Abstract
The debate over return of individual research results and incidental findings to research participants is a key frontier in research ethics and practice. Fundamentally, this is a problem of translational science, a question of when information about an individual that is generated in research should be communicated for clinical attention, as the technology itself is moving into clinical care. There is growing consensus that investigators should offer participants at least those individual findings of high clinical importance and actionability. Increasing attention to what information biobanks and secondary researchers owe people who provide data and samples offers an opportunity to treat these source individuals as research partners. Cutting-edge issues include return of results in pediatric populations and return to kin and family, including after death of the proband. Progress will require facing the continuum linking research and clinical care and developing standards and models for return.
Keywords: genetics, genomics, research ethics, bioethics, biobank
Introduction
The question of whether to return individual research results (IRRs) and incidental findings (IF) to participants in genetic and genomic research is now recognized as one of the most difficult challenges facing investigators. When our research group at the University of Minnesota began funded work on this problem in 2005, the return of results debate in genetics and genomics was in its infancy. Indeed, that project was framed comparatively: using the more advanced debate at the time over management and return of incidental findings in neuroimaging research and CT colonography research (as the latter images most of the torso and customarily reveals extracolonic IFs), our national project group launched into consideration of how to define, anticipate, manage, and return IFs in genetic and genomic research. We published consensus recommendations1 as part of a symposium offering papers on different pieces of this puzzle.
Concern over how to handle incidental findings in research was preceded by a long history of attention to the question of how to handle IFs in clinical care. Probably every clinician has had the experience of a patient presenting with a certain complaint and the clinician discovering an additional and unrelated pathology. Indeed, the term “incidentaloma” is defined in medical dictionaries as an occult adrenal tumor, accidentally discovered.2,3 Genetics has seen a long-standing debate on how to handle an incidental finding of misattributed paternity revealed by genetic testing.4
As this long-standing concern over clinical incidentalomas moved into the research sphere and edged toward genetics, the National Bioethics Advisory Committee (NBAC) published a report on stored tissue in 1999.5 That report included a brief section on “Reporting Research Results to Subjects” and offered several recommendations. NBAC urged that Institutional Review Boards (IRBs) develop guidelines and require that protocols address this issue. However, the committee recommended that disclosure should be “an exceptional circumstance,” and only if “the findings are scientifically valid and confirmed,” “the findings have significant implications for subjects’ health concerns,” and “a course of action to ameliorate or treat these concerns is readily available.” They also suggested that at the time of disclosure, “appropriate medical advice or referral should be provided,” though later recommendations instead have counseled that investigators should offer the finding as a research finding, with referral for clinical follow-up. (1) This contemplates a “hand-off” of information from the domain of research to that of the clinic, in part to avoid mistaking research for clinical care.
NBAC cited few sources to show earlier attention to the problem of return of research results. The most prescient was a short article by Reilly from 1980.6 As NBAC recounted, Reilly distinguished three types of findings: “1) ‘findings that are of such potential importance to the subject that they must be disclosed immediately’; 2) ‘data that are of importance to subjects…but about which [the investigator] should exercise judgment about the decision to disclose…[i]n effect, these are data that trigger a duty to consider the question of disclosure’; and 3) ‘data that do not require special disclosure.’”
By the time NBAC published its report, a significant literature was already emerging on how to manage incidental findings in imaging research, where IFs can be visually obvious and hard to overlook. In 1997, for example, Yue et al. published a study of IFs discovered in imaging the brain7 as did Katzman et al. in 1999.8 The literature on IFs in imaging research became voluminous. In 2005, Illes led a workshop including investigators and policy-makers from the National Institutes of Health (NIH) focusing on IFs in neuroimaging research, which led to progress on consensus recommendations.9 Consensus recommendations emerged for IFs discovered in CT colonography as well.10 And in 2008, our project published consensus recommendations bridging from imaging research to genetic and genomic research. (1)
In the fast-moving work of genetics and genomics, the 1990’s and even the mid-2000’s is now a long time ago. With increasing reliance on large-scale genomic research using biobanks and archived data sets, the emergence of whole exome sequencing (WES) and whole genome sequencing (WGS), their increasing speed and plummeting cost, and developments in informatics allowing increasingly automated analysis of potentially returnable variants and computer-supported communication to clinicians and even participants, the debate over return of IRRs and IFs has intensified. NIH, and especially the National Human Genome Research Institute (NHGRI), deserves great credit for recognizing the fundamental importance of these issues, committing significant funding to the research needed to build a strong evidence base for solutions, and speeding progress by linking funded investigators through a Return of Results (RoR) Consortium with targeted work groups.11
The importance of this issue has now been widely recognized. Both the professional literature (scientific, medical, ethics, and legal) as well as the popular media now regularly cover this unfolding story. A 2011 news article in Science reported that, “Whether to divulge results…, and how, is arguably the most pressing issue in genetics today.”12 In August 2012, the New York Times quoted NIH Director Francis Collins, calling the issue “one of the thorniest current challenges in clinical research.”13 An October 2012 report from the Presidential Commission for the Study of Bioethical Issues, which focused on the privacy challenges posed by the rise of WGS, included recommendations on return of IFs: “Researchers, clinicians, and commercial whole genome sequencing entities must make individuals aware that incidental findings are likely to be discovered in the course of whole genome sequencing. The consent process should convey whether these findings will be communicated….”14 Many in the genetics and genomics community now await the recommendations of the American College of Medical Genetics and Genomics (ACMG) Workgroup on return of IFs (which the Workgroup also calls “secondary findings”). In the group’s March 2012 preliminary report, they suggested a “minimum list of variants/conditions that labs should look for and return.”15
I have focused here on U.S. developments, but the debate over return of IFs and IRRs is international.16,17,18 This article concentrates largely on developments in the context of American policy and regulations, but genetic and genomic research cross national boundaries. Ultimately, international exchange on policy and best practices will be crucial, as a route toward international harmonization of policies and standards.
Definitions
In 2008, our project group offered a definition of an incidental finding as “a finding concerning an individual research participant that has potential health or reproductive importance and is discovered in the course of research but is beyond the aims of the study.” (1) Since this definition was offered, it has been widely recognized that not only health and reproductive importance, but also personal utility to the research participant may suggest possible return of an IF.19 Note that IFs discovered in the course of genetic or genomic research may not be limited to genetic findings. Screening individuals for possible enrollment in research, collecting baseline values on research participants, or gathering phenotypic information (for example, to search for genotype/phenotype associations) may yield a wide range of IFs such as abnormal blood pressure and other phenotypic findings.
In contrast to an IF, an individual research result is a finding concerning an individual research participant that has potential health or reproductive importance or personal utility and is discovered in the course of research on the focal variables under study in meeting the study’s aims. Thus, in genetic or genomic research, IRRs are likely to be genetic or genomic findings on this individual.
Of course, distinguishing IFs from IRRs may be more difficult in discovery-driven rather than hypothesis-driven research, as the aims in the former may be broad and the method inductive. (1) For example, in some genome-wide association studies (GWAS) that search widely across the genome for genotype/phenotype correlations, it may be hard to discern what findings are beyond the aims of the study. For this reason, distinctions between management of IFs and that of IRRs should be carefully justified, especially because research participants may find it difficult to distinguish these two types of findings. (19) Indeed, when commentators reference the “return of results,” they are typically referring to return of both IFs and IRRs, as I will in this review.
Both IFs and IRRs contrast with aggregate research results. These are findings concerning the research population (usually published) that are discovered in the course of research on the focal variables under study in meeting the study’s aims. Beskow et al. discuss ethical obligations to offer aggregate research results to research participants and the relationship to return of individual research results.20 Indeed, return of aggregate results to a research population (as in a newsletter or through a website) can lead individual participants to ask for their own findings.
There are a range of terms for the individuals whose findings are at issue. The literature variously calls them participants (or human subjects), donors, sources, and contributors. Some are indeed participants in research on human subjects as defined by the Common Rule, because they are a “living individual about whom an investigator…obtains data through intervention or interaction with the individual or identifiable private information”).21 However, much genetic and genomic research is performed on data and samples collected for clinical rather than research purposes and then deidentified. 22 Such research does not qualify as research on human subjects.23 Indeed, the source individuals (the term I will use here, though our group has also used “contributor” (19)) may not know their materials are being used in research (though possible changes to the Common Rule have been published for comment, which would require at least rudimentary consent from source individuals24). Thus, “donor” seems the wrong term, as it suggests a past donation.
A final definition of biobank is useful. As in much of the literature, I use the term here to refer to a range of structured collections of human biological materials and/or data, archived for ongoing use in research. (19) Others offer similar definitions,25 allowing for discussion of the role of biobanks in the return of results debate without getting lost in the welter of terms used for such structured collections, including biorepositories, tissue repositories, and DNA databanks.
Why has this issue become important?
Return of results has erupted into a major debate and focus for research. The importance of the issue stems in part from the gap between the preferences in favor of return that participants and the public appear to hold, and past research practice to avoid return. Research is still under way on the preferences of research participants, other individuals who serve as sources of data and specimens used in research, and the public. But data thus far indicate that most are interested in return. 26,27,28,29,30,31 Indeed, one survey found that “90% of…respondents wanted their genetic or risk information even when there was nothing that currently could be done with that information.”32 (p. 836) While more data and analysis are needed to understand preferences in a range of research contexts, as well as the impact of return on actual participants, the gap between apparent preferences and research practice has led to concern over the ethics of withholding individual research results of clinical significance.
This concern has arisen at a time of broader attention to the problem of how to earn and sustain the trust of individuals recruited for research as well as those source individuals whose data and specimens are used. As Trinidad et al. have noted, “A spate of recent events – including several…conflicts over newborn blood samples; the return of biospecimens to the Yanomamö people; and the bestselling account of the origins of the HeLa human cell line widely used in research – have raised questions about trustworthiness of the research process at a time when new approaches to genomic research place a premium on study participation.”33 (references omitted) Kohane et al. have argued that withholding data from research participants, makes them “passive purveyors of biomaterials and data,” not research partners.34 Illes et al. have similarly maintained that researchers should return IFs based on respect for participant autonomy and interests, as well as a duty of reciprocity to those who make research possible through their participation.35
Richardson and Belsky have offered ethical analysis to translate these concerns into investigator duties to return IFs to research participants.36,37,38 They argue that participants permit researchers access to their private data, specimens, and bodies, access that researchers otherwise would not have. This grant of access represents an act of partial entrustment (“partial” because participants are not fully entrusting their medical welfare to the researcher, as they would to a clinician). Richardson and Belsky maintain that the scope of this partial entrustment creates researcher duties of ancillary care. These are not the full duties of care borne by clinicians, but neither are researchers “pure scientists” with no duty of care. Richardson has argued that this duty of ancillary care embraces a duty to return IFs: “Having gotten the participants to waive these privacy rights, the researchers correspondingly come to have duties of care with regard to the pieces of information — and in particular the incidental findings — that fall in their hands by doing the research procedures.” (30)
Lurking here is a duty to warn or duty to rescue. Beskow and Burke explicitly embrace the notion of a duty to rescue, which they argue applies “when, in the course of research, an investigator discovers genetic information that clearly indicates a high probability of a serious condition for which an effective intervention is readily available.”39,40 Ossorio has questioned the extent of a duty to warn or rescue, at least a duty falling on secondary researchers, those most distant from any research interaction with participants. Yet even in the case of secondary researchers, she argues that there are cases in which the duty applies and return of results may be obligatory, as well as additional cases in which it may not be obligatory but would still be “morally superior to not doing so.”41
The question of whether researchers bear duties to return IFs and IRRs has proved particularly vexing because it straddles the worlds of research and clinical care, with their different norms and objectives. The core question is whether information discovered in the course of research should be conveyed to the individual participant in order to trigger clinical evaluation and follow-up. In that sense, the return of results is a “bridge” problem, because it bridges from the world of research (with its own norms and objectives) into the world of clinical care (with very different norms and objectives). On the research side of the bridge, investigators debate whether information acquired in the course of research should be communicated across that bridge to the domain of clinical care.
The problem of whether to return IFs and IRRs thus challenges the dichotomy between research and clinical care that ethics (and law, for that matter) has long embraced.42 On the clinical side, copious work on medical ethics as well as court decisions and legislation have established that the physician owes the patient a robust duty of clinical care. The physician’s goal is to serve the patient’s interests. A great deal follows from this, including informational obligations to disclose to the patient the diagnosis, treatment options, and other information material to treatment decisions. However, on the research side, the researcher’s core goal is to seek generalizable knowledge for the benefit of the many. The researcher owes a much thinner duty of clinical care, focused on averting and addressing research-caused harm. Researchers are obliged to seek research participants’ informed consent to be part of the research, but they currently have had no duty to seek consent from individuals whose clinically derived data and samples are used without identifiers. And what information the researchers should report back to the participant or individual source of data and specimens is the precise question posed by the return of results debate.
This dichotomous vision of the contrasting worlds of research and clinical care is rooted in the history of human subjects research. Traditionally, research asked narrow, circumscribed questions in time-limited investigation, aimed at advancing aggregate knowledge and welfare. In contrast, medical care addressed all of the patient’s health issues, extended over the patient’s life-time, and was provided by clinicians committed to advance the patient’s individual welfare. However, newer research realities now alter this contrast. Genetic and genomic research may now ask broad, uncircumscribed questions in GWAS discovery research and analysis of the full exome or genome. Research may no longer be time-limited, now that specimens and data sets are archived and re-analyzed indefinitely. Research technologies are so powerful that they routinely generate findings of potential clinical importance, and researchers may acquire data highly important to individual welfare.
The return of results problems is thus one of many signs that the old, dichotomous vision of research and clinical care widely separated will need to evolve into a new more translational vision of connected realms. The rise of genomic medicine and pharmacogenomics are interdigitating research and clinical care as well. Rather than relying on the old dichotomous vision, we may need to reconceptualize research and clinical care along a translational continuum. The problem of return of IFs and IRRs has become a central catalyst to forging this new vision.
How IRRs and IFs Arise
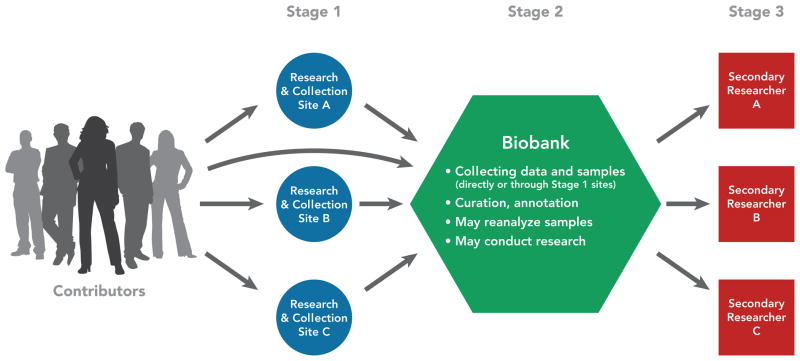
Both IFs and IRRs can arise throughout the course of research. This is true over the course of an individual study, starting at the beginning with recruitment and ascertaining eligibility. It is also true as data and specimens from multiple studies or those left over from clinical care are collected and aggregated, stored in biobanks or archived data sets, and used in secondary research. In 2012, our group published the results of a project on managing IFs and IRRs in genomic research involving biobanks and archived data sets. (19) Addressing this issue forced our project group to conceptualize how IFs and IRRs arise as data and specimens flow through what we called a biobank research system. Figure 1 depicts a biobank research system, comprised of three types of entities. At Stage 1, data and specimens from contributors are collected by primary research and collection sites. The initial collection may be in research or in clinical care. Research may itself occur at the Stage 1 sites.
Figure 1.
A biobank research system. (Reprinted with permission from Wolf et al. 2012 (19))
At Stage 2, the data and/or specimens are fed into a biobank for curation, annotation, storage, and making them available for subsequent research. (Note that some biobanks collect their own data/specimens, eliminating the Stage 1 collection sites.) The research on banked data and specimens may take place at the biobank, multiple secondary research sites, or both.
Those secondary research sites comprise Stage 3 of the biobank research system.
IFs and IRRs can arise at all three stages of this biobank research system. At Stage 1 primary research or collection sites, IFs may arise in ascertaining an individual’s eligibility to participate and collecting baseline information, as noted above. In addition, IFs and IRRs may arise in any subsequent research conducted at these Stage 1 sites.
At Stage 2 sites, where data and specimens are archived and processed to be made available for further research, IFs may arise in biobank processing of data/specimens. For example, a biobank that processes tumor specimens by reconfirming the reported pathology may discover an erroneous diagnosis (sometimes called a “discrepant diagnosis”). Biobank quality control (QC) is another potential source of IFs. For example, a biobank conducting routine QC by chromosomal confirmation that a contributor reported as female is indeed XX, may discover sex chromosome abnormalities and wonder whether these should be offered to the contributor or her physician. In addition, any research conducted at the biobank may yield IFs or IRRs. These may be discovered in the genetic data or in the phenotypic data about an individual, including in their electronic medical record, if that is used in the research.
At Stage 3 sites, secondary researchers using data/specimens obtained through the biobank may discover IFs or IRRs in the course of performing research. These are particularly challenging to handle, as secondary research may be far removed from data and specimen collection in time and geographically, secondary researchers may have no relationship with the source individuals, and the data and specimens are likely to be deidentified before being conveyed to the secondary researchers.
Conceptualizing the flow of data and specimens through the entire research system is important. It allows consideration of the proper stage for stripping identifiers, and what entity (if any of them) should hold the codes to allow reidentification. It also allows consideration of how the documents that structure the relationships between the Stage 1 sites and the Stage 2 biobank, and then the biobank and Stage 3 secondary researchers (documents including Material Transfer Agreements (MTAs) and Data Access Agreements (DAAs)) should address responsibilities for return of IFs and IRRs.
Most of the literature to date on IFs and IRRs in genetic and genomic research focuses on those that arise in Stage 1 primary research or does not specify the context in which the findings arise and must be addressed. However, much genomic research now occurs in a biobank research system and has to be addressed in this context. That was the focus of our 2012 consensus paper (19) and associated symposium. To address the more complex reality of genomic research conducted on a large scale through a biobank research system requires first examining the analysis that has emerged to date on how to handle IFs and IRRs in primary research.
Recommendations for Primary Research
The key questions that have structured the debate over return of IFs and IRRs in primary research have included:
What findings are we talking about?
What criteria should define returnable IFs and IRRs? Do returnable findings include only those of clinical significance? What about findings of reproductive significance (such as carrier status)? And what about findings of personal utility (such as a variant predicting serious illness and early death, that might prompt an individual to put their affairs in order and alter life decisions)? If a finding must be “actionable” to warrant return, how should “actionability” be defined?
How are these findings ascertained?
Do investigators have a duty to “hunt” for these findings, or should return of IFs and IRRs be limited to those that investigators and others stumble upon?
What should investigators do once they spot a suspected IF or IRR?
What personnel and procedures are needed to set up a responsible process for ascertaining these findings? Should the research team include (or arrange access to) a clinician with relevant expertise to examine the research findings of concern and confirm whether they warrant communication to participants for potential clinical evaluation and follow-up?
What further steps are needed to raise confidence in the finding to the level necessary for return?
Given that false positives occur even in clinical testing, what level of confidence in a research finding is required for return, given that return itself should then trigger clinical evaluation? Does return require confirmation of genetic IFs or IRRs in a lab certified to return findings for diagnosis or treatment use under the Clinical Laboratory Improvement Amendments (CLIA)? (19, 43) If so, how is this best accomplished?
To whom should return be offered?
Should return only be offered to research participants themselves? Are there circumstances under which return should be offered to the participant’s clinician, in addition or instead of the participant? Should return be offered only to participants who consent? Are there findings of such gravity and actionability that they should be returned even if the participant has not consented? How should participant consent for return be sought?
What systems and processes should be set up to support ethical handling of IFs and IRRs?
What should research protocols and consent forms say in advance about the likelihood of finding IFs and IRRs and how they will be handled? What should IRBs require? What should funders themselves require, and what funding is needed to support sound management of IFs and IRRs?
In our 2008 consensus recommendations for how to handle IFs, our project group concluded that investigators do shoulder duties to anticipate and manage IFs in their research. (1) We urged that they create a pathway for handling them, and offered a flowchart as well as description of that pathway. We suggested that researchers should address their plan for management of IFs in their proposed protocol and in the consent process, and obtain IRB approval. IRBs and funders should oversee fulfillment of these duties, assure the needed budget, and provide guidance.
In developing criteria for return, we distinguished three categories: (1) findings that should be returned, (2) findings that may be returned, and (3) those that should not be returned. This the 3-way division (which Reilly’s 1980 article anticipated (6)) has proven durable, with a number of subsequent recommendations (including those from Fabsitz et al. (42) and Berg et al.44) also distinguishing should return, may return, and (often) do not return. In our paper, we sorted findings into these three categories based on whether return potentially offered strong net benefit to the participant (should return), possible net benefit (may return), or unlikely net benefit (do not return). Thus, we made the ethical judgment that returnability should hinge on the importance of return from the perspective of the research participant. In “should return,” we included both findings of high clinical significance and those of high reproductive significance.
While the 3-way division has endured as well as the inclusion of findings of high clinical significance in the “should return” category, other features of our proposal have sparked more debate. A subsequent consensus paper by Fabsitz et al. stripped findings of reproductive significance out of “should return.” (42) That paper restricted “should return” to findings with important health implications, revealing established and substantial risks, when the findings were actionable, defined as having the potential to change the disease course. In addition, findings in this category had to be analytically valid, their disclosure had to comport with law (such as any applicable CLIA requirements), and the participant had to consent to receipt of the findings.
This was a more clinician-centered delineation of the “should return” category. The “actionability” requirement and definition meant that investigators had to conclude that clinicians could potentially use the returned finding to make a positive difference in the individual’s clinical course. This was a different ethical perspective that the one taken in our 2008 paper, which was instead guided by what information participants themselves would likely find valuable. This debate over whether to tether return to what clinicians can use versus what participants can use remains unresolved. It echoes a long-standing debate (the subject of seminal court cases such as Canterbury v. Spence45 as well as legislation) over whether informed consent in clinical care calls for disclosure of information whose scope is determined by professional custom or determined by what information patients are likely to find material.
Both our 2008 recommendations and those from Fabsitz et al. address return of individual findings in the context of research. Consequently, both have drawn objections from those who argue for maintaining a strict divide between research and clinical care. Key objections have been that return of IFs and IRRs requires time and resources, diverting personnel and funds from research.46 Another core concern has been that offering IFs and IRRs to participants may invite them to confuse research for clinical care. A third objection has been that guidelines recommending return of some IFs and IRRs may invite liability for failure to return.47
There are, as yet, few studies analyzing the cost of return, which is likely to vary considerably depending on study design, the types and frequency of IFs and IRRs identified, the size of the sample population, and the determination of what IFs and IRRs to actually return. A common recommendation, which our own consensus papers include, is that funders need to add to research budgets in order to support addressing IFs and IRRs. The reality is that ethics takes time and costs money, including basic informed consent.48
The possibility that research participants may mistake research for clinical care is a long-recognized problem that significantly predates the return-of-results debate. Researchers and IRBs now routinely address the “therapeutic misconception” and take steps to minimize this confusion. Addressing possible return of IFs and IRRs with research participants might actually be an opportunity to emphasize the distinction between research and clinical care, as participants need to understand the option of receiving findings generated in research that will then need to be pursued and clarified through clinical work-up.
Finally, concern over potential liability seems at least premature, if not misplaced.49 There have been no court cases as yet over return of results. However, guidelines that help move the research community toward a shared sense of what is owed to research participants may actually help avert potential liability, by articulating flexible standards. Without those, a research participant who is not offered a particular IF or IRR and arguably suffers harm caused by not receiving that finding will be freer to argue that lack of return was a compensable harm. With flexible guidelines in place, investigators can instead point to their reasonable use of those guidelines. None of the guidelines published to date state that investigators should return all possible IFs and IRRs. Instead, the guidelines customarily restrict “should return” to a small subset.
Despite the concerns articulated over return of IFs and IRRs, it is now difficult to find commentators who argue that absolutely no IFs and IRRs should be returned. The reality that some IFs and IRRs are clinically urgent is widely recognized. Indeed, consensus approaches to IFs in imaging research clearly recognize that some IFs are clinically urgent and categorize them this way.50
The progress that has been made on return of results in primary research is the necessary backdrop for the more complex debate over return of IFs and IRRs in research that involves biobanks. I turn next to that debate.
Recommendations for Biobanks & Secondary Research
Because biobanks are increasingly the engines of large-scale genomic research, determining how to handle return of IFs and IRRs in the simpler model of primary research is not enough. It is essential to grapple with how to manage IFs and IRRs as data and specimens move through all three stages of a biobank research system.
However, the conventional view has been that once data and specimens move beyond the primary research site to biobanks and then to secondary research sites (Stages 2 and 3 in the biobank research system), either no IFs and IRRs should be returned at all, or the biobank and secondary researchers should convey any IFs and IRRs to the primary site to determine whether any return should be undertaken. (51 19) This view minimizes or eliminates biobank and secondary researcher responsibilities to manage IFs and IRRs.
There is a growing recognition, however, that there are problems with this conventional view. (19, 50) First, some findings are so clinically urgent that failing to return them poses serious ethical challenges for biobanks. An example is biobank discovery in processing newly acquired tumor specimens that the pathology and diagnosis noted at the primary collection site (Stage 1) appears to be incorrect. This problem of “discrepant diagnosis” has led to a literature on how to manage and return what may be an IF of urgent clinical importance.52
A second set of problems with confining responsibility for addressing IFs and IRRs to primary research and collection sites is that they may lack the capacity to address the finding. In some scenarios, the primary site has merely supplied specimens and data collected in clinical care, and my not have the expertise to analyze the returnability of the genetic or genomic findings that the biobank and secondary researchers generate. Even if the primary site collected the data and specimens in research, the relevant investigator may now be gone and the investigator’s research project concluded.
More fundamentally, there is a strong argument for a systemic approach to the problem of how to manage IFs and IRRs that arise as data and specimens flow through a biobank research system. The flow of data and specimens is controlled by policies and documents such as MTAs and DAAs. (19) Those policies and agreements should address the responsibilities of primary research and collection sites, biobanks, and secondary researchers to manage IFs and IRRs. Only this kind of systemic approach will lead to harmonized expectations and clear notice to all of the actors as to their responsibilities.
Our 2012 consensus paper was the first to offer this kind of systemic analysis of how to approach the return of results problem in a biobank research system. We readily acknowledged that biobanks are varied. Some are population-based while others are diseased-based. They vary by source population, size, age of the collection, the range of data and specimens collected. They may aggregate data and specimens collected for clinical purposes and now deidentified, so that research on that material falls beyond the scope of “research on human subjects” under the Common Rule. (22, 23) On the other hand, data and specimens may have been collected for research or carry identifiers, so that this fundamental regulation of human subjects research applies.
Despite this variety, all biobanks and biobank research systems have the potential to discover IFs and IRRs. There is a need for guidance, especially guidance that offers the flexibility to tailor approaches to the realities of a given biobank research system. While some commentators have suggested that the sheer variety of biobanks counsels against general guidelines (30, 45), the virtue of offering guidance to biobanks is already recognized by publication of the influential Best Practices for Biorepositories issued by the National Cancer Institute’s Office of Biospecimen and Biorepository Research. (51) In addition to this, a substantial literature has emerged on the ethical responsibilities of biobanks, including duties of responsible custodianship. (29, 53,54,55) Leaving each biobank to grapple alone with the return of results problem, without even general guidelines, invites inefficiency, unnecessary cost, and unwarranted inconsistencies.
Biobanks are already beginning to address return of results issues, so the time is ripe for offering guidance and inviting debate over proposed policy. The eMERGE Network of biobank research sites has a network-level Return of Results Oversight Committee to offer general guidance, which individual sites can then tailor to their circumstances and needs.56 The Coriell Personalized Medicine Collaborative has an Informed Cohort Oversight Board (ICOB), a model suggested by Kohane et al. (23 57) The NIH Gene Environment Association (GENEVA) Studies use a Committee on Incidental Findings.58 Not all biobank research systems can return results; Vanderbilt’s BioVU is an example of a biobank that irretrievably strips identifiers, so that reidentification, and thus return, cannot be accomplished.59 However, this remains an unusual practice. More common is to retain a key code that allows reidentification. Indeed, in some research designs participants are fully identified and followed prospectively.
Our project offered consensus recommendations for return of results from biobank research systems. (19) The most fundamental recommendation we offered was to approach the issue of return of results systemically, by considering how IFs and IRRs can arise as data and specimens move through the entire system and by allocating among the key players within that system the responsibilities for dealing with return issues. We recognized that the biobank itself sits at the center of the 3-stage system, with relationships (including written agreements) extending both to primary research and collection sites and to secondary researchers. This puts biobanks in an important position to help ensure that the biobank research system as a whole addresses return of results issues.
To allocate responsibilities across the system, we identified four general steps involved in dealing with return of results: (i) Clarifying general criteria for what should be returned, may be returned, and should not be returned; (ii) Analyzing a particular finding in light of these criteria; (iii) Reidentifying the source individual; and (iv) Recontacting the individual to offer the finding. We summarized these four steps using the acronym CARR. We then offered specific recommendations for each step.
To clarify general criteria for return, we recommended that biobanks have a multidisciplinary committee such as an ICOB to work with an IRB on these return issues. As Fabsitz et al. also recommended (34), a nation-wide or central advisory committee would be helpful, to offer recommendations on the criteria for return and a periodically updated list of returnable variants. A given biobank research system might decide to deviate from those central recommendations, but at least would have a place to start.
To aid in formulating criteria for return, we offered a set of criteria similar to those for return in primary research, but with key caveats. Thus, we suggested that biobank research systems should return IFs and IRRs that reveal an established and substantial risk of a serious health condition, are actionable (offering a significant potential to alter the onset, course, or treatment of disease), are analytically valid and whose return complies with legal requirements (such as applicable CLIA requirements), and only if the source individual has consented to return. We went on to suggest that a biobank research system may return additional IFs and IRRs if they reveal an established and substantial risk of likely health or reproductive importance, or personal utility to the source and return is likely to provide net benefit from that person’s perspective.
However, among the caveats we offered was that, “The greater difficulty and cost of biobank return, the lower likelihood of benefit with lapse of time, and the reality that some contributors will not have consented to research, justify more restrictive criteria for return in biobank research than primary research.” Thus, although our 2008 consensus paper included some findings of reproductive importance in the “should return” category, the 2012 paper focusing on biobanks did not. We also noted that biobanks may hold data and specimens for so long that relocating and contacting the source individual may be challenging and the utility of return for that individual may be diminished. We also addressed the special challenges facing preexisting biobanks (as opposed to new biobanks that can consider return of results issues in their design). Older biobanks may hold data and specimens collected with consent forms that failed to address and seek consent for return or that stated there would be no return. We addressed options for recontacting source individuals for consent to return, but the need otherwise to respect the prior explicit agreement that there would be no return.
To analyze individual findings for potential return, we made a distinction. We urged that when IFs or IRRs arise in primary research, the primary researcher and institution should be responsible for handling them, working with their IRB. However, when IFs and IRRs arise later in the flow of data and samples through the biobank research system, the biobank itself has a crucial role to play. Thus, when IFs and IRRs arise in the biobank’s own collection of data or specimens (when these are collected by the biobank directly rather than through separate primary research and collection sites); when they arise in biobank quality control, processing, or research; or when they arise in seconday research on data and specimens supplied by the biobank, we urged that the biobank bear primary responsibility for analyzing whether a particular IF or IRR should be offered back to the source individual.
To reidentify the source individual, again a distinction is necessary. When only the primary researcher holds the key code to reidentify individuals, reidentification will need to occur at the primary research site. However, we urged that biobanks consider holding the key code or using a “trusted intermediary” to hold the code. (53, 60) This avoids relying entirely on the primary research site to maintain capacity for reidentification over the extended period of time during which biobank and secondary research is continuing. Planning for how to handle the return of results issue within a biobank research system thus requires planning how deidentification (if undertaken) will occur, how the key code allowing reidentification will be held, and thus what entity has the capacity to reidentify individuals as needed over time.
To recontact the individual to offer the finding, we suggested considering that in many cases the primary research or collection site may be best situated to perform recontact. The Stage 1 site may be the only site in the biobank research system that has had direct contact with the source individual (although in some biobank research systems, the biobank itself may collect data and specimens directly from these individuals and thus have direct contact). That history of direct contact may mean that the source individual is most directly familiar with the primary research or collection site, so the primary site would be the best entity to perform recontact. Thus, even if the biobank or a “trusted intermediary” performs reidentification, it may be the primary research or collection site that instead performs recontact.
This allocation of CARR responsibilities to different entities within the biobank research system demonstrates the importance of analyzing return of results systemically in genetic and genomic research involving biobanks. Our recommendations are sometimes misunderstood as thrusting all CARR responsibilities on biobanks themselves. (46) But that overlooks the systemic thrust of our analysis, distributing duties across the biobank research system, of which biobanks themselves are only one part.
Since we offered these recommendations, debate and research have continued. Bledsoe et al. have argued that the cost of return has the potential to be excessive. (46) Yet there is little work to date costing out return of results. (48) Getting a rigorous estimate of costs will be challenging, as cost will depend on the number of variants to be analyzed for potential return and the number to be returned, the method of sorting those variants to be returned, the size of the research populations, the method of return, and other variables. Indeed, the first of these – the number of variants to be analyzed for potential return and returned – itself remains a subject of research and debate.61,62,63,64 However, the fact that return of results requires expenditure of effort and funds is not itself an argument to avoid the practice. The reality is that ethics costs, including informed consent, IRB review, and the like. (48) If ethics calls for return, the key question will be how to scale return and develop procedures that make it feasible and compatible with achieving research objectives. (65,66 62)
Normative guidance on return of results will and should evolve as research contributes further to the evidence base. What we recommended was a middle course. There are some commentators and researchers who would be much more restrictive, and would offer little or even no return. (46, 47) There are others who would be far more generous, and offer considerably more than our criteria suggest, up to the possibility of offering a source individual their full data set. (34, 67) Thus, commentators from both sides can debate our proposals. We take an intermediate position.
Research continues on what findings source individuals wish to receive, what means of return are effective, and what consequences return has for those individuals, for their subsequent utilization of medical care, and for their health outcomes. Further research considers what genetics professionals consider to be returnable results and why. A good deal of effort is going into identifying a roster of returnable results with underlying criteria to justify the list. And researchers continue to debate how best to minimize false positives and create a process to restrict return to those findings whose meaning is adequately established. Of course, work is still required to reach consensus on what constitutes “adequately established” and how to best reconcile the effort to protect source individuals from false positives and data whose meaning is currently uncertain with the reality that some of these individuals want their data with accompanying indications of what is known and not, so that the individual can await further research to improve interpretation.
Further research addresses implementation of return of results, including the protocols, systems, informatics, consent processes, and costs involved. Getting a grip on these specifics and different models for return will be crucial to making progress.
Frontier issues prompting further research include how to approach return of results in pediatric populations.68,69,70 Issues include how to integrate return issues into pediatric assent and parent or guardian permission, to whom to offer pediatric IFs and IRRs, whether some findings (such as an IF of adolescent pregnancy) should be offered only to the adolescent, and how to handle disagreement between the pediatric proband and the parents or guardians on return of results issues. As WES and WGS move into research application to children and even newborns, the question arises whether to refrain from offering even to parents or guardians those findings that lack clinical utility in childhood.71 This would be in keeping with long-established guidelines urging that children only be tested for genetic variants with established clinical utility in childhood, preserving for the child the option to choose or refuse testing for other variants once the child achieves the age of majority.72
Another pressing issue is under what circumstances (if any) to offer return of IFs and IRRs to the participant’s or source individual’s kin or family. Kin or family may already receive a proband’s IFs and IRRs if the proband is a child or an adult without decision-making capacity. In these cases, the kin or family member receives results in his or her capacity as a source of permission for the child to participate in the research or as a source of consent for the adult without capacity. The further issue, however, is whether IFs or IRRs should ever be offered to kin or family members because of the potential implications for their own health or reproductive decision-making. Our research group is examining this issue collaboratively with investigators at the Mayo Clinic in the context of research based in a pancreatic cancer biobank.73 Because median life expectancy for probands diagnosed with pancreatic cancer remains short, the question arises whether to offer IFs and IRRs of significance to kin or family before or after death of the proband, whether proband consent is needed in order to share these findings, and what the utility and impact of sharing these findings are.74 Recent debate on returning results to kin and family after the proband’s death focuses on whether the shared familial nature of genetics makes a proband’s genetic findings a resource that should be available to kin and family75,76 and how this comports with the ethics and law that have traditionally protected individual privacy and confidentiality, including after death.77,78
Moving Into Clinical Care
The debate over return of IFs and IRRs that I have analyzed so far is a debate over the proper conduct of research. However, with the emergence of WES and WGS and their increasing integration into clinical care, concern has emerged over what to report to patients from the resulting flood of findings. This has led to the emergence of a literature that resembles the literature on return of research results and is often mistaken for guidance on return of research results, but actually addresses the question of what to return in a clinical context.
Thus, Berg et al. offer a schema for sorting WGS results into 3 “bins,” which correspond with a requirement to report; an option to report, depending on shared decision-making involving both patient and provider; and an imperative not to report. (44) However, this is all in the context of clinical deployment of WGS. Their Bin 1 (“should report”) covers results that are “known to cause disease or strongly predicted to disrupt function,” “medically actionable,” and have “direct clinical utility based on the current literature.” Their Bin 2 (“may report”) covers results that are “clinically valid but not directly actionable” in light of the recognition that some patients may want this information. They further subdivide Bin 2 into results of low risk and doubtful current utility (Bin 2A), medium risk but doubtful utility and may cause distress (Bin 2B), and may cause high distress (Bin 2C). Their Bin 3 (“should not report”) covers variants of no or unknown significance.
Although this proposal addresses return in the context of clinical use of WGS, there is an active debate over where WGS sits in the translational pipeline, whether WGS is ready for clinical use, and if so, for what indications. In 2012 the ACMG “recognize[d] that genomic sequencing approaches can be of great value in the clinical evaluation of individuals with suspected germ-line genetic disorders. Although this is an area that will continue to evolve with further research…, there are already instances in which genomic sequencing approaches can and should contribute to clinical care.”79 Yet a committee of the American Congress of Obstetricians and Gynecologists (ACOG) cautioned the same year that when personalized genomic tests are used to assess predictive risk, they “should be viewed as investigational at this time,” as there is need to assess their validity and utility.80 Writing in Science, Drmanac opined that WGS “is already a powerful research tool” and though doctors may “also use WGS for some of their patients (mostly with idiopathic disease or refractory cancers)…[but] usually as part of a clinical study.”81 Clearly, WGS is in transition into clinical use and for broadening indications.82
The ACMG 2012 policy statement in part addresses IFs in WGS and WES. The statement acknowledges that when WGS or WES is used for any purpose, IFs “are highly likely, if not inevitable.” (81) It goes on to say that labs and clinics need policies on disclosure of IFs, and should share that policy with patients. Before testing, individuals should be counseled on what “will or will not be disclosed.” The standards for disclosure should be sensitive to whether asymptomatic or affected individuals are undergoing testing. When screening asymptomatic individuals, standards for return should be high to avoid reporting multiple false-positives. However, when considering “diagnostic results that are clearly related to a patient’s phenotype or clinical condition…a lower threshold for reporting is appropriate.” Patients should be allowed to opt-out of receiving some IFs, although “exceptional” cases may arise.
As noted above, an ACMG Workgroup is now focusing on developing a “minimum list of variants/conditions that labs should look for and return,” though labs may return more. A preliminary report from that Workgroup in March 2012 indicated that they are focusing on findings of high penetrance and high positive predictive value, that are not detected in newborn screening, and for which an effective intervention is available. (15)
The Translational Future of Return of IRRs & IFs
The fact that recommendations emerging for return of results in clinical WGS/WES are so close to recommendations for return of IFs and IRRs in research suggests a way forward for the translational future of genetics and genomics. Recognizing that genetic and genomic analytic tools (including WES/WGS) move through time from research use into clinical care, we may be able to identify a core set of criteria that should distinguish findings to be considered for return. However, we should not underestimate the challenge. We will need to remain sensitive to differences between the research and clinical contexts, even as we transition from viewing them as separate domains to recognizing their translational linkage.
In refining criteria for return, we will need to identify how established and substantial the risk should be, how useful the return, whether that usefulness is best judged from the standpoint of what the clinician can offer (clinical actionability) or from the standpoint of what source individuals find useful (which is likely to be a broader set of findings, including some with reproductive and even personal utility). While work on returnability now customarily embraces actionability as a core criterion, it remains unclear exactly how actionability should be defined. Nor is it clear why actionability should be limited to findings with health implications, omitting findings with high and established reproductive importance. From the standpoint of source individuals, such a reproductive finding may be highly actionable.
In confronting the challenge of return of results, we are facing the translational nature of genetics and genomics. What is in the domain of research today is fast moving into the clinic. And it is unavoidable that in the course of conducting research, we will discover information about source individuals of clinical significance and even urgency. Imaging researchers have already confronted this reality.
The return of results debate thus forces us to re-think the traditional dichotomy in ethics (as well as in law) between the domain of research and the domain of clinical care. This old, static dichotomy was built on premises that are increasingly outmoded. Research on human genetics and genomics is translational science yielding insights that can move into clinical care with speed. And in a host of scenarios, researchers seek genetic and genomic answers to burdensome disease and disability, while helping individuals and families end their diagnostic odyssey, or while shedding light on any remaining interventional options for otherwise terminal disease. Research and clinical care are connecting along a translational continuum. Instead of a wall between the two, we now have a permeable membrane. The return of results is a debate about how to structure the flow of information through that membrane.
Conclusion
At the end of the day, the return of results debate is about people. It is about the research participant who does not know that she has a variant associated with malignant hyperthermia or Lynch syndrome, or that she has a BRCA 2 variant. It is about the family enrolling their child with a puzzling and devastating developmental disorder in genomic research, hoping that research to aid others will also yield some clue to the puzzle.
The debate is also about the investigator chafing at the custom of offering no information to participants, no matter how significant and actionable – the researcher troubled by the tradition of silence.83 Nearly 30 years ago Jay Katz published his classic study of the tradition of silence in the doctor-patient relationship.84 His most famous example was that of a physician, who finds himself disturbed shortly before performing a mastectomy on a young woman, troubled by information he had withheld from her. He went to her bedside to reveal what he had withheld, and it changed her choice of treatment. Katz was tracing the roots of a sea change in clinical care, the change that yielded a duty to share information with patients, to treat them as individual decision-makers entitled to material information about their condition.
We stand now at the brink of a change as profound in research. Research is not the same as clinical care. It seeks generalizable knowledge, in order to later yield diagnostics and treatments to benefit the many. But the only way to generate that knowledge is to earn and keep the trust of those people generous enough to participate in research. Even when research is conducted on data and specimens left over from clinical care, the trend increasingly is to recognize that these crucial materials derive from real people, who may continue to incur a privacy risk even if the materials are deidentified, who retain a stake in the responsible use of their materials, and who may benefit greatly in some cases from return of results.
Return of results is the next frontier in the challenge of treating the people whose data and specimens make research possible as partners. Much work remains to be done, to develop appropriate criteria for return, efficient and sustainable processes, the evidence base to shape model protocols, and approaches that make sense for individual research projects and biobank research systems. But the silence is broken. The effort has begun to treat research participants and source individuals as indispensible partners in the research enterprise and people with a real stake in learning individual findings of significance.
Acknowledgments
Preparation of this article was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) and National Human Genome Research Institute (NHGRI) grant #1-R01-CA154517 on “Disclosing Genomic Incidental Findings in a Cancer Biobank: An ELSI Experiment” (G. Petersen, B. Koenig, S.M. Wolf, PIs); Robert Wood Johnson Foundation (RWJF) Investigator Award # 69763 (S.M. Wolf, PI); and a Robina LaPPS Fund award from the University of Minnesota (S.M. Wolf, PI). The views expressed in this article are those of the author and not necessarily the views of NIH, NCI, NHGRI, RWJF, or the University of Minnesota. Thanks to Rebecca Boxhorn, J.D., Elliot Ferrel, J.D. candidate, and Elizabeth Oji, J.D., for research assistance. Any errors are my own.
Footnotes
Disclosure Statement
The author receives research support from the National Human Genome Research Institute (NHGRI) and National Cancer Institute (NCI) at NIH, as well as the Robert Wood Johnson Foundation and the University of Minnesota. She has received honoraria in the last year from the Mayo Clinic and Boston Scientific.
Literature Cited
- 1.Wolf SM, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med & Ethics. 2008;36:219–48. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stedman’s Medical Dictionary. 27. Philadelphia: Lippincott Williams & Wilkins; 2000. incidentaloma. [Google Scholar]
- 3.Grumbach MM, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”) Ann Intern Med. 2003;138:424–29. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 4.Friedman Ross L. Disclosing misattributed paternity. Bioethics. 1996;10(2):115–30. doi: 10.1111/j.1467-8519.1996.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 5.National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. I. Rockville, MD: National Bioethics Advisory Commission; 1999. pp. vi–vii.pp. 71–72. Available at: http://bioethics.georgetown.edu/nbac/pubs.html. [Google Scholar]
- 6.Reilly P. When should an investigator share raw data with the subjects? IRB. 1980;2(9):4–5. 12. [PubMed] [Google Scholar]
- 7.Yue NC, et al. Clinically serious abnormalities found incidentally at MR imaging of the brain: data from the Cardiovascular Health Study. Radiol. 1997;202:41–46. doi: 10.1148/radiology.202.1.8988190. [DOI] [PubMed] [Google Scholar]
- 8.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282:36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Illes J, et al. Incidental findings in brain imaging research. Science. 2006;311:783–84. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalis ME, et al. CT colonography reporting and data system: a consensus proposal. Radiol. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 11.Mjoseth J. NHGRI Funds Return of Results Studies. Forms Expert Consortium. Available at: http://www.genome.gov/27545526.
- 12.Couzin-Frankel J. What would you do? Science. 2011;331:662–65. doi: 10.1126/science.331.6018.662. [DOI] [PubMed] [Google Scholar]
- 13.Kolata G. Genes now tell doctors secrets they can’t utter. New York Times. 2012;2012(Aug 25):A1. [Google Scholar]
- 14.Presidential Commission for the Study of Bioethical Issues. Privacy and Progress in Whole Genome Sequencing. 8–9. Washington, DC: 2012. pp. 94–99. [Google Scholar]
- 15.Green R. Preliminary report of the Secondary Findings in Clinical Sequencing Workgroup. 2012 Mar 28; Available at: http://www.slideshare.net/erikanature/acmg-secondary-findings-open-forum-3-2812-final.
- 16.Zawati MH, Knoppers BM. International normative perspectives on the return of individual research results and incidental findings in genomic biobanks. Genet Med. 2012;14:484–89. doi: 10.1038/gim.2012.13. [DOI] [PubMed] [Google Scholar]
- 17.Knoppers BM, et al. Population studies: return of research results and incidental findings policy statement. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston C, Kaye J. Does the UK Biobank have a legal obligation to feedback individual findings to participants? Med Law Rev. 2008;12:239–67. [Google Scholar]
- 19.Wolf SM, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. 2012;14:361–84. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beskow LM, et al. Offering aggregate results to participants in genomic research: opportunities and challenges. Genet Med. 2012;14:490–96. doi: 10.1038/gim.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.45 C.F.R. § 46.102(f) (2012)
- 22.Office for Human Research Protection. Guidance on Research Involving Coded Private Information or Biological Specimens. 2008 Available at: http://www.hhs.gov/ohrp/policy/cdebiol.html.
- 23.Brothers KB, Clayton EW. “Human non-subjects research”: privacy and compliance. Am J Bioethics. 2010;10(9):15–17. doi: 10.1080/15265161.2010.492891. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services. . Human subjects research protections: enhancing protections for research subjects and reducing burden, delay, and ambiguity for investigators. Federal Register. 2011;76:44512–31. Available at: http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/html/2011-18792.htm. [Google Scholar]
- 25.Organisation for Economic Co-Operation and Development. OECD Guidelines on Human Biobanks and Genetic Research Databases. 2009:1. Available at: http://www.oecd.org/dataoecd/41/47/44054609.pdf.
- 26.Murphy J, et al. Public expectations for return of results from large-cohort genetic research. Am J Bioethics. 2008;8(11):36–43. doi: 10.1080/15265160802513093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez CV, et al. The return of research results to participants: pilot questionnaire of adolescents and parents of children with cancer. Pediatr Blood Cancer. 2007;48:441–46. doi: 10.1002/pbc.20766. [DOI] [PubMed] [Google Scholar]
- 28.Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med. 2002;162:1457–62. doi: 10.1001/archinte.162.13.1457. [DOI] [PubMed] [Google Scholar]
- 29.Beskow LM, Smolek SJ. Prospective biorepository participants’ perspectives on access to research results. J Empir Res Hum Res Ethics. 2009;4:99–111. doi: 10.1525/jer.2009.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Facio FM, et al. Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.179. 1038/ejhg.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bollinger JM, et al. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genet Med. 2012;14:451–57. doi: 10.1038/gim.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman D, et al. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10:831–39. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- 33.Trinidad SB, et al. Research practice and participant preferences: the growing gulf. Science. 2011;331:287–88. doi: 10.1126/science.1199000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohane IS, et al. Reestablishing the researcher-patient compact. Science. 2007;316:836–37. doi: 10.1126/science.1135489. [DOI] [PubMed] [Google Scholar]
- 35.Illes J, et al. Incidental findings in brain imaging research. Science. 2006;311:783–84. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson HS, Belsky L. The ancillary-care responsibilities of medical researchers. an ethical framework for thinking about the clinical care that researchers owe their subjects. Hastings Cent Rep. 2004;34(1):25–33. [PubMed] [Google Scholar]
- 37.Richardson HS. Incidental findings and ancillary-care obligations. J Law Med Ethics. 2008;36:256–70. doi: 10.1111/j.1748-720X.2008.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson HS. Moral Entanglements: The Ancillary Care Obligations of Medical Researchers. New York: Oxford University Press; 2012. [Google Scholar]
- 39.Beskow LM, Burke W. Offering individual genetic research results: context matters. Sci Transl Med. 2010:2. doi: 10.1126/scitranslmed.3000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greely HT. The uneasy ethical and legal underpinnings of large-scale genomic biobanks. Ann Rev Genomics Hum Genet. 2007;8:343–64. doi: 10.1146/annurev.genom.7.080505.115721. [DOI] [PubMed] [Google Scholar]
- 41.Ossorio P. Taking aims seriously: repository research and the limits on the duty to return individual research findings. Genet Med. 2012;14:461–66. doi: 10.1038/gim.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf SM. Incidental findings in neuroscience research: a fundamental challenge to the structure of bioethics and health law. In: Illes J, Sahakian B, editors. Oxford Handbook of Neuroethics. New York: Oxford University Press; 2011. [Google Scholar]
- 43.Fabsitz RR, et al. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung and Blood Institute working group. Circulation: Cardiovascular Genet. 2010;3:574–80. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genet Med. 2011;13:499–504. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- 45.Canterbury v. Spence, 464 F.2d 772 (D.C. Cir. 1972)
- 46.Bledsoe, et al. Return of results from genomic biobanks: cost matters. Genet Med. 2012 doi: 10.1038/gim.2012.105. Available at: http://www.nature.com/gim/journal/vaop/ncurrent/full/gim2012105a.html. [DOI] [PMC free article] [PubMed]
- 47.Clayton EW, McGuire AL. The legal risks of returning results of genomic research. Genet Med. 2012;14:473–77. doi: 10.1038/gim.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf SM. Return of results in genomic biobanks: ethics matters. Genet Med Letter. doi: 10.1038/gim.2012.162. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf SM. The role of law in the debate over return of research results and incidental findings: the challenge of developing law for translational science. Minn J Law Sci Technol. 2012;13:435–48. doi: 10.2139/ssrn.2117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim B, et al. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol. 2002;23:1674–77. [PMC free article] [PubMed] [Google Scholar]
- 51.National Cancer Institute, Office of Biorepositories and Biospecimen Research. Workshop Summary, 4 March 2011; Workshop on Release of Research Results to Participants in Biospecimen Studies; Bethesda, Maryland. 8–9 July 2010; 2011. Available at: http://biospecimens.cancer.gov/global/pdfs/NCI_Return_Research_Results_Summary_Final-508.pdf. [Google Scholar]
- 52.Lockhart NC, et al. Intersection of biobanking and clinical care: should discrepant diagnoses and pathological findings be returned to research participants? Genet Med. 2012;14:471–23. doi: 10.1038/gim.2012.11. [DOI] [PubMed] [Google Scholar]
- 53.International Society for Biological and Environmental Repositories. . best practices for repositories, collection, storage, retrieval and distribution of biological materials for research. Cell Preserv Technol. 2008;6:3–58. [Google Scholar]
- 54.Chadwick R, Berg K. Solidarity and equity: new ethical framework for genetic databases. Nature Rev Genet. 2001;2:318–21. doi: 10.1038/35066094. [DOI] [PubMed] [Google Scholar]
- 55.Winickoff DE, Winickoff RN. The charitable trust as a model for genomic biobanks. N Engl J Med. 2003;349:1180–84. doi: 10.1056/NEJMsb030036. [DOI] [PubMed] [Google Scholar]
- 56.Fullerton SM, et al. Return of individual research results from genome-wide association studies: experience of the Electronic Medical Records and Genomics (eMERGE) Network. Genet Med. 2012;14:424–31. doi: 10.1038/gim.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holm IA, Taylor PL. The Informed Cohort Oversight Board: from values to architecture. Minn J Law Sci & Technol. 2012;13:669–90. [PMC free article] [PubMed] [Google Scholar]
- 58.GENEVA Study. GENEVA Statement on Incidental Findings. Available at: https://genevastudy.org/Incidental_Findings_Files.
- 59.Pulley J, et al. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3:42–48. doi: 10.1111/j.1752-8062.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yassin R, et al. Custodianship as an ethical framework for biospecimen-based research. Cancer Epidemiol Biomarkers Prev. 2010;19:1012–15. doi: 10.1158/1055-9965.EPI-10-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassa CA, et al. Disclosing pathogenetic genetic variants to research participants: quantifying an emerging ethical responsibility. Genome Res. 2012;22:421–28. doi: 10.1101/gr.127845.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohane IS, Hsing M, Kong SW. Taxonomizing, sizing, and overcoming the incidentalome. Genet Med. 2012;14:399–404. doi: 10.1038/gim.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnston JJ, et al. Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. Am J Hum Genet. 2012;91:97–108. doi: 10.1016/j.ajhg.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westbrook MJ, et al. Mapping the incidentalome: estimating incidental findings generated through clinical pharmacogenomics testing. Genet Med. 2012 doi: 10.1038/gim.2012.147. Available at: http://www.nature.com/gim/journal/vaop/ncurrent/pdf/gim2012147a.pdf. [DOI] [PMC free article] [PubMed]
- 65.Berg JS, et al. An informatics approach to analyzing the incidentalome. Genet Med. 2012 doi: 10.1038/gim.2012.112. Available at: http://www.nature.com/gim/journal/vaop/ncurrent/full/gim2012112a.html. [DOI] [PMC free article] [PubMed]
- 66.Ball MP, et al. A public resource facilitating clinical use of genomes. PNAS. 2012;109:11920–27. doi: 10.1073/pnas.1201904109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thakuria J. Managing IFs and IRRs in genomic biobanks and archives. Presentation at working group meeting, Managing Incidental Findings and Research Results in Genomic Biobanks & Archives; Washington, DC. 2010. Jun, [Google Scholar]
- 68.Wilfond BS, Diekema DS. Engaging children in genomics research: decoding the meaning of assent in research. Genet Med. 2012;14:437–43. doi: 10.1038/gim.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenkins MM, et al. Ethical issues raised by incorporation of genetics into the National Birth Defects Prevention Study. Am J Med Genet Part C: Semin Med Genet. 2008;148C:40–46. doi: 10.1002/ajmg.c.30157. [DOI] [PubMed] [Google Scholar]
- 70.Kaufman D, et al. Ethical implications of including children in a large biobank for genetic-epidemiologic research: a qualitative study of public opinion. Am J Med Genet Part C: Semin Med Genet. 2008;148C:31–39. doi: 10.1002/ajmg.c.30159. [DOI] [PubMed] [Google Scholar]
- 71.Hens K, et al. Children and biobanks: a review of the ethical and legal discussion. Hum Genet. 2011;130:403–13. doi: 10.1007/s00439-011-1031-8. [DOI] [PubMed] [Google Scholar]
- 72.American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. . Points to consider: ethical, legal, and psychological implications of genetic testing in children and adolescents. Am J Hum Genet. 1995;57:1233–41. [PMC free article] [PubMed] [Google Scholar]
- 73.Disclosing genomic incidental findings in a cancer biobank: an ELSI experiment. 2011–16. NIH/NCI/NHGRI #1-R01-CA154517 (Petersen G, Koenig B, Wolf SM, PIs)
- 74.Ormondroyd E, et al. Disclosure of genetics research results after the death of the patient participant: a qualitative study of the impact on relatives. J Genet Counsel. 2007;16:527–38. doi: 10.1007/s10897-007-9088-1. [DOI] [PubMed] [Google Scholar]
- 75.Chan B, et al. Genomic inheritances: disclosing individual research results from whole-exome sequencing to deceased participants’ relatives. Am J Bioethics. 2012;12(10):1–8. doi: 10.1080/15265161.2012.699138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black L, McClellan KA. Familial communication of research results: a need to know? J Law Med Ethics. 2011;39:605–13. doi: 10.1111/j.1748-720X.2011.00627.x. [DOI] [PubMed] [Google Scholar]
- 77.Rothstein MA. Disclosing decedents’ research results to relatives violates the HIPAA privacy rule. Am J Bioethics. 2012;12(1):16–17. doi: 10.1080/15265161.2012.699588. [DOI] [PubMed] [Google Scholar]
- 78.Tassé AM. Biobanking and deceased persons. Hum Genet. 2011;130:415–23. doi: 10.1007/s00439-011-1049-y. [DOI] [PubMed] [Google Scholar]
- 79.American College of Medical Genetics and Genomics. Policy statement: points to consider in the clinical application of genomic sequencing. 2012 doi: 10.1038/gim.2012.74. Available at: http://www.acmg.net/AM/Template.cfm?Section=Policy_Statements&Template=/CM/HTMLDisplay.cfm&ContentID=6979. [DOI] [PubMed]
- 80.American College of Obstetricians and Gynecologists, Committee on Genetics. . Committee opinion, number 527. Personalized genomic testing for disease risk. Obstet Gynecol. 2012;119:1318–19. doi: 10.1097/AOG.0b013e31825af3c6. [DOI] [PubMed] [Google Scholar]
- 81.Drmanac R. The ultimate genetic test. Science. 2012;336:1110–12. doi: 10.1126/science.1221037. [DOI] [PubMed] [Google Scholar]
- 82.Biesecker LG. Opportunities and challenges for the integration of massively parallel genomic sequencing into clinical practice: lessons from the ClinSeq project. Genet Med. 2012;14:393–98. doi: 10.1038/gim.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biesecker L. Genet Med Letter In press. [Google Scholar]
- 84.Katz J. The Silent World of Doctor and Patient. New York: Free Press; 1984. [Google Scholar]