell subsets were analyzed based on subjects grouped by baseline CD4 counts ≤500 or >500 cells/mm3. In each group, similar increases in total CD4 T cells, as well as CM, EM RO and EM RA CD4 populations occurred. Subjects with entry CD4≤ 500 also showed significant increases in naïve cells (data not shown). For CD8 cells, subjects in each CD4 strata demonstrated significant decreases in CM and EM RO populations.
Week 48 lymphocyte subsets compared to controls
At week 48, mean total CD4 T cell counts were not significantly different from control individuals (p=0.0543) (Figure 2A). In subset analysis, cART resulted in normalizing of CD4 naïve cells to levels similar to controls (p=0.1946), CM cells remained lower (p<0.0001), while both EM RO and EM RA subsets were higher (p<0.0001 for both).
Total CD8 populations, as well as both EM RO and RA subsets were higher in subjects at week 48 than in controls (p<0.0001 for each), whereas naïve and CM subsets were similar. (Figure 2B) Although significant longitudinal decreases for some CD8 subsets (CM and EM RO) occurred in subjects, elevated CD8 T cells persisted at week 48 driven by higher EM RO and EM RA subsets.
Decreased but persistent CD8 activation despite virologic suppression
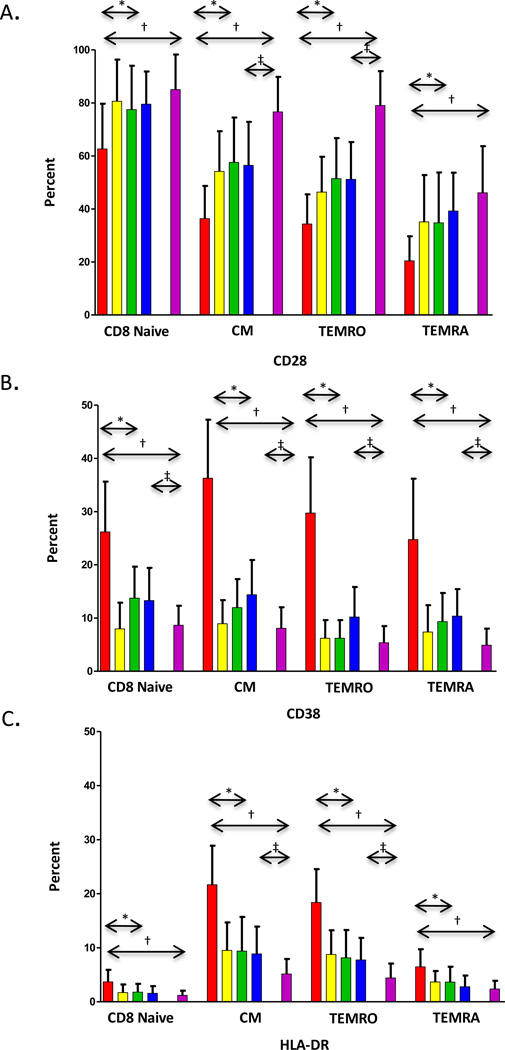
Immune activation within each CD8 T cell subset was assessed as percentage of cells expressing CD28, CD38 or HLA DR at week 48 relative to earlier time points (Figure 3). Control of VL resulted in significant longitudinal increases in the percentage of CD8 naïve, CM, EM RA, and EM RO subsets expressing CD28 (p<0.0001 for each). (Figure 3A) However, subjects at week 48 compared to controls had lower CD28 expression on naïve (p=0.0072), CM (p<0.0001), and EM RO (p<0.0001) CD8 subsets, consistent with persistent activation. Similar to down regulation of CD28, increased expression of CD38 and HLA DR reflects T cell activation. The percentage of cells expressing CD38 was higher at baseline but declined on naïve, CM, EM RO, and EM RA CD8 subsets within subjects (p<0.0001 for each time point post therapy). Expression of CD38 remained significantly higher for all CD8 subsets (p<0.0001) and failed to normalize even after 48 weeks of cART when compared to controls. Similarly, the percentage of cells expressing HLA DR decreased at week 48 for all CD8 subsets within subjects (p<0.0001 for all). These decreases led to percentages for naïve and EM RA CD8 subsets that were similar to controls, while CM (p<0.0001) and EM RO (p<0.0001) subsets remained higher. Although co-expression of CD38 and HLA-DR declined for each CD8 subpopulation (p<0.0001 for all, data not shown), percentage co-expression remained elevated at week 48 for all subpopulations (p<0.0001 for naïve, CM, and EM RO; p=0.0002 for EM RA) compared to controls. Assessment of activation markers on CD8 T cell subsets by MFI showed that by 48 weeks CD28 increased, while CD38 and HLA DR decreased significantly on naïve, CM, EMRA, and EMRO (p <0.001 for all) but failed to normalize compared to controls (p < 0.001) (Supplemental Table S1).
Figure 3. Trends in mean expression of T cell activation markers on CD8+ T cells.
Change in percent expression of CD28 (panel A), CD38 (panel B), and HLA-DR (panel C) are shown for each subpopulation. Bars represent baseline ( ), week 12 (
), week 12 ( ), week 24 (
), week 24 ( ), week 48 (
), week 48 ( ), and HIV− controls (
), and HIV− controls ( ). Significant changes over 48 weeks are shown with arrows. Changes from entry to 48 weeks among HIV+ subjects are represented with (*) (p<0.05, Wilcoxon Rank-Sum test). Significant differences between HIV+ subjects at baseline to HIV− controls and HIV+ subjects at 48 weeks to HIV− controls are shown with (†) and (‡), respectively (p<0.05, Wilcoxon Rank-Sum test). Error bar indicates 1 standard deviation.
). Significant changes over 48 weeks are shown with arrows. Changes from entry to 48 weeks among HIV+ subjects are represented with (*) (p<0.05, Wilcoxon Rank-Sum test). Significant differences between HIV+ subjects at baseline to HIV− controls and HIV+ subjects at 48 weeks to HIV− controls are shown with (†) and (‡), respectively (p<0.05, Wilcoxon Rank-Sum test). Error bar indicates 1 standard deviation.
Soluble factors of inflammation and activation
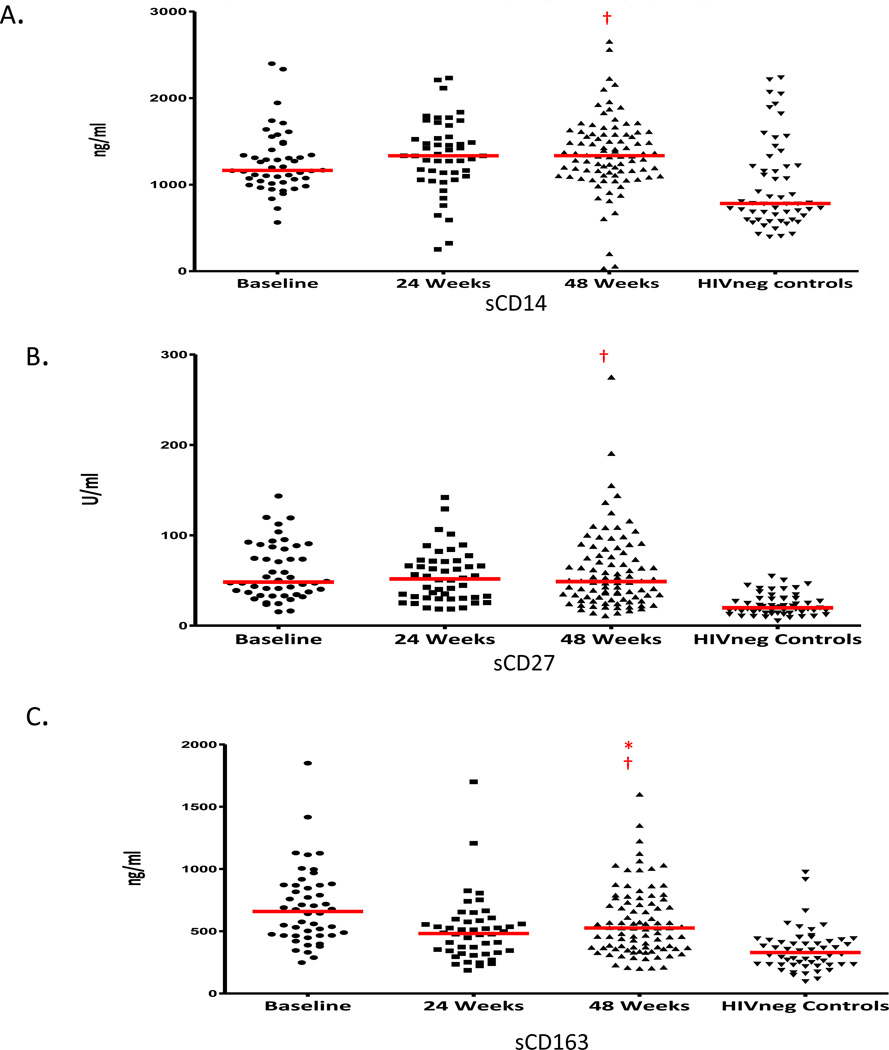
The impact of cART on macrophage activation was evaluated by assessing changes over 48 weeks of treatment in plasma levels of sCD14 and sCD163, markers of innate activation, along with sCD27, a soluble marker of lymphocyte activation. While sCD163 decreased, sCD14 and sCD27 remained similar to baseline levels (Figure 4), and each factor persisted at significantly higher levels compared to HIV-1 negative controls. The extent of elevation in sCD14, sCD163, and sCD27 did not correlate with the levels of CD8 T cell activation based on expression of HLADR or CD38 on naïve, CM, EMRA, or EMRO subsets either at entry or after 48 weeks (data not shown).
Figure 4. Trends in mean levels of plasma biomarkers of macrophage and lymphocyte activation.
Panels show levels of sCD14 levels (ng/ml) (panel A), sCD27 levels (U/ml) (panel B) and sCD163 levels (ng/ml) (panel C). The horizontal red line indicates mean values. Significant changes between baseline and 48 weeks in HIV+ subjects are represented with * (p<0.05, Wilcoxon Rank-Sum test). Significant differences between HIV+ subjects and HIV− controls are shown with † (p<0.05, Wilcoxon Rank-Sum test).
Discussion
Since the time that ATN 061 was initiated in 2008 , treatment guidelines have shifted to recommend cART at early stages of HIV infection as standard of care.3 As HIV incidence continues to be high in youth, especially ethnic minority young men who have sex with men (MSM), understanding the risks and benefits of early therapy in this population is essential.1,2 Treatment of young adults in early stage of disease, where there is a shorter interval from infection to treatment and higher capacity for immune reconstitution could result in preservation of T cell homeostasis,25,26 and lower the risk HIV inflammatory complications such as cardiovascular disease.4–12 In our study of youth who initiated treatment when CD4 counts were above 350 cells/mm3 or even greater than 500 cells/mm3, T cell reconstitution by 48 weeks was robust, T cell activation was diminished, while macrophage activation persisted. Thus, despite early treatment and successful viral control in this younger cohort, T cell activation and inflammation persisted similar to chronic infection in older adults.
The subjects in ATN 061 were therapy naïve, initiated treatment prior to immunologic deterioration, and were treated with the same antiretroviral regimen with close monitoring to assure documentation of viral suppression. At the time ATN 061 was implemented, atazanavir was a recommended first line component of cART. Subsequent availability of darunavir might have advantages for treatment of HIV-infected youth,27–29 although darunavir or atazanavir are similarly effective with similar metabolic profiles compared to efavirenz as part of cART,30,31 The HIV-uninfected controls and infected subjects in this study were similar for gender, age, and race, but co-existing conditions such as infection by herpes viruses, mycobacterium, or other sexually transmitted diseases that may be prevalent among the infected youth but were not captured as confounding variables, might contribute to immune activation. Although measures of immune activation and inflammation are not part of standard monitoring in the clinical setting, determination of levels in the research setting provides critical insights into the impact of therapy. In our study, viral suppression was maintained even with persistent immune activation. Conversely, immune activation was independent of viral breakthrough.
While viral replication differentially impacts T cell immune activation, primarily within CD8 CM and EM populations,23,32–34 control of viral replication should reestablish distribution of populations that are poised to return to normal levels.35 The capacity to rapidly express or down-regulate activation markers on CM and EM CD8 cell subsets suggests that assessment of discrete T cell populations has utility in monitoring immune activation in HIV-infected patients on cART as the extent of CD38 expression within CD8 subsets and levels of cell-associated virus predict disease progression in untreated HIV-infected adults.36 A surprising result among the youth with sustained viral suppression after 48 weeks of cART was continued elevation in CD38 on CM and EM CD8 T cells and the failure to normalize CD28 when compared to uninfected controls. Longer duration of optimal viral suppression may be needed before these signs of T cell activation decline in younger individuals.
In HIV-infected older adults who initiate therapy after CD4 counts decline to less than 350 cells/mm3, the extent to which CD4 cells normalize is much lower than in adolescents who initiate ART early in infection.4,5,37,38 Early therapy and persistence of thymic function is associated with improved immunologic outcomes in adults, children and adolescents treated with antiretroviral therapy.4–12,25,26 While gains in naïve CD4 cell T cells occur in most youth in our study, increases are most apparent among subjects with lower baseline CD4 counts at baseline; by 48 weeks of cART naïve CD4 T cells were similar to uninfected youth reflective of restored T cell homeostasis.
HIV-1 infection primes macrophages for activation and accentuates LPS-induced macrophage activation through TLR-4, as measured by the levels of sCD14 and sCD163 in the plasma.39–43 There was no correlation between changes in macrophage activation and the extent of T cell activation when expression of CD38 and HLADR expression was correlated to levels of sCD14 and sCD163. These results further illustrate independent mechanisms of innate and adaptive immune activation. Elevations in sCD14 and sCD163 persisted in spite of cART and were significantly higher than controls. Macrophage activation contributes to the long-term inflammatory complications of HIV-infection and appears to be independent of viral replication.44–46 Persistent macrophage mediated inflammation among youth who are asymptomatic with normal CD4 cell counts emphasizes the need for early interventions to reduce the long term inflammatory consequences of HIV infection.
sCD27 is a member of the tumor necrosis family released into the plasma following lymphocyte activation47 and like activation markers measure using flow cytometry, persistence of sCD27 in the plasma indicates ongoing immune activation.47–49 Elevated level of sCD27 when compared to controls further supports the observation that lymphocyte activation is not completely reversed by effective cART and provides another biomarker of lymphocyte activation that is not dependent on extended flow cytometry. Previous studies in adults and children show continued gains in CD4 cells and declines in inflammatory markers can be achieved with long term viral suppression but years of treatment are required.50–52
Immune activation during early infection is independently associated with CD4 cell loss in untreated individuals.53 Data in older adults indicate that the impact of CD8 cell activation on AIDS or non-AIDS events is confounded by age with those subjects over 50 more at risk for co-morbid events.54 CD38 expression on CD8 cells is associated with disease progression in adolescents as measured by CDC disease classification.34 The long term follow-up of the subjects enrolled in ATN 061 will prove whether expression of CD38 on CM and EM CD8 cells can predict viral failure while receiving cART and better understand the impact of initial immune activation and inflammation on treatment outcomes in young adults.
Supplementary Material
Acknowledgements
This work was supported by The Adolescent Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health [U01 HD 040533 and U01 HD 040474] through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (B. Kapogiannis), with supplemental funding from the National Institutes on Drug Abuse (K. Davenny) and Mental Health (P. Brouwers, S. Allison). It was also supported by R01 DA DA031017 (M. Goodenow and J. Sleasman). The protocol was co-endorsed by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group. Support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [U01 A1068632]. The study was scientifically reviewed by the ATN’s Therapeutic Leadership Group. Network, scientific and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at The University of Alabama at Birmingham. Network operations and analytic support was provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz, B. Driver, G. Price).
The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilberman, Julian), Children’s Hospital of Los Angeles (Belzer, Flores, Tucker), University of Southern California at Los Angeles (Kovacs, Homans, Lozano), Children’s National Medical Center (D’Angelo, Hagler, Trexler), Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County, and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California-San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children’s Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude’s Children’s Research Hospital (Flynn, Dillard), and Children’s Memorial (Garofalo, Brennan, Flanagan).
The following IMPAACT sites participated in the study: Children’s Hospital of Michigan – Wayne State (Moore, Rongkavilit, Hancock), Duke University Medical Center Pediatric CRS (Cunningham, Wilson), Johns Hopkins University (Ellen, Chang, Noletto), New Jersey Medical School CTU/CRS (Dieudonne, Bettica, Monti), St. Jude/Memphis CTU/CRS (Flynn, Dillard, McKinley), University of Colorado School of Medicine/The Children’s Hospital (Reirden, Kahn, Witte) University of Southern California Medical Center (Homans, Lozano), Howard University Hospital (Rana, Deressa),
Four of the ATN and IMPAACT sites utilized their General Clinical Research Center (GCRC)/Pediatric Clinical Research Center (PCRC) for the study. The centers were supported by grants from the General Clinical Research Center Program of the National Center for Research Resources (NCRR), National Institutes of Health, Department of Health and Human Services as follows: Children's National Medical Center, M01RR020359; Howard University Hospital, MO1-RR010284; University of California at San Francisco, UL1 RR024131; and University of Colorado School of Medicine/Children’s Hospital, UL1 RR025780. The University of Pennsylvania/Children's Hospital of Philadelphia utilized its Institutional Clinical and Translational Science Award Research Center (CTRC), supported by grant UL1 RR024134 from NCRR. The Tulane University Health Sciences Center utilized its Clinical and Translational Research Center (CTRC) for the study which was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP (RC/EEP - 06).
Atazanavir was provided by Bristol Myers Scribb with support from Jonathon Uy.
We thank the ATN Community Advisory Board and the youth who participated in the study. We also thank Susan Lukas for assistance with flow cytometry and ELISA testing and Theresa Considine for assistance with manuscript preparation.
Footnotes
Conflicts of Interest: None
References
- 1.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diagnoses of HIV Infection and AIDS in the United States and Dependent Areas. Centers for Disease Control and Prevention; 2010. [Accessed June 13, 2013]. at http://www.cdc.gov/hiv/library/reports/surveillance/2010/surveillance_Report_vol_22.html. [Google Scholar]
- 3. [Accessed June 13, 2013];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. at http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. [PubMed]
- 4.Li T, Wu N, Dai Y, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011;53:944–951. doi: 10.1093/cid/cir552. [DOI] [PubMed] [Google Scholar]
- 5.Kolte L, Dreves AM, Ersboll AK, et al. Association between larger thymic size and higher thymic output in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2002;185:1578–1585. doi: 10.1086/340418. [DOI] [PubMed] [Google Scholar]
- 6.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 7.Resino S, Seoane E, Perez A, Ruiz-Mateos E, Leal M, Munoz-Fernandez MA. Different profiles of immune reconstitution in children and adults with HIV-infection after highly active antiretroviral therapy. BMC Infect Dis. 2006;6:112. doi: 10.1186/1471-2334-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco JM, Leon-Leal JA, Leal M, et al. CD4+ and CD8+ T lymphocyte regeneration after anti-retroviral therapy in HIV-1-infected children and adult patients. Clin Exp Immunol. 2000;119:493–498. doi: 10.1046/j.1365-2249.2000.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engsig FN, Gerstoft J, Kronborg G, et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect Dis. 2010;10:1471–2334. doi: 10.1186/1471-2334-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico R, Yang Y, Mildvan D, et al. Lower CD4+ T lymphocyte nadirs may indicate limited immune reconstitution in HIV-1 infected individuals on potent antiretroviral therapy: analysis of immunophenotypic marker results of AACTG 5067. J Clin Immunol. 2005;25:106–115. doi: 10.1007/s10875-005-2816-0. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 13.Ryscavage P, Anderson EJ, Sutton SH, Reddy S, Taiwo B. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58:193–197. doi: 10.1097/QAI.0b013e31822d7564. [DOI] [PubMed] [Google Scholar]
- 14.Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One. 2012;7:20. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans D, Menezes C, Mahomed K, et al. Treatment Outcomes of HIV-Infected Adolescents Attending Public-Sector HIV Clinics Across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses. 2013;29:892–900. doi: 10.1089/aid.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudy BJ, Murphy DA, Harris DR, Muenz L, Ellen J. Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: a study of prevalence and interactions. AIDS Patient Care STDS. 2009;23:185–194. doi: 10.1089/apc.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudy BJ, Murphy DA, Harris DR, Muenz L, Ellen J. Prevalence and interactions of patient-related risks for nonadherence to antiretroviral therapy among perinatally infected youth in the United States. AIDS Patient Care STDS. 2010;24:97–104. doi: 10.1089/apc.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed SS, Balluz RS, Kabagambe EK, et al. Assessment of biomarkers of cardiovascular risk among HIV type 1-infected adolescents: role of soluble vascular cell adhesion molecule as an early indicator of endothelial inflammation. AIDS Res Hum Retroviruses. 2013;29:493–500. doi: 10.1089/aid.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleeberger CA, Lyles RH, Margolick JB, Rinaldo CR, Phair JP, Giorgi JV. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol. 1999;6:14–19. doi: 10.1128/cdli.6.1.14-19.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sleasman JW, Leon BH, Aleixo LF, Rojas M, Goodenow MM. Immunomagnetic selection of purified monocyte and lymphocyte populations from peripheral blood mononuclear cells following cryopreservation. Clin Diagn Lab Immunol. 1997;4:653–658. doi: 10.1128/cdli.4.6.653-658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oswald-Richter K, Grill SM, Leelawong M, et al. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3:e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed May 15, 2014];SAS OnlineDoc 9.1.3. at http://support.sas.com/onlinedoc/913/docMainpage.jsp )
- 24.Nichols SL, Lowe A, Zhang X, et al. Concordance between self-reported substance use and toxicology among HIV-infected and uninfected at risk youth. Drug and alcohol dependence. 2014;134:376–382. doi: 10.1016/j.drugalcdep.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudy BJ, Lindsey JC, Flynn PM, et al. Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: week 60 results from the PACTG 381 cohort. AIDS research and human retroviruses. 2006;22:213–221. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 26.Rudy BJ, Crowley-Nowick PA, Douglas SD. Immunology and the REACH study: HIV immunology and preliminary findings. Reaching for Excellence in Adolescent Care and Health. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2001;29:39–48. doi: 10.1016/s1054-139x(01)00288-9. [DOI] [PubMed] [Google Scholar]
- 27.Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. Aids. 2010;24:2365–2374. doi: 10.1097/QAD.0b013e32833dec20. [DOI] [PubMed] [Google Scholar]
- 28.Arribas JR, Horban A, Gerstoft J, et al. The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS. 2010;24:223–230. doi: 10.1097/QAD.0b013e3283348944. [DOI] [PubMed] [Google Scholar]
- 29.Arribas JR, Clumeck N, Nelson M, Hill A, van Delft Y, Moecklinghoff C. The MONET trial: week 144 analysis of the efficacy of darunavir/ritonavir (DRV/r) monotherapy versus DRV/r plus two nucleoside reverse transcriptase inhibitors, for patients with viral load < 50 HIV-1 RNA copies/mL at baseline. HIV Med. 2012;13:398–405. doi: 10.1111/j.1468-1293.2012.00989.x. [DOI] [PubMed] [Google Scholar]
- 30.Imaz A, Llibre JM, Navarro J, et al. Effectiveness of efavirenz compared with ritonavir-boosted protease inhibitor-based regimens as initial therapy for patients with plasma HIV-1 RNA above 100,000 copies/ml. Antiviral therapy. 2014 doi: 10.3851/IMP2736. [DOI] [PubMed] [Google Scholar]
- 31.Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. 2012;28:1184–1195. doi: 10.1089/aid.2011.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 33.Mildvan D, Bosch RJ, Kim RS, et al. Immunophenotypic markers and antiretroviral therapy (IMART): T cell activation and maturation help predict treatment response. J Infect Dis. 2004;189:1811–1820. doi: 10.1086/383277. [DOI] [PubMed] [Google Scholar]
- 34.Wilson CM, Ellenberg JH, Douglas SD, Moscicki AB, Holland CA. CD8+CD38+ T cells but not HIV type 1 RNA viral load predict CD4+ T cell loss in a predominantly minority female HIV+ adolescent population. AIDS Res Hum Retroviruses. 2004;20:263–269. doi: 10.1089/088922204322996482. [DOI] [PubMed] [Google Scholar]
- 35.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahnke YD, Song K, Sauer MM, et al. Early immunologic and virologic predictors of clinical HIV-1 disease progression. Aids. 2013;27:697–706. doi: 10.1097/QAD.0b013e32835ce2e9. [DOI] [PubMed] [Google Scholar]
- 37.Zeng M, Southern PJ, Reilly CS, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:5. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Brown JN, Kohler JJ, Coberley CR, Sleasman JW, Goodenow MM. HIV-1 activates macrophages independent of Toll-like receptors. PLoS One. 2008;3:2. doi: 10.1371/journal.pone.0003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 42.Hayes TL, Asmuth DM, Critchfield JW, et al. Impact of highly active antiretroviral therapy initiation on CD4(+) T-cell repopulation in duodenal and rectal mucosa. Aids. 2013;27:867–877. doi: 10.1097/QAD.0b013e32835d85b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. Aids. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 47.Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 48.Yin L, Rodriguez CA, Hou W, et al. Antiretroviral therapy corrects HIV-1-induced expansion of CD8+ CD45RA+ CD2-) CD11a(bright) activated T cells. J Allergy Clin Immunol. 2008;122:166–172. doi: 10.1016/j.jaci.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallet MA, Rodriguez CA, Yin L, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. Aids. 2010;24:1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR. T-cell subset distribution in HIV-1-infected patients after 12 years of treatment-induced viremic suppression. J Acquir Immune Defic Syndr. 2012;61:270–278. doi: 10.1097/QAI.0b013e31825e7ac1. [DOI] [PubMed] [Google Scholar]
- 51.Guihot A, Tubiana R, Breton G, et al. Immune and virological benefits of 10 years of permanent viral control with antiretroviral therapy. Aids. 2010;24:614–617. doi: 10.1097/QAD.0b013e32833556f3. [DOI] [PubMed] [Google Scholar]
- 52.Hughes RA, Sterne JA, Walsh J, et al. Long-term trends in CD4 cell counts and impact of viral failure in individuals starting antiretroviral therapy: UK Collaborative HIV Cohort (CHIC) study. HIV Med. 2011;12:583–593. doi: 10.1111/j.1468-1293.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 53.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 54.Lok JJ, Hunt PW, Collier AC, et al. The impact of age on the prognostic capacity of CD8+ T-cell activation during suppressive antiretroviral therapy. Aids. 2013;27:2101–2110. doi: 10.1097/QAD.0b013e32836191b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.