Abstract
We present a one-pot chemo-enzymatic microscale synthesis of NADPH with two different patterns of isotopic labels: (4R)-[Ad-14C,4-2H] NADPH and (4R)-[Ad-3H,4-2H] NADPH. These co-factors are required by an enormous range of enzymes, and isotopically labeled nicotinamides are consequently of significant interest to researchers. In the current procedure, [Ad-14C] NAD+ and [Ad-3H] NAD+ were phosphorylated by NAD+ kinase to produce [Ad-14C] NADP+ and [Ad-3H] NADP+, respectively. Thermoanaerobium brockii alcohol dehydrogenase was then used to stereospecifically transfer deuterium from C2 of isopropanol to the re face of C4 of NADP+. After purification by HPLC, NMR analysis indicated the deuterium content at the 4R position is more than 99.7 %. The labeled cofactors were then used to successfully and sensitively measure kinetic isotope effects for R67 dihydrofolate reductase, providing strong evidence for the utility of this synthetic methodology.
Keywords: isotopic labeling, nicotinamide adenine dinucleotide 2′ phosphate, labeled NADPH, kinetic isotope effect, dihydrofolate reductase, Thermoanaerobium brockii alcohol dehydrogenase
Introduction
Reduced β-nicotinamide adenine dinucleotide 2′ phosphate (NADPH, Scheme 1) and its oxidized form (NADP+) are essential cofactors for a huge range of enzymes, including dihydrofolate reductase (DHFR), glucose dehydrogenase (GDH), and others involved in crucial biosynthetic pathways. The development of efficient methods for the synthesis of a variety of NADPH molecules with different isotopic labelling patterns is of interest to a broad range of biochemical, pharmacological, and mechanistic research. For example, recent kinetic isotope effects studies (KIEs) of DHFR have used six different isotopic labelling patterns to investigate the dynamics of this important model enzyme.1–4
Scheme 1.
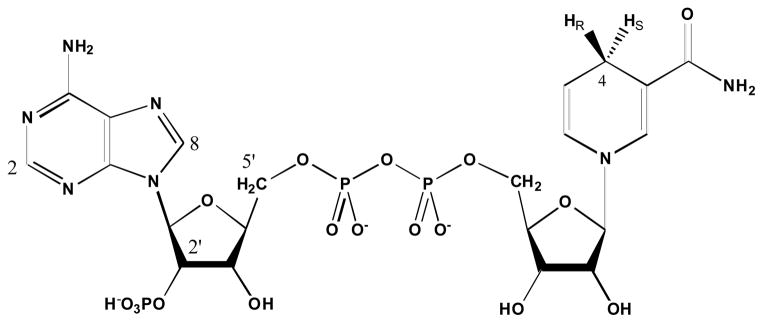
Structure of NADPH (HR = HS = 1H), (R)-[Ad-14C,4-2H]-NADPH (HR = 2H, HS = 1H), and (R)-[Ad-2,5′,8-3H,4-2H]-NADPH (HR = 2H, HS = 1H).
KIE studies have proved to be a valuable probe of the chemical step of enzymatic reactions, yielding information regarding fundamental catalytic processes like C-H bond activation.5 KIE measurements determine the relative reaction rates for isotopologuesϒ, and can be performed either non-competitively (where reaction rates are measured separately for each isotopologue) or competitively (isotopologues react together in one vessel). In comparison to non-competitive experiments, competitive KIE measurements are generally considered to be more reliable and sensitive, frequently due to the use of radiolabeled compounds.6 Competitive measurements of hydride transfer rate in type A (pro-R specific) NADPH-dependent enzymes, for example, utilize two isotopologues of NADPH, each labeled at the pro-R position of the C4 of the nicotinamide with one of the three isotopes of hydrogen, viz.1H (H), 2H(D) and 3H(T). Conventionally, the lighter isotopologue is traced using a 14C label at a position on the molecule remote from the reaction center, and 3H is used as the heavier isotope, and (depending upon the mixture of isotopes used) the measured KIEs can be kH/kT (H/T), kH/kD (H/D) or kD/kT (D/T). Other combinations are possible, but not commonly utilized.7
Given the ubiquity of NADPH-dependent enzymes, and the requirement of labeled NADPH molecules for kinetic measurements, there is a continuous need for efficient synthetic methods for the production of stereospecifically labeled NADPH. In particular, 4R deuterated NADPHs with remote radiolabeling are essential for competitive H/D and D/T KIE studies.8 However, the synthesis of radiolabeled 4R deuterated NADPH requires extra caution. Proton contamination at the 4R position can cause serious artifacts in H/D KIE measurements, so the deuterium content in the labeled compounds needs to be high enough so these artifact will be below the nise of the measurement (commonly > 99.5 % D). We have previously reported syntheses for (4S)-[4-3H]-NADPH, and (4R)-[4-3H]-NADPH using type B (pro-S specific) glucose dehydrogenase from Cryptococcus uniguttulatus (Schemes 2 and 3a).9 However, this procedure requires a long reaction time, and involves four separate enzymatic reactions, making it unappealing for the synthesis of deuterated compounds. We have also reported the synthesis of (4R)-[4-3H]-NADPH using type A alcohol dehydrogenase from Thermoanaerobactor brockii (tbADH) in a large scale reaction.10 Here we present an optimized procedure for a two-step, one-pot, chemo-enzymatic microscale synthesis of doubly isotopically labeled (4R)-[Ad-14C,4-2H]-NADPH, and (4R)-[Ad-3H,4-2H]-NADPH (Scheme 2, and 3b), and their use in the measurement of competitive primary (1°) KIEs. First, radiolabeled NAD+ was phosphorylated by NAD+ kinase to produce labeled NADP+. tbADH was then used to stereospecifically catalyze a deuteride transfer from d8-isopropanol (99.7 % D at the 2nd position) to the radiolabeled NADP+, followed by HPLC purification and preservation. The isotopic purity of the radioactive compounds was confirmed by NMR analysis (99.7 % D). Finally, measurements of H/D, and D/T 1° KIEs for plasmid-encoded R67 DHFR indicated that any contamination was low enough not to affect the measurements.
Scheme 2.
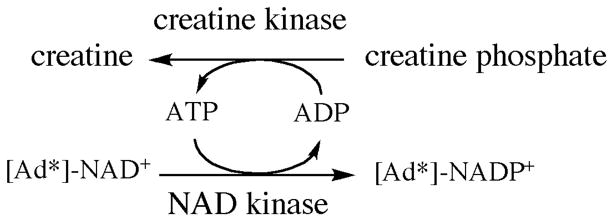
Phosphorylation of radiolabeled NAD+ using creatine recycling system.
Scheme 3.
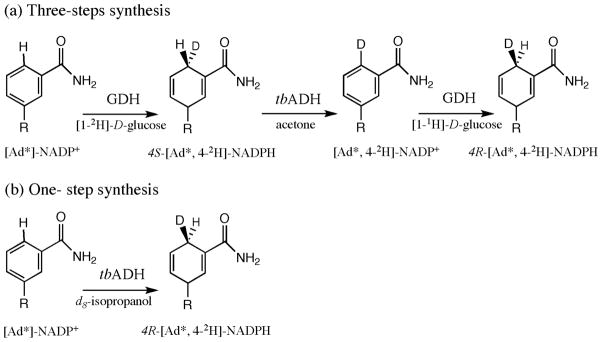
Chemoenzymatic synthesis of isotopically labeled (R)-[Ad-14C,4-2H]-NADPH, and (R)-[Ad-3H,4-2H]-NADPH. R represents adenine-2′-phosphate-ribose-5′pyrophosphate-ribose. (a) Three steps synthesis using Type B glucose dehydrogenase and alcohol dehydrogenase;15 (b) Onestep synthesis using tbADH.
Experimental
Materials
Reagent-grade chemicals were used as purchased unless specified otherwise. β-nicotinamide [Ad-14C] adenine dinucleotide ([Ad-14C]-NAD+; 267 mCi/mmol), and β-nicotinamide [2,5′,8-3H] adenine dinucleotide ([Ad-3H]-NAD+; 25.0 Ci/mmol) were purchased from Amersham Pharmacia. Monobasic potassium phosphate, TRIS, MgCl2, and Microcon YM-30 regenerated cellulose centrifugal filters (3000 MWCO) were purchased from Fisher. Ultima Gold liquid scintillation cocktail was purchased from PerkinElmer. All other materials were purchased from Sigma.
Methods
HPLC analysis and separation
Analytical HPLC separations were performed using an Agilent Technologies 1100 Series HPLC, fitted with a Supelco Discovery C-18 reverse-phase column (250 × 4.6 mm, 5 μm). A semi-preparative Supelco Discovery C18 reverse-phase column (250 × 10 mm, 5 μm) was used for the purification of the synthesized NADPH, and eluted peaks were analyzed using an online UV diode array detector or a Packard 500TR Series flow scintillation analyzer (HPLC-FSA). When more sensitive analysis was necessary, fractions were collected and analyzed by a Packard 2900 TR Series Liquid Scintillation Counter (LSC). The details of the HPLC method used have been described elsewhere. 11, 12
Synthesis of (R)-[Ad-14C,4-2H]-NADPH and (R)-[Ad-3H,4-2H]-NADPH
Radiolabeled NAD+ solution (500 μL, 12.5 μCi, for [Ad-14C]-NAD+, and 250 μL, 62.5 μCi, for [Ad-3H]-NAD+) was lyophilized, then the following were added to a total final volume of 450 μl: 250 mM Tris, 130 μM creatine phosphate disodium salt tetrahydrate, 800 μM NAD+, 20 mM MgCl2 (final concentrations). The pH was adjusted to 6.7, and ATP was added to a final concentration of 2.4 mM. 400 units of creatine phosphokinase, and 50 units of NAD+ kinase were then added to initiate the phosphorylation step. The reaction mixture was incubated with occasional mixing at 35 °C until the reaction was complete as determined by HPLC-FSA analysis.
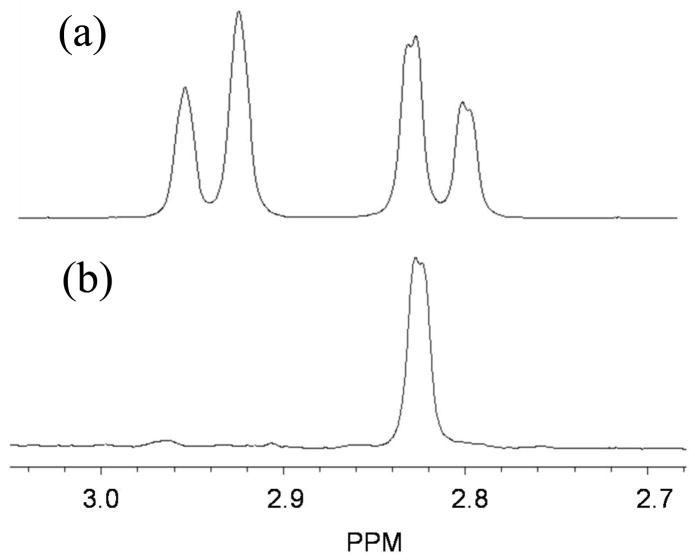
Upon completion of the phosphorylation step, the pH of the reaction mixture was adjusted to 8.0 (the intended product, NADPH, is more stable at this pH) and 5 μl of 2-propanol-d8 along with 7 units of tbADH were added. The reaction was incubated anaerobically at 43 °C for 15 min under argon. After the completion of the reaction, as determined by HPLC-FSA analysis, the enzymes were removed with a Microcon YM-30 centrifugal filter device at 4 °C. The product was purified via semi-preparative HPLC, and the total radioactivity was determined by LSC. The synthesized NADPH was lyophilized and stored at −80 °C. The proton contamination at the 4R position in both synthesized NADPHs was examined by 1H-NMR measurement using 600 MHz NMR for 10000 scans in D2O (Figure 1).
Figure 1.
1H-NMR spectrum of (a) NADPH, (b) (4R)-[Ad-14C,4-2H]-NADPH. The peaks shown represent the hydrogens at C4 of the product with the pro-R hydrogen at 2.935 PPM and the pro-S hydrogen at 2.822 PPM. In spectrum b, integration of the noise at and around 2.935 PPM indicated that up to 0.3 % 1H contamination would have been detectable if present. Since none is detected the H-contamination is less than 0.3 %.
Competitive KIE Experiments
For 1° D/T KIE experiments, (4R)- [Ad-14C,4-2H]-NADPH and (4R)-[4-3H]-NADPH were combined in a 1:6 DPM ratio of 14C:3H (to compensate for the lower efficiency of 3H scintillation counting), and copurified using reverse-phase HPLC to remove impurities. For 1° H/D KIE experiments, [Ad-14C] NADPH and (4R)-[Ad-3H,4-2H] NADPH were combined in either a 1:4, or 1:9 DPM ratio. The two ratios were examined in order to assure no experimental artifacts on the KIE values (theoretically these ratios should not affect the KIE, if the experiment is artifact free). The co-purified radiolabeled NADPH was divided into aliquots containing 360,000 DPM of 14C, and flash frozen for short-term storage (up to 3 weeks) at −80 °C. Measurements of KIEs on R67 DHFR were conducted as described elsewhere.13 The fractional conversion (f) of NADPH was determined as per equation 1, from the ratio of 14C in the product to the total amount of 14C in both product and reactant peaks:
| (1) |
The observed KIEs were calculated according to equation 2:14:
| (2) |
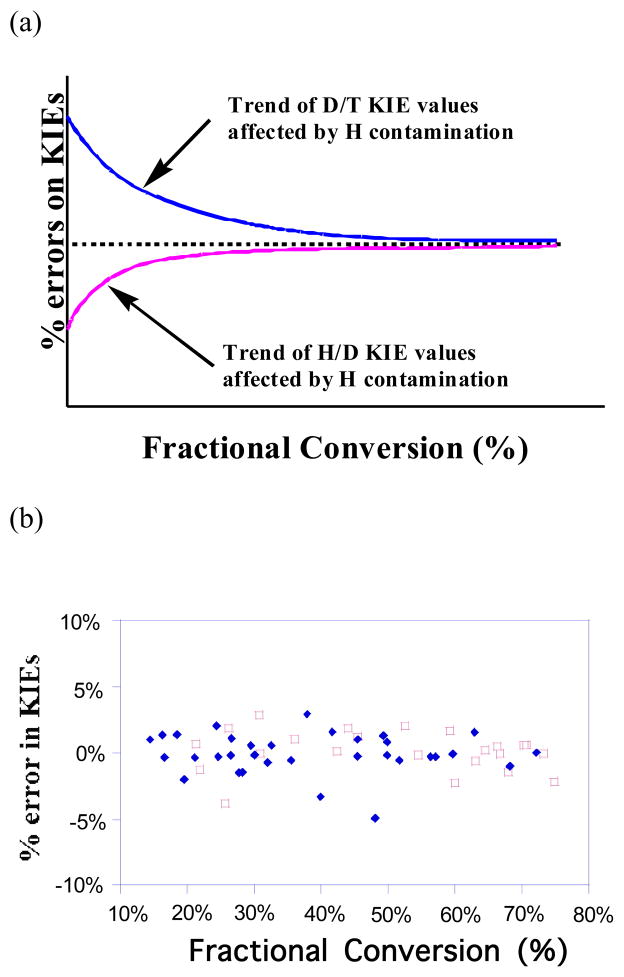
where Rt is the ratio of 3H to 14C in products at fractional conversion f, and R∞ is that ratio at 100 % conversion. The percentage error from the average KIE value at each temperature was plotted versus the fractional conversion, since most articacts would result in increasing or decreasing trends in such a plot (Figure 2a).
Figure 2.
Schematic illustration of the effects of H contamination on observed KIE values over fractional conversion. (a) Dotted line represents % error of KIEs with no H contamination (f independent KIEs). Solid lines represent the trends of affected H/D and D/T KIEs distribution due to the artifacts of H contamination. (b) Experimental data: Deviations of D/T (
 ) and H/D (
) and H/D (
 ) KIEs from their average values are plotted vs. fractional conversion (f).
) KIEs from their average values are plotted vs. fractional conversion (f).
Results and Discussion
Synthesis of (4R)-[Ad-14C,4-2H]-NADPH and (4R)-[Ad-3H,4-2H]-NADPH
(4R)-[Ad-14C, 4-2H]-NADPH and (4R)-[Ad-3H, 4-2H]-NADPH were each synthesized from the relevant NAD+ precursor in two steps, viz. phosphorylation of adenine-radiolabeled NAD+ to NADP+, followed by the stereospecific reduction of NADP+ with d8-isopropanol and tbADH. In the phosphorylation step, a combination of two enzymes was used in the reaction mixture. NAD+ kinase catalyzed the phosphorylation of NAD+ to form NADP+ by transferring phosphate from ATP (Scheme 2). Since one of the products, ADP, is a potent inhibitor of NAD+ kinase, the creatine phosphate/creatine phosphokinase system was concomitantly used to regenerate ATP, minimizing the amount of ATP required and preventing the accumulation of ADP. The completion (> 99 %) of the phosphorylation reaction is very important for subsequent applications, since NAD+ and NADPH cannot be well seperated in later purification steps.
Prior to the tbADH reaction, the pH was adjusted to 8.0 because NADPH is more stable at higher pH (> 7.5) while NAD+ and NADP+ are stable at lower pH. tbADH was then used to catalyze a deuteride transfer from commercially available deuterated isopropanol (< 0.3 % H on alcoholic carbon) to the re face of nicotiamide of radiolabeled NADP+ to form (4R)-[Ad-14C,4-2H]NADPH or (4R)-[Ad-3H,4-2H]NADPH in a single step. The overall yields in radioactivity for (4R)-[Ad-14C,4-2H]-NADPH and (4R)-[Ad-3H,4-2H]-NADPH was 70 %, and 60 %, respectively. Deuteration was over 99.7 % complete, as determined by NMR analysis (Figure 1). Our previously reported labeling procedure used type B glucose dehydrogenase and required three steps following the phosphorylation step (Scheme 3a), but this reduction by tbADH stereospecifically labels the 4R-position of radiolabeled NADPH in H2O with just one step after phosphorylation of the NAD+ (Scheme 3b).
Analysis of D/T and H/D KIEs values vs. Fractional Conversion
Competitive KIE measurements are a sensitive application that can identify the quality of the labeled materials. Almost any problem, like H-contamination, would result is a trend when using the linearized equation for KIEs (Eq. 2). In competitive KIE measurements, two different isotopically labeled substrates (3H and 14C) are mixed, and the fractionation of the light and heavy isotopes in the products is compared as a function of different fractional conversions. Competitive KIEs are a useful tool in studies of enzyme mechanisms, and in cases where the enzyme is NADPH-dependent, deuterated NADPH can be used with tritium or proton labeled NADPH to determine D/T and H/D KIEs, thus aiding the investigation of various mechanistic questions.8 In this case, in order to determine the extent of proton contamination at the 4R position in the deuterated products, both 1° H/D and 1° D/T KIE values were measured and plotted as function of f. The synthesized (4R)-[Ad-14C, 4-2H] NADPH was co-purified with (4R)-[4-3H]-NADPH, and the mixture used in the competitive measurement of 1° D/T KIEs, while the synthesized (4R)-[Ad-3H,4-2H]-NADPH was co-purified with [Ad-14C]-NADPH to determine 1° H/D KIEs in the reaction catalyzed by R67 DHFR. In case of significant H-contamination, observed KIE values should show a definite trend over a range of fractional conversions, since at lower fractional conversions it is expected that far more of the protonated material will have reacted than the deuterated (Figure 2a). Not only was such a trend not observed, but for H/D experiments, the different ratios of 3H :14C radioactivity did not appear to affect the measured KIEs. Furthermore, the errors on measured KIEs from six independent experiments for the D/T KIE, and five independent experiments for the H/D value, did not show any trends caused by proton contamination over the fractional conversion range. Figure 2b presents percentage errors vs. fractional conversion for both D/T and H/D KIEs. The measured KIEs are independent of fractional conversion within experimental error, indicating that the pro-R C4 position of NADPH is sufficiently deuterated in both cases.
Conclusion
We have successfully developed a two-step microscale chemo-enzymatic synthesis of doubly isotopically labeled (4R)-[Ad-14C,4-2H] NADPH, and (4R)-[Ad-3H,4-2H] NADPH. We have also demonstrated the utility of the synthesized materials by measuring the KIEs for the R67 DHFR catalyzed reaction: D/T and H/D KIEs were determined with high precision, and the lack of any observed trend in the relative KIE over a range of fractional conversions indicates that any H-contamination, if present, is too small to affect the sensitive competitive measurement. This labeling pattern can also be used, if necessary, in the kinetic study of many other NADPH dependent enzymes.
(4R)-[Ad-14C,4-2H]-NADPH, together with (4R)-[4-3H]-NADPH can be used to measure primary D/T KIEs, and (R)-[Ad-3H,4-2H]-NADPH together with [Ad-14C]-NADPH to measure primary H/D KIEs for any type A NADPH-dependent enzyme. Alternatively, these compounds can be used to measure secondary (2°) KIEs with any type B enzyme. The procedure described here can be easily modified to synthesize other labeled nicotinamides, including (R)-[carbonyl-14C,4-2H] NADPH, and 32P or 33P-labeled 2′-phosphate (4R)-[4-2H]NADPH.
Acknowledgments
Contract/grant sponsor: NIH; contact/grant number: R01 GM65368
Contract/grant sponsor: NSF; contract/grant number: CHE-0133117
Footnotes
Isotopologues are molecules that differ only in their isotopic composition.
References
- 1.Wang L, Tharp S, Selzer T, Benkovic SJ, Kohen A. Biochemistry. 2006;45:1383–1392. doi: 10.1021/bi0518242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Goodey NM, Benkovic SJ, Kohen A. Proceedings of the National Academy of Sciences of the USA. 2006;103:15753–15758. doi: 10.1073/pnas.0606976103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Goodey NM, Benkovic SJ, Kohen A. Philosophical Transactions of the Royal Society of London, Series B. 2006;361:1307–1315. doi: 10.1098/rstb.2006.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikorski RS, Wang L, Markham KA, Rajagopalan PTR, Benkovic SJ, Kohen A. Journal of the American Chemical Society. 2004;126:4778–4779. doi: 10.1021/ja031683w. [DOI] [PubMed] [Google Scholar]
- 5.Sen A, Kohen A. In: Quantum Tunneling in Enzyme Catalyzed Reactions. Allemann R, Scrutton N, editors. Royal Society of Chemistry; London, UK: 2008. [Google Scholar]
- 6.Parkin DW. In: Enzyme Mechanism from Isotope Effects. Cook PF, editor. CRC Press; Boca Raton, FL: 1991. pp. 269–290. [Google Scholar]
- 7.Northrop DB. In: Enzyme mechanism from isotope effects. Cook PF, editor. CRC Press; Boca Raton, FL: 1991. pp. 181–202. [Google Scholar]
- 8.Kohen A. In: Isotope effects in Chemistry and Biology. Kohen A, Limbach HH, editors. Taylor and Francis, CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- 9.McCracken JA, Wang L, Kohen A. Analytical Biochemistry. 2004;324:131–136. doi: 10.1016/j.ab.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal N, Kohen A. Analytical Biochemistry. 2003;322:179–184. doi: 10.1016/j.ab.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Markham KA, Sikorski RS, Kohen A. Analytical Biochemistry. 2003;322:26–32. doi: 10.1016/j.ab.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Markham KA, Kohen A. Current Analytical Chemistry. 2006;2:379–388. [Google Scholar]
- 13.Yahashiri A, Howell EE, Kohen A. ChemPhysChem. 2008;9:980–982. doi: 10.1002/cphc.200800067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melander L, Saunders HW. Reaction Rates of Isotopic Molecules. Krieger Pub. Co; Malabar, FL: 1987. [Google Scholar]
- 15.McCracken JA, Wang L, Kohen A. Anal Biochem. 2003;324:131–136. doi: 10.1016/j.ab.2003.09.025. [DOI] [PubMed] [Google Scholar]