Abstract
Developmental prosopagnosia (DP) is defined by severe face recognition problems resulting from a failure to develop the necessary visual mechanisms for processing faces. While there is a growing literature on DP in adults, little has been done to study this disorder in children. The profound impact of abnormal face perception on social functioning and the general lack of awareness of childhood DP can result in severe social and psychological consequences for children. This review discusses possible etiologies of DP and summarizes the few cases of childhood DP that have been reported. It also outlines key objectives for the growth of this emerging research area and special considerations for studying DP in children. With clear goals and concerted efforts, the study of DP in childhood will be an exciting avenue for enhancing our understanding of normal and abnormal face perception for all age groups.
Keywords: developmental prosopagnosia, childhood disorders, developmental disorders, face perception, vision
Madison’s parents first noticed her abnormal behaviour when she was around 18-months-of-age. She never seemed fearful of strangers, and she did not show the excitement or affection towards her parents that would be expected of a child her age- it was almost as if she did not know who was familiar and who was not. She also appeared confused or frightened by changes in emotions of other people. When Madison began speaking, she asked questions like, “Why did your face change?” and “What does that face mean?” When her mother picked her up after school, she noticed that Madison sometimes mistakenly walked over and greeted other parents who drove cars similar to hers. Madison’s mother distinctly recalled one day when the youngster happily approached a stranger who was visiting their next-door neighbor. This stranger had a very different face from the neighbor and was about 20–30 years younger. Confused, her mother asked Madison why she approached the man. Madison replied, “That’s our next-door neighbor.” When asked why she thought this man was her neighbor, Madison explained that she had used his eyeglasses to identify him. She did not even seem to notice that the stranger, who was quite tall with a large build, was not the short, stocky, man from next door.
Madison’s parents tried desperately to find a diagnosis that would explain their daughter’s unusual behaviours and her indifference to whether people were friends or strangers. Pervasive Developmental Disorder was considered, as was Asperger Syndrome (AS), but those diagnoses did not seem appropriate because Madison demonstrates relatively normal social functioning. In addition, she did not demonstrate other behaviours characteristic of AS, such as intensive specialized interests or stereotyped movements, and she only showed mild impairments within the domain of social communication and interaction. In fact, her mother noted that she made good eye contact, almost intently staring at people’s faces as if scrutinizing them. Specialists were baffled for years, and it was only when her parents came across the term “face blindness” that they had the “Aha!” moment they had been waiting for: Madison had developmental prosopagnosia.
Studying Developmental Prosopagnosia in Childhood
Developmental prosopagnosia (DP) is a neurodevelopmental condition characterized by severe face recognition problems that result from a failure to develop the necessary visual mechanisms for normal face processing (Duchaine & Nakayama, 2006b). It occurs in the absence of brain injury and is sometimes referred to as congenital prosopagnosia (Behrmann & Avidan, 2005). Although face recognition problems exist in the context of other disorders (e.g. autism), DP can exist in isolation from more general object agnosias or other developmental disorders. Although much work has been done to study DP in adults, there is a notable lack of research on DP in children.
The development of a deeper understanding of childhood DP is important for a number of reasons. First, DP is estimated to affect 2–2.9% of the population (Bowles et al., 2009; Kennerknecht et al., 2006). While these estimates were drawn from adult populations, this prevalence rate could represent millions of children worldwide. It is higher than prevalence rates reported for several other developmental disorders that receive a great deal of attention from the public and from the research community, such as autism (Yeargin-Allsopp et al., 2003), pointing to a need for similar attention in this developing field. Second, DP can have a profound impact during childhood, leading to difficulties making friends and participating in social activities in school, as well as increased levels of anxiety (Diaz, 2008). In addition, an inability to recognize faces increases the risk of being put in potentially dangerous situations with strangers, as is evident in Madison’s case. Thus, research aimed at understanding the disorder early in life and how to treat it is essential for the well-being of children with DP. Third, the impact of abnormal face processing on social functioning and the general lack of awareness of childhood DP can result in the misdiagnosis of children with DP as having other developmental disorders, such as autism spectrum disorders. Misdiagnosis could result in the application of interventions poorly matched to a child’s needs or ones that fail to address the child’s face processing difficulties altogether. The study of DP in children can provide answers to important questions about abnormal and normal face perception. Finally, learning about the etiology and progression of DP may also lead to insights about other selective developmental deficits such as dyslexia, dyscalculia, and specific language impairment by identifying whether commonalities exist between disorders.
In this review, we take the opportunity to outline some key objectives that we believe should be at the forefront of this emerging field of study. These include 1) increasing awareness of childhood DP, 2) the development of well-designed diagnostic tools, 3) the application of results from research on childhood DP to the study of theoretical issues related to DP in general, and 4) the development of empirically-driven treatment strategies for children and adults with DP.
The review begins with a discussion of possible etiologies of DP, including the contribution of genetics, innate mechanisms, experiential factors, and neurological factors. Next, we discuss the relatively small body of existing literature on childhood DP. We then outline the above-mentioned objectives, which we hope will help guide research on childhood DP in a productive direction, and important theoretical questions that can be answered through the study of DP in children. Finally, we summarize treatment strategies and special considerations for the study of DP in children. Ultimately, this review is intended to provide an overview of the current state of the field and to outline opportunities for future study of childhood DP. The development of normal face processing is beyond the scope of this review, but that information can be found elsewhere (e.g. Nelson, 2001; Pascalis et al., 2011)
It is important to note that throughout this review, the following three terms will be used in very specific ways: Face processing will be used as a non-specific reference to the processes involved in perceiving, encoding, and recalling a face; face perception refers specifically to the perceptual processing of a face (e.g. the ability to detect differences between faces or to encode a face); and face recognition to the behavioural act of recognizing a face. As an example of the importance of this distinction, DP is defined as failure of face recognition at a behavioural level, which could be the result of a failure of face perception or a failure of memory (cf. De Renzi, Faglioni, Grossi, & Nichelli, 1991), both of which are specific components of face processing.
Possible etiologies of DP
One of the specific advantages of investigating face recognition disorders early in life is the opportunity to evaluate possible causal mechanisms for prosopagnosia. There is little concrete knowledge of the etiology of DP, but major candidates include genetics, malfunctioning of innate face processing mechanisms, experiential factors, and neurological factors. We will discuss each of these in turn, though they are not mutually exclusive and instead are likely to interact in important and theoretically interesting ways.
Genetics
Research on familial cases of DP has resulted in clear evidence that DP tends to run in families, consistent with a genetic influence. The very first report on DP, written about a 12-year-old girl known as AB, indicated that AB’s mother experienced face recognition difficulties of her own, although she was not formally tested (McConachie, 1976). Since then, numerous reports have indicated the presence of DP in multiple family members (Behrmann, Avidan, Marotta, & Kimchi, 2005; de Haan, 1999; Diaz, 2008; Duchaine, Nieminenvon Wendt, New, & Kulomaki, 2003). Even some ‘celebrity’ cases of DP, such as primatologist Jane Goodall and neurologist Oliver Sacks, have reported family members who had suspected DP (Goodall & Berman, 2000; Sacks, 2010).
The first large-scale investigation into the genetics of DP revealed a familial link consistent with an autosomal dominant pattern of inheritance (Kennerknecht, et al., 2006). This study of German high school and university students involved questionnaire-based screening of 689 individuals. Based on responses to these questionnaires, 17 individuals were identified as having DP, consistent with a prevalence rate of 2.47%. A follow-up investigation of 14 of these individuals indicated that each case had a first-degree relative with suspected DP. These findings were based on subjective reports only, but the genetic basis of DP has since been supported by more objective tests of face processing. For example, studies using formal neuropsychological testing have confirmed the existence of DP in several family members (Duchaine, Germine, & Nakayama, 2007; Lee, Duchaine, Nakayama, & Wilson, 2010; Schmalzl, Palermo, & Coltheart, 2008). More recently, a twin study that measured face recognition in monozygotic and dizygotic twins drawn from the general population found that the correlation between scores of face recognition between monozygotic twins, who share 100% of their genes, was more than doubled the correlation found between dizygotic twins, who share only 50% of their genes (Wilmer et al., 2010). This strongly supports the presence of a genetic component to face recognition.
Taken together, the heritability of face processing and of DP is clear, but the nature of the heritability is not. Some individuals with DP report that they are the only member of their family with face recognition difficulties (Duchaine & Nakayama, 2005). Even within a family, heterogeneity may exist in terms of the subtype of DP, with different family members exhibiting different face-processing impairments (Lee, et al., 2010; Schmalzl, Palermo, & Coltheart, 2008). It is possible that some, but not all, subtypes of DP have a genetic basis, but this needs to be investigated further. As with all familial studies, shared family environment also needs to be taken into consideration.
Innate mechanisms
Innate mechanisms that cause preferential orienting to faces ensure that infants have experiences with faces that are necessary for the development of normal face processing (Morton & Johnson, 1991). In support of this notion, studies have shown that newborns have a preference to look at face-like patterns over non-face-like patterns (Fantz, 1963) and tend to orient both head and eyes towards faces to a greater degree than to matched non-face stimuli (Easterbrook, Kisilevsky, Hains, & Muir, 1999; Goren, Sarty, & Wu, 1975; Maurer & Young, 1983; Mondloch et al., 1999; Morton & Johnson, 1991). Once oriented, newborns typically fixate faces longer than non-face stimuli (Macchi Cassia, Simion, & Umilta, 2001) and even show a preference for faces with direct, compared to averted, gaze (Farroni, Csibra, Simion, & Johnson, 2002).
Interestingly, the tendency to orient to and discriminate faces without any experience with faces is maintained even after a delay. Monkeys denied exposure to faces for the first 6, 12, or 24 months of life still preferred to look at faces- monkey or human- compared to non-face stimuli when first exposed to face stimuli. In addition, after a short introduction to a selection of monkey and human faces, the monkeys were able to discriminate these faces from novel ones, demonstrating an expertise with faces despite their initial lack of experience with faces (Sugita, 2008).
Abnormal face perception in the context of DP could be the result of a failure of these innate face-orienting mechanisms. An infant’s failure to orient normally to faces from a very early age could lead to reduced or abnormal exposure to faces that could disrupt the normal development of face processing (Johnson, 2005). As will be discussed next, there is ample evidence that abnormal or reduced experience with faces can lead to deficits in face processing, making it reasonable to conclude that an early tendency to orient to faces is important for the development of normal face processing skills.
Experiential factors
Evidence from individuals who lack early visual experience with faces clearly shows that such deprivation can have long-term consequences for the development of normal face perception. For example, individuals with bilateral congenital cataracts that prevented any patterned visual input are impaired at certain aspects of face processing, indicating that early deprivation can affect later proficiency with faces (Le Grand, Mondloch, Maurer, & Brent, 2001, 2004; Ostrovsky, Andalman, & Sinha, 2006). One individual with bilateral cataracts removed at the age of 12 showed normal face discrimination and localization, but impaired face identification (Ostrovsky, et al., 2006). Even individuals who had their cataracts removed at very early age (e.g. < 2 months), show evidence of abnormal face perception (Le Grand, et al., 2001, 2004). They are impaired at detecting spacing between features and are not affected by composite face effects (i.e. their judgment of the top half of a face is not strongly affected by information from the bottom half of the face). This suggests a lack of sensitivity to second-order relations (i.e. relative distances between features) within faces and impaired holistic processing (i.e. integration of multiple features of a face into a single global percept). Importantly, despite the general nature of the visual deprivation, these effects appear to be specific to faces and do not seem to extend to other categories of objects (Ostrovsky, et al., 2006; Robbins, Nishimura, Mondloch, Lewis, & Maurer, 2010). For example, patients treated for bilateral congenital cataracts performed normally when asked to detect spacing changes between the local features (e.g. windows and doors) of houses (Robbins, et al., 2010).
Similar results were found with individuals who had left-eye-only congenital cataracts, but those with right-eye-only congenital cataracts appear to process faces normally (Le Grand, Mondloch, Maurer, & Brent, 2003). In the first 6 months of life the left eye projects almost exclusively to the right (vs. left) hemisphere (Lewis & Maurer, 1992) and inter-hemispheric integration of visual information has yet to develop (Liegeois, Bentejac, & de Schonen, 2000), suggesting that visual input to the right hemisphere is particularly important for the normal development of face perception (Le Grand, et al., 2003). The importance of the right hemisphere in the early stages of perceptual development is consistent with the well-established role of the right hemisphere in face processing (Benton, 1990; Kanwisher, McDermott, & Chun, 1997; Landis, Cummings, Christen, & Bogen, 1986; McCarthy, Puce, & Gore, 1997).
These findings from patients with congenital cataracts may seem to contradict the previously mentioned findings from monkeys deprived of visual exposure to faces (Sugita, 2008). While both groups were denied early exposure to faces, the cataract patients failed to develop normal face perception, yet the monkeys did. Comparisons of these studies allow speculation about which aspect(s) of visual experience are critical to the development of normal versus abnormal face processing. The patients with congenital cataracts were denied early visual patterned input, which happened to include faces, whereas the monkeys received normal visual input from non-face stimuli. This suggests that it may be general visual input, not exposure to faces per se, that is critical to the development of normal face processing. Follow-up studies are needed to further investigate this issue.
While visual deprivation is an extreme example of an experiential factor that can lead to the abnormal development of face perception, other more subtle variations from normal developmental experiences may lead to measurable face processing deficits. One such example is that shy children may be less sensitive to some cues for facial recognition (Brunet, Mondloch, & Schmidt, 2010). Children with high levels of temperamental shyness scored worse on a task that involved making same/different judgments about spacing differences between facial features. Children who are high in temperamental shyness have a tendency to avoid faces and eye contact from the time they are infants, suggesting differential experience with faces from their non-shy peers. That said, it is important to keep in mind the difficulties of inferring causation. Although one interpretation of this finding is that shyness leads to impaired face processing, it is also possible that children who lack proficiency with faces avoid them from an early age and consequently become shy.
A second example of a subtle experiential effect on face perception is evidence that children raised in institutionalized settings have impaired face memory (Pollak et al., 2010). Children raised in an institution, such as an orphanage, for a prolonged period of time performed worse on a face memory task than children who were adopted at an early age and children who were never institutionalized. Critical factors in institutional rearing of children include limited stimulation with toys, limited linguistic stimulation, and limited one-on-one interaction with caregivers (Nelson, 2007). It remains to be determined what specific aspects of institutionalized rearing may lead to face processing deficits. Also, it is unclear whether these deficits are the result of more general cognitive deficits, of which Pollak and colleagues reported many (e.g. deficits of spatial working memory, learning, and attention). Regardless, taken together with the above-mentioned findings, it is possible that the development of normal face processing may be contingent on several key factors that are present during a typical home rearing.
Neurological factors
Although in its infancy, research on the neural correlates of DP has led to several interesting findings about abnormal brain function and structure in individuals with DP. For example, research has shown functional as well as volumetric differences in the temporal lobe of adult participants with DP relative to controls (Behrmann, Avidan, Gao, & Black, 2007; Bentin, DeGutis, D’Esposito, & Robertson, 2007; Bentin, Deouell, & Soroker, 1999; Furl, Garrido, Dolan, Driver, & Duchaine, 2011; Garrido et al., 2009). Others have found decreased structural connectivity both in terms of density and volume of fibers in ventral occipito-temporal face networks (Thomas et al., 2009). Electrophysiological markers of DP include abnormal face-selectivity of the face-sensitive M170 magnetoencephalography component (Harris, Duchaine, & Nakayama, 2005), and abnormal neural responses to inverted faces indexed by the analogous ERP component, the N170 (Eimer, Gosling, & Duchaine, 2012) (for a more detailed discussion of electrophysiological studies of face processing in DP see Towler & Eimer, this issue). Although interesting, it is difficult to infer causation from these studies; abnormal brain function and structure could be the cause or the consequence of abnormal face processing.
One source of information about the neurobiological substrates of DP that may allow for more conclusive inferences about causation comes from research on other developmental disorders. Ramus (2004) has suggested that a particular neural correlate of dyslexia may similarly underlie other selective developmental disorders (SDDs), including DP. He proposed that ectopias, failures of neural migration that result in localized areas of cortical disorganization, may underlie some SDDs and that it may be the location of these ectopias that dictates the resulting behavioural deficits. For example, while evidence of abnormal cell migration has been found in the perisylvian cortex of dyslexics (Galaburda & Kemper, 1979; Galaburda, Sherman, Rosen, Aboitiz, & Geschwind, 1985; Humphreys, Kaufmann, & Galaburda, 1990), similar abnormalities in temporal and occipital regions that contribute to face processing could lead to the deficits that characterize DP.
Summary
Evidence exists for the role of genetic, experiential, and neurological factors in the etiology of DP. While no direct evidence exists for failures of innate mechanisms as a cause of DP, early orienting to faces is likely an important first step towards acquiring experience with faces. Although it is possible that each of these factors can alone lead to DP, it seems likely that there is interaction between them. For example, a genetic factor may contribute to abnormal development of the neural mechanisms of face processing, leading to face recognition deficits. In other cases, a genetic factor may lead to a failure of the innate mechanisms that cause orienting to faces, leading to abnormal experience with faces and a failure to develop normal face processing mechanisms. Even without a genetic contribution, abnormal experience with faces early in life may lead to abnormal development of the neural mechanisms underlying face processing. These are just a few possibilities, but the importance of each factor and the interactions among them remain to be explored.
Existing Studies of Children with DP
The cases reported here are summarized in Table 1.
Table 1.
Published cases of childhood DP
| AB | LG | TA | AL | K | A | N | I | P | T | |
|---|---|---|---|---|---|---|---|---|---|---|
| Reported in | McConachie 1976; deHaan & Campbell, 1991 | Ariel & Sadeh, 1996 | Jones & Tranel, 2001 | Joy & Brunsdon, 2002; Brunsdon et al, 2006 | Schmalzl et al, 2008; Wilson et al, 2010 | Wilson et al, 2010 | Wilson et al, 2010 | Wilson et al, 2010 | Wilson et al, 2010 | Wilson et al, 2010 |
| Age & Gender (M/F) | 1976: 12.75 yrs F 1991: ~28 yrs |
8 years M | 5.17 years M | 2002: 4 years M 2006: 8 years |
2008: 4 years F 2010: 7.5 years |
8.33 years M | 7.50 years M | 6.00 years M | 5.92 years M | 4.67 years F |
| IQ V = verbal P = performance RG = receptive grammar NVA = nonverbal ability* |
1976: VIQ 144 1976: PIQ 100 1980: VIQ 140 1980: PIQ 102 |
VIQ 142 PIQ 90 |
VIQ 140 PIQ 110 |
VIQ 109 PIQ 68 |
2008: Above average 2010: RG: 97, NVA: 41 |
RG: 99 NVA: 60 |
RG: 92 NVA: 63 |
RG: 69 NVA: 52 |
RG: 123 NVA: 52 |
RG: 100 NVA: 50 |
| Familiar faces | 1976: Impaired 1991: Impaired |
Impaired | Impaired | Impaired | 2008: Impaired | Anecdotally impaired | Anecdotally impaired | Anecdotally impaired | Anecdotally impaired | Anecdotally impaired |
| Unfamiliar faces | 1976: No false alarms 1991: Impaired |
b&w Impaired colour Normal | Normal at KABC Borderline impaired at facial discrimination |
Impaired | 2008: Impaired feature matching 2010: Impaired | Impaired | Impaired | Impaired | Impaired | Impaired |
| Detection | 1991: Normal- borderline impaired | ----- | Normal | Normal | 2008: Normal | ----- | ----- | ----- | ----- | ----- |
| Features | ----- | Impaired | ----- | Impaired | 2008: Impaired | ----- | ----- | ----- | ----- | ----- |
| Spacing | ----- | ----- | ----- | ----- | 2008: Normal | ----- | ----- | ----- | ----- | ----- |
| Age | 1991: Impaired | Impaired | ----- | Impaired | 2008: Normal | ----- | ----- | ----- | ----- | ----- |
| Gender | 1991: Impaired | Impaired | Impaired | Impaired | 2008: Impaired | ----- | ----- | ----- | ----- | ----- |
| Expression | 1991: Impaired | Impaired | Normal | Impaired | 2008: Impaired | ----- | ----- | ----- | ----- | ----- |
| Object perception | 1976: Some impairments, but no significant object agnosia 1991: impaired |
Impaired | Some impairments | Impaired | 2008: Impaired early visual analysis but intact basic level analysis 2010: ----- |
Normal on single shoe task | Impaired on single shoe task | ----- | Impaired on single shoe task | Normal on single shoe task |
| Autism | 1976: Solitary and studious, but no formal diagnosis | No | Poor eye contact but otherwise no formal diagnosis | ----- | 2008: Maybe 2010: Yes, (formally assessed) | No (formally assessed) | No (formally assessed) | Yes (formally assessed) | No (formally assessed) | No (formally assessed) |
| Other notes | 1976: EEG moderately abnormal on posterior right hemisphere 1991: No evidence of covert recognition from priming task |
“Blinking habit” | Normal covert recognition as measured by SCR | Strabismus; Successfully trained with familiar faces | Some family history of DP; 2008: Successfully trained with familiar faces | No known family history of DP | Some family history of DP, some motor dysfunction | No known family history of DP; pragmatic language disorder | Some family history of DP | Some family history of DP |
(----- indicates that this aspect was not reported)
Nonverbal ability is measured based on mean of 50 and standard deviation of 15.
Considering the dearth of research on childhood DP, it is surprising that the first published case study of DP was written some 36 years ago (McConachie, 1976). The report about a 12-year-old girl, AB, was brief, stating that despite normal intelligence and no known history of brain damage, she had severe difficulties recognizing faces in daily life, particularly her classmates who wore uniforms at school. When formally tested, AB was able to identify photographs of familiar faces, albeit hesitantly, and made no false-alarm identifications of unfamiliar faces as familiar. A 15-year follow-up with AB involved more extensive formal testing including tests of face perception, face memory, gender discrimination, expression matching, lip reading, and object recognition. These tests indicated that AB’s face recognition difficulties persisted, and additionally revealed deficits in the recognition of facial expression, gender, and within-class objects (de Haan & Campbell, 1991). Not only did AB demonstrate failures of overt face recognition, she also showed no evidence of covert processing of familiarity when tested with a method that was previously used to detect covert face processing in normal controls and in an individual with acquired prosopagnosia (Young, Hellawell, & de Haan, 1988). AB’s lack of covert processing is consistent with other studies that have shown a lack of evidence of covert recognition in adults with DP (Barton, Cherkasova, & O’Connor, 2001; Bentin, et al., 1999). However, Eimer, Gosling and Duchaine (2012) recently found electrophysiological evidence of covert processing in 6 of 12 of the individuals with DP whom they tested, suggesting that despite a lack of overt face recognition, for a certain subset of individuals with DP, familiarity may be processed at an unconscious level.
The next reported case of a child with DP, 8 year-old LG, came 20 years after McConachie’s report on AB (Ariel & Sadeh, 1996). LG, who was born at full term after a normal pregnancy and delivery, was described as social, with a good sense of humour, and significantly above average verbal intelligence (VIQ 142). LG had normal low-level visual processing, but was impaired at recognizing personally familiar faces. He performed normally when matching unfamiliar faces from colour photographs, but was impaired when images were presented in black and white. LG also demonstrated impairments when performing gender and age judgements. His face perception deficits were a part of a more general object agnosia as he also had severe difficulties identifying objects from photographs as well as more subtle difficulties with real objects. A more recent report on LG indicates that he has strong deactivation of mid-level visual areas (V2-V4), suggesting that he may be an atypical case of DP (Gilaie-Dotan, Perry, Bonneh, Malach, & Bentin, 2009). However, LG’s case is an interesting demonstration of how lower-level visual problems can lead to face recognition difficulties. It also highlights the importance of low-level testing and the value of imaging when conducting single case studies. It is useful to obtain a complete profile of each individual with DP to determine whether a particular individual is representative or atypical, and whether there exist clues to the case-specific etiology of the disorder.
Another reported case of DP in a child was that of a five-year-old boy known as TA (Jones & Tranel, 2001) who was impaired with personally familiar faces and had slight difficulty with unfamiliar face matching. TA showed deficits in discriminating gender, but his facial expression recognition was normal. He was reported to be intellectually gifted, like AB and LG who had similarly above-average verbal IQs (around 140, see Table 1). Interestingly, despite his poor overt recognition skills, TA showed normal covert recognition of faces as evidenced by his skin conductance response (a measure of autonomic arousal), which was more frequent and larger in amplitude in response to familiar faces compared to unfamiliar faces, indicating that they were being processed differently, but at an unconscious level. The contrast between TA’s covert face recognition and the absence of covert recognition in AB is consistent with Eimer et al’s (2012) findings that some, but not all individuals with DP process familiarity at a covert level.
Two training studies involving children with DP have been reported to-date (Brunsdon, Coltheart, Nickels, & Joy, 2006; Schmalzl, Palermo, Green, Brunsdon, & Coltheart, 2008). The details of the cases will be outlined here, but the training will be discussed later in the section on treatment. The first of these cases is AL, an 8-year-old boy with developmental face and object processing difficulties (Brunsdon, et al., 2006). AL had impairments in structural encoding of faces (i.e. integration of the features of the face with the global configuration to form a representation of the face, Bruce & Young, 1986), which affected his ability to recognize both familiar and unfamiliar faces. He was also impaired at making judgements about gender, age, and facial expression. Consistent with the concept of an impairment of structural encoding, AL showed deficits for feature perception (eye, nose, mouth), particularly when the features were in the context of a face. He was disproportionately impaired at eye and nose perception, with relatively good perception of mouths.
The second of the training cases involved a 4-year-old girl referred to as K (Schmalzl, Palermo, Green, et al., 2008), who had normal contrast sensitivity and normal face detection, indicating normal early visual analysis and sensitivity to first-order relations. In contrast, she was severely impaired at structural encoding of faces. While she detected spacing between facial features normally, she was deficient relative to controls for the detection of feature changes. She did not show a normal face inversion effect, with only minimal difference in accuracy for feature and spacing detection of upright compared to inverted faces. Systematic testing of feature discrimination indicated that K was impaired at encoding eye, nose, and mouth information, and that this was the case regardless of whether the features were presented in the context of a face or in isolation. Also, K was impaired at gender discrimination and expression recognition, but not for age judgements. In addition to the detailed assessment of K’s face perception abilities, her eye movements were recorded as she attempted to identify familiar faces. She made abnormally few fixations to the internal features of the face, particularly the eye region.
K was tested again more recently in a systematic evaluation of six 4–8 year-old children (Wilson, Palermo, Schmalzl, & Brock, 2010). In addition to K, youngsters known as N, A, I, T, and P were assessed with the Social Communication Questionnaire, 2-Alternative Forced-Choice (AFC) sequential face matching, 3-AFC simultaneous face matching, and a 2-AFC face memory task developed by Pellicano, Pimperton, and Duchaine, all using unknown faces. Although each case presented with face recognition difficulties, two of them, K and I, also met the criteria for autism spectrum disorder (ASD), and T had inconsistent face recognition. While the remaining 3 children had normal intellect and no evidence of ASD, two of them, N and P, had comorbid object recognition difficulties. Taken together, of the 6 children described in this report, A, an 8 year-old boy, was the only one who showed recognition problems restricted to faces, suggesting that the presence and nature of comorbid deficits and disorders is an important issue when studying DP in childhood. This will be discussed further in the section on special considerations.
Summary
Ten children with DP have been reported in the literature to date. Most of those reports describe a child’s relative strengths and weaknesses with face perception and basic visual perception as well as known comorbidities including diagnoses of autistic-type behaviours. Many of the youngsters (e.g. AB, LG, TA, K) have above average intelligence. An important consideration is that some of them also demonstrate autistic tendencies (Wilson, et al., 2010), or more general visual deficits including impaired object perception (e.g. AB, LG, TA, AL, K, N, P). Many have difficulties with other object classes outside of faces. One child, TA, showed evidence of covert face recognition in the absence of overt face recognition as measured by skin conductance response. In contrast, AB did not show evidence of covert face recognition when tested as an adult via visual priming. Given the heterogeneity of these cases, future work could be aimed at investigating how individual cases differ and the possibility that those with different comorbidities (e.g. DP with ASD or DP with object agnosia) represent different subtypes of the disorder.
Objectives for the study of DP in children
The summary of the research reported on children with DP highlights the paucity of work that has been done to study this disorder in a developing system. In addition to the small number of reported cases, there is a lack of consistency in the behavioural profiles presented in the reports. Qualitatively, some of the tests used have questionable reliability and/or validity. Quantitatively, the depth of testing is often insufficient to create meaningful behavioural profiles that will allow for a complete picture of the individual’s perceptual deficiencies. Much of the past work on DP in children has outlined cases of DP in childhood, but stopped short of speculating on theoretical questions important for understanding normal and abnormal face processing (e.g. causal factors). The study of DP in childhood affords an excellent opportunity for researchers to answer important questions about the etiology and progression of DP.
The primary shortcomings of the current research on childhood DP include a scarcity of cases, an absence of consistency and depth of testing, and a lack of theoretically motivated investigation. These deficiencies are understandable given that this is an emerging field of study, and highlight the value of identifying standard methods of study and key questions of theoretical interest early on. Here we identify four primary objectives that should be at the forefront of work done on childhood DP: 1) increasing awareness of childhood DP, 2) the development of well-designed diagnostic tools, 3) the application of results from research on childhood DP to the study of important theoretical issues related to DP in general, and 4) the development of empirically driven treatment strategies for children and adults with DP. These objectives and early efforts to reach them are discussed below.
Increasing awareness of childhood DP
As mentioned earlier, the estimated prevalence of DP in adult populations is in the range of 2–2.9% (Bowles, et al., 2009; Kennerknecht, et al., 2006). Given that many adults with DP report having experienced face recognition difficulties their entire lives (cf. Duchaine & Nakayama, 2006b), it is likely that millions of children are affected worldwide. One factor that limits the identification and recruitment of these children for scientific study is a lack of awareness about DP, among parents, as well as among educators and healthcare providers. Many parents who have contacted us describe long journeys through a series of diagnoses while they searched for one that would adequately explain their child’s abnormal behaviours. One parent who first heard about prosopagnosia through an Internet forum wrote to our group saying, “… even the educational psychologist I consulted and the special needs coordinator in [my son’s] new school needed to have the condition explained to them.” Given the implications of an inability to recognize faces on social functioning, many children are assigned a default diagnosis of autism spectrum disorder (ASD) or the catch-all diagnosis of PDD. Thus, an early goal of the field of childhood DP is to spread awareness to educators and health care providers such that they understand that this condition is an alternative to the usual go-to diagnoses, and to prepare them to identify children who have face recognition difficulties.
Knowledge about DP is slowly spreading through the placement of research findings in popular media. Websites such as faceblind.org have also been created to spread awareness and to allow individuals with self-reported face recognition difficulties to provide their contact information and express a willingness to take part in research on DP. Through this website we have been contacted by over 45 parents of children with suspected face recognition difficulties who range in age from 4–17 years of age. The Visual Perception Lab at the University of Minnesota has similarly been contacted by many parents who have come across their contact information through the lab website.
We, and others, have also been engaging in outreach programs designed to inform the public about DP. We have given presentations to students and staff at local schools, and partnered with a science museum to set up a temporary exhibit about childhood DP. Parents and educators are in general very interested to learn about DP. Some individuals have even commented that they, or someone they know, have had face recognition difficulties from an early age, but were unaware that these difficulties could be symptomatic of a disorder. Some of these individuals have since joined our list of self-reported cases of DP who are interested in participating in research.
The development of diagnostic tools
The lack of well-designed face recognition tests for children is one explanation for the small number of reports on childhood DP. What is needed is a standard set of criteria for the creation of tests designed to detect DP in children. First, because adults and children may be able to rely on extra-facial cues for recognition, diagnostic tests should be free of these kinds of cues. In other words, clothing, eye colour, hair, glasses, jewelry, and other superficial indicators should be removed so that these cues cannot be used to identify a particular face. Furthermore, given that some individuals may be able to match faces but not remember them, tests for DP should not allow for simultaneous matching of faces (with the exception of tests designed to measure perception). Finally, like with any other behavioural tests, care should be made to avoid floor and ceiling effects for various age groups so that the same tests can be used for many groups of children and developmental comparisons can be made.
Some tests of face recognition designed for children exist although many fail to meet the above criteria. General neuropsychological assessment batteries for children that include face recognition subtests have been developed (e.g. A Developmental Neuropsychological Assessment, (NEPSY, Korkman et al, 1998), Kaufman Assessment Battery for Children (KABC; Kaufman & Kaufman, 1983)) and may useful as a first pass for detecting face recognition difficulties in children. However, because they include some of the above-mentioned superficial cues for recognition such as hair and multiple races, as well as simultaneous presentation, there is a need for instruments without such limitations.
The development of tools to assess a range of face processing skills is critical not only to the ability to identify children with DP, but also for an adequate profiling of cases. Detailed profiling would be one step towards more in-depth investigations of the specific nature of the face processing deficits associated with this disorder. Also important is the use of a variety of tests that assess the same facets of face processing so as to provide converging evidence for the presence or absence of specific defects in a given individual. Converging measures are important in adult studies, but especially valuable when studying children given that children’s performance is typically less reliable (Bayley, 1949). Finally, the use of standardized measures would allow for comparison between cases reported from different research groups. As such, one goal for the emerging field of research on childhood DP is the design of a standard battery of sensitive, specific, and reliable measures of face perception for children.
Attempts at creating such a battery have been made before. Bruce et al. (2000) described 10 tests from a battery of face perception for children. These tests fulfilled many of the above-listed criteria. The battery included tests of a range of face processing abilities, such as identity and expression recognition, lip-reading, and gaze processing. There were at least two tests for each of these skills and good inter-correlations between many of the pairs of tests that were designed to test the same ability. Bruce et al. collected normative data from children from 4–10 years of age and reported that the tests ranged in difficulty for the youngest group tested (4–5 year olds, 57%–81%), but that most were at ceiling for the oldest group (9–10 year olds, 74% and 85%, but all others 94%–100%). Some tests approached ceiling for the 5–6 year olds (91% for one of the identity matching tasks and 96% for an expression task). Thus, many of the tests in this battery are unlikely to be sensitive enough to detect face recognition difficulties. All tests had simultaneous presentation of faces for unlimited durations, allowing for feature matching as a way of performing well. Most of the tasks used faces with hair and ears visible, allowing for the use of extrafacial cues. The two tests that produced mid-range scores for the 9–10 year olds were identity matching with hair and ears were masked. These tests are better examples of well-designed face recognition tasks, but due to the method of simultaneous presentation of the faces they still allow for feature matching between target and test items.
One test that improves on some of the limitations of this battery is a face memory test for children. Pellicano, Pimperton, and Duchaine developed a 2-AFC test version of the Cambridge Face Memory Test (CFMT), a test of memory for unknown faces that was designed and normed for adults and that does not allow for feature matching between target and test items (Duchaine & Nakayama, 2006a). The Visual Perception Lab at the University of Minnesota has collected data on typically developing children with this task for purposes of having an established comparison group for children with DP (Corrow, Chatterjee, Mathison, Nakayama, & Yonas, in prep). They found that the children’s version of the CFMT shows good test-retest reliability (r = 0.69, n=31) and that children who are reported to have face recognition difficulties in daily life tend to score poorly on the test. One limitation of this version of the CFMT is that it uses 2- rather than 3-AFC, which leads to ceiling effects in older children. It also has a chance level performance of 50%, which increases the risk of floor effects in children who do have face recognition difficulties. Another drawback is that this test uses adult faces rather than child faces. Given that children may be more interested in child faces than adult faces (Brooks & Lewis, 1976), and evidence that observers are better at recognizing own-age compared to other-age faces (Perfect & Moon, 2005; Wright & Stroud, 2002), it may be important that measures that are designed to study face recognition in children use child rather than adult faces. This in itself is an issue that needs to be addressed.
With combined efforts from multiple groups, the design of a complete battery of tests for use with children is underway. As a starting point, we have developed a database of photos of children for use in the diagnostic tests. Until now, no extensive, well controlled, database of child faces existed. The Dartmouth Database of Child Faces (DDCF) contains pictures of faces of over 80 children. The children were asked to make 8 different facial expressions (neutral, pleased, happy, sad, angry, afraid, surprised, disgusted), and their photos were taken from five different angles (60° left, 30° left, 0°, 30° right, 60° right) and under two different lighting conditions. Adults and children have rated the photos in the database in terms of the quality and intensity of the facial expressions, providing a measure of validity of the faces as stimuli for use in tests of face processing.
Using photos from the DDCF as well as from the Internet, we have designed several tests of face processing for children. These include tests of facial identity memory, facial identity perception, face detection, emotion perception, and object perception. Like the 2-AFC version of the CFMT that was designed for children, our face memory task is based on the original CFMT, a test that was designed for and normed with adults. Our version of this test, the Cambridge Face Memory Test - Kids (CFMT-Kids, Figure 1), follows the same format as the original CFMT, with 3-AFC items that test memory for six target faces, but the CFMT-Kids uses faces of children rather than adults. As noted, children may find child faces more interesting to look at than adult faces (Brooks & Lewis, 1976). There is also evidence that children are better at recognizing own-age faces compared to the faces of adults (own-age bias) (Anastasi & Rhodes, 2006; He, Ebner, & Johnson, 2011; Perfect & Moon, 2005) although others have found no age bias in children (Ebner & Johnson, 2009; Mondloch, Maurer, & Ahola, 2006) or even an adult-face bias (Macchi Cassia, 2011). Designing tests for children that use child faces will engage children in the tests and allow for future investigation of own-age biases in normal children and children with DP.
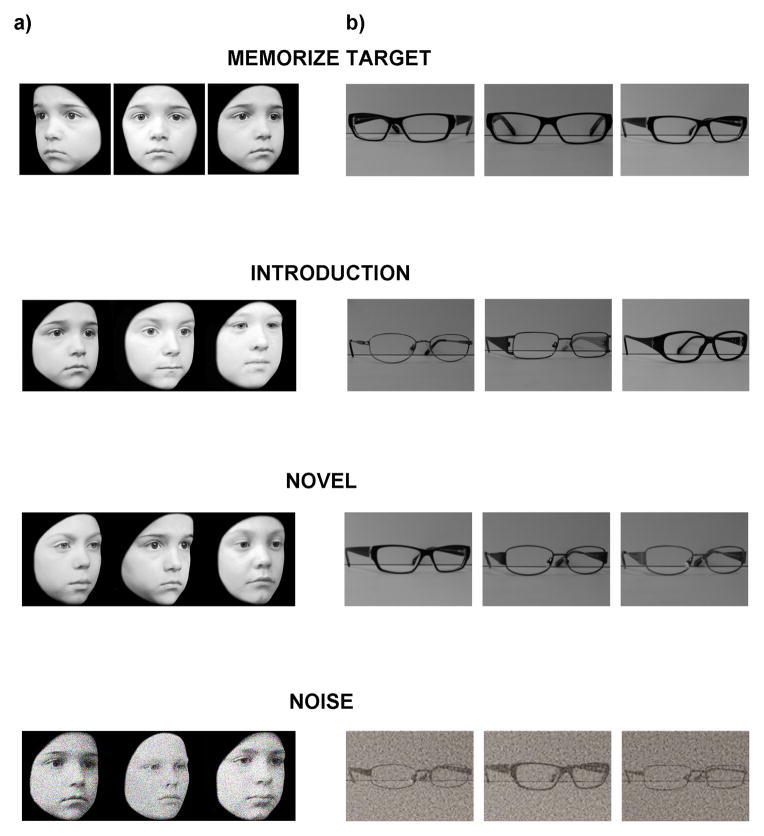
Figure 1.
a) Example stimuli from the new CFMT-Kids. The top panel shows study views of a target face. Study views are presented for 3 seconds each. The remaining panels are examples of the different test phases from the experiment. Children have an unlimited amount of time to choose the face that matches the target. In the Introduction phase, the target is introduced and followed by 3 test trials containing that target and two distractors before the next target is introduced. In the Novel phase, all 6 targets are displayed together for 20 seconds before a series of test images appear containing one of the target faces and two distractors. The Noise phase follows the same method as the Novel phase, but noise is added to the faces to increase task difficulty. b) Example stimuli from the eyeglasses object memory task that was designed to match the method of the CFMT-Kids.
We calibrated the difficulty of our version of the CMFT using data from a group of 30 11-year old children and found that the test has good internal consistency (α=0.83). We administered this test to Madison, the DP child who was introduced at the beginning of this review, and she scored more than 2 standard deviations below the mean, indicating that this test can identify children with face recognition difficulties.
Our test of face perception is based on the Cambridge Face Perception Test (CFPT) (Duchaine, et al., 2007), yet some key differences make it more appropriate for use with children. The original CFPT presents the test taker with a target face at the top of the screen and a series of similar faces below. These faces are taken from a morph continuum between the target face and another face and therefore vary in their similarity to the target face. The test taker is asked to sort the faces from most-to-least similar to the target face. Performance is measured by the number of errors in the final order of the faces. Preliminary results from the administration of the original CFPT to children indicate those around the age of 8 and younger fail to grasp the concept of sorting faces into a continuum. Rather, it seems that at least younger children attempt to sort the faces into two groups: faces that are similar to the target and faces that are not (Corrow, Chatterjee, Mathison, Nakayama, & Yonas, in prep). To compensate for this limitation, we have designed a face perception task that uses a 3-AFC method. As in the original CFPT, a target face is displayed at the top of the screen, yet in the child version of the task, below the target are three faces chosen from a morph continuum between the target face and another face. The child’s task is to select the face that is most similar to the target face. Like the CFMT-Kids, this test of face perception uses child faces drawn from the DDCF, so the presence of extra-facial cues is minimized.
To test the specificity of perceptual deficits in children with face recognition difficulties, there is a need for object perception tasks that are matched in method, difficulty, reliability and validity to the tests of face perception. The Visual Perception Lab at the University of Minnesota has made some progress in this regard, with a test of memory for eyeglasses that is matched to the 2-AFC CFMT for children. We are currently modifying the test to be a 3-AFC task (Figure 1), matching the new CFMT-Kids. A similar object recognition test using bicycles is also being prepared, also with the aim of matching the new CFMT-Kids.
A need also exists for alternate form versions of the tests that are reliable and valid, use similar methods, and have similar difficulty levels. Given that the next and possibly most important goal is the design of effective treatment strategies for children with DP, each test of face and object perception in the test battery needs a paired test that will allow for well-controlled pre- and post-training assessment. The design of tests matching those mentioned above is currently underway.
In addition to tests of face perception, children should be tested for comorbidities such as object agnosia, low-level visual problems, and ASD. Children with autism often also have face recognition difficulties although in these cases the face recognition difficulties are confounded with the social and perceptual impairments associated with ASD itself (Dawson, Webb, & McPartland, 2005; Elgar & Campbell, 2001). One useful diagnostic tool for ASD is the Autism Diagnostic Observation Schedule (A-DOS) (Lord, Rutter, DiLavore, & Risi, 1999), but it is time-consuming and needs to be administered by a trained clinician, making it potentially impractical for research purposes. An alternative, quicker, test for ASD is the Autism-Spectrum Quotient (AQ) test (Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001); however, it is not recommended for use as a formal diagnostic tool for ASD. It should be noted that, given the high sensitivity and low specificity of diagnostic tests of ASD and similar symptomatology between ASD and DP, a score in the autistic range on these tests does not necessarily rule out DP, making tests of DP that rule out ASD especially critical (see section on special considerations for further discussion of this topic).
Theoretical questions that may be addressed by studying childhood DP
The reliable identification of children with DP will make it possible to start addressing novel theoretical issues related to DP. These issues include, but are not limited to, identification of different phenotypes, the neural basis, the developmental trajectory, and the psychosocial consequences of DP. We outline some starting points for investigations here.
What are the different phenotypes of DP?
It is widely agreed that acquired prosopagnosia can be divided into two distinct phenotypes: individuals with impaired face memory versus individuals with impaired face perception (De Renzi, et al., 1991). These same distinctions may exist in DP, yet other behavioural bases for classification of different phenotypes exist, too. For example, face perception deficits in some individuals extend beyond impaired identity perception to problems with face detection (Garrido, Duchaine, & Nakayama, 2008), expression recognition (Duchaine, Yovel, Butterworth, & Nakayama, 2006) and gender discrimination (Duchaine, et al., 2006), while for others these abilities are normal (Bentin, et al., 2007; Duchaine, Parker, & Nakayama, 2003; Garrido, et al., 2008; Garrido, et al., 2009; Nunn, Postma, & Pearson, 2001). A report of the behavioural profiles of multiple cases of DP from a single family suggests that different phenotypes of DP may exist within the same family (Lee, et al., 2010). Furthermore, a comparison of the affected members of that family and those in another family (Duchaine, et al., 2007) indicated the existence of differences in phenotypes between families (Lee, et al., 2010). It is important to determine what phenotypes exist in order to understand individual differences in behavioural and neurological measures, as well as possible etiologies of DP.
What is the neural basis of DP?
While acquired prosopagnosia can typically be linked to damage to one or more components of the ventro-temporal face processing system (Dalrymple et al., 2011; Haxby, Hoffman, & Gobbini, 2000), findings regarding the neural correlates of DP are mixed. For example, some individuals in this population have normal face-selectivity of the M170 magnetoencephalography component, while others do not (Harris, et al., 2005); some show normal selectivity of the N170 component (Eimer, et al., 2012), while others do not (Bentin, et al., 2007; Bentin, et al., 1999; Harris, et al., 2005; Kress & Daum, 2003); some show normal fMRI activation in face-selective areas (Avidan, Hasson, Malach, & Berhmann, 2005), while others do not (Bentin, et al., 2007; Furl, et al., 2011), and some demonstrate covert face recognition (Eimer, et al., 2012; Jones & Tranel, 2001), while others do not (Barton, et al., 2001; E. H. de Haan & Campbell, 1991; Eimer, et al., 2012). Although interesting, one critical limitation of these findings is that, because this work has been done in adults, it is difficult to determine whether these neurological abnormalities are the cause or the consequence of impaired face perception in DP. Studying the neural basis of DP in children, possibly longitudinally, may shed light on this issue of cause versus effect.
As discussed in the earlier section about the etiology of DP, ectopias are failures of neural migration that result in localized areas of cortical disorganization and may underlie some SDDs, including DP (Ramus, 2004). While the possible link between ectopias and DP is an important theoretical issue of its own, the ectopia model of SDDs could also explain individual differences in the selectivity of deficits in DP. Highly localized instances of cortical disorganization could cause more specific deficits in face perception while more distributed instances of disorganization could also affect other abilities, like object recognition and navigation, mediated by neighboring brain areas (Duchaine & Nakayama, 2005). Comparing the neurodevelopment of individuals with highly specific DP to those of individuals with common comorbidities could provide answers about how neural organization affects the expression of DP.
What is the developmental trajectory of DP?
Is DP a stable condition or does it change over the lifespan? A cursory way to address this question is to look at prevalence rates. As mentioned previously, while prevalence of DP has been estimated to be between 2–2.9% (Bowles, et al., 2009; Kennerknecht, et al., 2006) in adult populations, no estimates have been made in children. Although it is possible that these prevalence rates are stable across age groups, it is also possible that they change. If childhood prevalence of DP is lower than the rate in adults, this would indicate DP emerges later in life, or exists early on but becomes more severe and therefore more easily detectable over time. If childhood rates are higher than adults, this could indicate that DP can spontaneously resolve later in life or that compensatory strategies can make DP less apparent. Each of these possibilities is of clear theoretical interest, and highlight the importance of studying DP in childhood.
Another approach to the investigation of the developmental trajectory of DP involves investigating the phenotypes of DP. Specifically, determining whether the same behavioural and neural dissociations that exist in adults also exist children with DP can inform us as to whether DP in adults is the same as DP in kids. Preliminary evidence from the case studies presented in Table 1 suggests this may be the case. For example, of the five single-case studies reported, some of the children showed a neurological impairment evident by imaging and electrophysiological measures, and others did not. Likewise, some of the children showed impairments in object recognition, and others did not. However, given that the measures used in this study are not standardized and the subject numbers are few, it is not possible to draw any firm conclusions. It may be the case that DP is a more general deficit early in life and that face recognition deficits persist into adulthood leading to more specificity later on. At this point we can only speculate, but determining whether these distinctions exist at an early age will be instrumental in understanding the development of DP, as well as the development of normal face processing.
What are the psychosocial consequences of DP?
Important issues surrounding DP in children concern social and clinical factors. Some adults and children with DP report serious social consequences associated with their inability to recognize faces, such as difficulty making and maintaining friendships and romantic relationships, complications at work/school, and in children, increased risk of being put in dangerous situations with strangers (Diaz, 2008; Yardley, McDermott, Pisarski, Duchaine, & Nakayama, 2008). Others also show elevated levels of social anxiety and feelings of inadequacy (Yardley, et al., 2008). Consequently, another avenue of research involves the investigation of long-term effects of DP on personality and on mental illness. Longitudinal studies investigating personality characteristics of children with DP as they mature can address the effects of DP on the big five personality factors (Norman, 1963), and other psychosocial processes such as resilience (Fonagy, Steele, Steele, Higgitt, & Target, 1994). Early testing for clinical disorders can lead to information regarding the presence and development of mental illness in DP.
Treatment
Research on childhood DP will hopefully lead to the development of empirically driven treatment strategies. As mentioned earlier, only two reports exist on training initiatives with children with DP, one with 8-year-old AL (Brunsdon, et al., 2006) and one with 4-year-old K (Schmalzl, Palermo, Green, et al., 2008). Both were based on Bruce and Young’s (1986) theoretical framework. AL’s treatment program focused on perception and analysis of facial features using photographs of familiar individuals. AL was first asked to identify an individual in a photograph and then taught to observe, discuss, and remember five defining characteristics (e.g. age, gender, defining facial features such as large nose) about that individual. Efforts were made to reduce his reliance on external features like hairstyle or glasses. After 14 treatment sessions over the course of 1 month, AL showed a large and sustained improvement for naming these familiar individuals. He also made fewer false-positive identifications of unfamiliar faces. These results generalized to other photographs of the same familiar individuals, but did not generalize to individuals who were not part of the training set. AL also improved his ability to discriminate features. The effects were still present at a 3-month follow-up. AL’s processing latency increased significantly for all features, suggesting that he may have engaged in a conscious analysis of features and that his improvement with familiar faces reflected a change in his face processing strategy, rather than a change to his underlying neural mechanisms. Regardless, the sustained improvement for recognizing friends and family represents an important perceptual gain for this child and provides promise for rehabilitation for other children with DP.
K’s training was very much like the training for AL, focusing on teaching her to recognize familiar faces by concentrating on specific characteristics of the internal facial features. As with AL, K was asked to remember five defining characteristics for each face, including whether it was male or female and whether it was an adult or a child. K’s training took one month and included nine sessions. Post-training assessment showed K was perfect at identifying the familiar individuals in photographs; however this ability did not generalize to the same familiar faces when they were presented at different angles. Interestingly, at follow-up 4 weeks later, not only had K maintained her ability to recognize the familiar individuals whom she had been trained to recognize, she was now able to identify them when the faces were presented at different angles. In terms of her post-training eye movements, K spent significantly more time looking at internal features compared to during her pre-training session. Specifically, she spent more time looking at the eyes post-training compared to pre-training. Interestingly, even though looking times increased for internal compared to external features for both familiar and unfamiliar faces, the increase in fixations on the eyes only occurred for the faces used in training. Although Schmalzl et al. (2008) were the first to report K’s case, she also featured in the Wilson et al. (2010) report when she was 7.5 years of age. Those authors noted continued maintenance of the benefits from K’s training and raised the possibility that she is on the autism spectrum.
Although these two training strategies were somewhat successful, AL’s gains in face recognition did not generalize to other faces (this was not tested in K). It seems that the strategy to focus on internal features may not promote holistic face processing, which some suggest is critical for normal face recognition (Avidan, Tanzer, & Behrmann, 2011; Bruce, 1988; Galton, 1879; Palermo et al., 2011; Tanaka & Farah, 1993). AL’s increased response times suggest the implementation of a very deliberate and intensive face recognition strategy, which may be artificial and impractical.
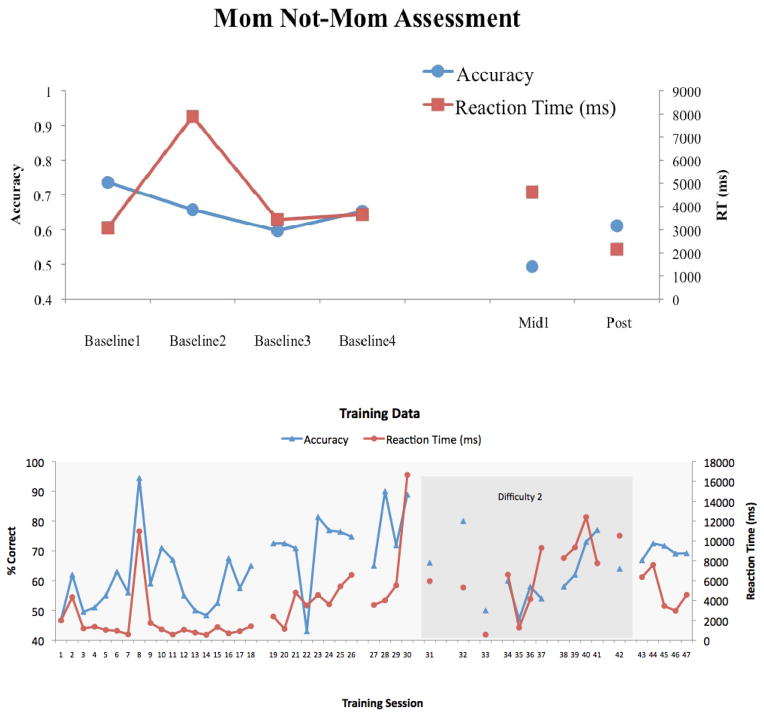
While these treatment outcomes were somewhat positive, anecdotal evidence suggests that many strategies used to-date have been unsuccessful. For example, DeGutis and his colleagues (personal communication, January 2012) used an individualized computer-based training program to train an adolescent boy (TM, age 12) with severe DP to recognize the face of his mother. In general, DP is accompanied by extremely slow and dysfunctional face learning abilities, but there is reason to believe that there may be some capacity for face learning (see Avidan & Behrmann, 2008; Bate, Haslam, Tree, & Hodgson, 2008). DeGutis and his colleagues reasoned that it may be possible, with enough practice on a single face and constant feedback, for a face to eventually be learned in some rudimentary way. To test this notion, they first assessed TM’s recognition of his mother by showing several different pictures of her as well as two age-matched Caucasian female foils. Though TM was slow to recognize his mother, he was significantly above chance during four baseline sessions and achieved a mean accuracy of 66.1% and mean reaction time of 4515 ms (see Figure 2). This group next created a ‘mom/not-mom’ training task using one image of TM mother’s face (different from the image used during assessment) and three age-matched Caucasian female foils different from the individuals used in the mom/not-mom assessment. TM had up to 15 seconds to make a ‘mom/not-mom’ decision on each training trial, at which point he received feedback about the accuracy of his response. TM graduated to a higher level of difficulty when he achieved better than 85% correct over two days. In order to ensure compliance with the training, he was rewarded points based on his performance during the training. These points could then be traded for pre-designated, age-appropriate toys (i.e. toy helicopter; iPod shuffle). TM performed 47 sessions of training over the course of 10 months, 35 at an introductory level of difficulty and 12 at second level of difficulty. Results from training and pre- and post- training assessment can be seen in Figure 2. At post-training assessment, TM showed no signs of improvement and was in fact at chance performance indicating that his improvements on the mom/not-mom training task did not generalize to the mom/not-mom assessment. After a 3-month break, TM commenced training on the introductory level a final time, but at assessment he showed no evidence of improvement relative to his pre-training performance. Thus overall, this particular individualized training with TM failed to improve his ability to recognize his mom. Although there could be several explanations for why this training was unsuccessful, including the severity of TM’s prosopagnosia, the intensity of training, and motivational factors (training was quite tedious), this program by DeGutis and colleagues provides cautionary evidence that even the simplest face training procedure may not be effective in some individuals with DP. They advise that others should be careful to test the generalizability of training-related improvements before investing extensive time and resources into any training program.
Figure 2.
a) TM’s assessment results (accuracy and RT) on “Mom/Not Mom” task across 4 baseline (pre-training) measurements, mid-training (midl), and post-training (post). b) TM’s accuracy on training task across 48 days of training. Lighter shading indicates training at the introductory difficulty level. Darker shading indicates training at a harder level of difficulty. A break in the line indicates a gap of more than 5 days between sessions.
In addition to this attempt by DeGutis and colleagues, there is one published account of an unsuccessful training program in a child with acquired prosopagnosia (Ellis & Young, 1988). A young girl, KD, tested from age 8 to 11.6 years, had a complicated neurological and neuropsychological history following meningococcal meningitis, but her face recognition difficulties were disproportionately severe. Her prosopagnosia was described as being perceptually based, and she showed no evidence of covert recognition. KD was trained and assessed in four stages over an 18-month period. These stages included simultaneous matching of photographs of familiar and unfamiliar faces, paired discriminations using schematic faces and digitized images of real faces, and learning face-name associations. The strategy was to have KD practice each given task. Although her performance on some of the tasks was slightly above chance at the beginning of training, she showed no improvement on any of the tasks. The authors concluded that KD’s face perception difficulties were unresolvable and that this may be related to the severity and/or the perceptual nature of her prosopagnosia.
Despite these accounts, the small number of training programs that have been successful shows potential for future training attempts. For example, DeGutis, Bentin, Robertson, D’Esposito (2007) reported remarkable behavioural and neural changes in an adult with DP, MZ, whom they trained over a period of 14 months. MZ was asked to sort faces into two categories based on spacing among internal components. Training improved her ability to identify faces both on laboratory tests and in real life. At a neural level, MZ had post-training changes in activity and connectivity in the ventral occipital temporal cortex, and the N170 component measured by event-related potentials showed a selectivity to faces that was previously absent. Unfortunately, MZ’s face recognition abilities were not sustained without training, and her N170 was no longer face selective 90 days post-training. Perhaps the implementation of similar training initiatives at a young age, in children with DP, will lead to more positive long-term outcomes.
A recent investigation of FaceSay, a computer-based program developed to train face processing skills in autistic children (face recognition, emotion recognition, and gaze following; Hopkins et al., 2011) is an example of a new strategy that may be useful for training youngsters with DP. In this task, children are asked to identify missing face parts, match face emotions, and follow the gaze of an avatar across three different tasks, respectively. A child known as B, who has severe face recognition difficulties in laboratory testing and in every day life, showed a consistent improvement in scores on the 2-AFC CFMT for children after two months of training (Corrow, Chatterjee, Mathison, Nakayama, & Yonas, in prep). These improvements were maintained even two months post-training, suggesting that the effects of training may be more long-lasting in children than in adult populations (see Degutis, et al., 2007). Future work will examine how long the effects of training can be maintained in a child with DP. Although these findings provide a certain degree of optimism regarding the possibility of training face recognition in children with DP, a few limitations of the program should be mentioned. First, due to the limited availability of face recognition tests for children, the same assessment was used for both pre- and post-training, and thus practice effects could account for the improvement. However, B showed consistent performance for 4 pre-test measures followed by an improvement immediately post-training. Furthermore, his improvement was maintained (but his performance did not further improve) at 1 month and 2 months post-testing, suggesting that practice effects are unlikely.
Let’s Face It! is another training program that was initially designed for individuals with ASD, and has not yet been formally tested with children with DP (Tanaka et al., 2010). This computerized program consists of a set of 7 interactive games that target different aspects of face perception, such as recognition of identity across changes in expression, viewpoint and features, analytic and holistic face processing, and attention to information from the eye region of faces. After 20 hours of training, children with autism or Asperger’s performed better on a Parts/Whole Identity test. Greatest improvements were in the processing of parts, particularly mouths, but there were also significant improvements in holistic processing, specifically with improvements of recognition of eyes in the context of the whole face. Given the success of this program on children with autism and Asperger’s, and its child-friendly format, it could be a good option for training children with DP. However, in a personal communication (June, 2012) the parent of a child with suspected DP reported that her 7-year-old daughter informally tried the Let’s Face It! program and was easily able to succeed at the tasks by using extra-facial cues, like hair. Although this is only one individual, it highlights the need for condition-specific treatment strategies and suggests that modifications may be necessary before the Let’s Face It! program is used to train children with DP.
Our group has plans for systematic training of children with DP aimed at improving the ability to represent facial identities. Because it is not yet entirely clear what aspects of face processing are deficient in individuals with DP and whether this deficit is consistent across affected individuals, a general training that simply builds on the task of recognizing faces may be most effective for treating DP in individuals who may have differing underlying deficits. Our method will involve creating morph continuums from pairs of faces. Children will be presented with a target face chosen from one of the pairs, and three choices of faces chosen from the morph continuum. The task will be to select the face that most resembles the target face. Trials will be easy at first, with faces that are taken from the ends of the continuum. As the child gains proficiency with the task, we will increase task difficulty by choosing faces from more intermediate locations along the continuum. We hope this method will promote the use of normal face recognition by having children gradually learn to process differences in facial identity.
Summary
The research reported to-date on children with DP is sparse, and what is reported shows a lack of qualitative and quantitative consistency in behavioural measures. We have outlined four primary objectives that should be at the forefront of work done on childhood DP including increasing awareness of childhood DP, the development of diagnostic tools to aid in the identification of children with DP, the study of theoretically important issues related to DP, and the treatment of individuals with DP. While some early steps have been taken towards reaching these objectives, there are numerous avenues for future study that require immediate and careful attention.
Special considerations
Although awareness of DP is growing, it is still relatively unknown even to individuals involved in early childhood education. As a result, a common complaint among parents of children with DP is that no resources are available at school or elsewhere to help their child. Anecdotally, we have noted that some parents express concerns that their child has been misdiagnosed with another developmental disorder, such as autism spectrum disorder, which may present similar symptoms such as social avoidance. As a result, there is a need to identify tests that can reliably differentiate between individuals with DP and individuals with other developmental disorders. Early and accurate diagnosis of childhood developmental disorders is critical for identifying and recruiting the appropriate resources to help children with these disorders.
By some definitions, DP excludes children and adults with a diagnosis of ASD (e.g. Behrmann & Avidan, 2005; Duchaine, Murray, Turner, White, & Garrido, 2009). In other words, those with a diagnosis of ASD would not be classified as having DP even if they presented a face recognition deficit. However, due to the many similarities between these disorders, further examination of the distinctions between them is essential.
The DSM-IV criteria for the diagnosis of Asperger’s Syndrome (AS), which is part of the umbrella term of autism spectrum disorders (ASD) and likely the most common form of misdiagnosis given its similarity with DP, requires that the child show a specified number of symptoms in each of the two following categories: “(1) qualitative impairment in social interaction, and (2) restricted repetitive and stereotyped patterns of behavior, interests, and activities” (American Psychiatric Association, 2000). There are several common screening tools (e.g. CAST; Scott, Baron-Cohen, Bolton, & Brayne, 2002) and diagnostic tests (e.g. Autism Diagnostic Observation Schedule (ADOS); Lord, et al., 1999) that are used to identify these symptoms in children with potential AS. These measures detect impairments in social interaction, communication, as well as stereotyped behaviours and specialized interests.
Interestingly, many behaviours that are characteristic of individuals with DP appear similar to those that are symptomatic of ASD. For example, a child with DP might show limited eye contact due to a general lack of interest in non-informative faces or because he or she is trying to identify an individual based on extra-facial information, such as hairstyle or clothing. Furthermore, social isolation is common in DP, but may be mistakenly interpreted as disordered communication and social skills in the context of ASD.
In contrast, a child with DP would be unlikely to show symptoms in the second category of the ASD diagnostic criteria. However, because some screening tools and diagnostic tests are designed with low specificity, and high sensitivity, a child need not demonstrate symptoms in both categories in order to be classified as being on the autism spectrum. In other words, these kinds of screening tools and diagnostic tests could lead to a misdiagnosis of a child with DP as having an ASD based on superficial similarities in behaviours from the first diagnostic category of ASD. This is consistent with several personal communications from parents of children with DP who were initially classified as having autism or Asperger’s before receiving a diagnosis of DP. Furthermore, the child known as B who was mentioned above in the discussion of interventions, shows little evidence of ASD (e.g. intact emotion recognition, no evidence of stereotyped interests or behaviours, etc.) and yet was classified as being on the autistic spectrum when evaluated using the ADOS. Interestingly, the examiner (who was unaware of his face recognition deficit) noted that he did not demonstrate clear behaviours classified by category 2 (above) other than perhaps adult-like speech. This case provides a clear example of what might be expected in an ADOS evaluation of a child with DP.
The main concern with a misdiagnosis is that intervention programs designed for training the social skills of children with autism are likely inappropriately suited for a child with DP. Central to helping a child attain normal social interactions with others is an understanding of why social interactions are abnormal to begin with. An inability to recognize faces leading to a persistent lack of familiarity with others is a fundamentally different issue than a more broad based deficit in social functioning. That is not to say that it is misguided to facilitate the social development of children with DP. Rather, the cause of the issue, in this case a face recognition impairment, needs to be addressed first and foremost.
Conclusions
Much work has been done to study DP in adults, but there has been little research involving children with DP. Possible causes of DP include genetics, failures of innate mechanisms, abnormal qualitative or quantitative experience with faces, and neurological factors. Clearly, we need further exploration of all of these factors, along with the interactions among them to understand how DP arises. In addition to questions about the possible causes of DP, other important theoretical issues need to be examined, such as the existence and classification of different phenotypes, the neural correlates, the developmental trajectory, and the psychosocial consequences of DP.
Even though little work has been done to investigate DP systematically in children, the advances that have been made are promising. The two published reports of training programs for children with DP were successful to a degree since gains in face recognition with familiar faces were sustained. While these results are encouraging, results for at least one of these children did not generalize beyond the faces that were used for training. Preliminary results from work with the child known as B suggest that other methods may lead to more widespread effects. Of critical importance for future investigations is the development of additional strategies (possibly like those used with B) that result in a more generalizable gain in face recognition. Ultimately, interventions should be designed to improve face processing skills (e.g. global processing) rather than simply promoting face recognition strategies (e.g. memorizing the salient features of a single person). The former type of intervention may lead to the recruitment and restructuring of the perceptual system, perhaps leading to more long-lasting effects.
Before these strategies can be developed, there is need for increased awareness of DP across health care providers and educators alike and for the creation of diagnostic tools that will provide reliable pre- and post- training measure of face processing. Such measures may also provide a means to test some of the theoretical questions regarding normal and abnormal face processing that arise from this review. It is our hope that there will be a strong and concerted effort to begin addressing them soon.
Acknowledgments
KAD was supported by an Economic and Social Research Council (UK) grant to BD. SC was supported by the Eva O. Miller Fellowship through the graduate school of the University of Minnesota and a pre-doctoral training grant provided by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5T32HD007151 from the NICHD. We would like to thank Madison and her mother for their correspondence and candid accounts of Madison’s experiences. Thank you to Joe DeGutis and Roger Mercado for providing detailed information about their experiences with TM.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Anastasi J, Rhodes M. Evidence for an own-age bias in face recognition. North American Journal of Psychology. 2006;8:237–253. [Google Scholar]
- Ariel R, Sadeh M. Congenital visual agnosia and prosopagnosia in a child. Cortex. 1996;12:76–82. doi: 10.1016/s0010-9452(96)80048-7. [DOI] [PubMed] [Google Scholar]
- Avidan G, Behrmann M. Implicit familiarity processing in congenital prosopagnosia. Journal of Neuropsychology. 2008;2:141–164. doi: 10.1348/174866407x260180. [DOI] [PubMed] [Google Scholar]
- Avidan G, Hasson U, Malach R, Berhmann M. Detail exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. Journal of Cognitive Neuroscience. 2005;17(7):1150–1176. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Avidan G, Tanzer M, Behrmann M. Impaired holistic processing in congenital prosopagnosia. Neuropsychologia. 2011;49:2541–2552. doi: 10.1016/j.neuropsychologia.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The Autism-Spectrum Quotient (AQ): Evidence from Asperget Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Barton J, Cherkasova M, O’Connor M. Covert recognition in acquired and developmental prosopagnosia. Neurology. 2001;57(7):1161–1168. doi: 10.1212/wnl.57.7.1161. [DOI] [PubMed] [Google Scholar]
- Bate S, Haslam C, Tree J, Hodgson T. Evidence of an eye movement-based memory effect in congenital prosopagnosia. Cortex. 2008;44:806–819. doi: 10.1016/j.cortex.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Bayley N. Consistency and variability in the growth of intelligence from birth to eighteen years. Journal of Genetic Psychology. 1949;75:165–196. doi: 10.1080/08856559.1949.10533516. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G. Congenital prosopagnosia: Face-blind from birth. Trends in Cogntive Sciences. 2005;9(4):180–187. doi: 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alteration in inferotemporal cortex in congenital prosopagnosia. Cerebral Cortex. 2007;17:2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Marotta J, Kimchi R. Detailed exploration of face-related processing in congenital prosopagnosia 1. Behavioral findings. Journal of Cognitive Neuroscience. 2005;17:1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- Bentin S, DeGutis J, D’Esposito M, Robertson L. Too many trees to see the forest: Performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative cogenital prosopagnosia. Journal of Cognitive Neuroscience. 2007;19(1):132–146. doi: 10.1162/jocn.2007.19.1.132. [DOI] [PubMed] [Google Scholar]
- Bentin S, Deouell L, Soroker N. Selective visual streaming in face recogntiion: evidence from developmental prosopagnosia. NeuroReport. 1999;10:823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- Benton A. Facial recognition. Cortex. 1990;26:491–499. doi: 10.1016/s0010-9452(13)80299-7. [DOI] [PubMed] [Google Scholar]
- Bowles D, McKone E, Dawel A, Duchaine B, Palermo R, Schmalzl L, et al. Diagnosing prosopagnosia: Effects of ageing, sex, and participant-stimulus ethnic match on the Cambridge Face Memory Test and the Cambridge Face Perception Test. Cognitive Neuropsychology. 2009;26:423–455. doi: 10.1080/02643290903343149. [DOI] [PubMed] [Google Scholar]
- Brooks J, Lewis M. Infants’ responses to strangers: Midget, adult, and child. Child Development. 1976;47:323–332. [PubMed] [Google Scholar]
- Bruce V. Recognizing faces. Hove: Lawrence Erlbaum Associates Ltd; 1988. [Google Scholar]
- Bruce V, Campbell R, Doherty-Sneddon G, Import A, Langton S, McAuley S, et al. Testing face processing skills in children. British Journal of Development Psychology. 2000;18:319–333. [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Brunet P, Mondloch C, Schmidt L. Shy children are less sensitive to some cues of facial recognition. Child Psychiatry and Human Development. 2010;41:1–14. doi: 10.1007/s10578-009-0150-0. [DOI] [PubMed] [Google Scholar]
- Brunsdon R, Coltheart M, Nickels L, Joy P. Developmental prosopagnosia. A case analysis and treatment study. Cognitive Neuropsychology. 2006;23(6):822–840. doi: 10.1080/02643290500441841. [DOI] [PubMed] [Google Scholar]
- Dalrymple K, Oruc I, Duchaine B, Pancaroglu R, Fox C, Iaria G, et al. The anatomic basis of the right face-selective N170 in acquired prosopagnosia: A combined ERP/fMRI study. Neuropsychologia. 2011;49:2553–2563. doi: 10.1016/j.neuropsychologia.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- de Haan E. A familial factor in the development of face recognition deficits. Journal of Clinical and Experimental Neuropsychology. 1999;21:312–315. doi: 10.1076/jcen.21.3.312.917. [DOI] [PubMed] [Google Scholar]
- de Haan EH, Campbell R. A fifteen year follow-up of a case of developmental prosopagnosia. Cortex. 1991;27:489–509. doi: 10.1016/s0010-9452(13)80001-9. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P, Grossi D, Nichelli P. Apperceptive and associative forms of prosopagnosia. Cortex. 1991;27:213–221. doi: 10.1016/s0010-9452(13)80125-6. [DOI] [PubMed] [Google Scholar]
- Degutis J, Bentin S, Robertson L, D’Esposito Functional plasticity in ventral temporal cortex following cognitive rehabilitation of a congenital prosopagnosic. Journal of Cognitive Neuroscience. 2007;19(11):1790–1802. doi: 10.1162/jocn.2007.19.11.1790. [DOI] [PubMed] [Google Scholar]
- Diaz A. Do I know you? A case study of prosopagnosia (face blindness) The Journal of School Nursing. 2008;24(5):284–289. doi: 10.1177/1059840508322381. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Germine L, Nakayama K. Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cognitive Neuropsychology. 2007;24:419–430. doi: 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Murray H, Turner M, White S, Garrido L. Normal social cognition in developmental prosopagnosia. Cognitive Neuropsychology. 2009;26(7):620–634. doi: 10.1080/02643291003616145. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. Dissociations of face and object recognition in developmental prosopagnosia. Journal of Cognitive Neuroscience. 2005;17(2):249–261. doi: 10.1162/0898929053124857. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results from neurologically intact individuals and an investigation of its validity using inverted stimuli and prosopagnosic participants. Neuropsychologia. 2006a;44(4):576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. Developmental prosopagnosia: A window to content-specific face processing. Current Opinion in Neurobiology. 2006b;16:166–173. doi: 10.1016/j.conb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nieminen-von Wendt T, New J, Kulomaki T. Dissociations of visual recognition in a developmental agnosic: Evidence for separate developmental processes. Neurocase. 2003;9:380–389. doi: 10.1076/neur.9.5.380.16556. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Parker H, Nakayama K. Normal recognition of emotion in a prosopagnosic. Perception. 2003;32:827–838. doi: 10.1068/p5067. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Yovel G, Butterworth E, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cognitive Neuropsychology. 2006;23(5):714–747. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- Easterbrook M, Kisilevsky S, Hains S, Muir D. Faceness or complexity: Evidence from newborn visual tracking of facelike stimuli. Infant Behavior and Development. 1999;22(1):17–35. [Google Scholar]
- Ebner N, Johnson M. Younger and older emotional faces: Are there group differences in expression identification and memory? Emotion. 2009;9:329–339. doi: 10.1037/a0015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Gosling A, Duchaine B. Electrophysiological markers of covert face recognition in developmental prosopagnosia. Brain. 2012;135:542–554. doi: 10.1093/brain/awr347. [DOI] [PubMed] [Google Scholar]
- Elgar K, Campbell R. The development of face-identification skills: What lies behind the face module? Infant and Child Development. 2001;10(1–2):25–30. [Google Scholar]
- Ellis A, Young A. Training in face-processing skills for a child with acquired prosopagnosia. Developmental Neuropsychology. 1988;4(4):283–294. [Google Scholar]
- Fantz R. Pattern vision in newborn infants. Science. 1963;140:296–297. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson M. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Steele M, Steele H, Higgitt A, Target M. The Emanuel Miller Memorial Lecture 1992: The theory and practice of resilience. Journal of Child Psychology and Psychiatry. 1994;35(2):231–257. doi: 10.1111/j.1469-7610.1994.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Furl N, Garrido L, Dolan R, Driver J, Duchaine B. Fusiform gyrus face selectivity reflects facial recognition ability. Journal of Cognitive Neuroscience. 2011;23(7):1723–1740. doi: 10.1162/jocn.2010.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A, Kemper T. Cytoarchitectomic abnormalities in developmental dyslexia: A case study. Annals of Neurology. 1979;6:94–100. doi: 10.1002/ana.410060203. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sherman G, Rosen G, Aboitiz F, Geschwind N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Annals of Neurology. 1985;18(2):222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Galton F. Composite portraits, made by combining those of many different persons into a single, resultant figure. Journal of the Anthropological Institute. 1879;8:132–144. [Google Scholar]
- Garrido L, Duchaine B, Nakayama K. Face detection in normal and prosopagnosic individuals. Journal of Neuropsychology. 2008;47:123–131. doi: 10.1348/174866407x246843. [DOI] [PubMed] [Google Scholar]
- Garrido L, Furl N, Draganski B, Weiskopf N, Stevens J, Tan G, et al. Voxel-based morphometry reveals reduced grey matter volume in the temporal cortex of developmental prosopagnosics. Brain. 2009;132:3443–3455. doi: 10.1093/brain/awp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Perry A, Bonneh Y, Malach R, Bentin S. Seeing with profoundly deactivated mid-level visual areas: Non-hierarchical functioning in the human visual cortex. Cerebral Cortex. 2009;19:1687–1703. doi: 10.1093/cercor/bhn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J, Berman P. Reason for hope: A spiritual journey. New York: Warner Books, Inc; 2000. [Google Scholar]
- Goren C, Sarty M, Wu P. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:545–549. [PubMed] [Google Scholar]
- Harris A, Duchaine B, Nakayama K. Normal and abnormal face selectivity of the M170 response in developmental prosopagnosics. Neuropsychologia. 2005;43:2125–2136. doi: 10.1016/j.neuropsychologia.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Haxby J, Hoffman E, Gobbini M. The distributed human neural system for face perception. Trends in Cogntive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- He Y, Ebner N, Johnson M. What predicts the own-age bias in face recognition memory? Social Cognition. 2011;29:97–109. doi: 10.1521/soco.2011.29.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins I, Gower M, Perez T, Smith D, Amthor F, Wimsatt F, et al. Avatar assistant: Improving social skills in students with ASD through a computer-based intervention. Journal of Autism and Developmental Disorders. 2011;41:1543–1555. doi: 10.1007/s10803-011-1179-z. [DOI] [PubMed] [Google Scholar]
- Humphreys P, Kaufmann W, Galaburda A. Developmental dyslexia in woman: Neuropathological findings in three patients. Annals of Neurology. 1990;28(6):727–738. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- Johnson M. Sub-cortical face processing. Nature Reviews Neuroscience. 2005;7:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Jones RD, Tranel D. Severe developmental prosopagnosia in a child with superior intellect. Journal of Clinical and Experimental Neuropsychology. 2001;23(3):265–273. doi: 10.1076/jcen.23.3.265.1183. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The Fusiform Face Area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Assessment Battery for Children. Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- Kennerknecht I, Grueter T, Welling B, Wentzek S, Horst J, Ewards S, et al. First report of prevalence of non-syndromic heredity prosopagnosia (HPA) American Journal of Medical Genetics, Part A. 2006;140A(15):1617–1622. doi: 10.1002/ajmg.a.31343. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Fellman V. NEPSY: A developmental neuropsychological assessment. San Antonio, Texas: The Psychological Corporation; 1998. [Google Scholar]
- Kress T, Daum I. Event-related potentials reflect impaired face recognition in patients with congenital prosopagnosia. Neuroscience Letters. 2003;352(2):133–136. doi: 10.1016/j.neulet.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Landis T, Cummings J, Christen L, Bogen J. Are unilateral right posterior cerebral lesions sufficient to cuase prosopagnosia? Clinical and radiological findings in six addtional patients. Cortex. 1986;22(2):243–252. doi: 10.1016/s0010-9452(86)80048-x. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch C, Maurer D, Brent H. Early visual experience and face processing. Nature. 2001;410:890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch C, Maurer D, Brent H. Expert face processing requires visual input to the right hemisphere during infancy. Nature Neuroscience. 2003;6(10):1108–1112. doi: 10.1038/nn1121. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch C, Maurer D, Brent H. Impairment in holistic face processing following early visual deprivation. Psychological Science. 2004;15(11):762–768. doi: 10.1111/j.0956-7976.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Duchaine B, Nakayama K, Wilson H. Three cases of developmental prosopagnosia from one family: Detailed neuropsychological and psychophysical investigation of face processing. Cortex. 2010;46:949–964. doi: 10.1016/j.cortex.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Lewis T, Maurer D. The development of temporal and nasal fields during infancy. Vision Research. 1992;32:903–911. doi: 10.1016/0042-6989(92)90033-f. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Bentejac L, de Schonen S. When does inter-hemispheric integration of visual events emerge in infancy? A developmental study on 19- to 28-month-old infants. Neuropsychologia. 2000;38:1382–1389. doi: 10.1016/s0028-3932(00)00041-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule- WPS (ADOS-WPS) Los Angeles, CA: Wester Psychological Sciences; 1999. [Google Scholar]
- Macchi Cassia V. Age biases in face perception: The effects of experience across development. British Journal of Psychology. 2011;102:816–829. doi: 10.1111/j.2044-8295.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- Macchi Cassia V, Simion F, Umilta C. Face preference at birth: The role of an orienting mechanism. Developmental Science. 2001;4:101–108. [Google Scholar]
- Maurer D, Young R. Newborn’s following of natural and distorted arrangements of facial features. Infant Behavior and Development. 1983;6:127–131. [Google Scholar]
- McCarthy G, Puce A, Gore J. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9(5):2188–2199. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- McConachie H. Develpmental prosopagnosia. A single case report. Cortex. 1976;12:76–82. doi: 10.1016/s0010-9452(76)80033-0. [DOI] [PubMed] [Google Scholar]
- Mondloch C, Lewis T, Budreau D, Maurer D, Dannemillier J, Stephens B, et al. Face perception during early infancy. Psychological Science. 1999;10(5):419–422. [Google Scholar]
- Mondloch C, Maurer D, Ahola S. Becoming a face expert. Psychological Science. 2006;17:930–934. doi: 10.1111/j.1467-9280.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson M. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Nelson C. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Nelson C. A neurobiological perspective on early human deprivation. Child Development Perspectives. 2007;1:13–18. [Google Scholar]
- Norman W. Toward an adquate taxonomy of personality attributes: Replicated factor structure in peer nomination personality ratings. Journal of Abnormal and Social Psychology. 1963;66:574–583. doi: 10.1037/h0040291. [DOI] [PubMed] [Google Scholar]
- Nunn J, Postma P, Pearson R. Developmental prosopagnosia: Should it be taken at face value? Neurocase. 2001;7(1):15–27. doi: 10.1093/neucas/7.1.15. [DOI] [PubMed] [Google Scholar]
- Ostrovsky Y, Andalman A, Sinha P. Vision following extended congenital blindness. Psychological Science. 2006;17(12):1009–1014. doi: 10.1111/j.1467-9280.2006.01827.x. [DOI] [PubMed] [Google Scholar]
- Palermo R, Willis M, Rivolta D, McKone E, Wilson C, Calder A. Impaired holistic coding of facial expression and facial identity in congenital prosopagnosia. Neuropsychologia. 2011;49:1226–1235. doi: 10.1016/j.neuropsychologia.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Martin de Vivies X, Anzures G, Quinn P, Slater A, Tanaka J, et al. Development of face processing. WIREs Cognitive Science. 2011;2:666–675. doi: 10.1002/wcs.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect T, Moon H. The own-age effect in face recognition. In: Duncan J, McLeod P, Phillips L, editors. Speed, control and age: In honour of Patrick Rabbitt. Oxford: Oxford University Press; 2005. pp. 317–340. [Google Scholar]
- Pollak S, Nelson C, Schlaak M, Roeber B, Wewerka S, Wiik K, et al. Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Development. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F. Neurobiology of dyslexia: A reinterpretation of the data. Trends in Neuroscience. 2004;27(12):720–726. doi: 10.1016/j.tins.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Robbins R, Nishimura M, Mondloch C, Lewis T, Maurer D. Deficits in sensitivity to spacing after early visual deprivation in humans: A comparison of human faces, monkey faces, and houses. Developmental Psychobiology. 2010;52:775–781. doi: 10.1002/dev.20473. [DOI] [PubMed] [Google Scholar]
- Sacks O. The Mind’s Eye. New York: Vintage Books; 2010. [Google Scholar]
- Schmalzl L, Palermo R, Coltheart M. Cognitive heterogeneity in genetically based prosopagnosia: A family study. Journal of Neuropsychology. 2008;2(1):99–117. doi: 10.1348/174866407x256554. [DOI] [PubMed] [Google Scholar]
- Schmalzl L, Palermo R, Green M, Brunsdon R, Coltheart M. Training of familiar face recognition and visual scan paths for faces in a child with congenital prosopagnosia. Cognitive Neuropsychology. 2008;25(5):704–729. doi: 10.1080/02643290802299350. [DOI] [PubMed] [Google Scholar]
- Scott F, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): Preliminary development of a UK screen for mainstream primary-school age children. Autism. 2002;6:9–31. doi: 10.1177/1362361302006001003. [DOI] [PubMed] [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no exposure to faces. Proceedings of the National Academy of Sciences. 2008;105(1):394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Farah M. Parts and wholes in face recognition. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1993;46A(2):225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Wolf J, Klaiman C, Koenig K, Cockburn J, Herlihy L, et al. Using computerized games to teach face recognition skills to children with autism spectrum disorder: The Let’s Face It! program. The Journal of Child Psychology and Psychiatry. 2010;51(8):944–952. doi: 10.1111/j.1469-7610.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung K, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nature Neuroscience. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer J, Germine L, Chabris C, Chatterjee G, Williams M, Loken E, et al. Human face recognition ability is specific and highly heritable. Proceedings of the National Academy of Sciences. 2010;107(11):5238–5241. doi: 10.1073/pnas.0913053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CE, Palermo R, Schmalzl L, Brock J. Specificity of impaired facial identity recognition in children with syspected developmental prosopagnosia. Cognitive Neuropsychology. 2010;27(1):30–45. doi: 10.1080/02643294.2010.490207. [DOI] [PubMed] [Google Scholar]
- Wright D, Stroud J. Age difference in lineup identification accuracy: People are better with their own age. Law and Human Behavior. 2002;25:641–654. doi: 10.1023/a:1020981501383. [DOI] [PubMed] [Google Scholar]
- Yardley L, McDermott L, Pisarski S, Duchaine B, Nakayama K. Psychosocial consequences of developmental prosopagnosia: A problem of recognition. Journal of Psychosomatic Research. 2008;65:445–451. doi: 10.1016/j.jpsychores.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernber N, Boyle C, Murphey C. Prevalence of autism in a US metropolitan area. Journal of the American Medical Association. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Young A, Hellawell D, de Haan EH. Cross-domain semantic priming in normal subjects and a prosopagnosic patient. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1988;40(3):561–580. doi: 10.1080/02724988843000087. [DOI] [PubMed] [Google Scholar]