Abstract
Hematopoietic stem cell transplantation (HSCT) is a highly effective procedure enabling long-term survival for patients with hematologic malignancy or heritable defects. Although there has been a dramatic increase in the success rate of HSCT over the last two decades, HSCT can result in serious, sometimes untreatable disease due to toxic conditioning regimens and Graft-versus-Host-Disease. Studies utilizing germline knockout mice have discovered several candidate genes that could be targeted pharmacologically to create a more favorable environment for transplant success. SHIP1 deficiency permits improved engraftment of hematopoietic stem-progenitor cells (HS-PCs) and produces an immunosuppressive microenvironment ideal for incoming allogeneic grafts. The recent development of small molecule SHIP1 inhibitors has opened a different therapeutic approach by creating transient SHIP1-deficiency. Here we show that SHIP1 inhibition (SHIPi) mobilizes functional HS-PC, accelerates hematologic recovery, and enhances donor HS-PC engraftment in both allogeneic and autologous transplant settings. We also observed the expansion of key cell populations known to suppress host-reactive cells formed during engraftment. Therefore, SHIPi represents a non-toxic, new therapeutic that has significant potential to improve the success and safety of therapies that utilize autologous and allogeneic HSCT.
Keywords: SHIP1, 3AC, Allogeneic BMT, Autologous BMT, Stem cell mobilization, SDF-1, MMP-9, NK cells, SHIPi
Highlights
-
•
SHIPi facilitates HS-PC mobilization.
-
•
SHIPi facilitates engraftment of autologous BM without myeloablation.
-
•
SHIPi enhances engraftment of allogeneic BM without cytotoxic effects on the host.
1. Introduction
Hematopoietic stem cell transplantation (HSCT) has historically been successful in treating patients with cancer, autoimmune disease (multiple sclerosis), and genetic disorders (thalassemia, sickle cell disease) (Li and Sykes, 2012). Prior to HSCT, an intense conditioning regimen is essential to reduce host tumor burden and/or auto-reactive lymphocytes. The pro-inflammatory state triggered by pre-transplant conditioning also enhances T and NK cell killing of residual tumor cells particularly in the allogeneic setting (Paulos et al., 2007). However, this same inflammatory milieu can promote donor or host T-cell reactions that culminate in Graft-versus-Host-Disease (GvHD) or in graft rejection (Shlomchik, 2007, Ferrara et al., 2009). Many patients who require HSCT do not have an appropriate human leukocyte antigen (HLA) matched donor available. Additionally, HLA mismatch can be beneficial for cancer patients as an HLA mismatch results in increased NK cell activity (Davies et al., 2002, Ruggeri et al., 2002). Therefore, a major goal for optimizing HSCT is to enhance the engraftment of HLA mismatched grafts, while preventing, or reducing the undesired side effects triggered by the graft, the intense preconditioning regimens, or both.
SHIP1 and SHIP2 are two SH2-domain containing inositol 5′ phosphatases that oppose the activity of PI3K by converting Phosphatidyl-Inositol(3,4,5)trisphosphate to Phosphadityl Inositol(3,4)bisphosphate. PI3K promotes the survival, proliferation and effector functions in a broad range of mammalian cell types, via activation of PDK1, Akt and Tec family kinases (Yuan and Cantley, 2008). SHIP1 and SHIP2 have also recently been shown to promote survival signals through the recruitment and activation of enzymes including Akt and Irgm1 (Brooks et al., 2010, Tiwari et al., 2009).
SHIP1 first emerged as a potential molecular target in HSCT when it was found that both acute bone marrow (BM) graft rejection and GvHD were compromised in SHIP1 deficient hosts (Wang et al., 2002). Improved allogeneic BM engraftment in SHIP1 deficient hosts results from a constellation of immune phenotypes that include compromised NK function (Wang et al., 2002, Wahle et al., 2006, Gumbleton et al., in press), decreased numbers of T-cells in mucosal tissues (Kerr et al., 2011, Park et al., 2014), and increased immunoregulatory cell numbers such as myeloid derived suppressor cells (MDSCs) (Ghansah et al., 2004, Paraiso et al., 2007), mesenchymal stem cells (MSC) (Iyer et al., 2014a, Iyer et al., 2014b), and Treg cells (Collazo et al., 2009, Kashiwada et al., 2006, Locke et al., 2009). Parallel studies of the hematopoietic stem cell (HSC) compartment in SHIP1−/− mice revealed that HSCs are spontaneously mobilized to the peripheral blood due to a combined effect of increased levels of granulocyte colony stimulating factor (G-CSF) and matrix metallopetidase 9 (MMP-9), and a decrease in stromal-cell derived factor 1 (SDF1) (Hazen et al., 2009). The loss of SHIP1 generates two positive outcomes in the context of HSCT: mobilization of Hematopoietic Stem-Progenitor Cells (HS-PCs) to the periphery for harvesting, and a flux in the BM microenvironment that results in a suitable milieu for incoming donor cell engraftment. In aggregate, these studies suggested that recently identified SHIP1 inhibitors (Brooks et al., 2010, Brooks et al., 2014, Fuhler et al., 2012) could eventually find utility in various aspects of both allogeneic and autologous HSCT.
Here, we investigated the potential of small molecule chemical inhibition of SHIP1 (SHIPi) in vivo with the aminosteroid inhibitor 3AC for facilitating HSCT. We found that SHIPi promotes beneficial effects that could find utility in both autologous and allogeneic transplant settings as well as mobilization of HS-PCs. We hypothesized that this instability in the bone marrow microenvironment would allow for significantly improved engraftment of an autologous BM graft following minimally ablative conditioning. As observed in genetic models, SHIPi increased immunoregulatory cell populations (MDSC and Treg cells), while disrupting NK cell effector function. The modulation of these immune cell types also enhanced engraftment of Major Histocompatibility Complex-I (MHC-I) mismatched BM following fully myeloablative radiation conditioning. Therefore, SHIPi shows promise as a potential non-toxic therapeutic to alter the immune environment for the purpose of successful HSCT.
2. Materials and Methods
2.1. Mouse Strains
C57BL/6-CD45.2 (B6.2), C57BL/6-CD45.1 (B6.1) and BALB/c (H2d) mice were purchased from Jackson Laboratories and Taconic and were at least 8 weeks old at the time of experimentation. All mice were housed at the Upstate Medical University Department of Laboratory Animal Resources Facility for at least one week prior to start of experimentations under conventional immunocompetent housing and feeding conditions. All experiments were performed with the approval of the Institutional Animal Care and Use Committee.
2.2. Synthesis and Verification of 3AC and K190 Purity
See Supplemental experimental procedures for synthesis of 3α-amino-5α-cholestane (3AC) and 3α-hydroxy-5α-cholestane (K190). See Fig. S1A for structures of small molecules.
2.3. SHIPi Treatment of Mice
3AC (26.5 mg/kg) and K190 (24.3 mg/kg) were freshly emulsified in vehicle immediately prior to administration to each mouse by intraperitoneal (i.p.) injection (100 μl per injection). Vehicle (0.3% (weight/volume) Hydroxypropyl cellulose (Sigma) in Phosphate Buffered Saline (1 ×PBS, Corning)) was filter sterilized and was also administered by i.p. injection (100 μl per injection) in vehicle control mice. All injections were performed once daily for 7 consecutive days unless otherwise indicated. SHIPi or vehicle treatment of mice was performed on age-matched and sex-matched groups.
2.4. Radiation Conditioning of Mice
Following SHIPi or vehicle treatment and prior to transplants, mice received 300 Rads for minimally ablative conditioning, or 1100 Rads (B6) or 800 Rads (BALB/c) for lethal irradiation conditioning by total body irradiation from an X-ray source (RadSource, RS2000).
2.5. ELISA Assays for G-CSF, MMP-9 and SDF1
Plasma levels of G-CSF, MMP-9 and SDF1-CXCL12 were determined by ELISA (R&D Systems) according to the manufacturer's instructions.
2.6. HS-PC Mobilization and Congenic White Blood Cell (WBC) Transplantation
Host B6.1 and BALB/c mice were treated with SHIPi or vehicle. On day 8, Red Blood Cell (RBC) lysis was performed on blood obtained from treated-mice, and live cells were counted. Untreated B6.2 and BALB/c host mice were lethally irradiated, and were each injected with 7.5 × 105 WBC from congenic vehicle-treated or SHIPi-treated donor mice. For all harvest, the number of WBC donors was equivalent or less than the number of hosts transplanted and did not compromise viability of donors. Survival of mice receiving the WBC grafts was monitored over a 4-month period.
2.7. Autologous, Congenic: Allogeneic and Allogeneic Bone Marrow Transplant (BMT) and Engraftment by Flow Cytometry
Host B6.2 mice were treated for 7 days with SHIPi or vehicle. On day 8, host mice were irradiated and received BMT as described in Table 1. Engraftment was measured by flow cytometry of RBC-lysed blood, stained with an antibody cocktail containing anti-CD45.2, anti-H2Dd or anti-CD45.1, anti-CD3ε, anti-Mac1, anti-GR1, and anti-CD19 for the different types of transplants as indicated in Table 1. For acute engraftment in congenic:allogeneic competitive BMT, host BM and splenocytes were harvested on day 5 post-transplant and stained with anti-H2Dd and anti-CD45.1 for analysis by flow cytometry. Complete description of each transplant is also described in Supplemental methods.
Table 1.
BM transplant schemes and engraftment analysis time frame.
| Setting | Host | Donor | Radiation | BM cells | Days of Txa | Engraftment analysis |
|---|---|---|---|---|---|---|
| Autologous | B6.2 | B6.1 | 300 Rads | 5 × 106 | 8, 9, 10 | 1–4 months |
| Congenic:allogeneic | B6.2 | B6.1 BALB/c |
1100 Rads | 5 × 106 5 × 106 |
8 | 5 days |
| Allogeneic | B6.2 | BALB/c | 1100 Rads | 1 × 106 or 2 × 106 |
8 | 1 week–4 months |
Tx: Transplants.
2.8. Kinetic Analysis of NK, MDSC and Treg Cells
10 week old female B6.2 mice were treated daily for 0 to 10 days with 3AC as described above. On each day (0 to 10 inclusively), 5 mice were sacrificed, splenocytes were harvested, RBCs were lysed using 1 × RBC Lysis buffer (eBioscience), counted and then stained with anti-NK1.1, anti-Mac1 and anti-Gr1, or with anti-CD3ε and anti-CD4 then fixed and stained with anti-FoxP3 as per manufacturer's recommendation (eBioscience). Cells were analyzed by flow cytometry as described below. Frequency and absolute numbers of live NK cells and MDSCs were compared to those observed in the splenocytes from the 5 uninjected mice harvested on day 0. Live CD4+FoxP3+ cells were expressed as frequency of CD3ε+ T-cells and compared to those on day 0.
2.9. Ex vivo NK Cell IFNγ Production Following Activating Receptor Crosslinking
Splenocytes were harvested from 6 day SHIPi or vehicle treated mice, red blood cells were lysed (1 × RBC Lysis buffer, eBioscience) and were incubated for 5 h alone (unstimulated), in anti-NK1.1 (PK136) antibody coated wells (NK1.1) or in the presence of 1.67 μg/ml Phorbol Myristate Acetate (PMA) and 1 μg/ml ionomycin. In all cases cells were incubated in the presence of GolgiPlug (BD Biosciences) Fc receptors were blocked (2.4G2, BD Biosciences), surface receptors were stained using anti-NKp46 or anti-DX5 and anti-CD3ε antibodies. Cells were fixed and permeabilized (BD Biosciences), Fc receptors were blocked (2.4G2, BD Biosciences), and cells were stained for IFNγ and analyzed via intracellular flow cytometry.
2.10. Flow Cytometry and Blood Recovery
Dead cells (positively stained for DAPI dye) were excluded from all the analyses, except for the NK cell analysis of INFγ and Treg cells that were stained with LiveDead Aqua (Invitrogen) for dead cell exclusion. Samples were acquired on an LSR-Fortessa or LSRII cytometers (BD Biosciences) and analyzed using FlowJo software. All antibodies were purchased from BD Biosciences or from eBiosciences. Blood component recovery was monitored by a Hemavet 950S automated blood cell analyzer (Drew Scientific).
2.11. Statistics
All statistical analyses were performed using GraphPad Prism 5.0.
2.12. Funding
This work was supported by grants from the NIH (RO1 HL072523, R01 HL085580, R01 HL107127) and the Paige Arnold Butterfly Run. S.F. is a postdoctoral fellow of the Crohn's and Colitis Foundation of America (CCFA). W.G.K. is the Murphy Family Professor of Children's Oncology Research, an Empire Scholar of the State University of NY and a Senior Scholar of the CCFA.
3. Results
3.1. Mobilization of an HS-PC Graft Capable of Long-Term, Multi-lineage Repopulation and Radioprotection
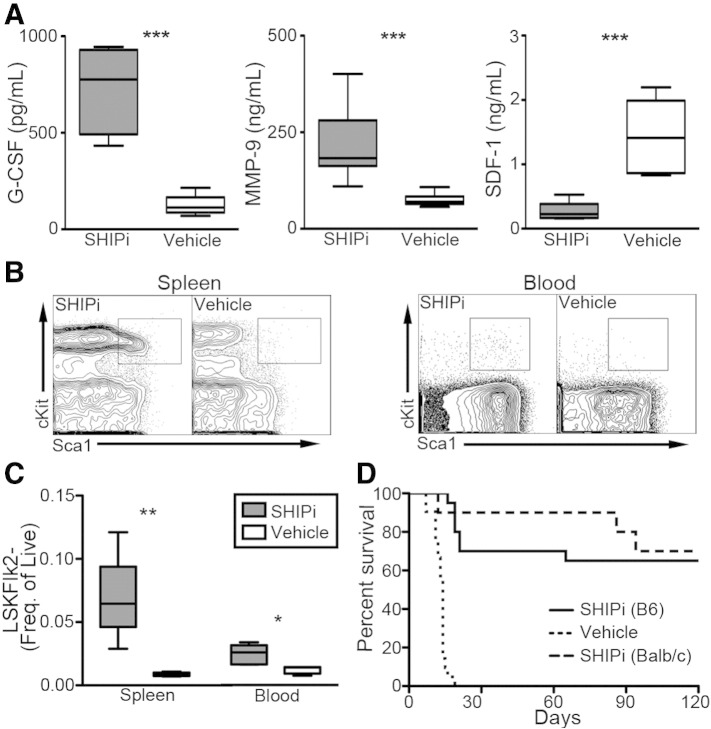
We previously found that SHIP1 has a significant role in HSC niche function and in BM retention of HSC (Desponts et al., 2006, Hazen et al., 2009, Iyer et al., 2014b). SHIP1-deficiency significantly reduces SDF1-CXCL12 expression by niche cells, increases G-CSF and MMP-9 production, and promotes significant mobilization of HSC to the periphery (Desponts et al., 2006, Hazen et al., 2009, Iyer et al., 2014b). We hypothesized that in vivo treatment with SHIPi would have a similar effect on the BM niche and HSC compartments. To determine this, wild type (WT) mice were treated with SHIPi and blood was collected for plasma cytokine analysis as well as phenotypic analysis of peripheral tissues by flow cytometry. Consistent with our observations in mice with germline SHIP1 deficiency (Hazen et al., 2009) G-CSF and MMP-9 levels were profoundly increased and SDF1-CXCL12 levels were significantly reduced following SHIPi (Fig. 1A). SHIPi also triggered significant mobilization of Lin−Sca1+cKit+Flk2− (LSKF-) HS-PC population (Christensen and Weissman, 2001) to the spleen and peripheral blood (Fig. 1B, C). To determine whether the HS-PCs mobilized through SHIPi treatment retained functional stem cell capabilities, we evaluated the capacity of peripheral blood from SHIPi treated donors to protect recipients from a lethal dose of radiation. Transplant of RBC lysed WBC from both SHIPi treated B6 or BALB/c donors afforded significant, long-term survival to 65% (13/20) and 70% (7/10) of their respective hosts (Fig. 1D). Importantly, WBC from vehicle treated donors exhibited no radioprotective capacity, consistent with the findings of Micklem et al. that murine peripheral blood lacks significant HSC activity (Micklem et al., 1975).
Fig. 1.
SHIP1 inhibition mobilizes hematopoietic stem-progenitor cells. (A) G-CSF, MMP-9 and SDF1-CXCL12 concentrations are significantly increased in the plasma of mice following 7-day treatment with SHIPi [n = 5, t-test ***p < 0.001]. (B) cKit+Sca1+ contour plots after gating on Lin−Flk2− stem-progenitor population in the spleen and blood of SHIPi and vehicle treated mice [representative from 2 independent experiments, n = 5]. (C) Lin−Sca1+cKit+Flk2− (LSKF−) HS-PC frequencies are significantly increased in the spleen and blood of SHIPi compared to vehicle controls [t-test, *p < 0.05 **p < 0.01]. (D) Enhanced survival of lethally irradiated hosts transplanted with 7.5 × 105 WBC from 3AC-B6, 3AC-BALB/c or vehicle (B6+BALB/c) treated donors [pooled from two experiments, n = 10 per strain per group].
3.2. Facilitation of Congenic Transplantation at Non-ablative Radiation Doses
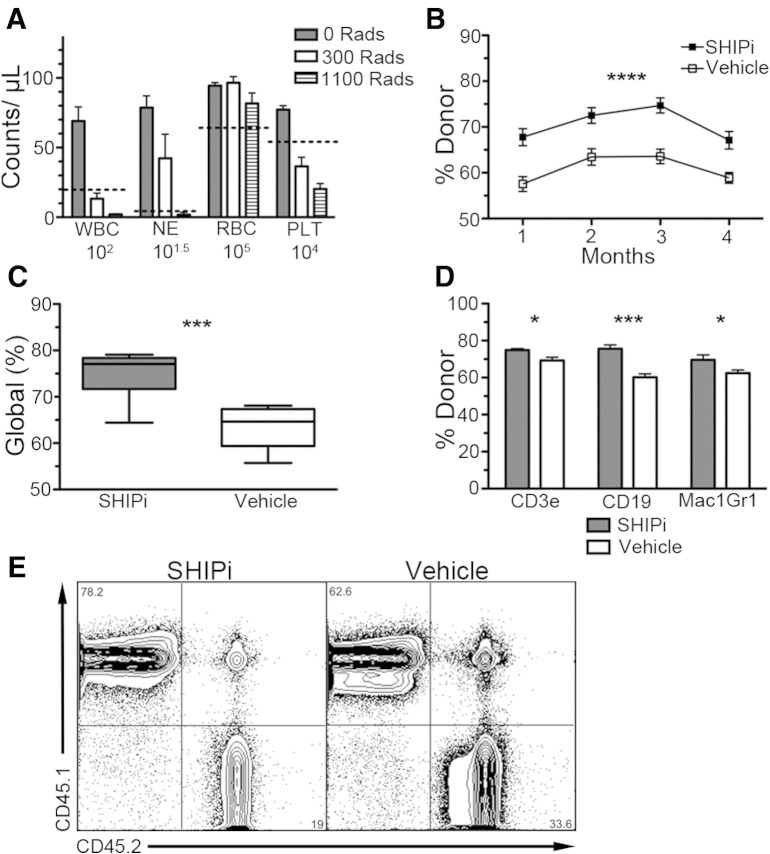
Intense pre-conditioning regimens are currently required to deplete the endogenous HSC compartment. This allows donor HSC to effectively compete for space in the BM HSC niche in order to rapidly reconstitute the hematolymphoid system. We considered that the mobilization of Sca1+c-Kit+Lin−Flk2− HS-PC induced by SHIPi (Fig. 1B, C) might create a space for engrafting HS-PC without the requirement for significant cytoablation. For this purpose, following SHIPi or vehicle treatment, we conditioned B6.2 host mice using a minimally ablative dose of 300 Rads (Fig. 2A) prior to transplant with congenic B6.1 BM. We found that even at these low radiation doses, SHIPi significantly improved long-term engraftment of congenic BM cells (Table 1, Fig. 2B–E), as both global and lineage-specific engraftment of congenic cells was increased in SHIPi relative to vehicle-treated hosts.
Fig. 2.
SHIP1 inhibition enhances autologous engraftment following minimally ablative conditioning. (A) Irradiation of mice at 300 Rads is minimally ablative such that key blood cell components [WBC, neutrophils (NE), RBC and platelets (PLT)] are not dramatically decreased at 7 days post-irradiation [dashed line indicated lower end of normal range, pooled from 2 independent experiments, 0 and 300 Rads n = 5, 1100 Rads n = 2, survival of all mice receiving 1100 Rad was < 15 days.] (B–E) B6.2 hosts were treated with SHIPi or vehicle for 7 days and irradiated at 300 Rads on day 8 prior to transplant with 5 × 106 B6.1 congenic BM cells on days 8, 9 and 10 [pooled from two experiments using n = 5]. (B) Long-term engraftment of congenic BM in the blood was significantly higher in SHIPi vs vehicle-treated mice as determined by flow cytometry [n = 9 SHIPi and n = 8 vehicle, mean ± SEM, 2-way ANOVA, p < 0.0001]. Enhanced global (C) and lineage specific (D) repopulation of host with congenic donor BM at 3 months post-transplant [t-test *p < 0.05, ***p < 0.001]. (E) Representative flow cytometry plots for engraftment at 3 months post-transplant.
3.3. SHIPi Increases MDSC and Treg Cells and Causes Hyporesponsiveness by the NK Cell Compartment
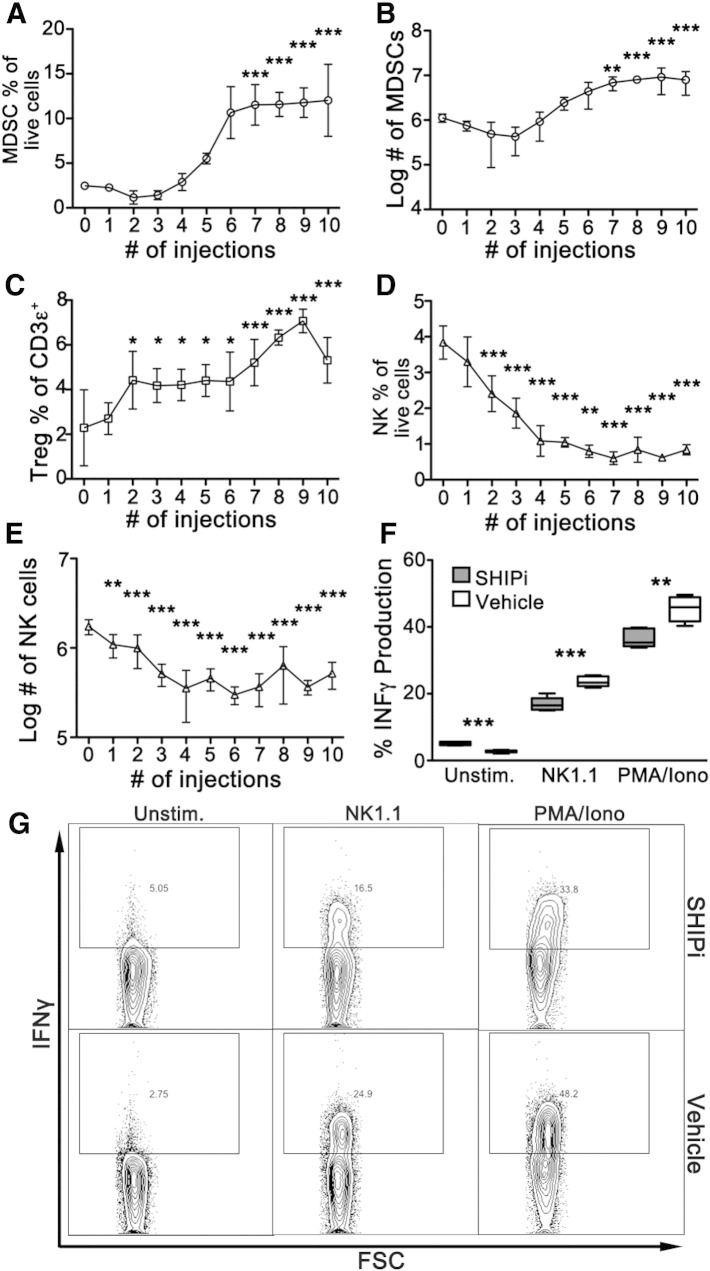
As mentioned previously, germline and induced SHIP-deficient mice are characterized by substantially increased numbers of MDSCs (Ghansah et al., 2004, Paraiso et al., 2007) and Tregs (Collazo et al., 2009) and display a severely compromised NK cell compartment (Wahle et al., 2006, Wang et al., 2002, Fortenbery et al., 2010). Moreover, we had previously shown that treatment of WT mice with SHIPi caused a dramatic increase in MDSCs in both the spleen and mesenteric lymph node (Brooks et al., 2010). To extend these analyses, we evaluated the effect of SHIPi treatment on splenic NK, Treg and MDSC cell numbers. For this purpose, B6 mice were treated daily for 10 consecutive days, and the frequency and absolute numbers of these cell types were observed. As shown in Fig. 3A and B, both the frequency and the absolute numbers of MDSC were significantly increased by SHIPi and reached a plateau after 7 days. While total T-cell numbers were slightly decreased during the course of SHIPi (data not shown), the frequency of CD4+FoxP3+ Treg cells was significantly increased over the treatment period (Fig. 3C). Concurrently, frequency and absolute number of NK cells were dramatically reduced following 2 daily injections of SHIPi and reached a nadir at 6–7 days as compared to controls (Fig. 3D, E). Cytokine production after NK cell activation is a major effector function of this cell type. NK cell effector function is compromised by SHIP1 deficiency in genetic mouse models, including when SHIP1 is selectively deleted in the NK cell lineage of NCR1CreSHIPflox/flox mice (Gumbleton et al., in press). To determine if SHIPi treatment results in NK cell hyporesponsiveness, mice were treated with SHIPi for 6 days. On the seventh day splenocytes were harvested and the ability of splenic NK cells to produce IFNγ following ex vivo crosslinking of an activating receptor was then assessed as a measurement of the functionality of the NK cell compartment (Biron et al., 1999). SHIPi treatment of mice resulted in significantly lower production of IFNγ after NK1.1 receptor crosslinking as compared to vehicle treated mice (Fig. 3F, G). Furthermore, NK cells from SHIPi treated mice also had significantly lower IFNγ production when treated with PMA and ionomycin. Therefore, the NK cells in SHIPi treated mice display lower levels of effector functions using both receptor-specific and unspecific stimulation. These findings suggest they are in a state of general hyporesponsiveness. Taken together, the cellular milieu promoted by SHIPi treatment could contribute to decreased allogeneic immune cell responses and enables better engraftment of allogeneic HS-PCs.
Fig. 3.
SHIPi treatment increases MDSCs, Tregs and decreases NK cell numbers and reduces their effector function. (A–E) B6.2 mice received indicated number of daily injection of SHIPi. 14 h after the last injection, splenocytes were harvested, counted and stained for Mac1, Gr1, and NK1.1 or fixed and stained for CD3ε, CD4, and FoxP3 and then analyzed by flow cytometry [mean ± SD, n = 5, 1-way ANOVA with Dunnett's Multiple Comparison Test between control (0 injections) and each time point, *p < 0.05, **p < 0.01, ***p < 0.001]. Mac1+Gr1+ double positive cells (MDSC) reached significantly higher frequencies (A) and absolute numbers (B) after 7 consecutive days of SHIPi treatment, as did the frequency of regulatory (CD4+FoxP3+) T-cells (C). NK1.1+ cells were significantly decreased starting at 2 daily injections and reached the lowest levels at 7 and 6 days of daily injections in terms of frequency (C) and absolute numbers (D), respectively. (E–F) IFNγ production was measured via intracellular flow cytometry on NKp46+CD3− splenocytes harvested from 6-day SHIPi or vehicle treated mice. Splenocytes were stimulated with NK1.1 (center), PMA and ionomycin (right) or left unstimulated (Unstim., left) for 5 h prior to IFNγ staining and analysis [representative experiment of three independent experiments is shown with n = 5, t-test **p < 0.01, *** p < 0.001]. (F) Representative flow cytometry plots from E.
3.4. SHIPi Facilitates Hematologic Recovery and Engraftment of MHC-I Unmatched BM Grafts
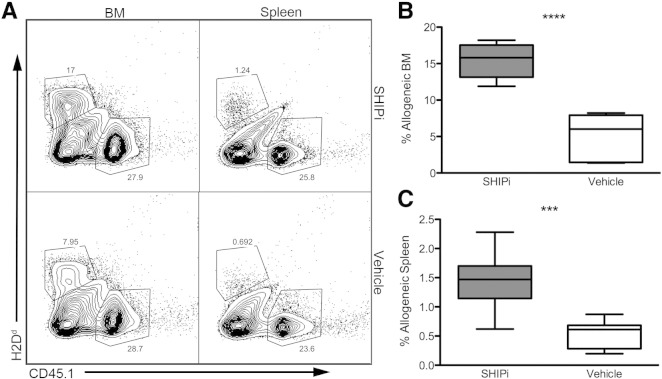
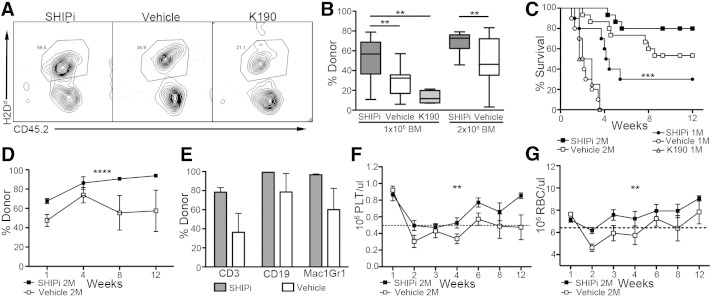
To assess the effects of SHIPi treatment on allogeneic BM engraftment, WT B6.2 hosts were treated with SHIPi or vehicle and were lethally irradiated prior to transplant with an equal mixture of congenic (B6.1) and MHC-I mismatched (BALB/c) BM cells (Table 1). The frequency of allogeneic cells found in BM and in the spleens of SHIPi or vehicle conditioned hosts was assessed by flow cytometry. Analysis of the acute engraftment of the mixed BM graft revealed that SHIPi treatment of host significantly increased frequencies of H2d+ cells found in both the BM (Fig. 4A left panels and B) and the spleen (Fig. 4A right panels and C) as compared to vehicle controls. These findings suggest that treatment with SHIPi reduced the acute rejection of the MHC-I mismatched BM graft in this fully ablative setting. We then assessed whether SHIPi facilitates engraftment of a mismatched MHC-I BM graft without a congenic co-graft following fully ablative conditioning. Using low doses of allogeneic BM (1 × 106 and 2 × 106 total cells, Table 1), we observed that both short (Fig. 5A, B) and long-term marrow repopulation activity (Fig. 5D) was improved relative to both vehicle and steroidal controls (Fig. S1A–C). Increased allogeneic engraftment at these low transplant doses was also reflected in significantly improved post-transplant survival (Fig. 5C), increased lineage specific repopulation (Fig. 5E) and in accelerated recovery of all major blood cell components (Fig. 5F–G, and Fig. S1D–E). Thus, SHIPi lowers the immune barrier to engraftment following allogeneic BMT, consistent with previous findings in genetically SHIP1-deficient hosts (Wang et al., 2002, Paraiso et al., 2007, Collazo et al., 2009, Wahle et al., 2006).
Fig. 4.
SHIP1 inhibition and myeloablative conditioning reduces the immune barrier to MHC mismatched BMT. Host B6.2 mice were treated daily for 7 days with SHIPi or vehicle and lethally irradiated (1100 Rads) on day 8, prior to transplant with 5 × 106 allogeneic BALB/c and 5 × 106 congenic B6.1 BM cells. (A) Representative flow cytometry plots of allogeneic (CD45.1−, H2d+) and congenic (CD45.1+, H2d−) engraftment in the BM (left) and spleen (right) in SHIPi (top) or vehicle (bottom) treated hosts at 5 days post-transplant. (B–C) Relative frequency of allogeneic cells in the BM (B) and spleen (C) of SHIPi and vehicle treated hosts [pooled from 2 experiments, n = 9 total, t-test ***p < 0.001, ****p < 0.0001]. No significant differences were observed in congenic engraftment (not shown).
Fig. 5.
SHIP1 inhibition and myeloablative conditioning enhances long-term engraftment and survival following fully MHC mismatched BMT. (A–G) Host B6.2 mice were treated for 7 days with SHIPi, vehicle, or steroidal control K190 and lethally irradiated (1100 Rads) on day 8, prior to transplant with 1 × 106 or 2 × 106 BALB/c BM cells. (A) Representative flow cytometry plots for engraftment in the blood at 7 days post-transplant of 1 × 106 allogeneic BM cells (H2Dd +, CD45.2+) in B6.2 hosts (H2Dd −, CD45.2+). (B) Percent engraftment in the blood at 7 days post-transplant measured by flow cytometry following BMT with 1 × 106 BALB/c BM cells [for 3AC vs vehicle pooled from 2 experiments with n = 5, K190 vs vehicle, n = 4, as in A] or 2 × 106 BALB/c BM cells [pooled from 3 experiments with n = 5] [t-test **p < 0.01]. (C) Enhanced survival of SHIPi vs vehicle or steroidal control-treated mice following myeloablative and fully allogeneic BMT Mantel-Cox log rank tests of significance from Kaplan–Meier survival curves, ***p < 0.001. Global (D) and lineage specific (E) repopulation at 3 months following myeloablative conditioning and allogeneic BMT in SHIPi vs vehicle conditioned hosts [2 × 106 BALB/c BM cells, 2 way-ANOVA ****p < 0.0001]. Enhanced recovery of PLT (F) and RBC (G) following myeloablative conditioning and allogeneic BMT in SHIPi vs vehicle conditioned hosts [2 × 106 BALB/c BM cells, 2 way-ANOVA **p < 0.01]. Dotted line marks lower end of normal range for each blood component.
4. Discussion
Our previous studies in mice with germline or induced ablation of SHIP1 expression suggested that chemical inhibition of SHIP1 in vivo might recapitulate genetic phenotypes that reduce the immune barrier to allogeneic HSCT and or enhance mobilization of HSC (Wang et al., 2002, Ghansah et al., 2004, Wahle et al., 2006, Desponts et al., 2006, Paraiso et al., 2007, Collazo et al., 2009, Hazen et al., 2009). The study presented here reveals that SHIPi does indeed modulate several key components of the immune system (NK cells were shown to be decreased and hyporesponsive, and MDSC and T regulatory cells were significantly increased) such that we observed considerably improved marrow repopulating activity following transplant of allogeneic BM. Consequently, inhibition of SHIP1 by small molecule therapeutics could have a profound impact on the utility of allogeneic HSCT. Indeed, brief inhibition of SHIP1 failed to display toxicity at doses that allowed for improved engraftment in fully mismatched MHC-I BM graft. By extension of these studies, eventually SHIP1 may prove to be a valuable molecular target that could help facilitate allogeneic grafts particularly for patients who lack an HLA matched donor.
Mobilization of HS-PCs by G-CSF administration as it is currently practiced is a very effective and safe procedure. However, since a subset of patients and donors fail to mobilize sufficient numbers of HS-PCs to permit engraftment, other alternatives such as CXCR4 antagonists (AMD-3100) have been developed (Broxmeyer et al., 2005). Clinical trials demonstrated that combining this antagonist, AMD3100-Plerixafor, with G-CSF improved stem cell harvest and reduced the incidence of failure (Dipersio et al., 2009a, Dipersio et al., 2009b). Nonetheless, even with this combination of mobilizing agents, failure rates of ~ 7% are still observed (Hosoba and Waller, 2014). New molecular targets are therefore actively sought to further improve HSC mobilization for HSCT and also in the treatment of vascular diseases (e.g. myocardial infarction, peripheral artery disease or coronary artery disease), where periodic HSC mobilization by G-CSF is believed to facilitate vascular repair (Poole et al., 2013, Seiler et al., 2001). Our data show that SHIPi can mobilize significant numbers of HS-PC such that a single blood draw from a donor can provide for radioprotection and long-term reconstitution in the majority of hosts. The SHIPi mobilized graft may therefore represent a new methodology to mobilize patient or donor HSC for HSCT therapies. This could be applied in settings where donors fail G-CSF mobilization, or be used in concert with G-CSF and-or AMD3100, to further increase the yield and efficiency of HS-PC mobilization procedures.
Minimally ablative procedures in allogeneic HSCT have seen increased utilization in various clinical settings, but there has also been a concomitant increase in graft failure (Mattsson et al., 2008). The mobilization of HS-PC triggered by SHIPi might also be used in lieu of such cytoablative regimens, or perhaps in combination with minimally ablative chemotherapeutic regimen to decrease the incidence of graft failure. In several diseases, and particularly severe autoimmune diseases, there is a growing effort to use autologous or allogeneic BMT to ‘reboot’ the patient's immune system (Li and Sykes, 2012). However, HSCT can be associated with significant morbidity and mortality and thus is only utilized in the most severe cases. As the SHIPi conditioning regimen we describe poses no significant risk to host viability (Brooks et al., 2010), we propose that SHIPi might eventually enable a great proportion of patients to benefit from these autologous BMT procedures, at least in part by creating space in the BM niche for engrafting HSC.
In our previous studies, it was observed that germline SHIP1 deficiency caused dramatic alterations in the receptor repertoire such that rejection of MHC-I mismatched BM was severely compromised (Wang et al., 2002, Wahle et al., 2006). More recently, NK cell-specific deletion of SHIP1 has been shown to alter the receptor repertoire and impair induction of INFγ upon receptor crosslinking (Gumbleton et al., in press). Moreover, SHIP1 deficiency in NK cells also enhances engraftment of an MHC-I unmatched BM graft (Gumbleton et al., in press). Interestingly, both receptor specific and general activation of NK cells are compromised by daily, extended treatment with SHIPi. Therefore, alterations of SHIP1 signaling within the NK cell prevent rejection of cells bearing mismatched MHC-I antigens. Furthermore, it is well documented that SHIP-deficiency, whether it be in germline (Ghansah et al., 2004), induced (Paraiso et al., 2007) or triggered by SHIPi (Brooks et al., 2010), causes a dramatic increase in MDSCs that can modulate T-effector functions. Overall, the effects of SHIPi on the both the NK and the myeloid cell compartments are believed to be the important factors in facilitating the observed increase in allogeneic BM engraftment. Finally, germline SHIP-deficiency has been shown to cause increased Treg cell numbers (Collazo et al., 2009, Ghansah et al., 2004, Paraiso et al., 2007), and splenocytes from SHIPi treated mice and SHIPi treated peripheral blood mononuclear cells (PBMC) display reduced priming of allogeneic responses in one-way mixed leukocyte reactions (Brooks et al., 2010). We propose that the significant increase in CD4+FoxP3+ regulatory T-cell frequency observed here also contributed to the immunoregulatory environment, which allowed for better engraftment of allogeneic BM grafts as host Treg cells can also promote MHC-I mismatched BM engraftment (Fujisaki et al. 2011).
In summary, similarly to that observed in germline SHIP1-deficient mice, chemical inhibition of SHIP1 was observed to significantly enhance HS-PC mobilization and engraftment in a variety of settings, including minimally ablative conditioning for autologous transplantation and in a fully ablative MHC-I mismatched setting. These findings further confirm a role for SHIPi in several aspects of HSCT and suggest that SHIPi could prove to be an attractive therapeutic target to improve HSCT outcomes.
Author contributions
S.F., R.B., M.G., and W.G.K. designed the research, analyzed the data and wrote the manuscript. S.F., R.B., M.G. and M.Y.P. performed experiments. S.F. and R.B. contributed equally. J.D.C., C.M.R. and K.T.H. synthesized 3AC and developed and synthesized K190.
Disclosures
W.G.K., S.F., R.B., M.G., and J.D.C. have patents, filed, pending and issued, concerning the analysis and targeting of SHIP1 in disease. The other authors have no conflicts to disclose.
Acknowledgments
The authors wish to thank B. Toms and C. Youngs for technical assistance, and S. Neelam and K.H. Miller for generating recombinant SHIP1 protein and in vitro enzymatic assays, respectively.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.02.004.
Appendix A. Supplementary data
Supplemental Figure 1, and Supplemental Experimental Procedures.
ARRIVE checklist
References
- Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Brooks R., Fuhler G.M., Iyer S., Smith M.J., Park M.Y., Paraiso K.H., Engelman R.W., Kerr W.G. SHIP1 inhibition increases immunoregulatory capacity and triggers apoptosis of hematopoietic cancer cells. J. Immunol. 2010;184:3582–3589. doi: 10.4049/jimmunol.0902844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R., Iyer S., Akada H., Neelam S., Russo C.M., Chisholm J.D., Kerr W.G. Coordinate expansion of murine hematopoietic and mesenchymal stem cell compartments by SHIPi. Stem Cells. 2014 doi: 10.1002/stem.1902. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., Hangoc G., Cooper S., Plett P.A., Liles W.C., Li X., Graham-Evans B., Campbell T.B., Calandra G., Bridger G., Dale D.C., Srour E.F. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J.L., Weissman I.L. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo M.M., Wood D., Paraiso K.H., Lund E., Engelman R.W., Le C.T., Stauch D., Kotsch K., Kerr W.G. SHIP limits immunoregulatory capacity in the T-cell compartment. Blood. 2009;113:2934–2944. doi: 10.1182/blood-2008-09-181164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.M., Ruggieri L., Defor T., Wagner J.E., Weisdorf D.J., Miller J.S., Velardi A., Blazar B.R. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- Desponts C., Hazen A.L., Paraiso K.H., Kerr W.G. SHIP deficiency enhances HSC proliferation and survival but compromises homing and repopulation. Blood. 2006;107:4338–4345. doi: 10.1182/blood-2005-12-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipersio J.F., Micallef I.N., Stiff P.J., Bolwell B.J., Maziarz R.T., Jacobsen E., Nademanee A., McCarty J., Bridger G., Calandra G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J. Clin. Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- Dipersio J.F., Stadtmauer E.A., Nademanee A., Micallef I.N., Stiff P.J., Kaufman J.L., Maziarz R.T., Hosing C., Fruehauf S., Horwitz M., Cooper D., Bridger G., Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- Ferrara J.L., Levine J.E., Reddy P., Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. PMCID: 2735047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenbery N.R., Paraiso K.H., Taniguchi M., Brooks C., Ibrahim L., Kerr W.G. SHIP influences signals from CD48 and MHC class I ligands that regulate NK cell homeostasis, effector function, and repertoire formation. J. Immunol. 2010;184:5065–5074. doi: 10.4049/jimmunol.0901862. [DOI] [PubMed] [Google Scholar]
- Fuhler G.M., Brooks R., Toms B., Iyer S., Gengo E.A., Park M.Y., Gumbleton M., Viernes D.R., Chisholm J.D., Kerr W.G. Therapeutic potential of SH2 domain-containing inositol-5′-phosphatase 1 (SHIP1) and SHIP2 inhibition in cancer. Mol. Med. 2012;18:65–75. doi: 10.2119/molmed.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J., Wu J., Carlson A.L., Silberstein L., Putheti P., Larocca R., Gao W., Saito T.I., Lo Celso C., Tsuyuzaki H., Sato T., Cote D., Sykes M., Strom T.B., Scadden D.T., Lin C.P. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghansah T., Paraiso K.H., Highfill S., Desponts C., May S., Mcintosh J.K., Wang J.W., Ninos J., Brayer J., Cheng F., Sotomayor E., Kerr W.G. Expansion of myeloid suppressor cells in SHIP-deficient mice represses allogeneic T cell responses. J. Immunol. 2004;173:7324–7330. doi: 10.4049/jimmunol.173.12.7324. [DOI] [PubMed] [Google Scholar]
- Gumbleton M., Vivier E., Kerr W.G. SHIP1 intrinsically regulates NK cell signaling and education resulting in tolerance of a MHC-I mismatched BM graft in mice. J. Immunol. 2015 doi: 10.4049/jimmunol.1402930. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen A.L., Smith M.J., Desponts C., Winter O., Moser K., Kerr W.G. SHIP is required for a functional hematopoietic stem cell niche. Blood. 2009;113:2924–2933. doi: 10.1182/blood-2008-02-138008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoba S., Waller E.K. New molecule for mobilizing marrow stem cells. Blood. 2014;123:310–311. doi: 10.1182/blood-2013-12-538249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S., Viernes D.R., Chisholm J.D., Margulies B.S., Kerr W.G. SHIP1 regulates MSC numbers and their osteolineage commitment by limiting induction of the PI3K/Akt/beta-catenin/Id2 axis. Stem Cells Dev. 2014;23:2336–2351. doi: 10.1089/scd.2014.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S., Brooks R., Gumbleton M., Kerr W.G. SHIP1-expressing mesenchymal stem cells regulate hematopoietic stem cell homeostasis and lineage commitment during aging. Stem Cells Dev. 2014 doi: 10.1089/scd.2014.0501. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwada M., Cattoretti G., Mckeag L., Rouse T., Showalter B.M., Al-Alem U., Niki M., Pandolfi P.P., Field E.H., Rothman P.B. Downstream of tyrosine kinases-1 and Src homology 2-containing inositol 5′-phosphatase are required for regulation of CD4+CD25 + T cell development. J. Immunol. 2006;176:3958–3965. doi: 10.4049/jimmunol.176.7.3958. [DOI] [PubMed] [Google Scholar]
- Kerr W.G., Park M.Y., Maubert M., Engelman R.W. SHIP deficiency causes Crohn's disease-like ileitis. Gut. 2011;60:177–188. doi: 10.1136/gut.2009.202283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.W., Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat. Rev. Immunol. 2012;12:403–416. doi: 10.1038/nri3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke N.R., Patterson S.J., Hamilton M.J., Sly L.M., Krystal G., Levings M.K. SHIP regulates the reciprocal development of T regulatory and Th17 cells. J. Immunol. 2009;183:975–983. doi: 10.4049/jimmunol.0803749. [DOI] [PubMed] [Google Scholar]
- Mattsson J., Ringden O., Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2008;14:165–170. doi: 10.1016/j.bbmt.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem H.S., Anderson N., Ross E. Limited potential of circulating haemopoietic stem cells. Nature. 1975;256:41–43. doi: 10.1038/256041a0. [DOI] [PubMed] [Google Scholar]
- Paraiso K.H., Ghansah T., Costello A., Engelman R.W., Kerr W.G. Induced SHIP deficiency expands myeloid regulatory cells and abrogates graft-versus-host disease. J. Immunol. 2007;178:2893–2900. doi: 10.4049/jimmunol.178.5.2893. [DOI] [PubMed] [Google Scholar]
- Park M.Y., Srivastava N., Sudan R., Viernes D.R., Chisholm J.D., Engelman R.W., Kerr W.G. Impaired T-cell survival promotes mucosal inflammatory disease in SHIP1-deficient mice. Mucosal Immunol. 2014;7:1429–1439. doi: 10.1038/mi.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos C.M., Wrzesinski C., Kaiser A., Hinrichs C.S., Chieppa M., Cassard L., Palmer D.C., Boni A., Muranski P., Yu Z., Gattinoni L., Antony P.A., Rosenberg S.A., Restifo N.P. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8 + T cells via TLR4 signaling. J. Clin. Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole J., Mavromatis K., Binongo J.N., Khan A., Li Q., Khayata M., Rocco E., Topel M., Zhang X., Brown C., Corriere M.A., Murrow J., Sher S., Clement S., Ashraf K., Rashed A., Kabbany T., Neuman R., Morris A., Ali A., Hayek S., Oshinski J., Yoon Y.S., Waller E.K., Quyyumi A.A. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:2631–2639. doi: 10.1001/jama.2013.282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L., Capanni M., Urbani E., Perruccio K., Shlomchik W.D., Tosti A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (New York, NY. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Seiler C., Pohl T., Wustmann K., Hutter D., Nicolet P.A., Windecker S., Eberli F.R., Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation. 2001;104:2012–2017. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- Shlomchik W.D. Graft-versus-host disease. Nat. Rev. Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- Tiwari S., Choi H.P., Matsuzawa T., Pypaert M., Macmicking J.D. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat. Immunol. 2009;10:907–917. doi: 10.1038/ni.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle J.A., Paraiso K.H., Costello A.L., Goll E.L., Sentman C.L., Kerr W.G. Cutting edge: dominance by an MHC-independent inhibitory receptor compromises NK killing of complex targets. J. Immunol. 2006;176:7165–7169. doi: 10.4049/jimmunol.176.12.7165. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Howson J.M., Ghansah T., Desponts C., Ninos J.M., May S.L., Nguyen K.H., Toyama-Sorimachi N., Kerr W.G. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295:2094–2097. doi: 10.1126/science.1068438. [DOI] [PubMed] [Google Scholar]
- Yuan T.L., Cantley L.C. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1, and Supplemental Experimental Procedures.
ARRIVE checklist