Abstract
The aim of the present study was the direct covalent coupling of the epidermal growth factor receptor (EGFR)-specific monoclonal antibody (mAb) to the surface of poly(lactide)-co-glycolide (PLGA)-polyethylene glycol (PEG) nanoparticles in order to achieve a cell type-specific drug carrier system against pancreatic cancer. The PLGA-PEG-NH2 diblock copolymer was synthesized by coupling reaction via amide linkage between PEG-diamine and activated PLGA. PLGA and PLGA-PEG-NH2 nanoparticles loaded with gemcitabine were prepared using the double-emulsion solvent evaporation method. PLGA-PEG immunonanoparticles were prepared by glutaraldehyde mediated cross-linking method. The conjugated antibody was analysed by transmission electron microscopy and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) analysis. Cell viability study was performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and cell uptake study was performed on fluorescein isothiocyanate-loaded formulations using confocal microscopy. The PAGE results indicated that mAb integrity was remained intact in the formulations after conjugation. Biological activity was confirmed under cell culture conditions: antibody-conjugated nanoparticles showed specific targeting to EGFR-overexpressing MIA PaCa-2 cell lines as shown in fluorescence image using confocal microscopy. The obtained data provide the basis for the development of stable and biologically active carrier systems for direct targeting of tumour cells using antibody-conjugated PLGA-PEG nanoparticles. Direct covalent coupling of antibodies to nanoparticles using glutaraldehyde as a cross-linker is an appropriate method to achieve cell type-specific drug carrier systems based on PLGA-PEG nanoparticles and the anti-EGFR-decorated PLGA-PEG nanoparticles have potentials to be applied for targeted chemotherapy against EGFR positive cancers.
Keywords: Adenocarcinoma, Epidermal growth factor receptor, Targeting, Gemcitabine, Multifunctional nanoparticles, MIA PaCa-2
Introduction
Adenocarcinoma of the exocrine pancreas is the fourth leading cause of cancer deaths in the USA (Berger et al. 2004). Currently, surgery is the only treatment although, due to its late presentation, only 9–15 % of the patients are suitable for surgery. The median survival for all stages of pancreatic cancer is 3–5 months from diagnosis (Shore et al. 2004). The underlying problem in the use of anticancer drugs is their toxicity and poor bioavailability (Langer 1998). It is expected that if a delivery system-bearing anticancer drugs can be delivered in a targeted fashion, inhibition of tumour growth with reduced systemic toxicity will occur. Therefore, new therapeutic strategies are necessary to combat this deadly disease. Antibodies are well-established systems to target drugs or colloidal carriers to specific cell types. This principle is based on a defined receptor ligand interaction which enables the surface binding and subsequent cellular internalisation of drugs or drug carriers conjugated to the antibody. Chemical modification of the antibody is necessary to enable conjugation with a drug carrier, but at the same time it is a prerequisite that while performing conjugation reactions, biological activity and full receptor binding of the antibody is maintained. Epidermal growth factor receptor (EGFR) monoclonal antibody is directed against the extracellular domain of the receptor. EGFR belongs to a receptor tyrosine-specific protein kinase family, the EGFR family, which consists of four EGF receptors, EGFR (ErbB1), ErbB2 (Neu), ErbB3, and ErbB4. Members of the EGFR family contain a cytoplasmic tyrosine kinase domain, a single transmembrane domain, and an extracellular domain that is involved in ligand binding and receptor dimerisation (Ferguson et al. 2003; Olayioye et al. 2000). Overexpression of EGFR at the cell surface may lead to a spontaneous formation of homodimers, but more likely the availability of the receptor for ligand-driven heterodimerisation increases (Jorissen et al. 2003). EGFR overexpression translates into signals that potentiate dysregulated growth, oncogenesis, metastasis, and possibly resistance against chemotherapeutic agents. EGFR is amplified at 50–70 % incidence in human pancreatic cancer (Dancer et al. 2007). The EGFR monoclonal antibody (mAb) acts in a cytostatic manner by blocking and downmodulation of the EGFR receptor and by antibody-dependent cellular cytotoxicity as major mechanism of antibody action (Ennis et al. 1991; Xiong and Abbruzzese 2002). It induces EGFR internalisation and degradation. EGFR mAb offers an excellent strategy for drug targeting to cancer cells because EGF is an easily accessible cell surface receptor overexpressed on the primary tumour as well as on metastatic sites (Bloomston et al. 2006). The internalisation ability of EGFR allows an efficient uptake of the antibody alone as well as conjugated to drugs or drug carrier systems.
Colloidal systems such as nanoparticles provide higher drug-carrying capacities than antibodies and, therefore, are especially suited for conjugation to antibodies. Poly(lactide)-co-glycolide (PLGA)-based nanoparticles represent a colloidal drug carrier system with high drug-loading capacity. These particles are biodegradable, non-antigenic, non-irritative for tissues, and nontoxic (Mundargi et al. 2008). Drugs can be incorporated within the particle matrix, adsorbed on the particle surface or bound by covalent linkage. Especially incorporation in the particle matrix protects the drug against degradation and enables controlled release.
The aim of the present study was the direct covalent coupling of the EGFR mAb to the surface of PLGA-polyethylene glycol (PEG) nanoparticles in order to achieve a cell type-specific drug carrier system against pancreatic cancer.
Materials and methods
Gemcitabine hydrochloride was generously gifted by Sun Pharma (SUPAC, Hyderabad). Poly (d,l-lactic-co-glycolic acid) 50:50, polyethylene glycol bis amine (PEG diamine), anti-EGFR mAb were purchased from Sigma Chemical Laboratories Pvt. Ltd. Mumbai. All other chemicals were purchased from HiMedia, Bombay and CDH Laboratory Reagents, New Delhi and were of analytical grade.
Cell line
The cell line, MIA PaCa-2 cell line (human pancreatic carcinoma) was obtained from National Centre for Cell Sciences, Pune, India. Cell line was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10 % (v/v) heat-inactivated foetal bovine serum and horse serum (2.5 %). Cells were maintained in 5 % CO2-humidified incubator at 37 °C. During subculture, cells were detached by trypsinization when they reached 80 % confluency and split (1:4). Growth medium was changed every 3 days.
Preparation of PLGA-PEG-NH2 diblock copolymer
The PLGA-PEG diblock copolymer was synthesized by coupling reaction via amide linkage between PEG diamine and activated PLGA (Esmaeili et al. 2008), with minor modifications. The carboxyl terminal end of the PLGA was first activated with N-hydroxysuccinimide (NHS) by using dicyclohexylcarbodiimide (DCC). Briefly, the PLGA (100 mg) was dissolved in DCM (5 ml); and then DCC (5 mg) and NHS (2.5 mg) were added under magnetic stirring. The activation reaction of carboxylic acid end group was accomplished for 12 h at room temperature. Insoluble dicyclohexyl urea was filtered and the resultant solution was precipitated by dropping into ice-cold anhydrous diethyl ether. The activated PLGA was completely dried under vacuum. The NHS ester of the PLGA was conjugated with PEG diamine via amide linkage. The activated PLGA (100 mg) was dissolved in DCM (5 ml) and excess of PEG diamine (35 mg) was added in to this solution. The coupling reaction was performed under magnetic stirring for 9 h at room temperature. An excess amount of PEG diamine was used to prevent the formation of PLGA-PEG-PLGA triblock copolymers. The amine-terminated PEG-PLGA diblock copolymer was precipitated with cold methanol and purified with excess of ethanol (to eliminate unconjugated PEG diamine) and finally dried under vacuum.
Preparation of nanoparticles
PLGA and PLGA-PEG-NH2 NPs loaded with gemcitabine were prepared using the double emulsion solvent evaporation method (Avgoustakis et al. 2002). PLGA polymer (40 mg) or PLGA-PEG-NH2 copolymer (40 mg) was dissolved in 4 ml DCM. An aqueous solution of gemcitabine (V = 1 ml, C = 5 mg/ml) was emulsified in solution while vortexing for 20 s and further sonicated using probe sonicator (ultrasonic liquid processor, Misonix, USA) at an amplitude of 40 W for 1 min to form primary water (w)/oil (o) emulsion. The emulsion was transferred dropwise to an aqueous solution of sodium cholate (V = 20 ml, 12 mM) and the mixture was probe sonicated at an amplitude of 60 W for 3 min to form w/o/w emulsion. The emulsion formed was gently stirred at room temperature until the evaporation of the organic phase was completed. The gemcitabine loaded PLGA NPs (GEM-PLGA NPs) and GEM-PLGA-PEG-NH2 NPs were purified by applying two cycles of centrifugation (22,000 rpm for 20 min) using refrigerated centrifuge (Laborzentrifugen, Sigma 3K30) and reconstituted with deionized and distilled water. Blank NPs were also prepared by the same method without adding gemcitabine at any stage of the preparation.
Characterization of nanoparticles
Characterization of NPs was done on the basis of size, zeta potential and entrapment efficiency. The method used for preparation of NPs was optimized on the basis of change in amount of polymer, concentration of drug and surfactant (Govender et al. 1999; Acharya et al. 2009).
Optimization of process parameters
Optimization of concentration of drug
NPs were optimized for different concentration of gemcitabine (5, 7, and 10 mg/ml) with respect to particle size and entrapment efficiency.
Optimization of amount of polymer
NPs were prepared varying the amount of polymer using 20, 40, and 60 mg of PLGA while keeping the concentration of drug and surfactant constant. The formulations were optimized on the basis of size and entrapment efficiency.
Optimization of concentration of surfactant
Different formulations were prepared using different concentrations of surfactants (8, 10, and 12 mM) while keeping concentration of drug and polymer constant. The formulations were evaluated for particle size and entrapment efficiency.
Size and zeta potential
The average particle size and zeta potential of optimized formulations were determined by photon correlation spectroscopy using Zetasizer (Beckman Coulter, UK). The samples were kept into the cuvettes of zetasizer and the size, polydispersity index (PDI), and zeta potential were measured.
Entrapment efficiency
The method adopted for estimating the entrapment efficiency was a slight modification to the extraction method followed (Blanco and Alonso 1997). The amount of gemcitabine entrapped in NPs was estimated by adding 5 mg of NPs in 1 ml of acetonitrile and shaking NPs vigorously in order to solubilise the polymer so as to extract out the drug followed by addition of 4 ml of PBS 7.4 to solubilise the drug. The amount of gemcitabine was measured by UV spectrophotometer (Shimadzu, Japan) using a detection wavelength of 270 nm. The percentage drug entrapment was measured as:
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to examine the shape and morphology of the NPs (Govender et al. 1999; Acharya et al. 2009). The NPs were placed on sample holder, coated with gold-palladium alloy (150–250 Å) using a sputter coater and then placed in scanning electron microscope.
In vitro drug release
The in vitro drug release profile of entrapped drug from NPs was studied using dialysis membrane. Each dialysis bag (pore size, 12 kDa; Sigma Chemical Co., USA) was loaded with lyophilized preparation equivalent to 2.5 mg of drug. The dialysis bag was then placed in 50 ml PBS (pH 7.4), containing 1 % tween 80, at 37 ± 1 °C and under 100 rpm stirring. Samples (5 ml) were withdrawn at predetermined time interval and replaced with the same volume of fresh dialyzing medium and the withdrawn samples were assayed for drug content by measuring absorbance at 270 nm against the blank using UV spectrophotometer (Li et al. 2001).
Preparation of PLGA-PEG immunonanoparticles
The PLGA-PEG immunonanoparticles (Ab-PLGA NPs) were prepared by two methods.
-
Glutaraldehyde mediated cross-linking.
The Ab-PLGA NPs were prepared with slight modifications in reported method (Hun and Zhang 2009). PLGA-PEG-NH2 NPs were prepared using optimized formulation of PLGA NPs. NPs (4 mg) were dispersed in PBS 7.4 containing glutaraldehyde (25 %) up to a final concentration of 1.25 %, for about 2 h. The excess of glutaraldehyde was separated from NPs using dialysis membrane (12 kDa). The presence of free aldehydic group of glutaraldehyde was detected by addition of Schiff reagent (25 μL of Schiff reagent was added to 250 μL of NPs formulation). Glutaraldehyde-activated NPs were further incubated with anti-EGFR antibody (1 mg/ml) for 12 h at 4 °C with continuous shaking. The anti-EGFR antibody conjugated NPs (glutaraldehyde Ab-PLGA NPs) were centrifuged at 22,000 rpm at 4 °C for 20 min to remove excess antibody and kept at 4 °C in PBS 7.4.
-
MBS cross-linking
The antibody conjugation was also performed by thiolation of mAb with slight modifications in reported method (Steinhauser et al. 2006). The PLGA-PEG-NH2 NPs were dispersed in 1 ml PBS (pH 7.2) and the cross-linker m-maleimidobenzoyl-N-hydroxysuccinimide (MBS; 3.14 mg) was dissolved in 1 ml of dimethyl sulfoxide (DMSO). MBS solution (100 μl) was added in 1 ml NPs dispersion and incubated for 30 min at 25 °C in shaker incubator. The excess of MBS was separated from activated NPs using sephadex G25. For thiolation of anti-EGFR antibody, 5.7 mg of 2-iminothiolane (Traut’s reagent) was dissolved in 5 ml PBS (pH 7.4) and 100 μl anti-HER-2 mAb was incubated with 4.2 μl of 2-iminothiolane for 2 h at 20 °C under constant shaking. Finally, 100 μl thiolated mAb was added in 100 μl MBS-activated NPs for 30 min at 25 °C under constant shaking for preparation of PLGA-PEG immunonanoparticles.
Characterization of Ab-PLGA NPs
Transmission electron microscopy
Transmission electron microscopy (TEM) was done for both the prepared Ab-PLGA NP conjugates (Acharya et al. 2009). Dispersions were visualized under TEM with an accelerating voltage of 100 kV. A drop of the sample was placed on to a carbon-coated copper grid to leave a thin film on the grid. The film was negatively stained with 1 % phosphotungastic acid solution and dried. A drop of the staining solution was added on to the film and the excess of the solution was drained off. The grid was allowed to dry at ambient temperature and samples were viewed under transmission electron microscope (Hitachi, Japan) and photographs were taken at suitable magnification.
Size and zeta potential
The size, PDI, and zeta potential of optimized formulations were determined by photon correlation spectroscopy method using zetasizer (Beckman Coulter). The samples were loaded into the cuvettes of zetasizer and the size, PDI, and zeta potential were measured (Acharya et al. 2009).
Entrapment efficiency
The amount of gemcitabine entrapped in NPs was estimated by adding 5 mg of NPs in 1 ml of acetonitrile and shaking NPs vigorously in order to solubilise the polymer so as to extract out the drug followed by addition of 4 ml of PBS 7.4 to solubilise the drug. The amount of gemcitabine was measured by UV spectrophotometer using a detection wavelength of 270 nm (Blanco and Alonso 1997).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Sodium dodecyl sulphate (SDS) gel electrophoresis is widely used for the analysis and characterization of protein samples. It is useful in molecular weight determination, relative estimation of purity, and amino acid sequencing of proteins. In the present study, conjugation efficiencies were evaluated by SDS-polyacrylamide gel electrophoresis (PAGE), allowing comparison of free mAb fragment vs. conjugated NPs (Flatman et al. 2007). SDS-PAGE was done for both the prepared conjugated NPs.
Optimized Ab-PLGA NPs
The method for the preparation of immunonanoparticles was optimized on the basis of morphology (TEM), size, zeta potential, and entrapment efficiency. The optimized formulation was used for cell line studies.
In vitro evaluation
In vitro evaluation of prepared formulations was carried out on cell lines using MIA PaCa-2 cell line (human pancreatic carcinoma). The studies included cell viability assay (using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye) and cellular uptake studies (using confocal microscopy).
Cell viability assay
Cell viability was tested using MTT assay (Wang et al. 1996; Acharya et al. 2009) based on the cleavage of yellow tetrazolium salt MTT by metabolically active cells to form an orange formazan dye which was quantified using ELISA reader (Biorad, USA, model 680). Cells were seeded in 96-well microtitre plates (2 × 104 cells/200 μL growth medium/well) followed by overnight incubation. Supernatants from the wells were aspirated out and fresh aliquots of growth medium (containing NPs, i.e., GEM-PLGA NPs, blank PLGA NPs, GEM-Ab-PLGA NPs, and blank Ab-PLGA NPs) in desired concentrations in the range of 2–32 μg/mL were added. Blank formulations were used as control. After 24 h, supernatants were aspirated out and the cell monolayers in the wells were washed with 200 μL PBS (0.1 M, pH 7.4). Subsequently, MTT reagent (150 μL, 0.8 mg/mL) was added in each well, incubated for 5 h. DMSO (100 μL) was added in each well after aspirating out the supernatant and incubated at 37 °C for 1 h. Absorbance at two wavelengths (570 nm for soluble dye and 630 nm for cells) were recorded using ELISA reader. Concentrations of samples showing 50 % reduction in cell viability (i.e., IC50 values) were then calculated. An optical density value of control cells (unexposed cells) was taken as 100 % viability (0 % cytotoxicity).
Cell uptake studies
Preparation of fluorescence marker loaded nanoparticles
The NPs encapsulating fluorescence marker (fluorescein isothiocyanate, FITC) was prepared by the double emulsification solvent evaporation technique as described earlier. Polymer or copolymer (40 mg) was dissolved in 4 ml dichloromethane. An aqueous solution of FITC (V = 1 ml, C = 1 mg/ml) was emulsified in solution while vortexing for 20 s and further sonicated using probe sonication (ultrasonic liquid processor, Misonix, USA) at 10 W for 1 min to form primary w/o emulsion. The emulsion was transferred dropwise to an aqueous solution of sodium cholate (V = 20 ml, 12 mM) and the mixture was probe sonicated at 18 W for 3 min to form w/o/w emulsion. The emulsion formed was gently stirred at room temperature until the evaporation of the organic phase was complete. The NPs were purified by applying two cycles of centrifugation (22,000 rpm for 20 min) using refrigerated centrifuge (Laborzentrifugen, Sigma 3 K30) and reconstituted with deionized-distilled water. The prepared NPs were further conjugated to anti-EGFR antibody using glutaraldehyde-mediated cross-linking described previously.
Procedure for cell uptake studies
EGFR positive MIA PaCa-2 cells were seeded on glass cover slips (18 × 18 mm) placed into 35 mm tissue culture plates at a density of 2 × 105 cells (in 2 mL growth medium). After overnight growth, supernatants from the culture plates were aspirated out and fresh aliquots of growth medium containing suspension of FITC-loaded PLGA NPs and FITC-loaded Ab-PLGA NPs were added at the concentration of 0.25 mg/ml and incubated at 37 °C for 6 h. Upon incubation for 6 h, cells were washed with PBS, fixed in 4 % formaldehyde for 20 min, and then washed twice with PBS and observed under confocal laser scanning microscope (CLSM) (Fluoview 1000, Olympus, USA) after excitation at 490 nm (Acharya et al. 2009).
Statistical analysis
All experiments were carried out three times, independently. The data obtained were expressed in terms of “mean ± standard deviation” values. Wherever appropriate, the data were also subjected to unpaired two-tailed Student’s t test. A value of p < 0.05 was considered as significant. Asterisk (*) in figure denotes a statistically significant difference compared to control (p < 0.05).
Results
Optimization parameters of PLGA NPs
Optimization of concentration of drug
Different concentrations of gemcitabine (5, 7.5, and 10 mg/ml) were used for preparation of NPs while amount of polymer and concentration of surfactant were kept constant. The concentration of drug was optimized on the basis of size and entrapment efficiency (Table 1). Formulation D2 (drug, 7.5 mg/ml) was found to be optimum for tumour delivery as this formulation showed the smallest size of 178 ± 8 nm. The size of the other two formulations was recorded to be higher. Also, formulation D2 showed maximum entrapment efficiency (35.17 ± 1.0 %) amongst all the three formulations.
Table 1.
Optimization of amount of drug (n = 3)
| Code | Amount (mg) | Size (nm) | Entrapment efficiency (%) |
|---|---|---|---|
| D1 | 5 | 254.9 ± 6.5 | 24.5 ± 1.3 |
| D2 | 7.5 | 178 ± 8 | 35.17 ± 1.0 |
| D3 | 10 | 216 ± 5.5 | 19.8 ± 0.8 |
Optimization of amount of polymer
The amount of polymer was optimized keeping drug concentration at its optimum level and surfactant concentration constant in order to obtain formulation of desirable size and entrapment efficiency. NPs were prepared using different amounts of polymer (20, 40, and 60 mg). Formulation P2 was found to be optimum on the basis of the lowest particle size of 178 ± 8.5 nm and highest entrapment efficiency of 34.9 ± 0.89 % (Table 2).
Table 2.
Optimization of amount of polymer (n = 3)
| Code | Amount (mg) | Size (nm) | Entrapment efficiency (%) |
|---|---|---|---|
| P1 | 20 | 206.6 ± 9.5 | 20.5 ± 1.4 |
| P2 | 40 | 178 ± 8.5 | 34.9 ± 0.89 |
| P3 | 60 | 392 ± 7 | 34 ± 0.94 |
Optimization of concentration of surfactant
Concentration of sodium cholate was optimized using optimized amount of polymer and drug. NPs were prepared using different concentrations of surfactant (8, 12, and 16 mM). The NPs prepared using 12 mM (formulation S2) were found to be optimum on the basis of lowest particle size of 178 ± 10 nm and highest entrapment efficiency of 33.6 ± 1.2 % (Table 3).
Table 3.
Optimization of concentration of surfactant (n = 3)
| Code | Concentration (mM) | Size (nm) | Entrapment efficiency (%) |
|---|---|---|---|
| S1 | 8 | 243 ± 7.5 | 22 ± 1 |
| S2 | 12 | 178 ± 10 | 33.6 ± 1.2 |
| S3 | 16 | 204 ± 9.5 | 30.6 ± 1.4 |
Morphological study (SEM)
The morphological appearance of the developed NPs was examined by SEM. The SEM images are shown in Fig. 1a,b which indicates that PLGA NPs and PLGA-PEG NPs were almost spherical in nature and free from debris. Table 4 shows the various parameters for optimized formulations of PLGA NPs and PLGA-PEG NPs.
Fig. 1.
a SEM photograph of PLGA NPs. b SEM photograph of PLGA-PEG NPs
Table 4.
Various parameters of optimized formulation of PLGA and PLGA-PEG-NH2 NPs
| S. No. | Parameters | PLGA nanoparticles | PLGA-PEG-NH2 nanoparticles |
|---|---|---|---|
| 1 | Size (nm) | 178 ± 8.5 | 132.5 ± 10 |
| 2 | Polydispersity index (PDI) | 0.095 ± 0.04 | 0.072 ± 0.03 |
| 3 | Zeta potential (mV) | −59.88 ± 3.5 | −38.43 ± 2.3 |
| 4 | % Drug entrapment efficiency | 35.16 ± 1.04 | 34.56 ± 1.35 |
In vitro release studies
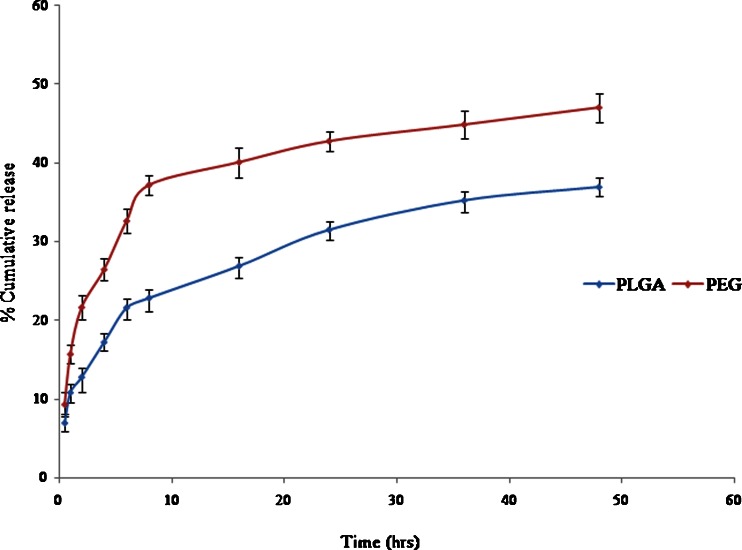
The in vitro release profile of entrapped drug from NPs was determined in PBS (pH 7.4) using dialysis bag. The release study of both PLGA and PLGA-PEG-NH2 NPs was performed three times to check the reproducibility. The release behaviours of both the formulations were comparatively different from each other. The release behaviour involved two different mechanisms, one was the diffusion of drug and the other was the degradation of polymer matrix. The burst release of drug was due to the drug which had been adsorbed at the surface of polymer, which diffused out easily during initial incubation time (Asadishad et al. 2010). It was observed that 6.93 ± 1.00 % of drug was released within initial 0.5 h and 36.9 ± 1.10 % was released in 48 h from PLGA NPs. In contrast to PLGA NPs, PLGA-PEG-NH2 NPs showed release of 9.3 ± 1.54 and 47 ± 1.80 % in 0.5 and 48 h, respectively. Figure 2 shows the release profile of both GEM-PLGA and GEM-PLGA-PEG-NH2 NPs.
Fig. 2.
In vitro release of PLGA and PLGA-PEG-NH2 nanoparticles
Characterization of Ab-PLGA NPs
Morphological study (TEM)
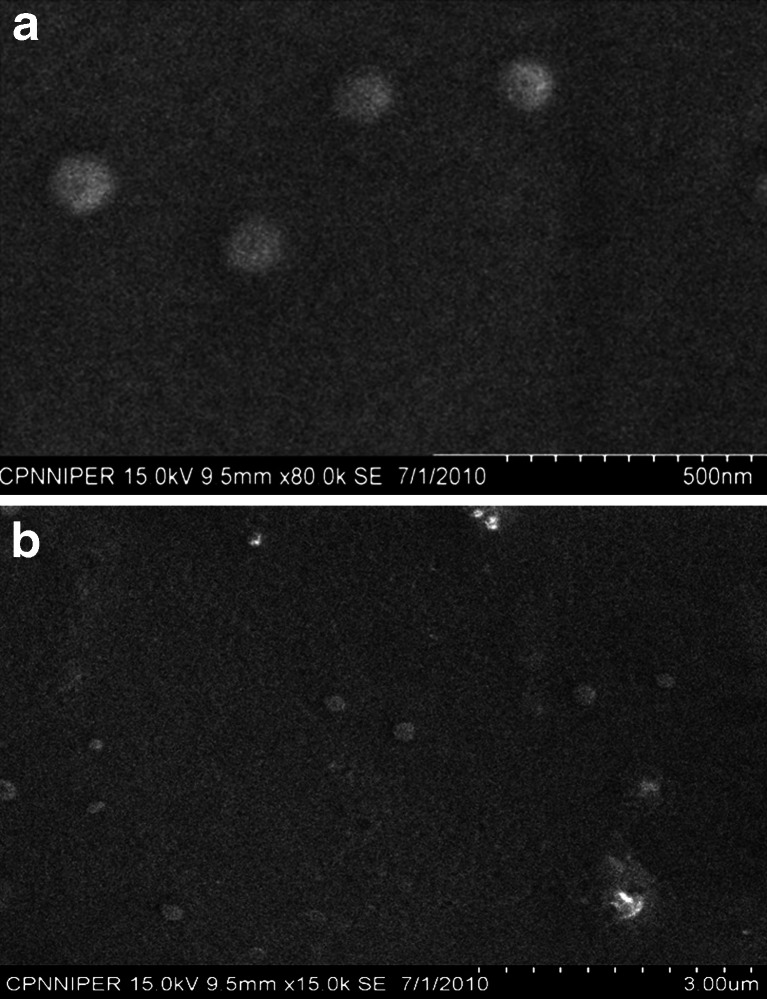
The conjugation of antibody to the surface of nanoparticle was confirmed by TEM. TEM was performed for PLGA NPs as well as Ab-PLGA NPs prepared by MBS-mediated cross-linking and glutaraldehyde cross-linking. The images revealed that conjugation was successfully done in both the conjugates but the conjugation with glutaraldehyde was more efficient than MBS crosslinked Ab-PLGA NPs, as more antibodies were conjugated to the PLGA-PEG NPs following glutaraldehyde conjugation. Figures 3 and 4a,b shows the TEM images of PLGA nanoparticle, glutaraldehyde, and MBS cross-linked Ab-PLGA NPs, respectively.
Fig. 3.
TEM image of PLGA NPs
Fig. 4.
a TEM image of Glu-Ab-PLGA NPs. b TEM image of MBS-Ab-PLGA NPs
Size and zeta potential
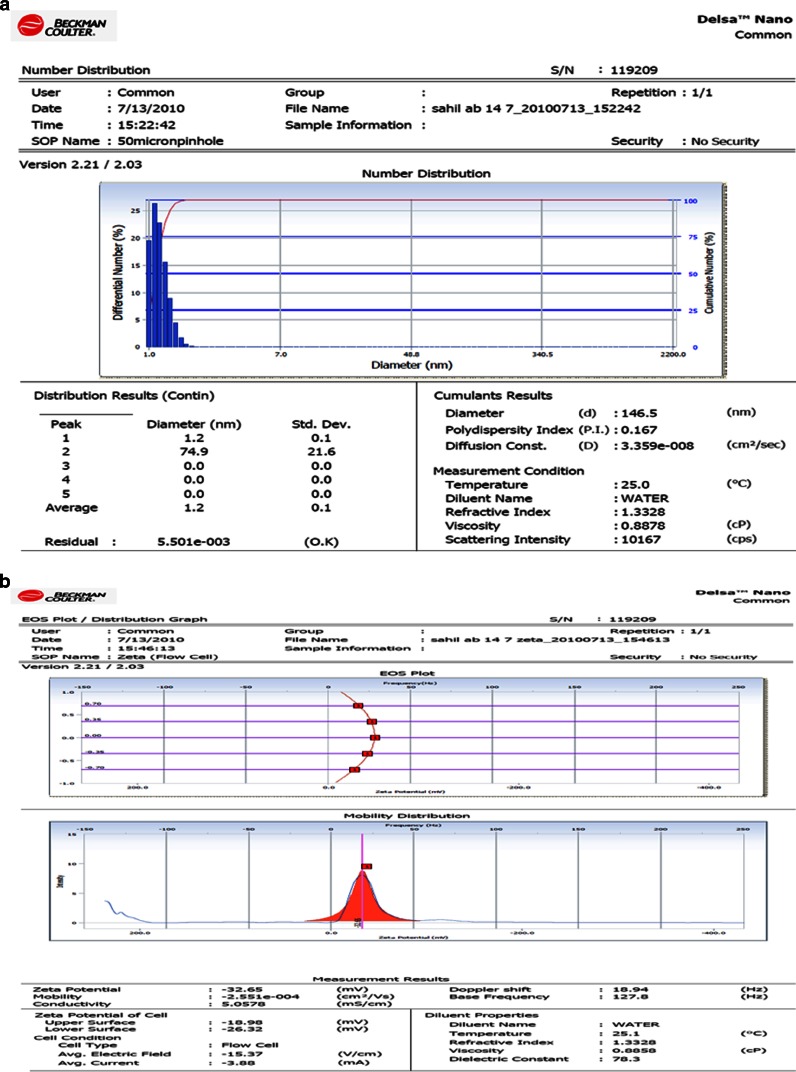
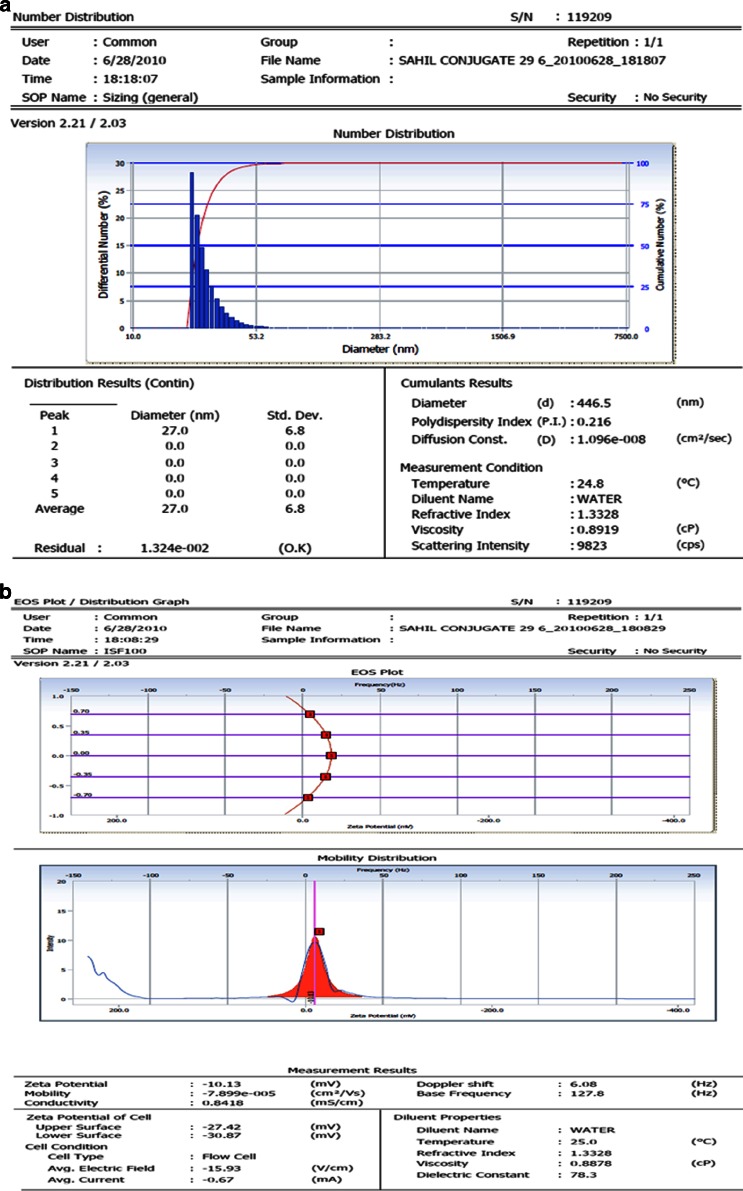
The size of prepared Ab-PLGA NPs should be in the suitable range for intravenous administration, after conjugation of the antibody on the NPs. Particle charge determines the stability of NPs. The particle size, size distribution, and zeta potential of the glutaraldehyde mediated Ab-PLGA NPs are shown in Fig. 5a,b, respectively. Figure 6a,b shows the size, size distribution, and zeta potential of MBS cross-linked Ab-PLGA NPs.
Fig. 5.
a The size and size distribution. b Zeta potential of Glu-GEM-Ab-PLGA NPs
Fig. 6.
a The size and size distribution. b Zeta potential of MBS cross-linked Ab-PLGA NPs
Entrapment efficiency
The entrapment efficiency of gemcitabine-loaded Ab-PLGA NPs was estimated and repeated three times. The formulation showed good reproducible results. Table 5 shows the entrapment efficiency of Ab-PLGA NPs along with other characterization parameters.
Table 5.
Characterization parameters of Ab-PLGA NPs
| Nanoparticle | Size (nm) | PDI | Zeta potential (mV) | %Drug entrapment efficiency |
|---|---|---|---|---|
| Glutaraldehyde-mediated Ab-PLGA NPs | 146.5 | 0.167 | −32.65 | 31.43 ± 1.58 |
| MBS mediated-Ab-PLGA NPs | 446.5 | 0.216 | −10.13 | 29.58 ± 2.14 |
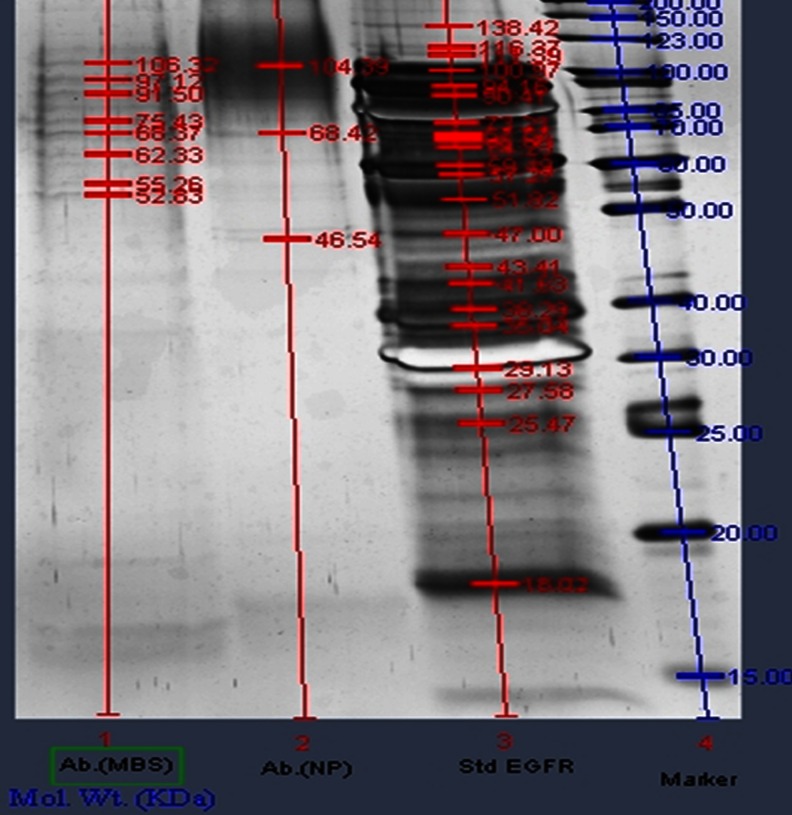
SDS-PAGE
The integrity of anti-EGFR antibody after conjugation on the NPs surface was analyzed by SDS-PAGE in comparison with the native anti-EGFR antibody (mAb). SDS-PAGE was run for both the conjugated NPs that is MBS crosslinked Ab-PLGA NPs and glutaraldehyde crosslinked Ab-PLGA NPs. Figure 7 shows SDS-PAGE of glutaraldehyde cross-linked and MBS cross-linked Ab-PLGA NPs. Lane 1 shows the SDS-PAGE for MBS crosslinked NPs, lane 2 represents PAGE for glutaraldehyde crosslinked NPs, and lane 3 shows PAGE run of standard anti-EGFR mAb. The PAGE results indicate that mAb integrity remained intact in both the samples after conjugation.
Fig. 7.
SDS-PAGE of antibody-conjugated PLGA nanoparticles. 1 The SDS-PAGE for MBS cross-linked NPs, 2 PAGE for glutaraldehyde cross-linked NPs, and 3 PAGE run of standard anti-EGFR mAb
Optimization of conjugation method
On the basis of morphology, size, and zeta potential, the conjugation method of antibody to the NPs was selected and used for further experimentation. Out of both the prepared Ab-PLGA NPs, glutaraldehyde cross-linked immunonanoparticle were found to be optimum and used further for cell line studies.
In vitro evaluation
Cell viability assay
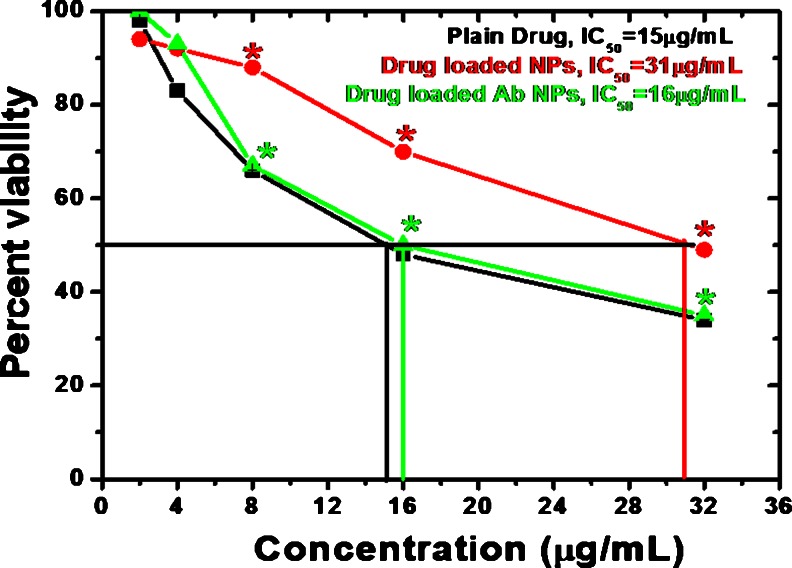
The cell viability assay was performed on MIA PaCa-2 cell lines. IC50 values were found to be 16 and 31 μg/ml for drug-loaded PLGA NPs and drug-loaded Ab-PLGA NPs, respectively. Plain drug showed an IC50 of 15 μg/ml (Fig. 8).
Fig. 8.
Percent viability measured by MTT assay on human pancreatic cells. An optical density value of unexposed cells, PLGA NPs, and Ab PLGA-PEG NPs was taken as 100 % viability (0 % cytotoxicity) for drug, drug-loaded PLGA, and drug-loaded Ab PLGA-PEG NPs, respectively. *p < 0.05, compared to plain drug
Cell uptake studies
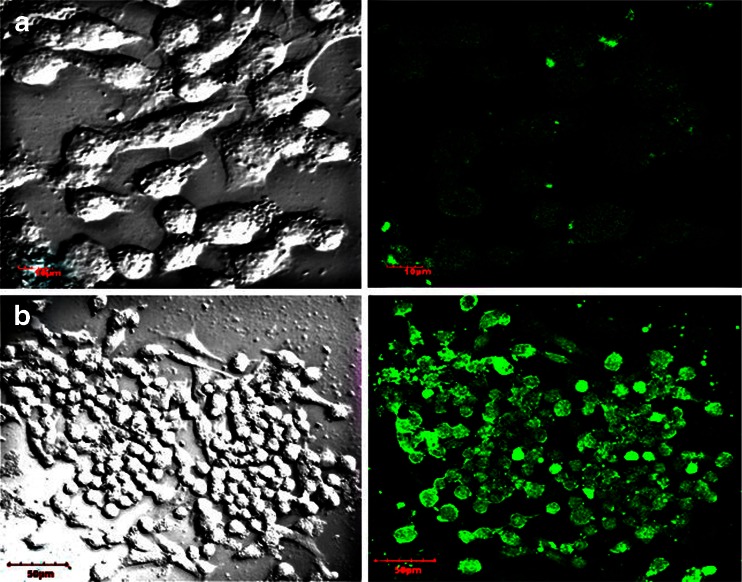
NPs encapsulated with fluorescent dye were used to study cellular uptake. FITC, fluorescent dye was incorporated in NPs as a marker for fluorescence microscopy to study the NPs uptake in cells. The fluorescence image shows the uptake of NPs in the cells. CLSM images of the MIA PaCa-2 cells after incubation of FITC loaded NPs and Ab-PLGA NPs suspension for 6 h at 37 °C are shown in Fig. 9.
Fig. 9.
Confocal laser scanning micrographs of MIA PaCa-2 pancreatic cancer cells after 6 h incubation with the FITC-loaded (a) PLGA NPs (b) Ab-PLGA NPs at 0.25 mg/ml nanoparticle concentration at 37 °C
Discussion
The prime objective of the study was to prepare and evaluate Ab-PLGA NPs derived from PLGA-PEG diblock copolymer. Both the polymer and copolymer were biocompatible and biodegradable biomaterial. The PLGA-PEG diblock copolymer was synthesized by coupling reaction between primary amine group of PEG diamine and terminal carboxylic end group of PLGA via amide linkage. The w/o/w double emulsification solvent evaporation method was used for encapsulating hydrophilic drug, gemcitabine. The method is reported to be the most suitable for encapsulating water-soluble drugs in polymeric carriers. An important step in the preparation of NPs was the formation of a stable homogenous primary emulsion, as the droplet size of dispersed phase in primary emulsion is directly related to the final NPs size. By applying sonication, which induced ultrasonic waves, emulsion droplets smaller than 0.5 μm were obtained leading to NPs of the nanosize. Several process parameters were varied to identify their influence on the particle size and entrapment efficiency keeping the other parameters constant (primary sonication amplitude (40 W) for 2 min and secondary sonication amplitude (60 W) for 4 min; volume of DCM (4 ml) and volume of sodium desoxycholate solution (20 ml)). The amount of drug, amount of polymer, and concentration of surfactant used in formulation were optimized.
The concentration of drug that is added in the formulation during emulsification process also affects the morphology and physicochemical properties of the resultant NPs. The drug that is added in the primary emulsion shows the tendency to rapidly partition out into the outer aqueous phase (being hydrophilic in nature), thus higher drug amount would result in low entrapment efficiency. The amounts of drug used for optimization were 5, 7.5, and 10 mg. Out of the three formulations prepared, i.e., D1, D2, and D3; formulation D2 was found to be most suitable on the basis of size as well as entrapment efficiency. The amount of polymer used for the preparation of NPs also caused variation in size and entrapment. Increasing the amount of polymer, beyond an optimum range would result in increase in size of the formulation and an additional amount of energy would be required to reduce the size to desired range. Thus the amount of polymer used for formulating NPs was also optimized and three formulations P1, P2, and P3 were prepared using 20, 40, and 60 mg of polymer. Of the prepared formulations, P2 responded well to the chosen parameters. The size of the formulation prepared using 40 mg of polymer was lesser and entrapment efficiency was higher of all the three formulations. Increasing the amount of polymer, i.e., 60 mg resulted in increase in size beyond acceptable range. Furthermore, the concentration of surfactant would result in variation in selected parameters. Surfactant in less concentration would not be able to stabilize the NPs whereas, in high concentration, it results in aggregation of NPs. Thus, for optimizing concentration of surfactant, again three formulations S1, S2, and S3 were prepared using 8, 12, and 16 mM of sodium desoxycholate. Of the three formulations, S2 was optimized on the basis of desired size and maximum entrapment of all the three formulations.
The optimized method was utilized to formulate the NPs using both PLGA and PLGA-PEG diblock copolymer for drug delivery. The use of PLGA-PEG diblock copolymer enables the preparation of non-aggregating particles without the need of an additional stabilizer such as poly (vinyl alcohol). Furthermore, PEG is an uncharged, highly flexible polymer that is known to decrease drug adsorption and cell interactions when present at the surface. PEG has outstanding physiochemical and biological properties including solubility in water and in organic solvents. Furthermore, it is non-toxic, non-antigenic, and non-immunogenic. Morphological studies were performed utilizing SEM. The particles were observed to be spherical, smooth and morphologically similar. The size of the optimized formulations was also found to be in nanorange, i.e., 178 ± 8.5 and 132.5 ± 10 nm for PLGA and PLGA-PEG NPs, respectively.
The drug entrapment in case of optimized formulations was also found to be more than other formulations and was 35.16 ± 1.04 % for PLGA and 34.56 ± 1.35 % for PLGA-PEG-NH2 NPs. The polydispersity index was also found to be very less, i.e., 0.095 and 0.072 for PLGA and PLGA-PEG-NH2 NPs, respectively, depicting a uniform size distribution for both the formulations. PLGA NPs exhibited highest zeta potential (−59.88 ± 3.5 mV) due to the presence of free carboxyl end group on particle surface whereas in case of PLGA-PEG-NH2 NPs the zeta potential (−38.43 ± 2.3 mV) was found to get shifted to lesser negative value due to the presence of amine terminated PEG chains because the coating layer shields and move the shear plane outwards from the particle surface. A marked difference in the surface charge between PLGA and PLGA-PEG-NH2 NPs was observed. The result confirms that the amine terminated PEG chains were situated on the surface of the NPs. The hydrophilic NH2-PEG moiety was oriented outside towards aqueous medium forming corona type, which was anchored to hydrophobic PLGA polymer backbone as a core material.
The in vitro release profile of PLGA and PLGA-PEG-NH2 NPs was determined in PBS (pH 7.4) at 37 °C using a constant shaking incubator. The drug release profile was studied for various time intervals, i.e., 0.5, 1, 2, 4, 6, 8, 16, 24, 36, and 48 h. Both formulations exhibited a biphasic release pattern that was characterized by an initial burst up to 10 h, followed by a slower sustained release and remained constant up to 48 h. At the end of 48 h, the PLGA-PEG-NH2 NPs showed higher release rate of drug than PLGA NPs. The PLGA-PEG-NH2 NPs degraded quickly and released the drug subsequently. This could be explained by the degradation mechanisms; the PEG chains (being hydrophilic) enhanced the water penetration into the matrix and the NPs were degraded homogeneously over time. In contrast, PLGA formulation released only 36.9 ± 1.1 % drug due to its hydrophobicity and hence poor degradation in aqueous environment. It is inferred that the gradual degradation of the polymer resulted in sustained release of the drug. However, the release of gemcitabine depends on several factors including the drug to polymer ratio, drug solubility inside the matrix, and the interaction between the core and drug.
The aim of this study was to prepare Ab-PLGA NPs derived from PLGA-PEG-NH2 block copolymers containing functional groups at the PEG chain end allowing NPs surface modification for targeting purposes. Amine groups were chosen to couple targeting units by using two different conjugation techniques, one involving glutaraldehyde as a cross-linker and other method involved MBS modification of NPs. The former involved applying aldehyde chemistry in aqueous media. NPs were prepared from PLGA-PEG-NH2 copolymers and the coupling of anti-EGFR mAb (as an antibody for pancreatic cell for targeting units) to glutaraldehyde-activated NPs at the surface was studied. The excess of glutaraldehyde was removed after the activation of NPs using dialysis membrane. The free aldehyde group present on the activated NPs was made to react with amine moiety of antibody. The removal of excess of glutaraldehyde was confirmed using Schiff reaction. At different time intervals, 250 μl of glutaraldehyde mixed formulation was taken and 25 μl of Schiff reagent was added, which turns deep purple if glutaraldehyde is present. The decrease in intensity of colour showed almost removal of excess glutaraldehyde. The second technique for conjugation involved activation of NH2 moiety of NPs using MBS and thiolation of mAb using Traut’s reagent, respectively. Later, both the active moieties were incubated for 30 min to allow conjugation of both the units.
Both the conjugated Ab-PLGA NPs were characterized on the basis of size, PDI, zeta potential, and entrapment efficiency. The size of MBS cross-linked NPs was much higher and was beyond acceptable range. Also, zeta potential depicted comparatively lower stability of MBS-conjugated NPs. The entrapment efficiency of former was also lower which might be due to dissolution of NPs because of use of DMSO as cosolvent during activation of PLGA-PEG-NH2. TEM images also revealed that favourably more antibody was coupled using glutaraldehyde as a cross-linker as compared to MBS-mediated conjugation.
The particle size of Ab-PLGA NPs was slightly increased than plain PLGA-PEG NPs. Moreover, polydispersity index of the Ab-PLGA NPs was higher. The reason could be that, during immobilization, a small fraction of the glutaraldehyde-activated NPs might have reacted with other NPs, leading to bridging between two particles. The anti-EGFR antibody attachment showed comparatively little effect on drug entrapment efficiency.
Anti-EGFR antibody might become inactive due to irreversible denaturation and aggregation during the immunonanoparticle preparation process. The integrity of anti-EGFR antibody after conjugation on the NPs surface was analyzed by SDS-PAGE in comparison with the native anti-EGFR antibody. The SDS-PAGE showed that the molecular weight of both the antibody conjugated NPs was shifted towards the higher side as compared to native anti-EGFR antibody. From the comparison of the native antibody and antibody conjugated on surface of PLGA-PEG NPs, it can be concluded that antibody conjugated on both the NPs surface was almost same in structure as the native EGFR antibody, which also validates the feasibility of Ab-PLGA NPs for EGFR overexpressed cancer targeting. On the basis of results obtained, the glutaraldehyde-mediated conjugation was selected for further in vitro cell line studies.
In cytotoxicity study, gemcitabine concentrations in all the formulations were adjusted to be the same. Percentage cell viability of both the formulations at different concentrations in MIA PaCa-2 cell line was determined. Ab-PLGA NPs showed significantly higher cytotoxic effect (IC50 16 μg/ml) than PLGA NPs (IC50, 31 μg/ml) after 24-h incubation period in MIA PaCa-2 cell line, which might be due to lower cellular uptake of PLGA NPs as compared to Ab-PLGA NPs. The anti-EGFR monoclonal antibody attached on the surface of NPs for targeting EGFR positive MIA PaCa-2 pancreatic cancer cells produced a synergistic effect with anticancer drug depicting possible role of antibody in blocking cellular signaling, thus leading to inhibition of cellular proliferation. In vitro cytotoxicity results of Ab-PLGA NPs (without drug) also support this finding. The blank PLGA-PEG NPs also exhibited low cytotoxicity in MIA PaCa-2 cell lines. It implies that these NPs could be useful as drug carriers without any significant cytotoxic effects. The significant cytotoxicity was shown by antibody alone and synergistic effect was shown along with gemcitabine.
NPs encapsulated with fluorescent dyes are frequently used to study cellular uptake. FITC, fluorescent dye, was incorporated in NPs as a marker for fluorescence microscopy to study the NPs uptake in cells. Aggregates of the green colour of FITC-loaded NPs in cell cytoplasm indicate that NPs were internalised by the cells. It can thus be concluded that the fluorescent NPs were located inside the cells. In the present study, the cellular uptake of Ab-PLGA NPs was higher than that of PLGA NPs as shown by high intensity fluorescence developed by antibody-conjugated NPs as compared to PLGA NPs, in EGFR-positive MIA PaCA-2 cells. The results reveal that anti-EGFR antibody-conjugated NPs were successfully developed for specifically targeting EGFR receptors in pancreatic cancer cells.
Conclusion
The study provide the basis for the development of stable and biologically active carrier systems for direct targeting of tumour cells using antibody-conjugated PLGA-PEG NPs. Direct covalent coupling of antibodies to NPs using glutaraldehyde as a cross-linker is an appropriate method to achieve cell type-specific drug carrier systems based on PLGA-PEG NPs and the anti-EGFR decorated PLGA-PEG NPs have potentials to be applied for targeted chemotherapy against EGFR-positive cancer. Such a nanoparticle system for drug delivery is multifunctional, which provides a platform to formulate anticancer drugs which reduces the side effects of the formulated anticancer drug, promotes synergistic therapeutic effects, and achieves targeted delivery for the therapy.
Acknowledgments
The authors acknowledge the chairman of the ISF College of Pharmacy, Moga for providing the necessary facilities required to carry out this research work.
References
- Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30(29):5737–5750. doi: 10.1016/j.biomaterials.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Asadishad B, Vossoughi M, Alamzadeh I. In vitro release behavior and cytotoxicity of doxorubicin-loaded gold nanoparticles in cancerous cells. Biotechnol Lett. 2010;32(5):649–654. doi: 10.1007/s10529-010-0208-x. [DOI] [PubMed] [Google Scholar]
- Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79(1–3):123–135. doi: 10.1016/S0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11(7):644–649. doi: 10.1245/ASO.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Blanco MD, Alonso MJ. Development and characterization of protein-loaded poly(lactide-co-glycolide) nanospheres. Eur J Pharm Biopharm. 1997;43(3):287–294. doi: 10.1016/S0939-6411(97)00056-8. [DOI] [Google Scholar]
- Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23(1–2):74–79. doi: 10.1159/000093497. [DOI] [PubMed] [Google Scholar]
- Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol Rep. 2007;18(1):151–155. [PubMed] [Google Scholar]
- Ennis BW, Lippman ME, Dickson RB. The EGF receptor system as a target for antitumor therapy. Cancer Invest. 1991;9(5):553–562. doi: 10.3109/07357909109018953. [DOI] [PubMed] [Google Scholar]
- Esmaeili F, Ghahremani MH, Ostad SN, Atyabi F, Seyedabadi M, Malekshahi MR, Amini M, Dinarvand R. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA-PEG-folate conjugate. J Drug Target. 2008;16(5):415–423. doi: 10.1080/10611860802088630. [DOI] [PubMed] [Google Scholar]
- Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11(2):507–517. doi: 10.1016/S1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Flatman S, Alam I, Gerard J, Mussa N. Process analytics for purification of monoclonal antibodies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(1):79–87. doi: 10.1016/j.jchromb.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 1999;57(2):171–185. doi: 10.1016/S0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Hun X, Zhang Z. Anti-epidermal growth factor receptor (anti-EGFR) antibody conjugated fluorescent nanoparticles probe for breast cancer imaging. Spectrochim Acta A Mol Biomol Spectrosc. 2009;74(2):410–414. doi: 10.1016/j.saa.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. doi: 10.1016/S0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10. [PubMed] [Google Scholar]
- Li Y, Pei Y, Zhang X, Gu Z, Zhou Z, Yuan W, Zhou J, Zhu J, Gao X. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release. 2001;71(2):203–211. doi: 10.1016/S0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125(3):193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19(13):3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S, Vimalachandran D, Raraty MG, Ghaneh P. Cancer in the elderly: pancreatic cancer. Surg Oncol. 2004;13(4):201–210. doi: 10.1016/j.suronc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Steinhauser I, Spankuch B, Strebhardt K, Langer K. Trastuzumab-modified nanoparticles: optimisation of preparation and uptake in cancer cells. Biomaterials. 2006;27(28):4975–4983. doi: 10.1016/j.biomaterials.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Chang CH, Lin CP, Tsai MC. Using MTT viability assay to test the cytotoxicity of antibiotics and steroid to cultured porcine corneal endothelial cells. J Ocul Pharmacol Ther. 1996;12(1):35–43. doi: 10.1089/jop.1996.12.35. [DOI] [PubMed] [Google Scholar]
- Xiong HQ, Abbruzzese JL. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002;29(5 Suppl 14):31–37. doi: 10.1053/sonc.2002.35645. [DOI] [PubMed] [Google Scholar]